Abstract
We report an approach to the design of reactive polymer films that can be functionalized post-fabrication to either prevent or promote the attachment and growth of cells. Our approach is based on the reactive layer-by-layer assembly of covalently crosslinked thin films using a synthetic polyamine and a polymer containing reactive azlactone functionality. Our results demonstrate (i) that the residual azlactone functionality in these films can be exploited to immobilize amine-functionalized chemical motifs similar to those that promote or prevent cell and protein adhesion when assembled as self-assembled monolayers on gold-coated surfaces, and (ii) that the immobilization of these motifs changes significantly the behaviors and interactions of cells with the surfaces of these polymer films. We demonstrate that films treated with the hydrophobic molecule decylamine support the attachment and growth of mammalian cells in vitro. In contrast, films treated with the hydrophilic carbohydrate D-glucamine prevent cell adhesion and growth almost completely. The results of additional experiments suggest that these large differences in cell behavior can be understood, at least in part, in terms of differences in the abilities of these two different chemical motifs to promote or prevent the adsorption of protein onto film coated surfaces. We demonstrate further that this approach can be used to pattern regions of these reactive films that resist the initial attachment and subsequent invasion of mammalian cells for periods of at least one month in the presence of serum-containing cell culture media. Finally, we report that films that prevent the adhesion and growth of mammalian cells also prevent the initial formation of bacterial biofilms when incubated in the presence of the clinically relevant pathogen Pseudomonas aeruginosa. The results of these studies, collectively, suggest the basis of general approaches to the fabrication and functionalization of thin films that prevent, promote, or pattern cell growth or the formation of biofilms on surfaces of interest in the contexts of both fundamental biological studies and a broad range of other practical applications.
Introduction
The ability to prevent or promote the attachment of cells and proteins on surfaces is critical in a wide range of biotechnological applications. For example, the unwanted adsorption of proteins or the growth of cells on the surfaces of implantable devices can lead to biofouling and compromise device function.1–4 Alternatively, surfaces that promote adsorption and adhesion in spatially defined locations are useful for the investigation of fundamental aspects of cell growth and communication5–8 and the development of surface-based formats for biochemical and drug-screening assays.9–11 Although significant advances have been made toward the design of surfaces that promote or prevent cell attachment and dictate cell behavior, there remains a general need for new materials-based platforms that are (i) chemically robust and stable in physiologically relevant environments, (ii) compatible with the introduction of a broad range of chemical functionality, and (iii) useful for the functionalization of surfaces in ways that are largely independent of the chemical or physical properties of the substrates on which they are deposited (that is, approaches that are substrate-independent). In this paper, we report a step toward these broad goals through the fabrication of covalently crosslinked and chemically reactive ultrathin films that can be modified readily to promote and/or prevent the adsorption of proteins and the attachment of cells. Our approach is based on methods for the reactive layer-by-layer fabrication of thin polymer films and exploits the versatility and robust reactivity of azlactone-functionalized polymers to fabricate surface coatings that can be functionalized and patterned post-fabrication to present a broad range of chemical or biological functionality.
The work reported here builds upon numerous past reports describing the design of materials that promote or prevent the adsorption or adhesion of proteins, mammalian cells, and/or bacterial cells on surfaces.12–16 Self-assembled monolayers (SAMs) of alkanethiols on gold-coated surfaces present well-established methods for the modification of surfaces with chemical functionality that can be used to probe the interactions of proteins and cells with surfaces.13,15,17,18 For example, SAMs displaying peptide sequences known to promote cell attachment (e.g., the RGD sequence) promote the attachment and growth of cells,13,19 whereas SAMs presenting oligo(ethylene glycol) functionality18,20,21 (or other hydroxyl-presenting groups)22,23 generally resist protein adsorption and cell adhesion. SAMs thus provide a useful tool for the in vitro investigation of fundamental cellular behaviors, such as the response of cells to the presentation of various chemical motifs and the attachment, growth, migration, and differentiation of cells on chemically patterned surfaces.13 However, concerns related to the long-term stability and biocompatibility of SAMs raise general questions about the suitability of these materials for use in vivo.18,24 Other alternative approaches include (i) the design of polymers and macromolecular assemblies with structures or functionalities that can promote or prevent cell adhesion when spread, assembled, or anchored onto surfaces,12,14–16,25 and (ii) the design of reactive polymers and reactive surfaces that can be further modified to present functionality that modulates protein or cell behavior.26–31 This latter approach presents several potential practical advantages, as it avoids the need to synthesize individually many different functionalized polymers and it permits facile and modular post-fabrication modification or patterning of surfaces with a range of different functionality.
The approach reported here makes use of methods developed for the layer-by-layer fabrication of thin polymer films on surfaces.32 Layer-by-layer assembly is a particularly useful method for fabricating surface coatings because it offers precise control over the thicknesses and compositions of multicomponent polymer films, and it can be used to deposit conformal coatings on the surfaces of topographically or topologically complex substrates.25,33–35 The majority of work in this area has focused, in large measure, on the design and characterization of films fabricated using positively or negatively charged water-soluble polymers (i.e., polyelectrolytes).25,33–35 These methods are typically aqueous, and have been used to fabricate thin films (or ‘polyelectrolyte multilayers’) of interest in a broad range of applications.25,33–35 Of particular relevance to the work reported here, several groups have demonstrated that polyelectrolyte multilayers can be designed to either promote or prevent the adsorption or adhesion of cells and proteins (for example, by tuning film stiffness or swellability).36–40
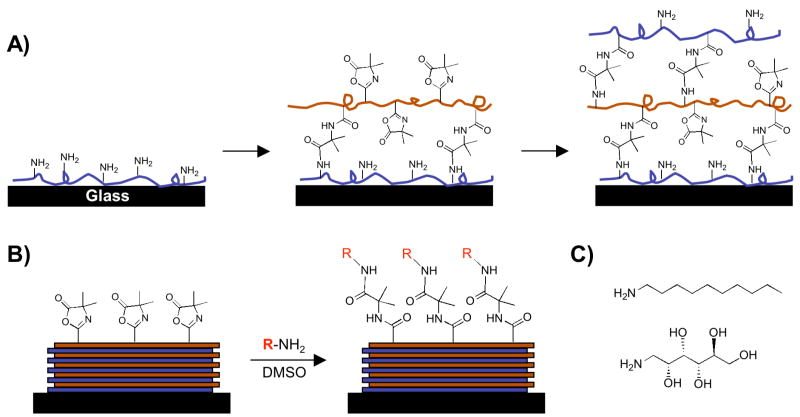
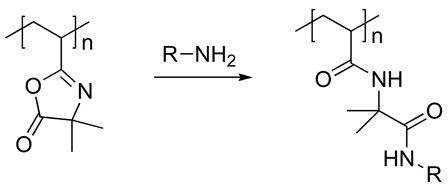
We recently reported a layer-by-layer approach to the fabrication of covalently crosslinked and chemically reactive thin films that has many of the same practical advantages of traditional, aqueous-based methods for the assembly of polyelectrolytes, but that can be performed entirely in organic solvents.41 The extension of layer-by-layer methods to the use of organic solvents broadens considerably the range of different polymer structures that can be used to include those that are either insoluble in water or that contain functionality that would otherwise react readily with water. Our past work demonstrates that reactive layer-by-layer assembly42 can be used to fabricate crosslinked thin films using the chemically reactive polymer poly(2-vinyl-4,4′-dimethylazlactone) (PVDMA).41 The azlactone functionality of PVDMA reacts with primary amines by a rapid, ‘click’-type reaction (Eq 1),43 and, thus, provides a basis for reactive layer-by-layer assembly with polymers containing primary amine groups. Figure 1A demonstrates schematically the basis of this approach.
Figure 1.
