Abstract
Postoperative nausea and vomiting (PONV) remains a significant problem in modern anesthetic practice, with an incidence in high-risk groups of up to 80%. In addition to being unpleasant and distressing for the patient, PONV has the potential to adversely affect patient and surgical outcomes. Advances in PONV prophylaxis over recent years include using non-pharmacological means to reduce baseline risk, a change to less emetogenic anesthetic techniques and the combination of multiple antiemetic drugs. The 5-hydroxytryptamine-3 (5-HT3) antagonists have proven a particularly valuable addition to the armamentarium against PONV. Palonosetron is a second-generation 5-HT3 antagonist that has recently been approved for prophylaxis against PONV. It has unique structural, pharmacological and clinical properties that distinguish it from other agents in its class. This review summarizes current evidence on PONV prophylaxis, reviews the 5-HT3 antagonists in particular and focuses on the established and future roles of palonosetron.
Keywords: palonosetron, antiemetics, 5-HT3 antagonists, postoperative nausea and vomiting
Management of postoperative nausea and vomiting: an overview
Postoperative nausea and vomiting (PONV) is the most common complication of surgery and anesthesia. Both health care professionals and patients rate its avoidance and control of similar importance to that of alleviating pain.1–4 In addition to patient dissatisfaction,5 PONV may have adverse consequences such as delayed recovery, unexpected hospital admission and delayed return to work of ambulatory patients. Rarely postsurgical morbidities such as wound dehiscence, pulmonary aspiration, surgical site bleeding and dehydration occur.6 Nausea occurs in approximately 20% of patients in the recovery room and in 50% thereafter, with vomiting in 5% and 25% respectively.7 Although children more than 3 years of age are at higher risk than adults,8 in some high-risk adult populations the incidence of PONV is 80% or more.9,10
It is difficult to quantify the risk of PONV for any individual patient both because of the many pre-, intra-and postoperative factors that contribute to PONV and uncertainty about the relative impact of these potential influences. Activation of the vomiting center or the sensation of nausea may result from stimulation of the chemoreceptor trigger zone (eg, drugs, metabolic stimuli), the vestibular apparatus (motion), visceral afferent inputs (eg, gut distension or stasis, surgical stimulation of viscera, cardiovascular disturbance) and cortical inputs (eg, anxiety, pain, hypoxia, sensory stimuli, psychological associations, raised intracranial pressure). At least 3 nerves and 7 neurotransmitters are involved, making prophylaxis and treatment complex. In general a number of patient, surgical and anesthetic factors affect the risk of PONV6 and various patient risk assessment scores have been developed. The best known and validated is a simple 4-point score based mainly on patient characteristics. These are female gender, non-smoking habit, past history of motion induced or postsurgical nausea and vomiting, and postoperative opioid requirement.11,12 Prediction of outcome in the individual patient is imperfect, but management based on risk stratification of surgical sub-populations can reduce overall institutional rates of PONV.13,14 The duration of surgery (and anesthesia) is also a risk factor and some surgical procedures (eg, laparoscopy, strabismus surgery) are thought to confer higher risk, especially for nausea.15,16 Other established factors are younger patient age,6,16 higher intra-and postoperative opioid requirement6,17 and the type of anesthetic. Regional anesthesia is associated with a much lower risk than general anesthesia,16 with significant risk factors for the latter being maintenance with volatile agents rather than propofol,18 use of nitrous oxide19–21 and inadequate intravenous fluid loading.22–24
Universal pharmacological prophylaxis against PONV is not warranted.25,26 If 30 of 100 people would feel sick or vomit after surgery and all 100 were given a prophylactic antiemetic drug, 10 would benefit and 90 would not, and 1 to 5 would suffer a mild side effect such as headache or sedation.27 Therefore non-pharmacological strategies to reduce the baseline risk of PONV should be considered. Level I evidence supports techniques such as acupuncture, acustimulation or acupressure from wrist-bands applied at the Chinese P6 (Neiguan or Nei-Kuan) point near the wrist or at a number of Korean acupressure points on the fingers. These produce up to a one-third reduction in PONV, making them more effective than ondansetron against nausea, and they have an excellent side effect profile.28–30 Avoidance of general anesthesia or minimizing opioid requirement through the use of regional anesthesia might be appropriate,16 intravenous fluid (eg, 2 mL/kg for each hour of fasting) can be considered in ambulatory and high-risk inpatients,24 nitrous oxide avoided,19,20 and propofol used for both induction and maintenance of general anesthesia (reciprocal of the absolute risk reduction or number-needed-to-treat [NNT] of 5 to prevent PONV within 6 hours of surgery).18 Using total intravenous anesthesia is as effective as giving a single antiemetic such as ondansetron.19,31 In addition a multimodal analgesic regimen should be used to minimize opioid dose requirements.17
The cost-benefit analysis of providing prophylaxis (rather than treatment) of PONV with antiemetic drugs is determined by the efficacy of the drug, its cost, and the consequences of the event. If the baseline risk is high, pharmacological intervention with a multimodal approach is justified.32,33 Approximately 20 drugs show efficacy, although only about 8 drugs are of proven reliability.27 Of these, there is little evidence that any one is better than another and most have a NNT of 4 to 7. This means, for example, that a reduction in risk of PONV from 80% to 60% represents a 25% relative risk reduction and a 20% absolute risk reduction or NNT of 5. When the baseline risk is only 10% a similar relative risk reduction produces an absolute risk reduction of only 2.5% and a NNT of 40.
Interventions that have proven ineffective for prophylaxis of PONV include ginger root and the cannabinoids,34,35 while intravenous (iv) metoclopramide 10 mg shows poor efficacy (no anti-nausea effect and NNT to prevent vomiting of 10).36,37 The antihistamine and phenothiazine drug classes (eg, promethazine 12.5–25 mg iv, dimenhydrinate 25–50 mg iv, prochlorperazine 5–12.5 mg iv, cyclizine 50 mg iv) show efficacy but clinical utility is limited, particularly in ambulatory surgical patients, because of sedation.38–43 Similar problems beset transdermal scopolamine44 which also requires application at least four hours pre-operatively due to its slow onset. Side effects such as visual disturbance (number-needed-to-harm [NNH] of 5), dry mouth (NNH 12), dizziness (NNH 50) and agitation (NNH 100) tend to persist and limit its value, particularly in the elderly.
The butyrophenone droperidol remains the most cost effective drug for the prophylaxis of PONV in adults,45–49 despite recent issues relating to a ‘Black Box’ regulatory warning from the US Federal Drug Administration (FDA) in relation to possible cardiac conduction delay. It is cheap, has an NNT of 5 for both nausea and vomiting (NNT of 3 when added to patient-controlled intravenous morphine), with administration at the end of prolonged surgery recommended because of its short duration of action. Droperidol is one of the few antiemetic drugs to show a dose-response relationship.27 Low doses (500 μg to 1.25 mg) are effective and minimize sedative and extrapyramidal side effects, both of which can be worrisome for children and ambulatory surgical patients.50 Cardiovascular events are extremely unlikely, because QT prolongation in the antiemetic dose range is not significant,51,52 and as such its FDA warning has now been downgraded.
Dexamethasone 4 to 5 mg iv is a cheap, long acting antiemetic drug that shows efficacy against both nausea and vomiting (NNT 4).53–55 Early administration is recommended because it can prevent both early and late (up to 24 hours) PONV. After a single dose, dexamethasone appears to have an excellent side effect profile, although its effects on immune function, and the potential for adverse outcomes such as wound infection, have not been studied.
