Abstract
Background
Injection drug users (IDUs) have estimated mortality rates over 10 times higher than the general population; much of this excess mortality is HIV associated. Few mortality estimates among IDUs from developing countries, including India, exist.
Methods
IDUs (1158) were recruited in Chennai from April 2005 to May 2006; 293 were HIV positive. Information on deaths and causes was obtained through outreach workers and family/network members. Mortality rates and standardized mortality ratios were calculated; multivariate Poisson regression was used to identify predictors of mortality.
Results
We observed 85 deaths over 1998 person-years (p-y) of follow-up [incidence rate (IR) 4.25 per 100 p-y; 95% confidence interval (CI) 3.41, 5.23]. The overall standardized mortality ratio was 11.1; for HIV-positive IDUs, the standardized mortality ratio was 23.9. Mortality risk among HIV-positive IDUs (IR 8.88 per 100 p-y) was nearly three times that of negative IDUs (IR 3.03 per 100 p-y) and increased with declining immune status (CD4 cells > 350: 5.44 per 100 p-y vs. CD4 cells ≤ 200: 34.5 per 100 p-y). This association persisted after adjustment for potential confounders. The leading causes of mortality in both HIV negative and positive IDUs were overdose (n = 22), AIDS (n = 14), tuberculosis (n = 8) and accident/trauma (n = 9).
Conclusion
Substantial mortality was observed in this cohort with the highest rates among HIV-positive IDUs with CD4 cells less than 350. Although in these 2 years, non-AIDS deaths outnumbered AIDS-related deaths, the relative contribution of AIDS-associated mortality is likely to increase with advancing HIV disease progression. These data reinforce the need for interventions to reduce the harms associated with drug use and increase HAART access among IDUs in Chennai.
Keywords: HIV, India, injection drug users, mortality, overdose
Introduction
There are an estimated 15.9 million injection drugs users (IDUs) globally; over 75% are believed to reside outside of the developed world [1]. IDUs are at high risk for premature mortality, sometimes as much as 13 times that of the general population [2]. Much of this excess mortality has historically been linked to HIV [3–5]. However, with the advent of highly active antiretroviral therapy (HAART), AIDS-related mortality has decreased significantly in the industrialized world [6,7], with non-HIV-related causes [e.g., hepatitis C virus (HCV] accounting for larger proportion of mortality [8]. This trend is likely not shared by IDUs in developing country settings where the burden of HIV is increasing and access to HAART is limited [1]. Further, in developing countries, there are multiple factors, including a high background burden of other infectious diseases (e.g., tuberculosis) that may contribute to increased mortality among IDUs [9]. To date, mortality among IDUs has not been well characterized in the developing world.
Injection drug use in India has been best characterized in the Northeast [10], but with increased production of heroin in Afghanistan and new trafficking routes running through India [11], there are emerging epidemics of HIV among IDUs from other regions in India [12]. It is estimated that there are between 165 000 and 1.1 million IDUs in India, and Mumbai and New Delhi are believed to have the largest IDU populations in the world [1,13]. Chennai, the capital city of the southern state of Tamil Nadu, is estimated to have 10 000–15 000 IDUs [14] with HIV prevalence approximately 30% [15,16]. Although the government of India introduced a free antiretroviral therapy (ART)-roll out program in 2004 [17], reports suggest that few IDUs access these programs [18]. We know of no mortality estimates among IDUs in India.
Accordingly, we characterize the rate, risk factors and causes of mortality among a cohort of IDUs in Chennai, India.
Methods
Study population
Between April 2005 and May 2006, 1158 IDUs were recruited into a longitudinal cohort in Chennai, India. The Madras Injection Drug Users and AIDS Cohort Study (MIDACS) operates through the YR Gaitonde Centre for Substance Abuse-Related Research (YRGCSAR) in north Chennai [16]. YRGCSAR was established in November 2004 to provide HIV voluntary counseling and testing (VCT) services to marginalized populations and to conduct longitudinal assessments of HIV incidence and the natural history of drug abuse among IDUs in Chennai. A convenience sample of IDUs was recruited through extensive community outreach as previously described [16]. Briefly, field staff, who were predominantly former IDUs, recruited participants from locales in all zones of Chennai where IDUs were known to congregate (e.g., shooting galleries, drug treatment centers, etc.). Once participants were recruited into the study, they were invited to refer their friends or acquaintances or both who injected drugs, including those who they injected with (i.e. network partners). Participants were eligible if they provided written informed consent, were at least 18 years of age, and injected at least once in the prior 6 months by self-report. This study was approved by the YR Gaitonde Centre for AIDS Research and Education (YRGCARE) and Johns Hopkins Bloomberg School of Public Health Institutional Review Boards.