Schematic illustration showing the layer-by-layer assembly (A) and the subsequent chemical functionalization (B) of the reactive, covalently-crosslinked, ultrathin polymer films used in this study. (A) Branched poly(ethylene imine) (PEI) is first adsorbed onto a glass substrate followed by treatment with a solution of an azlactone-containing polymer (PVDMA). Repetition of this process results in the layer-by-layer build-up of covalently crosslinked multilayers containing residual azlactone functionality. (B) A broad range of surface functionality can be accessed via post-fabrication modification of residual azlactone functionality by exposure of the films to primary amine-containing nucleophiles. (C) Chemical structures of the amine-functionalized small molecules decylamine (top) and D-glucamine (bottom) used to modify the surfaces of azlactone-containing films in this study.
 |
(1) |
In contrast to the conventional, aqueous layer-by-layer assembly of polyelectrolyte-based films, which is driven by electrostatic interactions between oppositely charged polymers, film growth in the system reported here is mediated by fast, interfacial chemical reactions that occur between the primary amines of the polyamine and the pendant azlactone functionality of PVDMA to generate covalent diamide crosslinks between the polymers.41 If this process occurs in a manner that leaves some fraction of azlactone unreacted, an additional layer of polyamine can be deposited and reacted at the surface (Figure 1A). Iterative repetition of this procedure yields thin films composed of covalently crosslinked polyamine and PVDMA with thicknesses and internal compositions that can be tuned by control over the number of layers added or the structures of the polymers that are used. We recently demonstrated the general feasibility of this approach using films fabricated from PVDMA and branched poly(ethylene imine) (PEI), a synthetic polymer containing primary, secondary, and tertiary amine functionality.41 This past work also demonstrated that the residual azlactone functionality that remains in these films can be exploited to functionalize the films post-fabrication (Figure 1B) simply by exposure of the films to amine-functionalized fluorophores in solution, or by using conventional soft lithography techniques.41
This current study sought to determine whether the reactivity of the azlactone-functionalized films described above could be exploited to design ultrathin surface coatings that promote and/or prevent the adhesion and growth of cells. We demonstrate here that it is possible to functionalize these polymer films post-fabrication to modulate the behaviors of cells by reaction with amine-functionalized chemical motifs demonstrated to promote or prevent cell adhesion when assembled as SAMs on gold-coated surfaces. For example, films functionalized using the carbohydrate D-glucamine resist the attachment and/or invasion of mammalian cells in treated areas for time periods exceeding one month in the presence of serum in vitro. In contrast, unfunctionalized films and films functionalized using hydrophobic amines support cell attachment, spreading, and growth. We demonstrate further that these large differences in cell behavior can be understood in terms of differences in the abilities of these different chemical motifs to either promote or prevent the adsorption of proteins, opening new possibilities for the patterning of both protein adsorption and cell adhesion using these materials. Finally, we demonstrate that films designed to prevent the attachment of mammalian cells can also be used to prevent or reduce substantially the growth of bacterial biofilms of the clinically relevant pathogen Pseudomonas aeruginosa.
Materials and Methods
Materials
Poly(2-vinyl-4,4′-dimethylazlactone) (PVDMA, Mn ~50,000, PDI = 4.3) was a kind gift from Dr. Steven M. Heilmann (3M Corporation, Minneapolis, MN). Branched poly(ethylene imine) (PEI; MW = 25,000), reagent grade DMSO, and acetone were purchased from Aldrich Chemical Company (Milwaukee, WI). Glass microscope slides were purchased from Fischer Scientific (Pittsburgh, PA). Decylamine and D-glucamine were purchased from TCI America (Portland, OR). Dulbecco’s modified Eagle Medium (DMEM), Opti-MEM I reduced serum medium, Calcein AM fluorescent cell stain, and FITC-labelled bovine serum albumin were purchased from Invitrogen (Carlsbad, CA). African green monkey kidney fibroblasts (COS-7 cells) and murine fibroblasts (NIH/3T3 cells) were obtained from ATCC (Manassas, VA). Tween 20 surfactant was purchased from Fischer Scientific (Pittsburgh, PA). All materials were used as received without further purification unless noted otherwise. Compressed air used to dry films and coated substrates was filtered through a 0.4 μm membrane syringe filter.
General Considerations
Silicon (10 mm × 50 mm) and glass (10 mm × 30 mm) substrates were cleaned with acetone, ethanol, methanol, and deionized water and dried under a stream of compressed air prior to the fabrication of multilayered films. Silicon substrates used for reflective infrared (IR) spectroscopy experiments were prepared by depositing thin layers of titanium (10 nm) and gold (200 nm) sequentially onto clean silicon wafers using an electron-beam evaporator (Tek-Vac Industries, Brentwood, NY). The optical thicknesses of films deposited on silicon were determined using a Gaertner LSE ellipsometer (632.8 nm, incident angle = 70°). Data were processed using the Gaertner Ellipsometer Measurement Program. Relative thicknesses were calculated assuming an average refractive index of 1.58 for the thin films. Thicknesses were determined in at least five different standardized locations on each substrate. Static contact angle measurements were made using a Dataphysics OCA 15 Plus instrument. Polarization–modulation infrared reflectance–absorbance spectroscopy (PM–IRRAS) was conducted in analogy to previously reported methods.44,45 Optical and fluorescence microscopy images were acquired using an Olympus IX70 microscope and analyzed using the Metavue version 4.6 software package (Universal Imaging Corporation).
Layer-by-Layer Fabrication of Films
Solutions of PEI or PVDMA were prepared in acetone (20 mM with respect to the molecular weight of the polymer repeat unit). Films were deposited layer-by-layer on silicon or glass substrates manually according to the following general protocol: 1) Substrates were submerged in a solution of PEI for 30 seconds, 2) substrates were removed and immersed in an initial acetone bath for 30 seconds followed by a second acetone bath for 30 seconds, 3) substrates were submerged in a solution of PVDMA for 30 seconds, and 4) substrates were rinsed in the manner described above. This cycle was repeated until the desired number of PEI/PVDMA layers was reached. Films were either characterized or used in subsequent experiments immediately or were dried under a stream of filtered, compressed air and stored in a vacuum desiccator until use. All films were fabricated at ambient room temperature.
Post-Fabrication Functionalization of Thin Films
PEI/PVDMA films were functionalized post-fabrication by immersing film-coated substrates in solutions of a designated amine-functionalized nucleophile (e.g., decylamine or glucamine, 50 mM in DMSO) at room temperature for ~16 hours. Films were soaked in DMSO for ~1 hour after functionalization; the DMSO was exchanged at least once during the rinse. Films were stored in a vacuum desiccator after fabrication and functionalization until further use. PEI/PVDMA films patterned with small, circular spots of glucamine were treated with a drop (1 μL) of a glucamine solution (20 mg/mL in DMSO) for two hours. Films were soaked in DMSO for one hour, rinsed with EtOH, and dried under air. Films functionalized with glucamine spots were then either placed in the desiccator for later use or immersed in a solution of decylamine (50 mM in DMSO) to functionalize the azlactone groups remaining after treatment with glucamine. All modified films were rinsed with EtOH following the surface reaction and dried with compressed air.
Characterization of Adhesion and Growth of Mammalian Cells on PEI/PVDMA Films
Experiments designed to investigate the attachment and proliferation of mammalian cells on unmodified and modified PEI/PVDMA films were performed using films fabricated on transparent glass substrates. All films were sterilized prior to seeding cells by rinsing liberally with EtOH (~10 mL), followed by drying with compressed air. Treated and untreated PEI/PVDMA films were placed individually into the wells of tissue culture-treated polystyrene culture plates. For experiments using COS-7 cells, cells were seeded on films at initial densities of 50,000 or 75,000 cells/mL in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. For experiments using 3T3 fibroblasts, cells were seeded on films at an initial density of 110,000 cells/mL in DMEM containing 10% (v/v) calf bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. For both cell types, cells were seeded directly on the films in ~0.2 mL of DMEM or Opti-MEM and allowed to adsorb to the surface for 20 minutes at room temperature, after which an additional 2–3 mL of the appropriate growth medium was added. Cells were incubated with substrates for at least 24 hours at 37 °C to allow for cell attachment and growth. At specified times (e.g., after 24 or 48 hours), cells were stained with 2 mL of a Calcein AM staining solution (1 μg/mL in PBS) for 30 minutes at 37 °C. Following incubation, the staining solution was aspirated and replaced with 2 mL of DMEM. Cells were imaged by optical light microscopy and fluorescence microscopy without removal of the glass substrates from the culture wells.