The 5-hydroxytryptamine-3 (5HT3) receptor antagonists are popular prophylactic drugs and are considered in more detail in the next section. The neurokinin-1 (NK1) receptor antagonists (aprepitant, rolapitant and casopitant) have an established indication for the prevention of chemotherapy-induced nausea and vomiting and are now also undergoing evaluation for PONV. These drugs appear particularly useful in preventing emesis. Aprepitant has recently been approved by the FDA for prophylaxis of PONV and preoperative oral aprepitant 40 mg has greater efficacy than ondansetron 4 mg IV against vomiting.56
Patients thought to be at moderate or high risk of PONV should receive multimodal prophylaxis57,58 that includes 2 or 3 antiemetic drugs from different drug classes. Each drug is likely to result in a similar relative risk reduction, giving an additive but declining absolute effect.19 Many studies confirm the value of combining two or more antiemetic drugs and this has led to the propagation of evidence-based guidelines.59–64 Nevertheless, data on optimal dose combinations are scarce and lower doses than used for monotherapy may be effective.65
The treatment of established PONV should be modified based on previous preventative measures and prophylactic drug therapies. Before management with antiemetic drugs, it may be possible to reduce symptoms by changing to an alternative analgesic or by adding adjuncts that reduce opioid dose consumption.66 Surgical, mechanical or incidental causes of nausea and emesis should also be excluded. If the patient has not received prophylactic antiemetic drugs, many of these drugs will show efficacy as treatment at lower dosage than when used for prophylaxis (eg, iv ondansetron 1 mg or iv promethazine 6.25 mg).67,68 In general a rescue dose with a drug of the same class should not be given within 6 hours, and dexamethasone or scopolamine should not be repeated.62 Although potentially effective in some circumstances,69,70 sedative and anxiolytic drugs such as midazolam (1–2 mg and then 1–2 mg/h) or propofol (15–20 mg and then 15–20 μg/kg/min) are infrequently used for prophylaxis. However, they offer valuable treatment options71–73 and are as efficacious as iv ondansetron.70,73 If PONV appears specifically opioid-induced, low-dose naloxone (eg, 0.25 μg/kg/h) is also effective, without reversing analgesia.74
5-hydroxytryptamine antagonists in the management of PONV
The potential value of 5-hydroxytryptamine (serotonin) receptor antagonists was discovered through the study of metoclopramide in the 1980s. The finding that, at high doses, metoclopramide showed activity at serotonin ‘M’ receptors (now known as 5-hydroxytryptamine type 3 [5-HT3] receptors) led to the development of specific receptor antagonists. Ondansetron was the first drug to become commercially available for PONV and has been followed by many others, including granisetron, dolasetron, tropisetron, ramosetron, azasetron and palonosetron. The 5-HT3 antagonists compare favorably with other antiemetic drugs,75 showing a NNT of 5 to 6 for prophylaxis against vomiting and 6 to 7 against nausea.67,76 Their efficacy is similar to droperidol or dexamethasone for the prevention of vomiting in adults,19 and their favorable side-effect profile has made them a popular choice in both adult and pediatric surgical populations. Because each of the 5-HT3 antagonists shows a generally similar efficacy and side effect profile, the choice of drug is often governed by local availability and cost considerations.77–80 However knowledge of the differences in their pharmacokinetics, receptor affinity and pharmacogenetically-influenced responses allows a more objective approach to drug selection.
Mechanism of action
5-HT3 receptors are found in the gut and in areas of the central nervous system associated with the regulation of nausea and vomiting, being abundant in the chemoreceptor trigger zone of the area postrema81,82 which has projections to the vomiting center located in the lateral reticular formation of the medulla oblongata. Stimulation of these receptors initiates the vomiting reflex.83 Peripheral 5-HT3 receptors are located in vagal nerve terminals, which are linked to the vomiting center via the nucleus tractus solitarius.83 Competitive antagonism with 5-HT3 receptor antagonists at these sites, and probably others, can block initiation of the vomiting reflex caused by emetogenic stimuli.
Pharmacokinetics
Azasetron (Serotone®, Yoshitomi Pharmaceuticals) is licensed for PONV in Japan and Argentina. It is a benzamine derivative which exhibits potent and selective 5-HT3 receptor antagonism. It has a terminal half-life of 6 to 8 hours and 60% to 70% of the active drug is excreted unchanged in the urine.84
Dolasetron (Anzemet®, Sanofi-Aventis) is a prodrug that is rapidly metabolized by carbonyl reductase (elimination half-life [t1/2β] less than 10 minutes) to the active form hydrolosetron. Hydrolosetron (t1/2β 7 hours) is predominantly metabolized in the liver via the cytochrome P450 enzyme CYP2D6. Hydrolosetron is mainly (53%) excreted unchanged in the urine.85
Granisetron (Kytril®, Roche) is unique among the 5-HT3 antagonists in that its liver metabolism is by the cytochrome P450 CYP3A isoenzyme. Its t1/2β is 5–8 hours, and 12% is excreted unchanged in the urine.86
Ondansetron (Zofran®, GlaxoSmithKline) has a relatively short t1/2β (3–5 hours) and undergoes extensive liver metabolism, primarily via the CYP3A4 isoenzyme, although CYP2D6 is an important secondary pathway. Some of its metabolites exhibit pharmacological activity but their plasma concentrations are too low to be clinically important. Five percent is excreted unchanged in the urine.87
Ramosetron (Nasea®, Astella Pharma) is licensed for use in Japan and Thailand, and has an additional indication for treatment of irritable bowel disease. It has a t1/2β of 4 to 9 hours, but a high receptor affinity prolongs its duration of action.88
Tropisetron (Navoban®, Novartis) undergoes extensive is liver metabolism by the CYP2D6 isoenzyme. Its t1/2β is 8 hours, and 8% is excreted unchanged in the urine.89
Pharmacodynamics
The duration of therapeutic effect of the various 5-HT3 antagonists is influenced by factors other than the elimination half-life, and appears more closely associated with their binding affinity for the 5-HT3 receptor.90 Skin-flare testing, in which inhibition of cutaneous 5-HT3 receptors is used as a surrogate marker, shows that some drugs have a longer clinical effect than their elimination half-life might suggest. For example cutaneous 5-HT3 inhibition lasts 9 hours after ondansetron and more than 24 hours after a single intravenous dose (40 μg/kg) of granisetron.91 This probably reflects granisetron’s high receptor affinity, demonstrated in vitro by the displacement of ondansetron but not granisetron by high receptor concentrations of 5-HT. Other 5-HT3 antagonists with insurmountable receptor binding are tropisetron and palonosetron.92,93 Half-lives and receptor affinities for the various 5-HT3 antagonists are shown in Table 1.
Table 1.
Half-lives and 5-HT3 receptor binding affinity for 5-HT3 antagonists
| Drug | Binding affinity (p Ki) | Half-life in healthy adult volunteers (h) |
|---|---|---|
| Azasetron | No data | 6–8 |
| Dolasetrona | 9.8b | 6.9–7.3 |
| Granisetron | 8.42 | 4.9–7.7 |
| Ondansetron | 8.07 | 3.5–5.5 |
| Ramosetron | 8.5–9.0b | 4.3–9.0 |
| Tropisetron | 8.81 | 8 |
| Palonosetron | 10.4 | 40 |
Values are those of the active metabolite hydrolosetron.
Antagonist affinity (pA2).
Pharmacogenetics
Various genetic factors influence an individual’s response to drugs and genetic polymorphism plays a role in the metabolism, transport and receptor binding of the 5-HT3 antagonists.
CYP2D6 genetic polymorphism
Phase I metabolism of the 5-HT3 antagonists occurs in the liver by the cytochrome P450 enzyme system, the most important isoenzyme for which is cytochrome P4502D6 (CYP2D6). This isoenzyme is responsible for the metabolism of many drugs94 and there is significant inter-individual variability in its activity. The gene encoding CYP2D6 lies on chromosome 22q13.195,96 and gene variants can alter enzymic activity, such that individuals can be classified as poor, intermediate, extensive and ultra-rapid metabolizers. Most of the population have the ‘wild-type’ 2D6 allele and are extensive (normal) metabolizers.97 Ultrarapid metabolizers typically display gene duplications and the resultant increase in enzyme activity may lead to sub-therapeutic plasma concentrations despite usual doses.98 Ethnic variability in the prevalence of ultra-rapid metabolizers is pronounced, varying from a low incidence in Caucasians (2%), 7% in parts of Spain (possibly due to Moorish colonization prior to the 15th century), to 20% to 29% in Arabic countries and Ethiopia.94,99–101
Although the CYP2D6 system is the dominant metabolic pathway for the 5-HT3 antagonists such as dolasetron, tropisetron and palonosetron, it is less influential for ondansetron which is primarily metabolized by CYP3A4. Granisetron’s metabolism is entirely independent of CYP2D6, undergoing transformation by CYP3A (Table 2). Genetic polymorphisms of CYP2D6 can influence clinical efficacy. Higher rates of vomiting occur in ultrarapid metabolizers treated with tropisetron (and to a lesser extent ondansetron) than in extensive or poor metabolizers.102,103 In contrast granisetron is unaffected by ultrarapid metabolizer status, and despite palonosetron undergoing CYP2D6 metabolism a small study found no difference in efficacy between poor and extensive metabolizers (although no ultrarapid metabolizers were investigated).90 Genetic testing may identify individuals who are less likely to respond to certain 5-HT3 antagonists, but screening is only likely to be helpful in high-risk ethnic populations.98
Table 2.