Of 1208 IDUs screened, 1172 (97%) consented and 1158 (95.8%) provided a baseline blood specimen and responded to a behavioral questionnaire. All HIV-negative participants (n = 865) were invited to return for semi-annual follow-up visits. HIV-positive participants (n = 293; 25.3%) were permitted to continue their semi-annual MIDACS follow-up visits but were simultaneously referred to an on-site HIV clinic and are under follow-up in a parallel HIV-positive clinical cohort[19].
Data collection
HIV status was ascertained at baseline and each follow-up visit by testing for antibodies to HIV by duplicate enzyme-linked immunosorbent assay (ELISA) (Murex HIV-1.2.O, Abbott Murex, UK and Vironostika HIV Uni-form II Ag/Ab, bioMérieux, Boxtel, The Netherlands). At baseline, hepatitis C serostatus was diagnosed by the presence of antibodies to HCV (anti-HCV) using the Murex Anti-HCV kit (Abbott Murex, Republic of South Africa), and chronic hepatitis B virus (HBV) infection was diagnosed by the presence of hepatitis B surface antigen (HBsAg) (Hepanostika HBsAg Uniform II, bioMérieux, The Netherlands).
Information on death and cause of death was obtained through reports from participants in the study and field workers who actively tracked participants when visits were missed. Participants were tracked through contacts that were provided at entry into the study and all subsequent follow-up visits; contacts included family members, friends and/or injecting (network) partners. All MIDACS HIV-negative participants were actively tracked by field workers within 1 month of a missed study visit. Field workers also tracked HIV-positive participants who had not had a MIDACS study visit or clinic visit for more than 6 months at the time of the analysis. Reasons for missed visits/loss to follow-up including mortality were recorded [20].
Self-reported information from family/network members was used to classify cause of death. Cause was grouped into six categories: AIDS related, tuberculosis, overdose, accidents (including suicides), chronic disease and nonspecific (cause not known). Deaths attributed to tuberculosis in HIV-positive IDUs with a CD4 cell count less than 350 cell/μl and deaths among HIV-positive IDUs with CD4 cells 200 cells/μl or less and a nonspecific cause were classified as AIDS related. Nonspecific causes of death in HIV-positive patients with CD4 cells less than 200 cells/μl included one inpatient death (CD4 cells: 48 cells/μl), and three cases in which the cause of death was reported as ‘sick’ (CD4 cells: 167, 184, and 185 cells/μl).
A risk assessment questionnaire was administered at each study visit by trained interviewers and included questions on drug (injection, noninjection and alcohol) use, injection and sexual practices. The interviewers provided pretest and posttest counseling. HIV-positive IDUs received absolute CD4 cell count estimation once every 3–6 months. HIV-positive participants who satisfied Indian guidelines for HAART initiation (CD4 cells < 200 cells/μl or CD4 cells < 350 cells/μl with an opportunistic infection or AIDS-defining illness at any CD4 cell count) were referred to the nearest ART rollout center for HAART.
Statistical analysis
Mortality rates per 100 person-years (p-y) were calculated. Person time at risk was calculated as the time between study enrollment and the last semi-annual visit date for HIV-negative IDUs. For HIV-positive IDUs, it was calculated as the time between study enrollment and the last completed clinic or semi-annual visit, whichever was later. The last semi-annual visit date also included a date of contact for participants who did not return to the clinic for their visit but whose whereabouts were known through tracking. For the 84 HIV-positive persons and 39 HIV-negative participants with no follow-up information after the baseline visit, 1 year of follow-up was provided. IDUs who were lost to follow-up were similar to those without any follow-up on major predictor of mortality [20]. Follow-up visits through June 2008 were included in this analysis. Sensitivity analyses were performed across a range of different assumptions (e.g., no follow-up, 6 months of follow-up and 2 years of follow-up), but overall inferences did not change. Standardized mortality ratios (SMRs) were calculated using the reference mortality rates among urban male patients residing in Tamil Nadu [21]. Additionally, SMRs were calculated against reference 2-year mortality rates among IDUs enrolled in the AIDS Linked to Intravenous Experience (ALIVE) cohort of IDUs between 1988–1989 in Baltimore, USA [22]. Multivariate Poisson regression was used to identify independent predictors of all-cause mortality. Variables included in the multivariate models included all those identified a priori to have an effect on mortality. Statistical analyses were performed using STATA Version 10.0 (College Station, Texas, USA).