For experiments designed to evaluate cell adhesion, growth, and migration over longer time periods (see text), PEI/PVDMA films functionalized with small, circular spots of glucamine (prepared as described above) were placed in cell culture wells and seeded with COS-7 cells at 50,000 cells/mL. Cells were imaged periodically using optical microscopy every 48–72 hours. Cells were also stained with Calcein AM every three or four days and imaged using fluorescence microscopy. For these longer-term experiments, cell culture media was removed and exchanged with fresh media every 48 hours for the first three weeks and every 12–24 hours thereafter.
Experiments to determine the extents to which films could be re-used after the detachment of cells by trypsin (see text) were performed using films fabricated onto aminosilane-treated glass to stabilize the films during repeated drying and rehydrating steps associated with the trypsinization procedure. Silanization was performed by immersing the glass substrates in a 1% (v/v) solution of 3-aminopropyltriethoxysilane in EtOH for 30 minutes. The substrates were then rinsed with EtOH (~10 mL) and dried prior to film fabrication. For these experiments, films were fabricated by depositing a first layer of PVDMA, followed by iterative deposition of layers of PEI and PVDMA (as described above) to produce films of desired thicknesses with a terminal layer of PVDMA. These films were then functionalized with small, circular spots of glucamine using methods described above, placed in the wells of 4-well chamber slides, and seeded with COS-7 cells at 75,000 cells/mL. After three days of incubation, cells were stained with Calcein AM and imaged by fluorescence microscopy. After imaging, the substrates were rinsed with 0.5 mL PBS, incubated twice with 0.5 mL of 0.25% trypsin-EDTA at 37 °C for 10 minutes, and rinsed a final time with PBS. The substrates were imaged using optical microscopy to verify the detachment of cells, and then re-seeded with COS-7 cells at 75,000 cells/mL and incubated for three days prior to repeating staining and imaging procedures. This process was repeated iteratively for at least six seeding/trypsinization/re-seeding cycles.
Characterization of Protein Adsorption on PEI/PVDMA Films
PEI/PVDMA films were fabricated on silicon substrates and modified with either decylamine or glucamine using procedures outlined above. A drop (1 μL) of an aqueous solution of FITC-labelled bovine serum albumin (10 μM, PBS buffer, pH 7.4) was spotted onto modified and unmodified films and allowed to sit for 1.75 hours. Films were placed in a humidified environment (i.e., films were placed in a petri dish with a water-saturated strip of filter paper, covered, and placed on a bench at room temperature) during this period to minimize the evaporation of water from the protein solutions. Films were then rinsed liberally by flowing a stream of water over the surface, followed by shaking in a 1% (v/v) solution of Tween 20 three times for 15, 5, and 5 minutes each. Films were rinsed again with distilled water, dried using compressed air, and characterized by fluorescence microscopy.
Bacterial Strain and Preparation of Media
The P. aeruginosa strain used for this study (PAO1 pTdK-GFP) was generously provided by Professor Barbara Iglewski at the University of Rochester.46 All media, reagents, and salts were purchased from commercial sources (Acros, Aldrich, Fluka, Fisher, and Sigma) and used as received. Freezer stocks and overnight cultures were maintained with standard Luria Bertani (LB) media at pH 7.35. Static biofilms were grown in a modified M9 minimal medium consisting of M9 salts (47.7 mM anhydrous Na2HPO4, 21.7 mM KH2PO4, 8.6 mM NaCl, 18.7 mM NH4Cl, 1 mM anhydrous MgSO4 and 0.1 mM CaCl2). Sodium succinate hexahydrate at a concentration of 0.2% (w/v) was added as the main carbon source, as it has shown to induce more homogenous and flat biofilms.47 The medium was further supplemented with 0.4% L-arginine, which has been shown to induce a biofilm-stimulatory effect in P. aeruginosa, and 0.01 mM FeSO4-7H2O, which is within the reported optimal range of Fe salts for biofilm growth.48–50 Stock solutions of sodium succinate hexahydrate, MgSO4, CaCl2, and FeSO4-7H2O were prepared and autoclaved separately and added after cooling to room temperature to minimize precipitation of salts. All media were supplemented with 300 μg/mL carbenicillin for plasmid maintenance and buffered to pH 7.35 to mimic biological pH.
Characterization of Bacterial Biofilm Growth on Modified PEI/PVDMA Films
Experiments designed to investigate the growth of bacterial biofilms on chemically modified PEI/PVDMA films were performed using films fabricated on transparent glass substrates. The substrates were sterilized prior to static biofilm assays by soaking in EtOH (3 mL) for five minutes and then drying at 37 °C. An overnight culture of PAO1 pTdK-GFP was grown by inoculating LB medium (10 mL) supplemented with carbenicillin (300 μg/mL) in a sterile 25 mL Erlenmeyer flask. The overnight culture was grown in an incubator-shaker (200 RPM) at 37 °C for approximately 15 hours to an OD600 of approximately 0.75. Aliquots were delivered to 50 mL Falcon tubes as needed and centrifuged (3,000 RPM, five minutes, room temperature) to remove extracellular matter. The supernatant was removed, and M9 medium supplemented with carbenicillin was added to effect a 1:10 dilution and an OD600 of approximately 0.100. Portions of this diluted culture (5 mL) were added to wells of a 6-well plate (Costar 3516) containing film-coated substrates or bare glass substrates. M9 media supplemented with carbenicillin (without bacteria) was added to wells containing substrates to be used as negative controls. Sterile deionized H2O (1 mL) was added to the remaining empty wells of each plate to minimize evaporation during the assay. The plates were incubated at 37 °C for 48 h under static conditions (i.e., without shaking), after which the slides were carefully transferred by lifting and lowering into a new well using sterile forceps, while maintaining the substrate horizontally. This transfer protocol proved to be less disrupting to the biofilm than other traditional methods of handling biofilm (e.g., removing the culture with a pipette). The forceps were rinsed with EtOH between sample transfers to prevent contamination and transfer of biomass. The transferred slides were soaked twice in M9 media (3 mL) for five minutes to remove non-biofilm adhered bacteria. Care was taken to not disrupt biofilms on the surfaces of the substrates when removing soak-media with a 1000 μL micropipette. After the second soaking, the media was carefully removed and the 6-well plate was incubated at 37 °C overnight (w/o shaking) to dehydrate and fix the biofilm to the surface of the substrate.
A modified staining procedure was adopted from Harrison-Balestra et al.51,52 to visualize biofilm-related exopolysaccharide (EPS) matrices on the surfaces of each substrate. Staining reagents and solutions were prepared as follows: Cetylpyridinium chloride monohydrate solution (CpCl; 10 mM), saturated aqueous (1 g/25 mL) Congo red solution (CR; 1 g in 25 mL), and 10% Tween 80 (v/v) (Sigma P5188) stock solutions were prepared. The final Congo red staining solution was prepared by making a 2:1 (v/v) saturated CR to 10% Tween 80 (v/v) stock. To rigidify the EPS and facilitate staining, dehydrated and fixed biofilms were pretreated with CpCl (5 min, at room temperature) by applying 150 μL to the top face of the substrate. After five minutes, the CpCl was tipped off the substrate and removed, and the substrates were heat dried at 37 °C for 30 minutes. EPS staining was accomplished by soaking the top face of the substrate in 150 μL CR solution for 15 minutes at room temperature. Uncomplexed dye was removed by washing the substrates twice with deionized H2O. The substrates were allowed to dry at 37 °C overnight and were imaged using a digital camera.
