Abstract
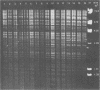
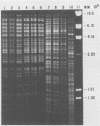
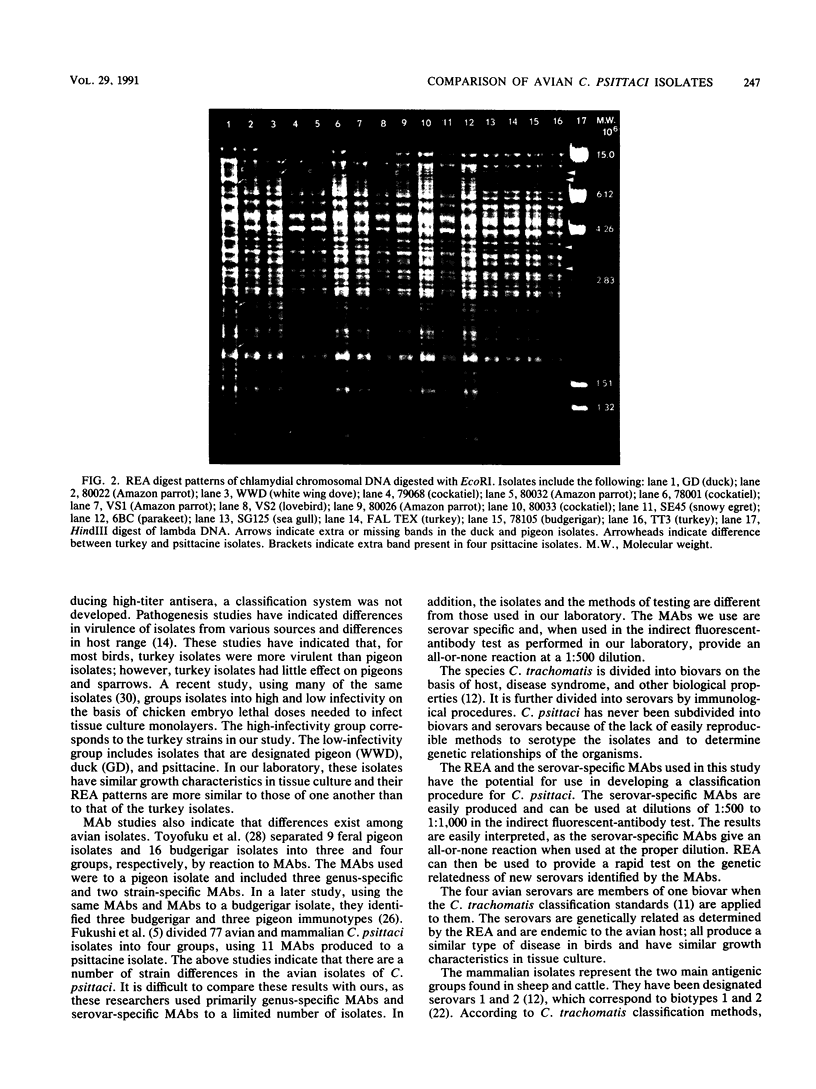
Avian Chlamydia psittaci isolates were examined by restriction endonuclease analysis and serovar-specific monoclonal antibodies and compared with ovine abortion and polyarthritis isolates. The avian isolates were divided into four serovars (turkey, psittacine, pigeon, and duck) based on their reactivity to the monoclonal antibodies. The DNA digest patterns were similar across the four avian serovars; most bands were identical when the isolates were tested with PstI, BamHI, and EcoRI restriction endonuclease enzymes. The turkey group restriction endonuclease analysis patterns were distinguished from those of the other avian strains by three to four band differences with all enzymes. The duck and pigeon isolates showed only minor DNA pattern differences when compared with the psittacine isolates. Four psittacine isolates from various locations in Texas had an extra band with the EcoRI restriction enzyme, suggesting that they were from a common source; however, they were indistinguishable from the other psittacine isolates when examined with the monoclonal antibodies. The avian isolates were distinctly different from either abortion or polyarthritis isolates by both restriction endonuclease analysis and monoclonal antibody analysis. The data demonstrate that the avian isolates form a distinct group or separate biovar with at least four serovars.