Results
Description of study population
The median age at baseline was 35 years [interquartile range (IQR) 29–40], and all but three IDUs were male patients. The majority were married (64%); 27.9% had no formal education and 59.4% had only a primary or secondary level of education. Sixty-five% reported injecting drugs for nonmedical purposes for at least 5 years. Seventy-six percent reported injection drug use in the prior month and 78.9% alcohol use. Compared with HIV-negative participants, HIV-positive participants were less likely to be married and report alcohol use or recent sexual intercourse but had a longer duration of injection drug use, tended to be heavier injectors and were more likely to be injecting heroin (Table 1 Table 1). They also tended to more often report injecting at a dealer’s place.
Table 1.
Demographics and risk behaviors at baseline stratified by HIV status of injection drug users enrolled in the Madras Injection Drug Users and AIDS Cohort Study cohort, Chennai, India (n = 1158)
| Variable | Entire cohort (n = 1158) | HIV positivea (n = 293) | HIV negativea (n = 865) |
|---|---|---|---|
| [0,1–4]Age | |||
| Median (IQR) | 35 (29 – 40) | 35 (30 – 38) | 35 (29–40) |
| [0,1–4]Marital statusb | |||
| Married or live-in partner | 745 (64.3) | 161 (55) | 584 (67.5) |
| Single | 356 (30.7) | 105 (35.8) | 251 (29) |
| Separated | 41 (3.5) | 22 (7.5) | 19 (2.1) |
| Divorced/widowed | 16 (1.4) | 5 (1.7) | 11 (1.3) |
| [0,1–4]Highest level of education | |||
| None | 323 (27.9) | 87 (29.7) | 236 (27.3) |
| Primary | 393 (33.9) | 101 (34.5) | 292 (33.8) |
| Secondary | 295 (25.5) | 74 (25.3) | 221 (25.5) |
| High school/University/professional | 147 (12.7) | 31 (10.6) | 116 (13.4) |
| [0,1–4]Frequency of alcohol consumptionb | |||
| Never | 244 (21.1) | 93 (31.7) | 151 (17.5) |
| Light (≤2 drinks/day) | 684 (59.1) | 166 (56.7) | 518 (59.9) |
| Heavy (>3 drinks/day) | 230 (19.9) | 34 (11.6) | 196 (22.6) |
| [0,1–4]Years of injection drug useb | |||
| ≤1 year | 78 (6.7) | 7 (2.4) | 71 (8.2) |
| Greater than 1 – 5 years | 330 (28.5) | 57 (19.5) | 273 (31.6) |
| Greater than 5 – >10 years | 307 (26.5) | 87 (29.7) | 220 (25.4) |
| >10 years | 443 (38.3) | 142 (48.5) | 301 (34.8) |
| [0,1–4]Frequency of injection in prior monthb | |||
| None | 281 (24.3) | 57 (19.5) | 224 (25.9) |
| 1 – 30 times | 688 (59.4) | 163 (55.6) | 525 (60.7) |
| >30 times | 189 (16.3) | 73 (24.9) | 116 (13.4) |
| [0,1–4]Drugs injected in prior monthb | |||
| None | 281 (24.3) | 57 (19.5) | 224 (2.6) |
| Heroin only | 609 (52.6) | 199 (67.9) | 410 (47.4) |
| Buprenorphine only | 175 (15.1) | 16 (5.5) | 159 (18.4) |
| Heroin and buprenorphine | 93 (8.0_ | 21 (7.2) | 72 (8.3) |
| [0,1–4]Injected at dealers place in prior monthb | |||
| No | 1028 (88.8) | 239 (81.6) | 789 (91.2) |
| Yes | 130 (11.2) | 54 (18.4) | 76 (8.8) |
| [0,1–4]Incarceration in prior 6 months | |||
| No | 1010 (87.2) | 248 (84.6) | 762 (88.1) |
| Yes | 148 (12.8) | 45 (15.4) | 103 (11.9) |
| [0,1–4]Sexual intercourse in prior monthb | |||
| No | 636 (54.9) | 204 (69.6) | 432 (49.9) |
| Yes | 522 (45.1) | 89 (30.4) | 433 (50.1) |
IQR, interquartile range.
All numbers reported represent n (%) unless otherwise specified.
Statistically significant (P-value<0.05).