Results and Discussion
We demonstrated recently that reactive layer-by-layer assembly42 could be used to fabricate covalently crosslinked ultrathin films (e.g., ~150 nm thick) using PEI and PVDMA.41 This general approach permits rapid film fabrication in common organic solvents (e.g., acetone) and results in films with linear growth profiles and smooth, uniform surfaces, as characterized by ellipsometry and atomic force microscopy (AFM). We also demonstrated using reflective infrared (IR) spectroscopy that these films contain residual azlactone functionality, and that the presence of these residual reactive groups could be used to functionalize and pattern these materials post-fabrication by treatment with primary amine-containing small molecules (e.g., amine-functionalized fluorophores).41 This current investigation sought to determine whether the well-known reactivity of these ‘spring-loaded’ azlactone groups with primary amines43,53–56 could be exploited to design thin films and coatings that promote and/or prevent the adhesion of cells.
We performed an initial series of studies to determine whether structural motifs that promote or prevent the adhesion of cells when assembled as SAMs on gold-coated surfaces could also be used to impart these properties to our azlactone-containing films. Numerous past studies demonstrate that SAMs decorated with poly(ethylene glycol) or oligo(ethylene glycol) functionality can prevent the adsorption of proteins and the adhesion of cells.18,20,21 However, the performance of these and other related materials has been reported to erode over time, possibly arising from the susceptibility of oligo- and poly(ethylene glycol) to auto-oxidation and/or hydrolytic degradation.22,57,58 We note that a recent study from our group demonstrated that the treatment of topographically patterned bulk films of azlactone-containing polymers with amine-terminated poly(ethylene glycol) could be used to pattern regions that prevent the initial attachment and proliferation of cells.59 We chose the carbohydrate D-glucamine (Figure 1C) as a cell- and protein-resistant motif for use in this study for several reasons: (i) past studies demonstrate that D-glucamine can prevent protein and cell attachment when used as a functional element of SAMs,23,60 (ii) recent reports have demonstrated that SAMs functionalized with mannitol (a structurally-related carbohydrate) can resist cell attachment for periods of time longer than SAMs decorated with oligo(ethylene glycol) functionality,22 and (iii) the structure of D-glucamine contains a nucleophilic primary amine group that should react rapidly and selectively with azlactone groups and, thus, facilitate the functionalization of our films. We selected the hydrophobic primary amine n-decylamine (Figure 1C) as an adhesion-promoting motif based on numerous past studies demonstrating that hydrophobic surfaces tend to promote non-specific cell adhesion.18,21 For these and all other experiments described below, reactive PEI/PVDMA films were assembled layer-by-layer on transparent glass substrates to facilitate characterization of cell adhesion and growth by optical and fluorescence microscopy. Film growth and thickness were estimated by characterizing otherwise identical films fabricated on the surfaces of planar silicon substrates using ellipsometry, as described in our past studies.41
PEI/PVDMA films were functionalized by completely immersing films ~150 nm thick (fabricated by the deposition of 15 PEI/PVDMA layer pairs, or ‘bilayers’) in solutions of decylamine or D-glucamine (50 mM, DMSO) for approximately 16 hours. Characterization of the surfaces of the resulting functionalized films by contact angle goniometry revealed large differences in the water-contact angles of the films. For example, the average contact angle of untreated, azlactone-containing films was measured to be ~62°, while the average contact angle of films functionalized with decylamine was ~100°. The contact angles of films functionalized with glucamine were significantly lower (e.g., estimated to be ~10°), but were difficult to measure with certainty as a result of significant wetting and drop spreading. These results reveal large changes in surface properties upon chemical modification of PEI/PVDMA films that are consistent with differences in the hydrophobicity and hydrophilicity of decylamine and glucamine, respectively. Additional characterization of both decylamine- and glucamine-functionalized films by polarization-modulation infrared reflectance-absorbance spectroscopy (PM-IRRAS) revealed the disappearance of the characteristic carbonyl absorbance peak (at 1828 cm−1) associated with unreacted azlactone functionality. These results are consistent with the results of our past studies41 and provide additional support for the view that these materials are functionalized by the formation of covalent bonds between the azlactone functionality of these small-molecule amines. We note that it was not possible to observe directly by IR spectroscopy the generation of carbonyl functionality associated with the formation of new amide bonds in these materials, most likely as a result of overlap of these new amide absorbance peaks with the amide crosslinks already present in the films (e.g., see Figure 1A).41
Characterization of Cell Adhesion and Protein Adsorption on Functionalized Films
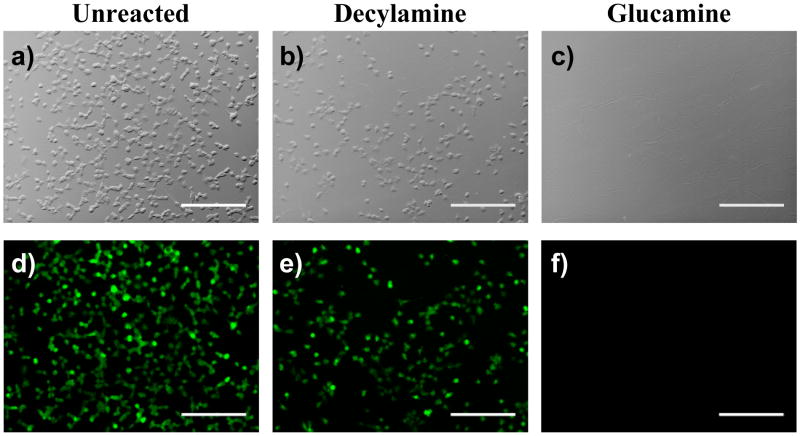
We next performed a series of cell-based experiments to evaluate the initial biocompatibility of these azlactone-containing films and determine whether the functionalization of these materials with decylamine and glucamine resulted in changes in the ability of mammalian cells to attach, grow, and migrate on the surfaces of coated substrates. For these experiments, African green monkey kidney fibroblasts (COS-7 cells) were seeded in serum-containing cell culture media on the surfaces of PEI/PVDMA films that were either unmodified (i.e., azlactone-containing films) or functionalized exhaustively with decylamine or glucamine. Cells were stained with Calcein AM, a small molecule stain that fluoresces when cleaved by esterases in viable cells,61 after 24 hours to provide a measure of cell viability and aid in characterization of the locations and morphologies of cells. Figures 2A and 2D show representative phase contrast and fluorescence microscopy images, respectively, of cells seeded on an unmodified PEI/PVDMA film ~150 nm thick. Inspection of these images reveals (i) that the cells have attached and grown to near-confluence on the surface of the azlactone-containing film, and (ii) that the cells are viable, as indicated by the observation of bright green fluorescence in nearly every cell. Figures 2B and 2E show phase contrast and fluorescence microscopy images of cells incubated with a PEI/PVDMA film functionalized with decylamine. These results are similar to those observed using unmodified films, and demonstrate that cells attach, grow, and remain viable on the surfaces of these hydrophobically-modified surfaces. These results are consistent with the results of past reports demonstrating that SAMs presenting aliphatic alkyl chains promote cell attachment and proliferation.18,21 Although additional experiments will be required to evaluate more completely the biocompatibility of these PEI/PVDMA films, the results of these initial experiments demonstrate that these unmodified and modified films do not influence cell viability significantly. We return to these observations and considerations again in the discussion below.
Figure 2.
Phase contrast (top row; a–c) and fluorescence (bottom row; d–f) microscopy images of COS-7 cells 24 hours after seeding on (a,d) an unmodified (i.e., azlactone-containing) PEI/PVDMA film, (b,e) a PEI/PVDMA film treated with decylamine, and (c,f) a PEI/PVDMA film treated with glucamine. Cells were treated with Calcein AM prior to imaging. Scale bars = 300 μm.