Metabolism of 5-HT3 antagonists
| Drug | Primary pathway | Secondary pathway |
|---|---|---|
| Dolasetrona | CYP2D6 | CYP3A |
| Granisetron | CYP3A | CYP3A4 |
| Ondansetron | CYP3A4 | CYP1A2
CYP2D6 CYP2E1 |
| Ramosetron | CYP1A2 | CYP2D6
CYP141 |
| Tropisetron | CYP2D6 | CYP3A4
CYP2E1 |
| Palonosetron | CYP2D6 | CYP3A4
CYP1A2 |
Values are those for the active metabolite hydrolosetron.
5-HT3 receptor genetic polymorphism
The 5-HT3 receptor is a ligand-gated cation channel with a pentameric structure. Five subunits enclose an ionopore modulating passage of ions such as calcium when activated by binding of serotonin. A number of polymorphisms of the gene coding for the 5-HT3B subunit exist, and oncology patients who are homozygous for an AAG deletion have a poorer response to tropisetron and ondansetron.104 The extent to which receptor polymorphism influences the efficacy of other 5-HT3 antagonists remains unclear, another study finding no difference in the antiemetic efficacy among patients with different polymorphisms of the 5-HT3A receptor subunit.105
ABCB1 transporter genetic polymorphism
The adenosine triphosphate-binding cassette subfamily B member 1 (ABCB1) transporter (also known as P-glycoprotein or MDR-1) functions as a transmembrane efflux pump in many tissues. It is responsible for the physiological transportation of a variety of drugs, including the 5-HT3 antagonists. A single-nucleotide polymorphism at position 3435 of the ABCB1 gene has shown limited influence on 5-HT3 antagonist efficacy. One study of granisetron, ondansetron and tropisetron found improved short-term (with a trend towards long-term) efficacy of granisetron in ABCB1 3435 TT individuals compared to ABCB1 3435 CC or CT genotypes.106 This finding may reflect higher CNS levels of granisetron due to improved drug transport, but further studies are required.
Clinical efficacy
The anti-vomiting effect of this class of drugs is greater than the anti-nausea effect76 and there is a 25% overall risk reduction for PONV.19,67 This makes the 5-HT3 antagonists cost effective for prophylaxis in high-risk patients, and although droperidol is cheaper and equally effective in adults, ondansetron prevents vomiting more effectively in children.37
The maximum recommended doses for single drug prophylaxis are 8 mg iv or 16 mg orally for ondansetron,107 12.5 mg IV for dolasetron,108,109 1 mg iv for granisetron110 and 5 mg iv for tropisetron.111 If the patient has not received prophylaxis a smaller iv dose (eg, ondansetron 1 mg, granisetron 0.1 mg or tropisetron 0.5 mg) is recommended for treatment.63,67 The NNT to prevent another episode of nausea or vomiting within 24 hours is 4 to 5.112
Adverse effects
The 5-HT3 antagonists have an enviable safety profile, with most side effects (eg, headache, constipation and asthenia) mild and transient. The NNH for ondansetron is 36 for headache, 31 for elevated liver enzymes and 23 for constipation.76
The cardiovascular and ECG effects are of particular interest since the saga of the (now reversed) FDA “Black Box” warning about droperidol and cardiac risk due to prolongation of the QT interval. All 5-HT3 antagonists block cardiac sodium ion channels in vitro113 and thus have the potential to alter cardiac conduction. Safety studies in healthy volunteers indicate a transient increase in PR, QRS and QTc intervals after dolasetron114 and prolonged QTc intervals after ondansetron,52 however a single dose of a 5-HT3 antagonist is considered unlikely to cause cardiovascular effects81 and meta-analysis shows mono-therapy or combined therapy has a similar safety profile to droperidol or dexamethasone.115
Palonosetron
Background
Palonosetron (Aloxi®, MGI Pharma) is the latest 5-HT3 antagonist licensed and the only drug of its class approved for prophylaxis against both acute and delayed chemotherapy-induced nausea and vomiting (CINV). Its unique properties have led to it being described as the first of a ‘second-generation’ of 5-HT3 antagonists. Far higher receptor affinity and a much longer half-life than other 5-HT3 antagonists confer a prolonged duration of action. Following successful Phase III clinical trials the FDA approved its use for prevention of PONV in March 2008.
Chemical structure and binding
Traditional 5-HT3 antagonists are based on a 3-substituted indole ring which mimics the structure of serotonin. In contrast palonosetron is a single stereoisomer isoquinoline based on a fused tricyclic ring system attached to a quinuclidine moiety (Figure 1). This novel chemical structure may explain some of the differences in its receptor affinity, interaction and binding.
Figure 1.
Structures of palonosetron and other 5-HT3 antagonists.
Reproduced with permission from Rojas C, Grunberg S, Rosti G. 2007. Creating real benefit for patients at risk of nausea and vomiting: palonosetron-from bench to bedside. Clin Adv Hematol Oncol, 5(12 Suppl 19):1–20.122 Copyright © 2007 Millenium Medical Publishing.
Pharmacokinetics
The pharmacokinetic profile of palonosetron has been evaluated in healthy volunteers116,117 and cancer patients.118 A single dose of 10 μg/kg iv is widely distributed in the tissues (mean ± SD volume of distribution 8.3 ± 2.4 L/kg). Palonosetron is moderately bound to plasma proteins (62%)119 and despite its extensive distribution, little is sequestered in erythrocytes.117
In keeping with most 5-HT3 antagonists, the metabolism of palonosetron is primarily in the liver by the cytochrome P450 enzyme system, with CYP2D6 the predominant isoenzyme and CYP3A4 and CYP1A2 of secondary importance.119 The main metabolites, N-oxide-palonosetron, 6-(S)-hydroxypalonosetron and small amounts of 6-keto-N-oxo-palonosetron display less than 1% of palonosetron’s activity at 5-HT3 receptors.117 Although a small study (n = 6) comparing poor against extensive metabolizers of CYP2D6 substrates found no difference in efficacy,90,119 palonosetron has not been studied in ultrarapid metabolizers so it is possible that in this genotype it has reduced efficacy.
Following initial rapid distribution, iv palonosetron undergoes a slow elimination phase, primarily handled by the kidney, with 83% of a 10 μg/kg dose being recovered from the urine after 240 hours117 and 40% of the administered dose excreted unchanged. Total body clearance of palonosetron in healthy subjects is approximately 160 ml/h/kg, with renal clearance approximately 66.5 mL/h/kg. This slow elimination results in a long terminal half-life of approximately 40 hours,116,117 which contrasts with previous 5-HT3 antagonists such as ondansetron (3–5 hours) and granisetron (5–8 hours) (see Table 1).
Pharmacokinetic studies show that the characteristics of palonosetron in healthy volunteers and elderly patients with cancer are similar116–118 and widespread clinical experience in the CINV setting confirms that no dose adjustment is necessary in elderly patients.119,120 In addition, mild to moderate renal impairment or hepatic impairment do not affect its pharmacokinetic parameters and dose modification is unnecessary.119
There is currently no clinical experience with palonosetron in pregnant or lactating women. Studies of teratogenicity in animal models show no evidence of interference with fertility or fetal development, but caution is advised until safety in these populations is established.119 There is little experience to date to determine the safety of palonosetron in children, however emerging evidence suggests that it is effective and appears safe.121
Pharmacodynamics
Receptor binding is thought to be the most important factor influencing the duration of action of the 5-HT3 antagonists. Palonosetron shows avid binding to the 5-HT3 receptor, with a pKi of 10.4,93 which far exceeds other 5-HT3 antagonists. This binding affinity is more than 30 times the potency of granisetron and 100 times that of ondansetron (Table 1). In addition, in isolated specimens binding is insurmountable by the addition of increasing concentrations of agonist, which suggests that palonosteron is not simply a competitive antagonist at the 5-HT3 receptor.93 High receptor affinity is accompanied by high selectivity, with low affinity (pKi < 6.0) demonstrated for various other receptors including 5-HT1A,1D,2A,2C.93 This makes it unlikely that palonosetron will produce unwanted effects at other receptor sites.