Mortality
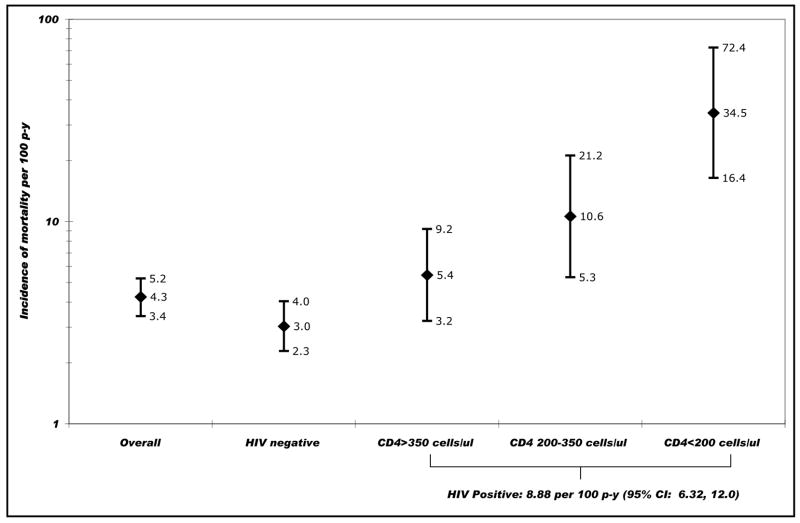
Eighty-five deaths were observed over 1998 p-y of follow-up; 48 deaths were observed among HIV-negative IDUs [IR 3.03 per 100 p-y; 95% confidence interval (CI) 2.29, 4.03] and 37 among HIV-positive IDUs (IR 8.88 per 100 p-y; 95% CI 6.32, 12.0). Among HIV-positive IDUs, the mortality rate increased with decreasing baseline CD4 cell counts (Fig. 1 Fig. 1).
Fig. 1.
Incidence of all-cause mortality in the Madras Injection Drug Users and AIDS Cohort Study cohort of injection drug users in Chennai, India (April 2005 to June 2008). gr1
CD4, absolute CD4+ T-lymphocyte count at baseline in cells/μl; CI, confidence interval; HIV negative, negative for HIV antibodies by double ELISA; HIV positive, positive for HIV antibodies by double ELISA; p-y, person-years.
The expected number of deaths for this cohort of IDUs using the age-specific mortality rates of urban men in Tamil Nadu was 7.67; SMR was 11.1. Thirty-seven deaths were observed against an expected 1.55 deaths among HIV-positive IDUs equating to an SMR of 23.9; among HIV-negative IDUs, the SMR was 7.83. Compared with the IDUs enrolled in the ALIVE cohort in 1988–1989, the SMRs for HIV-positive and HIV-negative IDUs were 2.80 and 2.91, respectively.
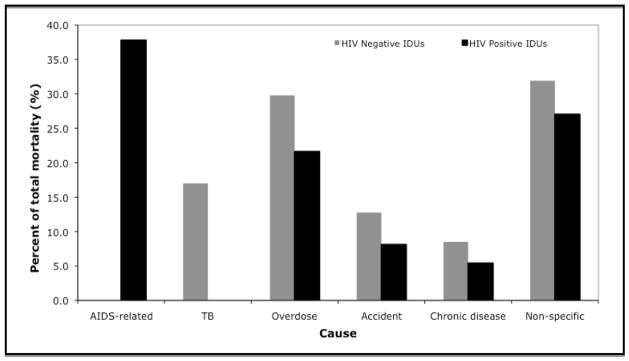
The distribution of cause of death by HIV serostatus is presented in Fig. 2 Fig. 2. Among HIV positive patients, 38% (n = 14) of deaths were AIDS related. The primary cause of non-AIDS mortality among HIV positive patients was drug overdose (n = 8), followed by accidents/suicides (n = 3) and chronic disease (n = 2). Among HIV negative patients, the leading cause of death was drug overdose (n = 14), followed by tuberculosis (n = 8), accidents/suicides (n = 6) and chronic disease (n = 4). Of the nine deaths attributable to accidents, four were reported as suicides.
Fig. 2.
Causes of mortality in the Madras Injection Drug Users and AIDS Cohort Study cohort of injection drug users in Chennai, India stratified by HIV status (April 2005–June 2008). gr2
The category accident also includes deaths due to suicides. IDUs, injection drug users.
Predictors of mortality
In univariate analysis, mortality rates were higher among persons who were HIV positive and immunocompromised and among anti-HCV-positive IDUs. IDUs who were older or were separated from their spouses also had higher mortality rates. Mortality rates were lower among persons who reported heavy alcohol use or recent sexual intercourse (Table 2 Table 2).
Table 2.