In contrast to the results of experiments using unmodified films or decylamine-treated films, PEI/PVDMA films functionalized with glucamine prevented almost completely the attachment and growth of cells. Figures 2C and 2F show representative images of PEI/PVDMA films functionalized with glucamine 24 hours after seeding with cells, and reveal the nearly complete absence of cells growing on these surfaces. The results of additional experiments in which cell attachment was characterized at shorter time periods after seeding (e.g., between 1–5 hours; data not shown) demonstrated that the absence of cells in these images was the result of the inability of cells to attach after seeding, and not the result of substrate toxicity (that is, cells did not attach initially and then subsequently detach over time). The results of experiments conducted using NIH-3T3 fibroblasts, a highly adherent murine cell line used routinely to characterize the adhesion of cells to surfaces, on unmodified films and decylamine- and glucamine-functionalized films were similar to the results shown in Figure 2 (data not shown). The results of these experiments demonstrate that it is possible to use design principles and structural motifs that have emerged from past studies of SAMs on gold-coated surfaces22,23,60 to functionalize reactive, azlactone-containing films in ways that capture, at least in part, some of the important functions of these more conventional, well-studied materials.
The attachment and proliferation of cells on surfaces is often facilitated by the non-specific adsorption of proteins.62 Because the experiments described above were performed using cell culture media supplemented with 10% serum (v/v), it is likely that the ability (or inability) of cells to attach to the surfaces of these PEI/PVDMA films could be dictated, to some extent, by the abilities of these films to either promote or prevent the adsorption of proteins. For example, past work has demonstrated the ability of SAMs functionalized with alkyl groups or glucamine to promote and prevent, respectively, the non-specific adsorption of proteins to gold-coated surfaces.18,21 In addition, past reports have demonstrated that azlactone functionality reacts readily with the primary amine groups of surface-exposed lysine residues in proteins.43,54,55 Thus, while decylamine-functionalized surfaces could promote the non-specific adsorption of proteins, unmodified azlactone-containing films could also facilitate cell attachment by providing platforms for the rapid and covalent attachment of protein upon immersion in serum-containing media.
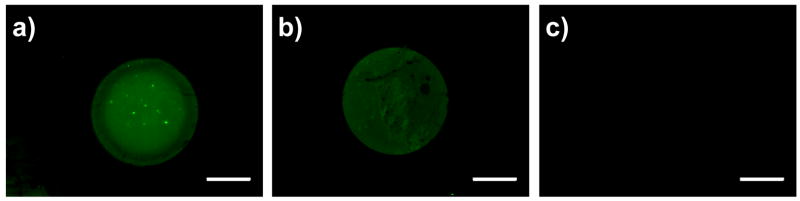
To investigate the relationship between protein adsorption and the behavior of cells in the experiments described above, we characterized the ability of unmodified PEI/PVDMA films, decylamine-functionalized films, and glucamine-functionalized films to promote or prevent the adsorption of FITC-labeled bovine serum albumin (FITC-BSA), a fluorescently-labeled model protein. For these experiments, a small droplet of a solution of FITC-BSA (1 μL of a 10 μM solution in PBS) was spotted onto the surface of each film and allowed to sit for ~2 hours. Figure 3 shows fluorescence micrographs of an unmodified film (A), a decylamine-functionalized film (B), and a glucamine-functionalized film (C) after gentle rinsing with a 0.1% (v/v) Tween 20 surfactant solution (see Materials and Methods for details). Figures 3A and 3B show circular areas of green fluorescence corresponding to the presence of FITC-BSA on the surfaces of azlactone-containing or decylamine-functionalized films. However, Figure 3C, which presents data obtained using a glucamine-functionalized film, shows little or no green fluorescence, suggesting that FITC-BSA does not adhere significantly to these surfaces. These results are consistent with the results of past studies described above for alkyl-functionalized and glucamine-functionalized SAMs and provide general support for the view that our functionalized films promote or prevent the attachment and growth of cells, at least in part, by promoting or preventing the adsorption of protein. The adsorption and attachment of protein at the surfaces of unmodified, azlactone-containing films could also play a role in increasing the apparent cytocompatibility of these reactive films by reacting with azlactone groups that could otherwise react with components of cell membranes and compromise cell viability. In addition, we note that the ability to covalently attach and immobilize proteins in defined locations on the surfaces of these reactive films (e.g., Figure 3A) could present additional opportunities to chemically pattern protein and/or cell attachment on surfaces.
Figure 3.
Fluorescence micrographs of (a) an unmodified PEI/PVDMA film, (b) a decylamine-treated PEI/PVDMA film, and (c) glucamine-treated PEI/PVDMA film after treatment with a small drop of a FITC-labeled bovine serum albumin solution, followed by washing (see text). Scale bars = 500 μm.
Characterization of Cell Attachment and Growth on Patterned Films
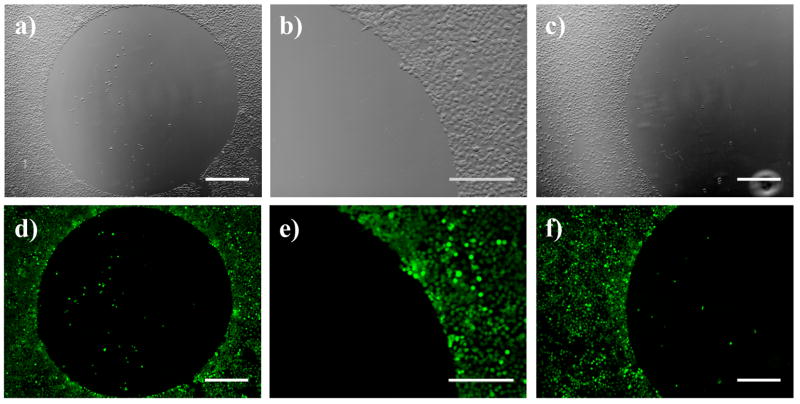
We next sought to determine whether decylamine and glucamine could be used in combination to pattern azlactone-containing films with spatially defined regions that prevent or promote cell adhesion and growth. To explore the feasibility of this approach, we first patterned a cell-resistant region by depositing a small drop of a solution of glucamine (1 μL of a 20 mg/mL solution in DMSO) for two hours, followed by liberal rinsing with DMSO and ethanol. The areas surrounding these circular glucamine-treated regions were either left unmodified (i.e., presenting azlactone functionality) or were functionalized with decylamine by immersing the entire glucamine-spotted substrate into a solution of decylamine. Figures 4A and 4D show optical and fluorescence micrographs of COS-7 cells 48 hours after seeding on a glucamine-spotted film (with the remainder of the film untreated). As described above, cells were stained using calcein AM to aid in characterization of cell behavior. Inspection of these images reveals a circular area on the film in which very few cells have attached, surrounded by a population of cells that is nearly confluent. Figures 4B and 4E show magnified views of one edge of the glucamine spot, and reveal a clear and distinct boundary between the glucamine-treated area of the film and the cells growing on the unmodified, azlactone-presenting area of the film. These images show clearly that cells attach and grow in areas surrounding the glucamine spot, but that they largely avoid attaching in areas of the film functionalized with glucamine.
Figure 4.
Phase contrast (top row; a–c) and fluorescence (bottom row; d–f) microscopy images of COS-7 cells 48 hours after seeding on chemically-treated PEI/PVDMA films. Images (a) and (d) show images of cells seeded on a PEI/PVDMA film treated with a small drop of a solution of glucamine (20 mg/mL in DMSO). Images (b) and (e) show higher magnification portions of the edges of the glucamine-treated regions shown in (a) and (d). Images (c) and (f) show cells seeded on a PEI/PVDMA film treated with a small drop of glucamine followed by subsequent treatment of the entire film with decylamine. Cells were treated with Calcein AM prior to imaging. (a,c,d,f): scale bars = 500 μm; (b,e): scale bars = 300 μm.