Emerging evidence indicates that palonosetron interacts at the 5-HT3 receptor in a different manner to previous 5-HT3 antagonists. The chemical structure is dissimilar to serotonin, so palonosetron may bind to the 5-HT3 receptor at an allosteric site, different to other antagonists that bind at the orthosteric site occupied by serotonin.122 This interaction at the allosteric site may prevent attachment of serotonin at its orthosteric site, explaining the insurmountable binding noted in vitro. Furthermore studies of calcium influx in specimens exposed to and then washed clear of palonosetron show continued receptor occupation well beyond that predicted by controls and far in excess of that shown by granisetron and ondansetron.123 The investigators ascribe this to possible internalization of the 5-HT3 receptor following exposure to palonosetron.
Adverse effects
Side effects
Observation of side effects during the clinical development of palonosteron indicated a similar safety profile to other 5-HT3 antagonists, the most common side effects being non-serious and short duration headache (9%), constipation (5%) and dizziness (1%)119 (Table 3). Post-marketing surveillance data after over 1 million patient exposures confirms of the safety of palonosetron, with few serious adverse events reported (n = 81, 0.0061%), most frequently headache (n = 13), hypersensitivity reactions (n = 8) and injection site burning or discomfort (n = 8).124
Table 3.
Adverse reactions of palonosetron, ondansetron and dolasetron
| Event | Palonosetron 0.25 mg (n = 633) | Ondansetron 32 mg (n = 410) | Dolasetron 100 mg (n = 194) |
|---|---|---|---|
| Headache | 60 (9%) | 34 (8%) | 32 (16%) |
| Constipation | 29 (5%) | 8 (2%) | 12 (6%) |
| Diarrhea | 8 (1%) | 7 (2%) | 4 (2%) |
| Dizziness | 8 (1%) | 9 (2%) | 4 (2%) |
| Fatigue | 3 (<1%) | 4 (1%) | 4 (2%) |
| Abdominal pain | 1 (<1%) | 2 (<1%) | 3 (2%) |
| Insomnia | 1 (<1%) | 3 (1%) | 3 (2%) |
Data reproduced with permission from MGI Pharma.119
Cardiac conduction
The potential for a delay in cardiac conduction, in particular QTc prolongation, was evaluated in Phase III studies. In common with other 5-HT3 antagonists, palonosetron slightly increases QTc intervals, the mean increase after a bolus dose lying between 1 and 3 ms.125–127 This compares favorably with a 5 ms increase after ondansetron125,127 and a 5.4 ms increase after dolasetron.126 Palonosetron has been safely administered to many patients with cardiac impairment although the prescribing information advises caution in patients at risk of QTc prolongation.119
Drug interactions
Palonosetron does not cause inhibition or induction of the main hepatic enzyme systems including CYP2D6, CYP1A2 and CYP3A4/5, so the risk of significant drug interactions is low.119 However an adverse reaction with apomorphine that presented as profound hypotension and altered consciousness has been reported, so concomitant use is contraindicated.128
Therapeutic efficacy
The following definitions have been used in trials describing palonosetron’s therapeutic efficacy: complete response (CR) – no rescue medication, no emesis; complete control (CC) – no rescue medication, no emesis, no more than mild nausea; treatment failure – episode of emesis, or rescue medication administered; early nausea and vomiting – 0 to 24 hours; delayed nausea and vomiting – 24 to 120 hours.
Chemotherapy-induced nausea and vomiting
Most clinical experience with palonosetron has been in the setting of the management of CINV, with over 5 million doses having been prescribed. For the purpose of this review, key outcomes only are described. In a phase II dose-ranging study118 complete response rates were highest in the 3 to 90 μg/kg groups and there was no dose-related increase in side effects. Consequently 0.25 mg and 0.75 mg (equivalent to 3 and 10 μg/kg respectively) doses were evaluated in phase III trials as minimum effective doses. The recommended initial treatment dose for CINV is now 0.25 mg.
For the control of early CINV (ie, 0–24 hours), phase III studies found that palonosetron compared favorably with dolasetron and ondansetron. Palonosetron 0.25 mg or 0.75 mg or dolasetron 100 mg resulted in similar complete response rates of 63%, 57% and 53% respectively. However, the palonosetron 0.25 mg group had fewer episodes of emesis and more patients free of emesis compared with the dolasetron group.126 In a comparison of palonosetron 0.25 mg, 0.75 mg or ondansetron 32 mg, palonosetron 0.25 mg was associated with a higher early complete response rate than ondansetron and fewer emetic episodes.125
In the 24–120 hour period palonosetron 0.25 mg and 0.75 mg were superior to dolasetron 100 mg for complete response rates (54%, 57% and 39%, respectively) and for complete control (48%, 52% and 36%, respectively).126 The complete control rates were higher with palonosetron on days 2 and 3 post administration and on day 4 after the higher dose. The number of patients free of nausea was also higher in both palonosetron groups and time to first emetic episode or treatment failure was longer, with most patients not requiring rescue medication until more than 2 days after their single dose. Similar results were seen in the comparison with ondansetron 32 mg.125 A pooled analysis of these trials found early CINV was an important predictor for delayed CINV129 but that patients without early CINV receiving palonosetron were less likely to get delayed CINV compared with dolasetron and ondansetron. Conversely patients who experienced early CINV despite palonosetron were more likely to be protected against delayed CINV (23%) than those taking dolasetron or ondansetron (12%). Therefore, as well as showing early efficacy, palonosetron seems to confer additional protection against delayed CINV.
Combination therapy using palonosetron with antiemetic drugs of other classes appears safe and effective.130 Palonosetron 0.25 mg and dexamethasone 8 mg produced high early complete response rates (84%), falling to 59% for late CINV. Only 3% to 13% of patients complained of more than mild nausea during days 0 to 5. In one study comparing palonosetron with ondansetron the early complete response rate did not differ overall, but among those also given dexamethasone complete response rates were improved in the 24- to 120-hour period in the palonosetron group.127 Triple therapy prophylaxis using aprepitant, dexamethasone and palonosetron 0.25 mg resulted in high rates of early efficacy (complete response 88%, no emesis 93% and no nausea 71%). These benefits extended into the ‘delayed CINV’ period and only 0% to 5% of patients rated their nausea as severe.131 A double-blinded, placebo-controlled, randomized pilot study was terminated after an interim analysis showing unacceptable early and delayed CINV in patient groups receiving palonosetron and dexamethasone in whom aprepitant was not also given.132 Good efficacy has been reported with this triple therapy combination given on day 1 only, the incidence of no emesis reported as 97% to 100% over 5 days.133
Multiple-day dosing with palonosetron 0.25 mg on alternate days appears effective but has not been adequately evaluated compared with a single dose.134,135
Palonosetron for PONV
Optimum dosing
Two placebo-controlled randomized studies have evaluated palonosetron across a range of doses for prophylaxis against PONV. Three hundred and eighty-one women undergoing major gynecological surgery were randomized to doses between 0.1 μg/kg and 30 μg/kg or placebo.136 1 μg/kg and 30 μg/kg doses produced a significantly better complete response in the first 24 hours (44% (p = 0.004) and 45% (p = 0.002) vs 19%) and a lower incidence of nausea during the same period. The second study compared 0.025 mg, 0.05 mg, and 0.075 mg doses of palonosetron in 546 patients with a simplified Apfel risk score for PONV of ≥2 9,11 undergoing laparoscopic surgery.137 Only the highest 0.075 mg dose showed a significantly improved rate of complete response compared with placebo in the 0–6 hour, 0–24 hour and 0–72 hour periods (49% vs 37%; 43% vs 26%; 39% vs 24% for each period respectively, p < 0.05). Patients receiving 0.075 mg were also less likely to report functional interference (eg, with appetite, enjoyment of life, social life) because of PONV experienced in the first 24 hours. Based on these two studies, the minimum effective dose of palonosetron in the setting of PONV is 0.075 mg, and this dose has been approved by the FDA for PONV prophylaxis.