Risk factors for all-cause mortality among 1158 injection drug users in Chennai, India (2005–2008)
| Variable | Unadjusted incidence rate ratio (95% CI) | Adjusted incidence rate ratio (95% CI) |
|---|---|---|
| [0,1–3]HIV and absolute CD4 cell count | ||
| Negative | 1 | 1 |
| Positive, CD4 cells > 350 cells/μl | 1.79 (0.99, 3.25) | 1.32 (0.68, 2.56) |
| Positive, CD4 cells 201 – 350 cells/μl | 3.5 (1.65, 7.39) | 2.64 (1.18, 5.90) |
| Positive, CD4 cells < 200 cells/μl | 11.4 (5.1, 25.1) | 7.85 (3.31, 18.6) |
| [0,1–3]HBsAg status | ||
| Negative | 1 | 1 |
| Positive | 1.33 (0.70, 2.50) | 1.37 (0.67, 2.79) |
| [0,1–3]Anti-HCV status | ||
| Negative | 1 | 1 |
| Positive | 2.03 (1.28, 3.22) | 1.48 (0.81, 2.68) |
| [0,1–3]Age | ||
| Per 5-year increase | 1.19 (1.04, 1.36) | 1.18 (0.98, 1.42) |
| [0,1–3]Current marital status | ||
| Married/live-in partner | 1 | 1 |
| Single | 1.02 (0.63, 1.65) | 0.72 (0.40, 1.27) |
| Separated | 2.6 (1.18, 5.74) | 1.11 (0.42, 2.91) |
| Divorced/widowed | 0.88 (0.12, 6.38) | 0.64 (0.09, 4.71) |
| [0,1–3]Highest level of education | ||
| None | 1 | |
| Primary | 0.90 (0.53, 1.52) | |
| Secondary | 0.53 (0.28, 1.02) | |
| High school/University/professional | 1.23 (0.65, 2.31) | |
| [0,1–3]Frequency of alcohol consumption | ||
| Never | 1 | 1 |
| Light (<2 drinks/day) | 0.42 (0.26, 0.68) | 0.66 (0.39, 1.11) |
| Heavy (>3 drinks/day) | 0.48 (0.26, 0.89) | 0.86 (0.43, 1.74) |
| [0,1–3]Years of injection drug use | ||
| Per 2-year increase | 1.03 (0.97, 1.09) | 0.97 (0.89, 1.05) |
| [0,1–3]Frequency of injection in prior month | ||
| None | 1 | 1 |
| Less than daily | 0.90 (0.53, 1.52) | 0.98 (0.55, 1.74) |
| At least daily | 1.58 (0.86, 2.92 | 1.52 (0.77, 3.0) |
| [0,1–3]Drugs injected in prior month | ||
| Heroin only | 1 | |
| Buprenorphine only | 0.47 (0.21, 1.04) | |
| Heroin and buprenorphine | 1.21 (0.59, 2.46) | |
| [0,1–3]Injected at dealer’s place in prior months | ||
| No | 1 | |
| Ye | 1.09 (0.57, 2.09) | |
| [0,1–3]Incarceration in prior 6 months | ||
| No | 1 | |
| Yes | 0.97 (0.52, 1.84) | |
| [0,1–3]Sexual intercourse in prior month | ||
| No | 1 | 1 |
| Yes | 0.54 (0.37, 0.80) | 0.63 (0.37, 1.08) |
Blank cells indicate variables that were not included in the multivariate Poisson regression model. 95% CI, 95% confidence interval; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
In multivariate analysis, HIV-associated immunosuppression remained the only predictor significantly associated with increased mortality risk. Compared with those who were HIV uninfected, no significant increased risk was observed among HIV-positive IDUs with CD4 cell counts more than 350 cells/μl [incidence rate ratio (IRR) 1.32; 95% CI 0.68, 2.56]. However, compared with HIV-negative persons, those with CD4 cell counts 200–350 cells/μl had 2.64 times the risk of mortality (95% CI 1.18, 5.90), and those with CD4 cell counts less than 200 cells/μl had 7.85 times the risk of mortality (95% CI 3.31, 18.6) after adjusting for age, duration and frequency of drug use, anti-HCV status, HbsAg status, alcohol consumption, marital status and history of sexual intercourse.
When the multivariate model was restricted to HIV-negative patients, the effects of age (IRR 1.27; 95% CI 1.04, 1.56) and greater than daily injection frequency (IRR 2.15; 95% CI 0.95, 4.88) strengthened. Further, among HIV-negative IDUs, those who combined heroin and alcohol use were 2.18 times (95% CI 0.93, 5.13; P-value = 0.07) more likely to die compared with those who combined other drugs or did not combine drugs with alcohol.