Figures 4C and 4F show images of a film that was first spot-functionalized with glucamine, followed by a second treatment of the remainder of the azlactone-containing film with decylamine to create a hydrophobic background. Similar to the observations described above, cells were observed to attach and proliferate in regions surrounding the glucamine-treated spot, but not within the glucamine-treated spot itself. This result demonstrates that surfaces coated with these reactive PEI/PVDMA films can be modified in a sequential manner with two, or potentially more, chemical agents that confer different cellular responses without compromising the function of previously deposited chemical functionality. We note in this context that our past studies demonstrate that the surfaces of these films can be chemically patterned using conventional methods of PDMS-mediated microcontact printing.41 Our current results thus suggest opportunities for the microscale or nanoscale printing of peptide ligands, small molecules, or other features that could influence the attachment, growth, or differentiation of cells in more complex ways. Finally, we note that the results shown in Figure 4 were obtained using PEI/PVDMA films ~150 nm thick fabricated by the layer-by-layer deposition of 15 bilayers of PEI and PVDMA. The results of additional experiments demonstrated that thinner films (including films fabricated from as little as one single bilayer of PEI and PVDMA) could promote or prevent cell adhesion in decylamine- and glucamine-functionalized regions at levels similar to those using thicker films (data not shown).
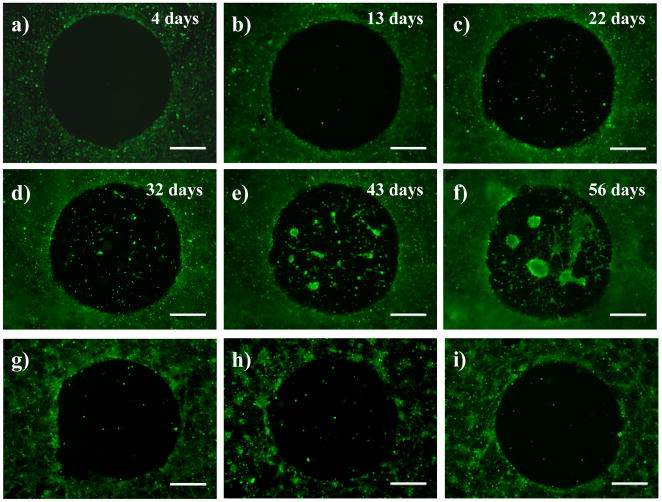
One challenge associated with the development of materials that modulate the behavior of cells on surfaces is to design materials that are stable and maintain their function for prolonged periods in physiologically relevant environments. To investigate the longer-term performance of our glucamine-functionalized films in vitro, we functionalized PEI/PVDMA films with glucamine spots (with the remainder of film untreated), similar to films discussed in the experiments described above. COS-7 cells were then seeded on these functionalized films in serum-containing cell culture media and allowed to attach, grow, and proliferate continually for 56 days. For these experiments, cells were stained with Calcein AM every three or four days to visualize cells and determine cell viability, and cell culture media was exchanged with fresh media periodically (see Materials and Methods for details). Figures 5A–5F show representative fluorescence microscopy images of cells at various time points over this 56-day experiment (images were acquired every few days; intermittent time points not shown). Figure 5A shows cells four days after seeding, and shows results similar to those shown in Figure 4D; cells were observed to avoid the glucamine-treated spot, but have proliferated to a near-confluent monolayer of cells in areas surrounding the spot. Figures 5B and 5C show results after 13 days and 22 days, respectively. Inspection of these images reveals that cells in the untreated area of the film remained viable and that they continued to grow and proliferate (additional characterization of cells by phase contrast microscopy suggested the presence of multilayers of cells that were many cell layers thick; data not shown). However, despite this additional activity and the prolonged exposure of these materials to serum-containing media, cells did not migrate into or invade the glucamine-functionalized region of the film.
Figure 5.
Fluorescence micrographs of COS-7 cells seeded on a PEI/PVDMA film treated with a small drop of a solution of glucamine. Cells were allowed to grow continuously on the substrates for almost two months (see text for details). Images in (a–f) correspond to cells growing on the surface of this film (a) 4 days, (b) 13 days, (c) 22 days, (d) 32 days, (e) 43 days, and (f) 56 days after seeding. Cells were treated with Calcein AM prior to imaging. Images in (g–i) are fluorescence micrographs of COS-7 cells 72 hours after seeding on a glucamine-treated PEI/PVDMA film that was treated with trypsin and subsequently re-seeded with cells (g) one time, (h) two times, and (i) three times (see text). Scale bars = 500 μm.
The cell-resistant properties of the glucamine-functionalized spot were maintained for at least 32 days, at which point small numbers of cells were observed to be present in the darker glucamine-treated region of the film (Figure 5D). We note here, however, that the proximity of these isolated cells to the center of the spot and the general maintenance of the clearly-defined border around the glucamine-functionalized region suggests that these cells did not migrate into this region, but rather that they may have detached from portions of the surrounding cell multilayers and landed in this location. In addition, we note that the majority of these cells appeared to be loosely attached and did not, in general, spread and proliferate in this region. Many of these cells appeared to detach and resettle elsewhere over time, as evidenced by significant changes in the day-to-day numbers and locations of cells in glucamine-patterned areas. At longer time periods (e.g., 43 days or 56 days; Figures 5E and 5F) larger clusters of cells were observed in the glucamine-treated area of the film, but the perimeter of this area remained relatively well defined, suggesting that cells were largely unable to invade or migrate into this region over periods of time lasting up to almost two months. Glucamine-treated areas of films that were exposed to serum-containing media (in the absence of cells) for periods ranging from one to four weeks prior to cell seeding also resisted cell attachment and growth for at least three weeks (data not shown). These results suggest that the ability of these materials to prevent non-specific adsorption of protein, as described above, may also be maintained over extended periods.
The results above contrast to the results of past studies investigating the cell-resistance of gold surfaces patterned with SAMs presenting oligo(ethylene glycol) functionality, for which the overgrowth of cells into oligo(ethylene glycol)-patterned regions has been reported to occur in as little as one week.22 However, our results are comparable to those reported recently using SAMs functionalized with mannitol, a carbohydrate that is structurally similar to glucamine.22 Patterned SAMs presenting regions of mannitol functionality have been demonstrated to resist the attachment and invasion of 3T3-Swiss fibroblasts in vitro for at least 25 days in the presence of serum-containing media.22 Our results also compare favorably with those of recent studies of chemically-crosslinked and patterned polyelectrolyte multilayers, which have been reported to resist the adhesion or invasion of cells into patterned regions for up to one month.37,63 We note that the PEI/PVDMA films used in the experiments above remained intact and adherent to their glass substrates without significant peeling or wrinkling (as determined by visual inspection and by optical microscopy) over the 56-day duration of these experiments. However, in other experiments that required periodic removal of film-coated substrates, intermittent washing steps, and/or drying (e.g., as described below), we observed some time-dependent wrinkling or peeling of the films. We were able to prevent this wrinkling and peeling, and improve the general performance and durability of these materials, by depositing films onto glass substrates that were aminosilane-treated to present primary amine functionality (see Materials and Methods for additional details). We speculate that the reaction of these covalently-bound primary amines with the azlactone functionality of PVDMA serves to anchor these films to their substrates more effectively through the formation of covalent bonds. In general, the apparent physical and functional stability of these covalently crosslinked PEI/PVDMA thin films could prove useful in applications that require surfaces with longer-term durability in physiologically relevant environments or other harsh media.
The results of a final set of cell-based experiments demonstrated that the ability of decylamine- and glucamine-functionalized films to promote and prevent cell adhesion is largely preserved when these films are treated with trypsin and re-used as cell substrates in multiple different experiments. These experiments were conducted by allowing COS-7 cells to grow to confluence on the surfaces of glucamine-spotted films deposited on aminosilane-treated glass (e.g., similar to Figure 5A), followed by treatment with trypsin to remove cells from the surface of the films (as determined by optical microscopy). Cells were then re-seeded on these trypsin-treated substrates and allowed to grow to confluence again. Figures 5G–I show images of Calcein AM-stained COS-7 cells on a glucamine-spotted film after one (G), two (H), and three (I) cycles of trypsinization and re-seeding. Inspection of these images reveals that, after each treatment with trypsin, new cells seeded on the films attached and grew on the azlactone-presenting areas surrounding the glucamine-functionalized area, but that they did not attach or grow into the glucamine-treated area itself. We were able to repeat this process of trypsinization and re-seeding for at least six different cycles without a significant loss of pattern fidelity (data for cycles 4–6 not shown). These results further demonstrate the chemically and physically robust nature of these crosslinked films.