Early PONV
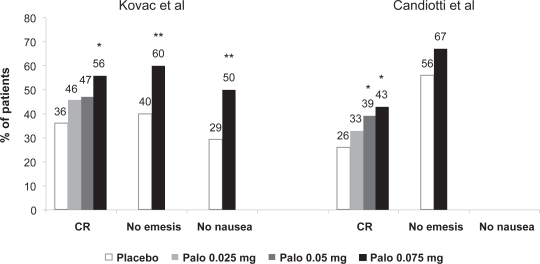
Two identically designed multi-center double-blind placebo-controlled Phase III efficacy trials were published recently.138,139 Both trials included early PONV amongst their primary and secondary endpoints. In one study138 544 patients who were at least a moderate risk of PONV (Apfel risk score ≥ 2) undergoing inpatient gynecological or breast surgery were given palonosetron 0.025 mg, 0.05 mg, 0.075 mg or placebo. All patients received nitrous oxide as part of their anesthetic and no other prophylactic antiemetic drugs. There was a dose-dependent increase in complete response in the 0-to 24-hour period, with rates for the placebo, palonosetron 0.025 mg, 0.05 mg and 0.075 mg groups being 36%, 46% (p = 0.073), 47% (p = 0.069) and 56% (p = 0.001), respectively (Figure 2). The incidence of emesis was significantly reduced in the palonosetron 0.075 mg group compared with placebo (40.0% vs 60.3%, p = 0.001), as was the incidence of nausea (49.6% vs 70.6%, p = 0.001). The severity of nausea (graded as none, mild, moderate, severe) was lower with all three doses of palonosetron.
Figure 2.
0- to 24-hour PONV: complete response (CR), no nausea and no emesis.
*p< 0.017; **p < 0.05.
Data derived from Kovac et al 2008138 and Candiotti et al 2008.139
Abbreviations: Palo, palonosetron; PONV, postoperative nausea and vomiting.
In the other study139 574 patients with Apfel score ≥ 2 and undergoing day-case laparoscopy received prophylaxis against PONV with palonosetron 0.025 mg, 0.05 mg, 0.075 mg or placebo. Nitrous oxide was used but no other prophylactic antiemetics were administered. A similar dose-dependent increase in complete response was observed, with rates in the 0- to 24-hour period for the placebo, palonosetron 0.025 mg, 0.05 mg and 0.075 mg groups of 26%, 33% (p = 0.187), 39% (p = 0.017) and 43% (p = 0.004), respectively (Figure 2). The incidence of early emesis was lower in the palonosetron 0.075 mg group compared with placebo (33% vs 44%, p = 0.075), as was the severity of nausea (p = 0.036).
These studies confirm that palonosetron 0.075 mg provides effective prophylaxis against acute early PONV. The relative risk reduction of 20% to 30% is of a magnitude comparable to that with other single-agent interventions.140
Delayed PONV
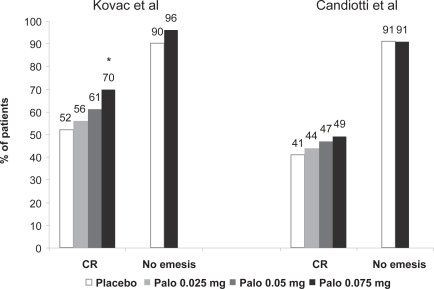
Both the phase III studies detailed above also evaluated the incidence of PONV in the delayed (24- to 72-hour) and ‘postdischarge’ (6- to 72-hour) periods. Kovac et al found complete response rates for placebo, palonosetron 0.025 mg, 0.05 mg and 0.075 mg of 43%, 53%, 52% and 66% (p < 0.05) respectively during the 6- to 72-hour period, and 52%, 56%, 61% and 70% (p = 0.002), respectively, during the 24–to 72-hour period138 (Figure 3). The time to treatment failure was significantly prolonged in the palonosetron 0.075 mg group (p = 0.004), and the median time to first emesis was more than 72 hours after palonosetron 0.05 mg (p = 0.014) and 0.075 mg (p = 0.002) (compared with 3.9 hours after placebo). The severity of nausea was less in the 6- to 72-hour period for the palonosetron 0.075 mg group (p = 0.011).
Figure 3.
24- to 72-hour PONV: complete response (CR) and no emesis.
*p< 0.017.
Data derived from Kovac et al 2008138 and Candiotti et al 2008.139
Abbreviations: Palo, palonosetron; PONV, postoperative nausea and vomiting.
Complete response rates in the Candiotti et al study for placebo, palonosetron 0.025 mg, 0.05 mg and 0.075 mg were not significantly different over the 24- to 72-hour period (Figure 3), and 34%, 38%, 39% and 45% (p = 0.064) respectively for the 6- to 24-hour period.139 The dose-dependent increase in complete response with increasing palonosetron dosage did not reach statistical significance but was present with respect to delay of treatment failure. Although the incidence of late emesis did not differ between the groups, there was a reduction in the intensity of nausea in the palonosetron 0.075 mg group for the 6- to 72-hour period (p = 0.036).
These findings confirm that palonosetron, at a dose of 0.075 mg, improves the control of nausea and vomiting into the second and third days post operatively, an effect that may be most marked after major operations requiring inpatient stay. Palonosetron 0.075 mg also reduces the severity of delayed nausea, which may be of particular relevance to the day-surgery population for whom it is difficult to identify those at risk of postdischarge PONV and for whom early return to normal activities is important.141 Of note, palonosetron also seems to have a prolonged effect in reducing the severity of nausea, a feature not shared by other 5-HT3 antagonists. However the magnitude of effect against PONV appears to be similar to that of other established drugs following inpatient surgery in moderate- or high-risk groups, and modest against delayed PONV in ambulatory surgical patients with shorter and lower postoperative opioid requirements, so more evidence is required before a role against postdischarge PONV in the day-care setting can be recommended.
Discussion
Approval of palonosetron for the prevention of PONV provides another therapeutic intervention in the arsenal against the ‘big little problem’.142 The prolonged half-life and very strong affinity of palonosetron for the 5-HT3 receptor provide the pharmacological basis for a long duration of action that appears to far exceed that of other 5-HT3 antagonists. Clinical effectiveness into the fifth day after chemotherapy has been demonstrated, and after surgery prolonged effectiveness is also of potential value because PONV often presents late or after discharge.141 Palonosetron is an established antiemetic drug in oncology medicine, where it shows better efficacy against both early and delayed CINV than other 5-HT3 antagonists. This prolonged clinical effect combined with superior efficacy against PONV mitigates a traditional obstacle for a newly developed drug – its cost. A recent theoretical evaluation suggested its cost effectiveness compared favorably with ondansetron.143 However the etiology of CINV, which involves a large release of serotonin from the enterochromaffin cells in the small intestine in response to chemotherapeutic agents, is different to that of PONV, which has multi-factorial aetiology. It remains to be seen whether the same degree of efficacy can be expected in the postsurgical setting.
The role of combination therapy in patients at high risk of PONV has been well established.19,57,58 On the basis of promising results for combination therapy with palonosetron in CINV, similar studies in the surgical population will no doubt be undertaken. The effectiveness of palonosetron and dexamethasone, particularly against nausea, may dovetail well with the antiemetic properties of the neurokinin-1 antagonists such as aprepitant. Future research needs to be directed towards comparisons of the efficacy of palonosetron and other 5-HT3 antagonists, towards establishing suitable drug and drug dose combinations to prevent PONV in high-risk patient groups (including cost-effectiveness evaluation). Investigation of its efficacy for the treatment of PONV is also required. Although the clinical value of palonosetron in this setting has yet to be established, the pre-marketing evidence suggests it may be a valuable addition to the pharmacological armamentarium.
Footnotes
Disclosures
Dr Neil Muchatuta has received financial assistance for continuing education activities from Schering-Plough and Professor Michael Paech has been a clinical consultant to Schering-Plough, Hospira and Xenome.