Discussion
IDUs in Chennai have high risk of premature mortality. The all-cause mortality rate observed in this cohort (4.3 per 100 p-y) was more than twice that estimated in a recent meta-analysis among IDUs (1.86 per 100 p-y) [23], but the majority of studies in this meta-analysis were initiated prior to the AIDS epidemic, and all were conducted in developed countries. To date, few reports of mortality derive from IDUs in the developing world. Two reports from Thailand have suggested mortality rates among HIV-negative IDUs to be 1.4 per 100 p-y and 3.85 per 100 p-y [24,25], the latter of which is comparable to the estimate from our cohort.
HIV has historically been the leading cause of death among IDUs. However, the introduction of HAART in 1996 dramatically reduced HIV-related mortality among IDUs. [26,27] For example, in the US ALIVE cohort, mortality declined in the HAART era with maximal benefit observed among IDUs with lower CD4+ cell counts [27]. Among IDUs with a CD4+ cell count less than 50 cells/μl at enrollment, the mortality rate declined from 44.25 per 100 p-y in the pre-HAART era to 11.53 per 100 p-y in the HAART era. By contrast, the HAART era mortality rate among HIV-positive IDUs with CD4 cells less than 200 cells/μl in our cohort was 34.5 per 100 p-y. Further, the SMR compared with HIV-positive IDUs in ALIVE reflected nearly three-fold higher mortality risk. This is particularly concerning because the reference rates from ALIVE are from the pre-HAART era (1988–1991). Possible explanations for the higher mortality rate in Chennai include limited access to VCT or HIV medical care or both. Although HAART has been available via free government rollout programs since 2004, none of the IDUs in MIDACS was receiving any HIV medical care at enrollment.
The high mortality rate among persons who entered the cohort with a CD4 cells le4sws than 350 cells/μl further highlights the lack of access to HAART. In fact, HIV-positive IDUs who were not immunosuppressed at baseline had mortality rates comparable to HIV-negative IDUs, suggesting that effective HAART use in this population would substantially impact mortality. In spite of referring eligible IDUs to the government rollout centers for initiation of HAART (HAART is not available free-of-charge at YRGCSAR), only a small proportion actually sought care and remained adherent to their medications.
Even though HIV-associated immunosuppression was the leading risk factor for mortality, AIDS-related deaths accounted for a lower proportion of mortality than has been observed in other cohorts of IDUs [26]. This might reflect the short follow-up; competing risks from overdose or accidents/suicides; stigma to report HIV as a cause of death; and/or relatively high CD4 cell counts at baseline (median: 461 cells/μl; only 29% had CD4 cell counts < 350 cells/μl). It is likely that the proportion of AIDS-related deaths will increase as HIV disease progresses if HAART access does not improve.
Despite the relatively low proportion of AIDS-related deaths, HIV may have also contributed to non-AIDS mortality. The most common cause of death was heroin overdose (26%), which is consistent with other reports [28]. The high rates of overdose mortality in this cohort may reflect the impact of polydrug use, in particular heroin and other central nervous system (CNS) depressants such as alcohol, synthetic/semi-synthetic opiates and/or benzodiazepines; variability in heroin purity; or the effect of HIV. Fatalities due to heroin overdose occur primarily because of respiratory depression, which is further accentuated when combined with CNS depressants such as alcohol [29,30]. Although no independent effect of alcohol was found, we observed high levels of alcohol use as well as an increased risk of mortality among HIV-negative IDUs who combined heroin with alcohol (7% of the population). Further, users have reported that levels of purity are generally low but that from time to time when a higher grade of heroin enters the market, overdoses follow (data not shown). Finally, HIV itself has been suggested as a risk factor for heroin overdose via different mechanisms including pulmonary coinfections that accentuate respiratory depression caused by heroin/opiates, and severe liver disease particularly among IDUs coinfected with HIV and HCV that can affect heroin metabolism [28]. Limited sample size and lack of markers of liver disease stage, respectively, restricted our abilities to test these hypotheses.
TB was another important cause of death in this cohort. Proportionate mortality due to TB would have likely been higher (11.8%) if deaths due to TB among HIV-positive persons with CD4 cells less than 350 cells/μl had not been classified as AIDS related. High rates of TB mortality may be explained by active injection drug use itself as has been seen in Russia [9] or delayed access to care and/or suboptimal adherence to anti-TB medications as has been shown with HAART [31,32].
Mortality due to chronic conditions such as liver or cardiovascular disease was relatively low in this cohort; this trend reflects mortality patterns in developed countries in the pre-HAART era. Similar patterns of increases in proportionate mortality attributable to chronic diseases could follow when access to HAART improves. Of particular concern is liver disease given the high prevalence of HCV, high rates of alcohol consumption and high burden of underlying comorbidities, the management of which includes hepatotoxic agents (e.g., nevirapine for HIV or rifampin for TB).