Characterization of Bacterial Biofilm Growth on Functionalized Films
In addition to the fouling of surfaces by mammalian cells and adsorbed protein, the function and performance of implants, biomedical devices, industrial tools, and other objects can also be compromised significantly by bacterial biofilms, or complex exopolysaccharide (EPS)-encased sessile colonies of bacteria.64–66 The eradication of biofilms of pathogenic bacteria is of significant interest in the context of biomedical applications, but general and robust strategies for the inhibition of biofilm growth remain scarce.67,68 Motivated by this broader challenge, we sought to determine if the general approach described above could also be used to attenuate bacterial biofilm growth. The results of a series of experiments using a static biofilm growth assay (Figure 6) suggest that chemical functionalization of PEI/PVDMA films can be used to design films that promote or prevent the initial formation of bacterial biofilms on the surfaces of glass substrates. For these experiments, PEI/PVDMA films (~150 nm thick) post-functionalized with either decylamine or glucamine were incubated in growth media in the presence of the bacterial pathogen P. aeruginosa (see Materials and Methods for additional details). After 48 hours, these substrates were stained with a solution of Congo red (CR), a red-colored small molecule dye that stains carbohydrate components of biofilm EPS matrices,69 to aid in the visualization and identification of bacterial biofilms on these substrates.
Figure 6.
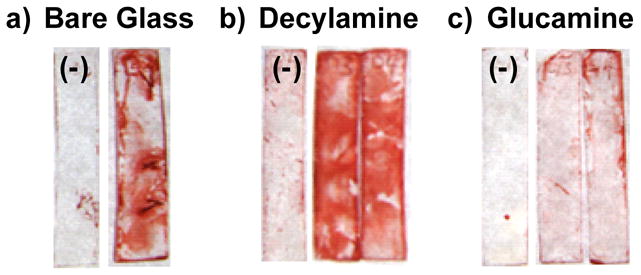
Images of Congo red-stained (a) bare glass and (b,c) film-coated glass substrates after incubation under static conditions in the presence or absence of P. aeruginosa for 48 hours. Images show relative levels of biofilm-related exopolysaccharide (EPS) matrices on the surfaces of glass slides (e.g., 10 mm × 30 mm) coated with (a) no film (bare glass), (b) a PEI/PVDMA film treated with decylamine, and (c) a PEI/PVDMA film treated with glucamine. Substrates marked with a (−) denote samples grown in the absence of bacteria (media only) used as negative controls.
Figure 6 shows images of glass substrates coated with (A) no film (bare glass), (B) decylamine-functionalized films, and (C) glucamine-functionalized films that were incubated in growth media in either the absence or presence of P. aeruginosa. Visual inspection of these images reveals the presence of red, CR-stained biofilm-related EPS on both the surface of the bare glass substrate (Figure 6A) and, to a greater extent, on the decylamine-treated films (Figure 6B). In contrast, the intensity of CR stain in the images shown in Figure 6C is substantially lower, suggesting that these glucamine-functionalized films are able to prevent or reduce substantially the growth of bacterial biofilm (relative to growth on either bare glass or decylamine-treated films) over this initial 48-hour incubation period. The results of similar experiments conducted using a crystal violet-based stain, which, in contrast to CR, stains and directly identifies bacterial cells rather than EPS,70 were similar to those shown in Figure 6 (data not shown) and provide further support for this general conclusion. Additional work will be required to characterize differences in the interactions of bacteria with these functionalized films, quantify differences in levels of biofilm growth, and determine the range of different conditions under which these decylamine- and glucamine-functionalized films do (or do not) promote or prevent biofilm growth. However, the results of these current studies suggest that it may be possible to design functionalized PEI/PVDMA films that either prevent, promote, or pattern the growth of biofilms on surfaces of interest in the contexts of both fundamental biological studies and a broad range of other practical applications.
Summary and Conclusions
We have reported an approach to the layer-by-layer assembly of reactive, azlactone-containing polymer films that can be functionalized post-fabrication to either promote or prevent the attachment and growth of cells. Our results demonstrate (i) that the residual azlactone functionality in these films can be used to immobilize amine-functionalized chemical motifs similar to those that have been demonstrated to promote or prevent cell and protein adhesion when assembled as SAMs on gold-coated surfaces, and (ii) that the immobilization of these motifs can be used to change significantly the behaviors and interactions of cells with these surfaces. For example, whereas films treated with decylamine supported the attachment and growth of mammalian cells, films treated with D-glucamine prevented cell adhesion and growth almost completely. Our results also suggest that these large differences in cell behavior can be understood in terms of differences in the abilities of these two different chemical motifs to promote or prevent the adsorption of proteins onto the surfaces of these films. The approach reported here can be used to pattern regions of these reactive films that resist the initial attachment and subsequent invasion of mammalian cells in vitro for at least one month in the presence of serum-containing cell culture media. Finally, we have extended the potential significance of this general approach by demonstrating that films that prevent or promote the adhesion and growth of mammalian cells can also prevent or promote the initial formation of bacterial biofilms when incubated in the presence of the bacterial pathogen P. aeruginosa. The results of these studies, when combined, suggest opportunities for the microscale or nanoscale patterning or printing of peptides, proteins, small molecules, or other features that could influence the attachment, growth, or differentiation of mammalian and bacterial cells in more complex ways.
The results of this investigation demonstrate that it is possible to use design principles and structural motifs that have emerged from past studies of SAMs on gold-coated surfaces to functionalize reactive, azlactone-containing films in ways that capture some of the functions of these more well-studied surfaces. The primary conclusions of this study are limited to those related to the design of surfaces that promote or prevent cell adhesion, and additional work will be required to elucidate structure/property relationships and characterize the range of differences (or potential similarities) between the properties of these two very different types of materials. However, if these initial observations are found to be general, it may also prove possible to use these azlactone-containing thin films as platforms for the functionalization of surfaces with other chemical motifs that have been found to be useful for the functionalization of gold-coated surfaces in a broad range of other applications.
Acknowledgments
Financial support to D. M. L. was provided by the National Institutes of Health (EB006820), the Alfred P. Sloan Foundation, the 3M Corporation, and the University of Wisconsin. Financial support to H. E. B. was provided by the National Institutes of Health (AI063326-01), the Office of Naval Research (N000140710255), and the Burroughs Welcome Fund. H. E. B. and D. M. L. are Research Fellows of the Alfred P. Sloan Foundation. M. E. B. was funded, in part, by an NIH Chemistry-Biology Interface Training Grant (NIGMS T32 GM008505). We thank Dr. Steven M. Heilmann and Dr. Jerald K. Rasmussen (3M) for providing samples of poly(2-vinyl-4,4-dimethylazlactone) and for many helpful discussions, and Prof. Barbara Iglewski (University of Rochester) for donation of the P. aeruginosa strain and advice on its manipulation. We thank Eric M. Saurer for assistance with mammalian cell culture experiments, imaging, and many helpful discussions.