References
- 1.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89(3):652–8. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Gan T, Sloan F, Dear Gde L, El-Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg. 2001;92(2):393–400. doi: 10.1097/00000539-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 3.van den Bosch JE, Bonsel GJ, Moons KG, Kalkman CJ. Effect of postoperative experiences on willingness to pay to avoid postoperative pain, nausea, and vomiting. Anesthesiology. 2006;104(5):1033–9. doi: 10.1097/00000542-200605000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Gin T, Lau AS, Ng FF. A comparison of patients’ and health care professionals’ preferences for symptoms during immediate postoperative recovery and the management of postoperative nausea and vomiting. Anesth Analg. 2005;100(1):87–93. doi: 10.1213/01.ANE.0000140782.04973.D9. [DOI] [PubMed] [Google Scholar]
- 5.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84(1):6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 6.Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102(6):1884–98. doi: 10.1213/01.ANE.0000219597.16143.4D. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78(1):7–16. doi: 10.1213/00000539-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 Suppl 1):24S–32S. doi: 10.1093/bja/69.supplement_1.24s. [DOI] [PubMed] [Google Scholar]
- 9.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Paech MJ, Pavy TJ, Evans SF. Single-dose prophylaxis for postoperative nausea and vomiting after major abdominal surgery: ondansetron versus droperidol. Anaesth Intensive Care. 1995;23(5):548–54. doi: 10.1177/0310057X9502300503. [DOI] [PubMed] [Google Scholar]
- 11.Apfel CC, Greim CA, Haubitz I, Goepfert C, Usadel J, Sefrin P, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol Scand. 1998;42(5):495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 12.Apfel CC, Kranke P, Eberhart LH, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2000;88(2):234–40. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 13.Apfel CC, Kranke P, Greim CA, Roewer N. What can be expected from risk scores for predicting postoperative nausea and vomiting? Br J Anaesth. 2001;86(6):822–7. doi: 10.1093/bja/86.6.822. [DOI] [PubMed] [Google Scholar]
- 14.Biedler A, Wermelt J, Kunitz O, Muller A, Wilhelm W, Dethling J, et al. A risk adapted approach reduces the overall institutional incidence of postoperative nausea and vomiting. Can J Anaesth. 2004;51(1):13–9. doi: 10.1007/BF03018540. [DOI] [PubMed] [Google Scholar]
- 15.Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003;98(1):46–52. doi: 10.1097/00000542-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted. Anesthesiology. 1999;91:109–118. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101(5):1343–8. doi: 10.1213/01.ANE.0000180204.64588.EC. [DOI] [PubMed] [Google Scholar]
- 18.Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88(5):659–68. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 19.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350(24):2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tramer M, Moore A, McQuay H. Omitting nitrous oxide in general anaesthesia: meta-analysis of intraoperative awareness and postoperative emesis in randomized controlled trials. Br J Anaesth. 1996;76(2):186–93. doi: 10.1093/bja/76.2.186. [DOI] [PubMed] [Google Scholar]
- 21.Leslie K, Myles PS, Chan MT, Paech MJ, Peyton P, Forbes A, et al. Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide-based vs nitrous oxide-free anaesthesia. Br J Anaesth. 2008;101(4):498–505. doi: 10.1093/bja/aen230. [DOI] [PubMed] [Google Scholar]
- 22.Yogendran S, Asokumar B, Cheng DC, Chung F. A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth Analg. 1995;80(4):682–6. doi: 10.1097/00000539-199504000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Magner JJ, McCaul C, Carton E, Gardiner J, Buggy D. Effect of intraoperative intravenous crystalloid infusion on postoperative nausea and vomiting after gynaecological laparoscopy: comparison of 30 and 10 ml kg(−1) Br J Anaesth. 2004;93(3):381–5. doi: 10.1093/bja/aeh219. [DOI] [PubMed] [Google Scholar]
- 24.Maharaj CH, Kallam SR, Malik A, Hassett P, Grady D, Laffey JG. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. 2005;100(3):675–82. doi: 10.1213/01.ANE.0000148684.64286.36. [DOI] [PubMed] [Google Scholar]
- 25.White PF, Watcha MF. Postoperative nausea and vomiting: prophylaxis versus treatment. Anesth Analg. 1999;89(6):1337–9. doi: 10.1097/00000539-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Alkaissi A, Gunnarsson H, Johnsson V, Evertsson K, Ofenbartl L, Kalman S. Disturbing post-operative symptoms are not reduced by prophylactic antiemetic treatment in patients at high risk of post-operative nausea and vomiting. Acta Anaesthesiol Scand. 2004;48(6):761–71. doi: 10.1111/j.0001-5172.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 27.Carlisle JB, Stevenson CA. rugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2006;3:CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee A, Done ML. The use of nonpharmacologic techniques to prevent postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 1999;88(6):1362–9. doi: 10.1097/00000539-199906000-00031. [DOI] [PubMed] [Google Scholar]
- 29.White PF, Issioui T, Hu J, Jones SB, Coleman JE, Waddle JP, et al. Comparative efficacy of acustimulation (ReliefBand) versus ondansetron (Zofran) in combination with droperidol for preventing nausea and vomiting. Anesthesiology. 2002;97(5):1075–81. doi: 10.1097/00000542-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Rowbotham DJ. Recent advances in the non-pharmacological management of postoperative nausea and vomiting. Br J Anaesth. 2005;95(1):77–81. doi: 10.1093/bja/aei125. [DOI] [PubMed] [Google Scholar]
- 31.Visser K, Hassink EA, Bonsel GJ, Moen J, Kalkman CJ. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: postoperative nausea with vomiting and economic analysis. Anesthesiology. 2001;95(3):616–26. doi: 10.1097/00000542-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Habib AS, White WD, Eubanks S, Pappas TN, Gan TJ. A randomized comparison of a multimodal management strategy versus combination antiemetics for the prevention of postoperative nausea and vomiting. Anesth Analg. 2004;99(1):77–81. doi: 10.1213/01.ANE.0000120161.30788.04. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Wu CL, Elkassabany N, Krug CE, Parker SD, Fleisher LA. Does the routine prophylactic use of antiemetics affect the incidence of postdischarge nausea and vomiting following ambulatory surgery? A systematic review of randomized controlled trials. Anesthesiology. 2003;99(2):488–95. doi: 10.1097/00000542-200308000-00033. [DOI] [PubMed] [Google Scholar]
- 34.Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84(3):367–71. doi: 10.1093/oxfordjournals.bja.a013442. [DOI] [PubMed] [Google Scholar]
- 35.Lewis IH, Campbell DN, Barrowcliffe MP. Effect of nabilone on nausea and vomiting after total abdominal hysterectomy. Br J Anaesth. 1994;73(2):244–6. doi: 10.1093/bja/73.2.244. [DOI] [PubMed] [Google Scholar]
- 36.Henzi I, Walder B, Tramer MR. Metoclopramide in the prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized, placebo-controlled studies. Br J Anaesth. 1999;83(5):761–71. doi: 10.1093/bja/83.5.761. [DOI] [PubMed] [Google Scholar]
- 37.Domino KB, Anderson EA, Polissar NL, Posner KL. Comparative efficacy and safety of ondansetron, droperidol, and metoclopramide for preventing postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 1999;88(6):1370–9. doi: 10.1097/00000539-199906000-00032. [DOI] [PubMed] [Google Scholar]
- 38.Loeser EA, Bennett G, Stanley TH, Machin R. Comparison of droperidol, haloperidol and prochlorperazine as postoperative anti-emetics. Can Anaesth Soc J. 1979;26(2):125–7. doi: 10.1007/BF03013781. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed AB, Hobbs GJ, Curran JP. Randomized placebo-controlled trial of combination antiemetic prophylaxis for day-case gynaecological laparoscopic surgery. Br J Anaesth. 2000;85(5):678–82. doi: 10.1093/bja/85.5.678. [DOI] [PubMed] [Google Scholar]
- 40.Chia YY, Lo Y, Liu K, Tan PH, Chung NC, Ko NH. The effect of promethazine on postoperative pain: a comparison of preoperative, postoperative, and placebo administration in patients following total abdominal hysterectomy. Acta Anaesthesiol Scand. 2004;48(5):625–30. doi: 10.1111/j.1399-6576.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 41.Silverman DG, Freilich J, Sevarino FB, Paige D, Preble L, O’Connor TZ. Influence of promethazine on symptom-therapy scores for nausea during patient-controlled analgesia with morphine. Anesth Analg. 1992;74(5):735–8. [PubMed] [Google Scholar]
- 42.Cholwill JM, Wright W, Hobbs GJ, Curran J. Comparison of ondansetron and cyclizine for prevention of nausea and vomiting after day-case gynaecological laparoscopy. Br J Anaesth. 1999;83(4):611–4. doi: 10.1093/bja/83.4.611. [DOI] [PubMed] [Google Scholar]
- 43.Johns RA, Hanousek J, Montgomery JE. A comparison of cyclizine and granisetron alone and in combination for the prevention of postoperative nausea and vomiting. Anaesthesia. 2006;61(11):1053–7. doi: 10.1111/j.1365-2044.2006.04794.x. [DOI] [PubMed] [Google Scholar]