One of the major limitations of this study was ascertainment of cause of death. Although autopsy reports/death certificates remain the gold standard, less than 14% of all deaths in India are medically certified [21]. Therefore, reported cause of mortality by relatives/friends remains the only viable option. The strong rapport between field staff and participants, as well as between participants, ensured the highest quality of data that could be achieved in this setting. The lack of consistent clinical information on all IDUs (e.g., TB status, liver disease stage) limited our ability to include these factors in multivariate analysis. Finally, as most of the HIV-positive IDUs were followed only as part of the clinical cohort, risk behavior information was available only at baseline. Given the short duration of follow-up, it is unlikely that accounting for time-varying behaviors would have affected our findings.
Long-term follow-up of this cohort and further data collection (e.g., comorbidities) will further clarify the role of infectious and chronic diseases on mortality outcomes among IDUs in Chennai. For now, the importance of improving access to harm reduction interventions and healthcare, which have been successful in other settings, is reflected by the high degree of premature mortality observed in this cohort. The alarmingly high mortality rate among HIV-positive IDUs who entered the study with advanced immunosuppression reinforces the need for initiatives to improve access to HIV medical services and HAART in this population. These data may also provide insight into similar situations in other developing countries with burgeoning HIV and IDU epidemics including Central Asia and the Former Soviet Union countries. Sustainable global strategies targeted at IDUs are urgently needed.
Acknowledgments
The authors would like to thank the staff at YRGCSAR and YRGCARE who helped with the implementation of this study, especially the field staff. This study was supported in part by the National Institutes of Health, USA (DA12568, DA18577, DA15616) and the Indian Council of Medical Research (ICMR), India, as part of the US-India Bilateral Collaborative Research on the Prevention of HIV/AIDS, and the Fogarty International Center/USNIH: (Grant # 2D 43 TW000010-20-AITRP). Most importantly, we would like to thank the participants without whom this article would not have been possible. S.S.S. and S.H.M. contributed to study design, statistical analysis and drafting of the manuscript; D.D.C., S.S., M.S.K. and G.M.L. contributed to study design and critical review of the manuscript; design; A.K.S. and C.K.V. recruited participants and contributed to study design; S.A. contributed to data analysis. There was no conflict of interest.
References
- 1.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 2.Hulse GK, English DR, Milne E, Holman CD. The quantification of mortality resulting from the regular use of illicit opiates. Addiction. 1999;94:221–229. doi: 10.1046/j.1360-0443.1999.9422216.x. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JR, Ronald PJ, Raab GM, Ross AJ, Parpia T. Deaths, HIV infection, abstinence, and other outcomes in a cohort of injecting drug users followed up for 10 years. BMJ. 1994;309:369–372. doi: 10.1136/bmj.309.6951.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Copeland L, Budd J, Robertson JR, Elton RA. Changing patterns in causes of death in a cohort of injecting drug users, 1980–2001. Arch Intern Med. 2004;164:1214–1220. doi: 10.1001/archinte.164.11.1214. [DOI] [PubMed] [Google Scholar]
- 5.Bargagli AM, Sperati A, Davoli M, Forastiere F, Perucci CA. Mortality among problem drug users in Rome: an 18-year follow-up study, 1980–97. Addiction. 2001;96:1455–1463. doi: 10.1046/j.1360-0443.2001.961014559.x. [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 7.Palella FJJ, Jr , Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 8.Lumbreras B, Jarrin I, del Amo J, Perez-Hoyos S, Muga R, Garcia-de la Hera M. Impact of hepatitis C infection on long-term mortality of injecting drug users from 1990 to 2002: differences before and after HAART. AIDS. 2006;20:111–116. doi: 10.1097/01.aids.0000196164.71388.3b. [DOI] [PubMed] [Google Scholar]
- 9.Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MKJ., Jr Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis. 2006;10:1224–1230. [PubMed] [Google Scholar]
- 10.Sarkar S, Das N, Panda S, Naik TN, Sarkar K, Singh BC. Rapid spread of HIV among injecting drug users in north-eastern states of India. Bull Narc. 1993;45:91–105. [PubMed] [Google Scholar]
- 11.United Nations Office on Drugs and Crime (UNODC) [Accessed on 18 November 2008];World Drug Report 2007. at http://www.unodc.org/pdf/research/wdr07/WDR_2007.pdf.
- 12.National AIDS Control Organization (NACO), India. [Accessed 18 November 2008];HIV Sentinel Surveillance and HIV Estimation in India 2007 - A Technical Brief. at http://www.nacoonline.org/upload/Publication/M&E%20Surveillance,%20Research/HIV%20Sentinel%20Surveillance%20and%20HI %20Estimation%202007_A%20Technical%20Brief.pdf.