Full text
PDF

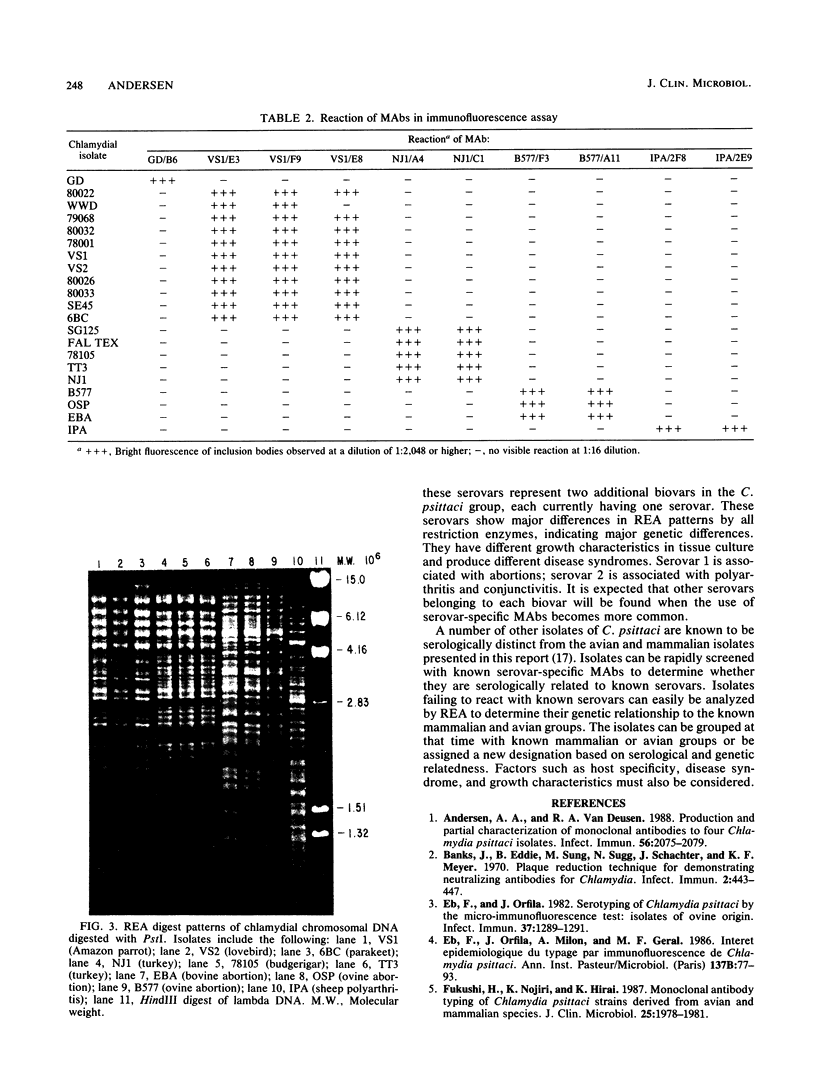

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. A., Van Deusen R. A. Production and partial characterization of monoclonal antibodies to four Chlamydia psittaci isolates. Infect Immun. 1988 Aug;56(8):2075–2079. doi: 10.1128/iai.56.8.2075-2079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J., Eddie B., Sung M., Sugg N., Schachter J., Meyer K. F. Plaque reduction technique for demonstrating neutralizing antibodies for Chlamydia. Infect Immun. 1970 Oct;2(4):443–447. doi: 10.1128/iai.2.4.443-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eb F., Orfila J., Milon A., Géral M. F. Intérêt épidémiologique du typage par immunofluorescence de Chlamydia psittaci. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):77–93. [PubMed] [Google Scholar]
- Eb F., Orfila J. Serotyping of Chlamydia psittaci by the micro-immunofluorescence test: isolates of ovine origin. Infect Immun. 1982 Sep;37(3):1289–1291. doi: 10.1128/iai.37.3.1289-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Nojiri K., Hirai K. Monoclonal antibody typing of Chlamydia psittaci strains derived from avian and mammalian species. J Clin Microbiol. 1987 Oct;25(10):1978–1981. doi: 10.1128/jcm.25.10.1978-1981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez J. A., Storz J. Antigenic diversity of Chlamydia psittaci of mammalian origin determined by microimmunofluorescence. Infect Immun. 1985 Dec;50(3):905–910. doi: 10.1128/iai.50.3.905-910.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., KISSLING R. E., CHAMBERLAIN R. W., EIDSON M. E. Isolation of a psittacosis-like agent from the blood of snowy egrets. Proc Soc Exp Biol Med. 1951 Dec;78(3):696–698. doi: 10.3181/00379727-78-19185. [DOI] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia: isolates of bovine origin. Infect Immun. 1975 May;11(5):904–907. doi: 10.1128/iai.11.5.904-907.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Chlamydia psittaci: growth characteristics and enumeration of serotypes 1 and 2 in cultured cells. J Infect Dis. 1979 Dec;140(6):959–967. doi: 10.1093/infdis/140.6.959. [DOI] [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Takashima I., Hashimoto N. Immunotyping of Chlamydia psittaci by indirect immunofluorescence antibody test with monoclonal antibodies. Microbiol Immunol. 1988;32(3):251–263. doi: 10.1111/j.1348-0421.1988.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Thiermann A. B., Handsaker A. L., Moseley S. L., Kingscote B. New method for classification of leptospiral isolates belonging to serogroup pomona by restriction endonuclease analysis: serovar kennewicki. J Clin Microbiol. 1985 Apr;21(4):585–587. doi: 10.1128/jcm.21.4.585-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku H., Takashima I., Arikawa J., Hashimoto N. Monoclonal antibodies against Chlamydia psittaci. Microbiol Immunol. 1986;30(10):945–955. doi: 10.1111/j.1348-0421.1986.tb03025.x. [DOI] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor D. K., Jr, Grimes J. E. Relationship between infectivity and cytopathology for L-929 cells, membrane proteins, and antigenicity of avian isolates of Chlamydia psittaci. Avian Dis. 1988 Jul-Sep;32(3):421–431. [PubMed] [Google Scholar]