- 44.Kranke P, Morin AM, Roewer N, Wulf H, Eberhart LH. The efficacy and safety of transdermal scopolamine for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2002;95(1):133–43. doi: 10.1097/00000539-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 45.Hill RP, Lubarsky DA, Phillips-Bute B, Fortney JT, Creed MR, Glass PS, et al. Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo. Anesthesiology. 2000;92(4):958–67. doi: 10.1097/00000542-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Henzi I, Sonderegger J, Tramer MR. Efficacy, dose-response, and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anaesth. 2000;47(6):537–51. doi: 10.1007/BF03018945. [DOI] [PubMed] [Google Scholar]
- 47.Fortney JT, Gan TJ, Graczyk S, Wetchler B, Melson T, Khalil S, et al. A comparison of the efficacy, safety, and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. S3A-409 and S3A-410 Study Groups. Anesth Analg. 1998;86(4):731–8. doi: 10.1097/00000539-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Tang J, Watcha MF, White PF. A comparison of costs and efficacy of ondansetron and droperidol as prophylactic antiemetic therapy for elective outpatient gynecologic procedures. Anesth Analg. 1996;83(2):304–13. doi: 10.1097/00000539-199608000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Tang J, Chen X, White PF, Wender RH, Ma H, Sloninsky A, et al. Antiemetic prophylaxis for office-based surgery: are the 5-HT3 receptor antagonists beneficial? Anesthesiology. 2003;98(2):293–8. doi: 10.1097/00000542-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Lim BS, Pavy TJ, Lumsden G. The antiemetic and dysphoric effects of droperidol in the day surgery patient. Anaesth Intensive Care. 1999;27(4):371–4. doi: 10.1177/0310057X9902700407. [DOI] [PubMed] [Google Scholar]
- 51.White PF, Song D, Abrao J, Klein KW, Navarette B. Effect of low-dose droperidol on the QT interval during and after general anesthesia: a placebo-controlled study. Anesthesiology. 2005;102(6):1101–5. doi: 10.1097/00000542-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102(6):1094–100. doi: 10.1097/00000542-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Liu K, Hsu CC, Chia YY. The effect of dose of dexamethasone for antiemesis after major gynecological surgery. Anesth Analg. 1999;89(5):1316–8. [PubMed] [Google Scholar]
- 54.Lee Y, Lai HY, Lin PC, Lin YS, Huang SJ, Shyr MH.A dose ranging study of dexamethasone for preventing patient-controlled analgesia-related nausea and vomiting: a comparison of droperidol with saline Anesth Analg 20049841066–71.table of contents. [DOI] [PubMed] [Google Scholar]
- 55.Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg. 2000;90(1):186–94. doi: 10.1097/00000539-200001000-00038. [DOI] [PubMed] [Google Scholar]
- 56.Diemunsch P, Schoeffler P, Bryssine B, Cheli-Muller LE, Lees J, McQuade BA, et al. Antiemetic activity of the NK1 receptor antagonist GR205171 in the treatment of established postoperative nausea and vomiting after major gynaecological surgery. Br J Anaesth. 1999;82(2):274–6. doi: 10.1093/bja/82.2.274. [DOI] [PubMed] [Google Scholar]
- 57.Scuderi PE, James RL, Harris L, Mims GR. 3rd. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth Analg. 2000;91(6):1408–14. doi: 10.1097/00000539-200012000-00020. [DOI] [PubMed] [Google Scholar]
- 58.Paech MJ, Lee BH, Evans SF. The effect of anaesthetic technique on postoperative nausea and vomiting after day-case gynaecological laparoscopy. Anaesth Intensive Care. 2002;30(2):153–9. doi: 10.1177/0310057X0203000205. [DOI] [PubMed] [Google Scholar]
- 59.McKenzie R, Tantisira B, Karambelkar DJ, Riley TJ, Abdelhady H. Comparison of ondansetron with ondansetron plus dexamethasone in the prevention of postoperative nausea and vomiting. Anesth Analg. 1994;79(5):961–4. doi: 10.1213/00000539-199411000-00024. [DOI] [PubMed] [Google Scholar]
- 60.Eberhart LH, Morin AM, Bothner U, Georgieff M. Droperidol and 5-HT3-receptor antagonists, alone or in combination, for prophylaxis of postoperative nausea and vomiting. A meta-analysis of randomised controlled trials. Acta Anaesthesiol Scand. 2000;44(10):1252–7. doi: 10.1034/j.1399-6576.2000.441011.x. [DOI] [PubMed] [Google Scholar]
- 61.Tramer MR. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part II. Recommendations for prevention and treatment, and research agenda. Acta Anaesthesiol Scand. 2001;45(1):14–9. doi: 10.1034/j.1399-6576.2001.450103.x. [DOI] [PubMed] [Google Scholar]
- 62.Habib AS, Gan TJ. Evidence-based management of postoperative nausea and vomiting: a review. Can J Anaesth. 2004;51(4):326–41. doi: 10.1007/BF03018236. [DOI] [PubMed] [Google Scholar]
- 63.Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97(1):62–71. doi: 10.1213/01.ane.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 64.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105(6):1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 65.Paech MJ, Rucklidge MW, Lain J, Dodd PH, Bennett EJ, Doherty DA. Ondansetron and dexamethasone dose combinations for prophylaxis against postoperative nausea and vomiting. Anesth Analg. 2007;104(4):808–14. doi: 10.1213/01.ane.0000258768.76093.16. [DOI] [PubMed] [Google Scholar]
- 66.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102(6):1249–60. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. A quantitative systematic review of ondansetron in treatment of established postoperative nausea and vomiting. BMJ. 1997;314(7087):1088–92. doi: 10.1136/bmj.314.7087.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moser JD, Caldwell JB, Rhule FJ. No more than necessary: safety and efficacy of low-dose promethazine. Ann Pharmacother. 2006;40(1):45–8. doi: 10.1345/aph.1G282. [DOI] [PubMed] [Google Scholar]
- 69.Lee Y, Wang JJ, Yang YL, Chen A, Lai HY. Midazolam vs ondansetron for preventing postoperative nausea and vomiting: a randomised controlled trial. Anaesthesia. 2007;62(1):18–22. doi: 10.1111/j.1365-2044.2006.04895.x. [DOI] [PubMed] [Google Scholar]
- 70.Kim SI, Han TH, Kil HY, Lee JS, Kim SC. Prevention of postoperative nausea and vomiting by continuous infusion of subhypnotic propofol in female patients receiving intravenous patient-controlled analgesia. Br J Anaesth. 2000;85(6):898–900. doi: 10.1093/bja/85.6.898. [DOI] [PubMed] [Google Scholar]
- 71.Gan TJ, El-Molem H, Ray J, Glass PS. Patient-controlled antiemesis: a randomized, double-blind comparison of two doses of propofol versus placebo. Anesthesiology. 1999;90(6):1564–70. doi: 10.1097/00000542-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Di Florio T, Goucke CR. The effect of midazolam on persistent postoperative nausea and vomiting. Anaesth Intensive Care. 1999;27(1):38–40. doi: 10.1177/0310057X9902700107. [DOI] [PubMed] [Google Scholar]
- 73.Unlugenc H, Guler T, Gunes Y, Isik G. Comparative study of the anti-emetic efficacy of ondansetron, propofol and midazolam in the early postoperative period. Eur J Anaesthesiol. 2004;21(1):60–5. doi: 10.1017/s0265021504001103. [DOI] [PubMed] [Google Scholar]
- 74.Gan TJ, Ginsberg B, Glass PS, Fortney J, Jhaveri R, Perno R. Opioid-sparing effects of a low-dose infusion of naloxone in patient-administered morphine sulfate. Anesthesiology. 1997;87(5):1075–81. doi: 10.1097/00000542-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Loewen PS, Marra CA, Zed PJ. 5-HT3 receptor antagonists vs traditional agents for the prophylaxis of postoperative nausea and vomiting. Can J Anaesth. 2000;47(10):1008–18. doi: 10.1007/BF03024875. [DOI] [PubMed] [Google Scholar]
- 76.Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997;87(6):1277–89. doi: 10.1097/00000542-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 77.White PF, Tang J, Hamza MA, Ogunnaike B, Lo M, Wender RH, et al. The use of oral granisetron versus intravenous ondansetron for antiemetic prophylaxis in patients undergoing laparoscopic surgery: the effect on emetic symptoms and quality of recovery. Anesth Analg. 2006;102(5):1387–93. doi: 10.1213/01.ane.0000208967.94601.cd. [DOI] [PubMed] [Google Scholar]
- 78.Gan TJ, Coop A, Philip BK. A randomized, double-blind study of granisetron plus dexamethasone versus ondansetron plus dexamethasone to prevent postoperative nausea and vomiting in patients undergoing abdominal hysterectomy. Anesth Analg. 2005;101(5):1323–9. doi: 10.1213/01.ANE.0000180366.65267.F6. [DOI] [PubMed] [Google Scholar]
- 79.Zarate E, Watcha MF, White PF, Klein KW, Sa Rego M, Stewart DG. A comparison of the costs and efficacy of ondansetron versus dolasetron for antiemetic prophylaxis. Anesth Analg. 2000;90(6):1352–8. doi: 10.1097/00000539-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 80.Paech MJ, Rucklidge MW, Banks SL, Gurrin LC, Orlikowski CE, Pavy TJ. The efficacy and cost-effectiveness of prophylactic 5-hydroxytryptamine3 receptor antagonists: tropisetron, ondansetron and dolasetron. Anaesth Intensive Care. 2003;31(1):11–7. doi: 10.1177/0310057X0303100102. [DOI] [PubMed] [Google Scholar]
- 81.Gan TJ. Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting: are they all the same? CNS Drugs. 2005;19(3):225–38. doi: 10.2165/00023210-200519030-00004. [DOI] [PubMed] [Google Scholar]
- 82.Ho KY, Gan TJ. Pharmacology, pharmacogenetics, and clinical efficacy of 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin Anaesthesiol. 2006;19(6):606–11. doi: 10.1097/01.aco.0000247340.61815.38. [DOI] [PubMed] [Google Scholar]
- 83.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77(1):162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 84.Tsukagoshi S. [Pharmacokinetics of azasetron (Serotone), a selective 5-HT3 receptor antagonist] Gan To Kagaku Ryoho. 1999;26(7):1001–8. [PubMed] [Google Scholar]
- 85.Sanofi-Anentis 2006 Anzemet (dolasetron) prescribing information [online]. Accessed 25 August 2008. URL: http://products.sanofi-aventis.us/Anzemet_Injection/anzemet_injection.html.