- 13.Aceijas C, Friedman SR, Cooper HL, Wiessing L, Stimson GV, Hickman M. Estimates of injecting drug users at the national and local level in developing and transitional countries, and gender and age distribution. Sex Transm Infect. 2006;82(Suppl 3):iii10–iii17. doi: 10.1136/sti.2005.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorabjee J, Samson L. A multicentre rapid assessment of injecting drug use in India. Int J Drug Policy. 2000;11:99–112. doi: 10.1016/s0955-3959(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 15.Panda S, Kumar MS, Lokabiraman S, Jayashree K, Satagopan MC, Solomon S. Risk factors for HIV infection in injection drug users and evidence for onward transmission of HIV to their sexual partners in Chennai, India. J Acquir Immune Defic Syndr. 2005;39:9–15. doi: 10.1097/01.qai.0000160713.94203.9b. [DOI] [PubMed] [Google Scholar]
- 16.Solomon SS, Srikrishnan AK, Mehta SH, Vasudevan CK, Murugavel KG, Thamburaj E. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49:327–332. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon S, Solomon SS, Ganesh AK. AIDS in India. Postgrad Med J. 2006;82:545–547. doi: 10.1136/pgmj.2006.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aceijas C, Oppenheimer E, Stimson GV, Ashcroft RE, Matic S, Hickman M. Antiretroviral treatment for injecting drug users in developing and transitional countries 1 year before the end of the ‘Treating 3 million by 2005. Making it happen. The WHO strategy’ (‘3 by 5’) Addiction. 2006;101:1246–1253. doi: 10.1111/j.1360-0443.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 19.Solomon SS, Hawcroft CS, Narasimhan P, Subbaraman R, Srikrishnan AK, Cecelia AJ. Comorbidities among HIV-infected injection drug users in Chennai, India. Indian J Med Res. 2008;127:447–452. [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon SS, Celentano DD, Srikrishnan AK, Vasudevan CK, Murugavel KG, Iqbal SH, et al. Low HIV incidence and declining risk behaviors in a cohort of injection drug users (IDUs) in Chennai, India; 15th Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2008. [Google Scholar]
- 21.Central Bureau of Health Intelligence (CGHI), India. [Accessed 18 November 2008];Mortality Statistics in India 2006. at http://www.cbhidghs.nic.in/writereaddata/mainlinkfile/File976.pdf.
- 22.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 23.Degenhardt L, Hall W, Warner-Smith M. Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS. Sex Transm Infect. 2006;82(Suppl 3):iii56–iii63. doi: 10.1136/sti.2005.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanichseni S, Kitayaporn D, Mastro TD, Mock PA, Raktham S, Des Jarlais DC. Continued high HIV-1 incidence in a vaccine trial preparatory cohort of injection drug users in Bangkok, Thailand. AIDS. 2001;15:397–405. doi: 10.1097/00002030-200102160-00013. [DOI] [PubMed] [Google Scholar]
- 25.Quan VM, Vongchak T, Jittiwutikarn J, Kawichai S, Srirak N, Wiboonnatakul K. Predictors of mortality among injecting and noninjecting HIV-negative drug users in northern Thailand. Addiction. 2007;102:441–446. doi: 10.1111/j.1360-0443.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferreros I, Lumbreras B, Hurtado I, Perez-Hoyos S, Hernandez-Aguado I. The shifting pattern of cause-specific mortality in a cohort of human immunodeficiency virus-infected and noninfected injecting drug users. Addiction. 2008;103:651–659. doi: 10.1111/j.1360-0443.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- 27.Galai N, Vlahov D, Bareta JC, Wang C, Cohn S, Sterling TR. Prognostic factors for survival differ according to CD4+ cell count among HIV-infected injection drug users: pre-HAART and HAART eras. J Acquir Immune Defic Syndr. 2005;38:74–81. doi: 10.1097/00126334-200501010-00014. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Vlahov D, Galai N, Cole SR, Bareta J, Pollini R. The effect of HIV infection on overdose mortality. AIDS. 2005;19:935–942. doi: 10.1097/01.aids.0000171407.30866.22. [DOI] [PubMed] [Google Scholar]
- 29.Hickman M, Lingford-Hughes A, Bailey C, Macleod J, Nutt D, Henderson G. Does alcohol increase the risk of overdose death: the need for a translational approach. Addiction. 2008;103:1060–1062. doi: 10.1111/j.1360-0443.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 30.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–972. [PubMed] [Google Scholar]
- 31.Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 32.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]