References
- 1.Anderson JM. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 2.Castner DG, Ratner BD. Surf Sci. 2002;500:28–60. [Google Scholar]
- 3.Ratner BD, Bryant SJ. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 4.Thevenot P, Hu WJ, Tang LP. Curr Top Med Chem. 2008;8:270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, Ingber DE. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 6.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 8.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DG, Levenberg S, Langer R. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 10.Angres B. Expert Rev Mol Diagn. 2005;5:769–779. doi: 10.1586/14737159.5.5.769. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Mochon JJ, Tourniaire G, Bradley M. Chem Soc Rev. 2007;36:449–457. doi: 10.1039/b511848b. [DOI] [PubMed] [Google Scholar]
- 12.Elbert DL, Hubbell JA. Annu Rev Mater Sci. 1996;26:365–394. [Google Scholar]
- 13.Mrksich M. Chem Soc Rev. 2000;29:267–273. [Google Scholar]
- 14.Nath N, Hyun J, Ma H, Chilkoti A. Surf Sci. 2004;570:98–110. [Google Scholar]
- 15.Senaratne W, Andruzzi L, Ober CK. Biomacromolecules. 2005;6:2427–2448. doi: 10.1021/bm050180a. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Weinman CJ, Ober CK. J Mater Chem. 2008;18:3405–3413. [Google Scholar]
- 17.Mrksich M, Whitesides GM. Annu Rev Biophys Biomol Struct. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 18.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 19.Perlin LMS, Rimmer S. Soft Matter. 2008;4:2331–2349. [Google Scholar]
- 20.Prime KL, Whitesides GM. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 21.Ostuni E, Yan L, Whitesides GM. Colloids Surf B. 1999;15:3–30. [Google Scholar]
- 22.Luk YY, Kato M, Mrksich M. Langmuir. 2000;16:9604–9608. [Google Scholar]
- 23.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. Langmuir. 2001;17:5605–5620. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 24.Flynn NT, Tran TNT, Cima MJ, Langer R. Langmuir. 2003;19:10909–10915. [Google Scholar]
- 25.Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Adv Mater. 2006;18:3203–3224. [Google Scholar]
- 26.Lahann J, Balcells M, Rodon T, Lee J, Choi IS, Jensen KF, Langer R. Langmuir. 2002;18:3632–3638. [Google Scholar]
- 27.Jerome C, Gabriel S, Voccia S, Detrembleur C, Ignatova M, Gouttebaron R, Jerome R. Chem Commun. 2003:2500–2501. doi: 10.1039/b307728d. [DOI] [PubMed] [Google Scholar]
- 28.Lahann J, Balcells M, Lu H, Rodon T, Jensen KF, Langer R. Anal Chem. 2003;75:2117–2122. doi: 10.1021/ac020557s. [DOI] [PubMed] [Google Scholar]
- 29.Feng CL, Zhang ZZ, Forch R, Knoll W, Vancso GJ, Schonherr H. Biomacromolecules. 2005;6:3243–3251. doi: 10.1021/bm050247u. [DOI] [PubMed] [Google Scholar]
- 30.Feng CL, Vancso GJ, Schonherr H. Adv Funct Mater. 2006;16:1306–1312. [Google Scholar]
- 31.Lahann J. Polym Int. 2006;55:1361–1370. [Google Scholar]
- 32.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 33.Bertrand P, Jonas A, Laschewsky A, Legras R. Macromol Rapid Commun. 2000;21:319–348. [Google Scholar]
- 34.Hammond PT. Adv Mater. 2004;16:1271–1293. [Google Scholar]
- 35.Peyratout CS, Dahne L. Angew Chem Int Ed. 2004;43:3762–3783. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 36.Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI, Rubner MF. Biomacromolecules. 2003;4:96–106. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
- 37.Yang SY, Mendelsohn JD, Rubner MF. Biomacromolecules. 2003;4:987–994. doi: 10.1021/bm034035d. [DOI] [PubMed] [Google Scholar]
- 38.Richert L, Boulmedais F, Lavalle P, Mutterer J, Ferreux E, Decher G, Schaaf P, Voegel JC, Picart C. Biomacromolecules. 2004;5:284–294. doi: 10.1021/bm0342281. [DOI] [PubMed] [Google Scholar]
- 39.Schneider A, Francius G, Obeid R, Schwinte P, Hemmerle J, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Langmuir. 2006;22:1193–1200. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 40.Picart C. Curr Med Chem. 2008;15:685–697. doi: 10.2174/092986708783885219. [DOI] [PubMed] [Google Scholar]
- 41.Buck ME, Zhang J, Lynn DM. Adv Mater. 2007;19:3951–3955. [Google Scholar]
- 42.Bergbreiter DE, Liao KS. Soft Matter. 2009;5:23–28. [Google Scholar]
- 43.Heilmann SM, Rasmussen JK, Krepski LR. J Polym Sci Part A. 2001;39:3655–3677. [Google Scholar]
- 44.Yang K, Cadwell K, Abbott NL. J Phys Chem B. 2004;108:20180–20186. [Google Scholar]
- 45.Zhang J, Fredin NJ, Lynn DM. J Polym Sci Part A. 2006;44:5161–5173. [Google Scholar]
- 46.Appl Environ Microbiol. 2001;67:1865–1873. doi: 10.1128/AEM.67.4.1865-1873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. Mol Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 48.Caiazza NC, O’Toole GA. J Bacteriol. 2004;186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musk DJ, Banko DA, Hergenrother PJ. Chem Biol. 2005;12:789–796. doi: 10.1016/j.chembiol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Anderson GG, Moreau-Marquis S, Stanton BA, O’Toole GA. Infect Immun. 2008;76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison-Balestra C, Cazzaniga AL, Davis SC, Mertz PM. Dermatol Surg. 2003;29:631–635. doi: 10.1046/j.1524-4725.2003.29146.x. [DOI] [PubMed] [Google Scholar]
- 52.Allison DG, Sutherland IW. J Microbiol Methods. 1984;2:93–99. [Google Scholar]
- 53.Guichard B, Noel C, Reyx D, Thomas M, Chevalier S, Senet JP. Macromol Chem Phys. 1998;199:1657–1674. [Google Scholar]
- 54.Xie SF, Svec F, Frechet JMJ. Biotechnol Bioeng. 1999;62:30–35. doi: 10.1002/(sici)1097-0290(19990105)62:1<30::aid-bit4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Cullen SP, Mandel IC, Gopalan P. Langmuir. 2008;24:13701–13709. doi: 10.1021/la8024952. [DOI] [PubMed] [Google Scholar]
- 56.Barringer JE, Messman JM, Banaszek AL, Meyer HM, Kilbey SM. Langmuir. 2009;25:262–268. doi: 10.1021/la802925g. [DOI] [PubMed] [Google Scholar]
- 57.Crouzet CDC, Marchal J. Makromolek Chem-Macromolec Chem Phys. 1976;177:145–157. [Google Scholar]
- 58.Wieland B, Lancaster JP, Hoaglund CS, Holota P, Tornquist WJ. Langmuir. 1996;12:2594–2601. [Google Scholar]
- 59.Fredin NJ, Broderick AH, Buck ME, Lynn DM. Biomacromolecules. 2009;10 doi: 10.1021/bm900045c. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orner BP, Derda R, Lewis RL, Thomson JA, Kiessling LL. J Am Chem Soc. 2004;126:10808–10809. doi: 10.1021/ja0474291. [DOI] [PubMed] [Google Scholar]
- 61.Bischof JC, Padanilam J, Holmes WH, Ezzell RM, Lee RC, Tompkins RG, Yarmush ML, Toner M. Biophys J. 1995;68:2608–2614. doi: 10.1016/S0006-3495(95)80445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 63.Berg MC, Yang SY, Hammond PT, Rubner MF. Langmuir. 2004;20:1362–1368. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- 64.Bryers JD. Biotechnol Bioeng. 2008;100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain A, Gupta Y, Agrawal R, Khare P, Jain SK. Crit Rev Ther Drug Carrier Syst. 2007;24:393–443. doi: 10.1615/critrevtherdrugcarriersyst.v24.i5.10. [DOI] [PubMed] [Google Scholar]
- 66.Hall-Stoodley L, Costerton JW, Stoodley P. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 67.Musk DJ, Hergenrother PJ. Curr Med Chem. 2006;13:2163–2177. doi: 10.2174/092986706777935212. [DOI] [PubMed] [Google Scholar]
- 68.Huigens RW, Richards JJ, Parise G, Ballard TE, Zeng W, Deora R, Melander C. J Am Chem Soc. 2007;129:6966–6967. doi: 10.1021/ja069017t. [DOI] [PubMed] [Google Scholar]
- 69.Teather RM, Wood PJ. Appl Environ Microbiol. 1982;43:777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]