- 86.Roche 2005 Kytril (granisetron) prescribing information [online]. Accessed 25 August 2008. URL: http://www.rocheusa.com/products/kytril/pi_injection.pdf.
- 87.GlaxoSmithKline 2006 Zofran (ondansetron) prescribing information [online]. Accessed 25 August 2008. URL: http://us-gsk.com/products/assets/us_zofran.pdf.
- 88.Ramosetron Rabasseda X. a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc) 2002;38(2):75–89. doi: 10.1358/dot.2002.38.2.820104. [DOI] [PubMed] [Google Scholar]
- 89.Novartis 2008 Navoban (tropisetron) prescribing information [online]. Accessed 25 August 2008. URL: http://www.novartis.com.au/PI_PDF/nav.pdf.
- 90.Aapro M. 5-HT(3)-receptor antagonists in the management of nausea and vomiting in cancer and cancer treatment. Oncology. 2005;69(2):97–109. doi: 10.1159/000087979. [DOI] [PubMed] [Google Scholar]
- 91.Upward JW, Arnold BD, Link C, Pierce DM, Allen A, Tasker TC. The clinical pharmacology of granisetron (BRL 43694), a novel specific 5-HT3 antagonist. Eur J Cancer. 1990;26(Suppl 1):S12–5. [PubMed] [Google Scholar]
- 92.Newberry NR, Watkins CJ, Sprosen TS, Blackburn TP, Grahame-Smith DG, Leslie RA. BRL 46470 potently antagonizes neural responses activated by 5-HT3 receptors. Neuropharmacology. 1993;32(8):729–35. doi: 10.1016/0028-3908(93)90180-b. [DOI] [PubMed] [Google Scholar]
- 93.Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L, et al. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol. 1995;114(4):851–9. doi: 10.1111/j.1476-5381.1995.tb13282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cascorbi I. Pharmacogenetics of cytochrome p4502D6: genetic background and clinical implication. Eur J Clin Invest. 2003;33(Suppl 2):17–22. doi: 10.1046/j.1365-2362.33.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 95.Eichelbaum M, Baur MP, Dengler HJ, Osikowska-Evers BO, Tieves G, Zekorn C, et al. Chromosomal assignment of human cytochrome P-450 (debrisoquine/sparteine type) to chromosome 22. Br J Clin Pharmacol. 1987;23(4):455–8. doi: 10.1111/j.1365-2125.1987.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gough AC, Smith CA, Howell SM, Wolf CR, Bryant SP, Spurr NK. Localization of the CYP2D gene locus to human chromosome 22q13.1 by polymerase chain reaction, in situ hybridization, and linkage analysis. Genomics. 1993;15(2):430–2. doi: 10.1006/geno.1993.1082. [DOI] [PubMed] [Google Scholar]
- 97.Touw DJ. Clinical implications of genetic polymorphisms and drug interactions mediated by cytochrome P-450 enzymes. Drug Metabol Drug Interact. 1997;14(2):55–82. [PubMed] [Google Scholar]
- 98.Janicki PK. Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005;11(10):RA322–8. [PubMed] [Google Scholar]
- 99.Dahl ML, Johansson I, Bertilsson L, Ingelman-Sundberg M, Sjoqvist F. Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther. 1995;274(1):516–20. [PubMed] [Google Scholar]
- 100.Agundez JA, Ledesma MC, Ladero JM, Benitez J. Prevalence of CYP2D6 gene duplication and its repercussion on the oxidative phenotype in a white population. Clin Pharmacol Ther. 1995;57(3):265–9. doi: 10.1016/0009-9236(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 101.McLellan RA, Oscarson M, Seidegard J, Evans DA, Ingelman-Sundberg M. Frequent occurrence of CYP2D6 gene duplication in Saudi Arabians. Pharmacogenetics. 1997;7(3):187–91. doi: 10.1097/00008571-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Candiotti KA, Birnbach DJ, Lubarsky DA, Nhuch F, Kamat A, Koch WH, et al. The impact of pharmacogenomics on postoperative nausea and vomiting: do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology. 2005;102(3):543–9. doi: 10.1097/00000542-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay PB, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol. 2002;20(12):2805–11. doi: 10.1200/JCO.2002.09.064. [DOI] [PubMed] [Google Scholar]
- 104.Tremblay PB, Kaiser R, Sezer O, Rosler N, Schelenz C, Possinger K, et al. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003;21(11):2147–55. doi: 10.1200/JCO.2003.05.164. [DOI] [PubMed] [Google Scholar]
- 105.Kaiser R, Tremblay PB, Sezer O, Possinger K, Roots I, Brockmoller J. Investigation of the association between 5-HT3A receptor gene polymorphisms and efficiency of antiemetic treatment with 5-HT3 receptor antagonists. Pharmacogenetics. 2004;14(5):271–8. doi: 10.1097/00008571-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 106.Babaoglu MO, Bayar B, Aynacioglu AS, Kerb R, Abali H, Celik I, et al. Association of the ABCB1 3435C> T polymorphism with antiemetic efficacy of 5-hydroxytryptamine type 3 antagonists. Clin Pharmacol Ther. 2005;78(6):619–26. doi: 10.1016/j.clpt.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 107.Helmers JH, Briggs L, Abrahamsson J, Soni J, Moodley J, Forrler M, et al. A single i.v. dose of ondansetron 8 mg prior to induction of anaesthesia reduces postoperative nausea and vomiting in gynaecological patients. Can J Anaesth. 1993;40(12):1155–61. doi: 10.1007/BF03009605. [DOI] [PubMed] [Google Scholar]
- 108.Kovac AL, Scuderi PE, Boerner TF, Chelly JE, Goldberg ME, Hantler CB, et al. Treatment of postoperative nausea and vomiting with single intravenous doses of dolasetron mesylate: a multicenter trial. Dolasetron Mesylate PONV Treatment Study Group. Anesth Analg. 1997;85(3):546–52. doi: 10.1097/00000539-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 109.Philip BK, McLeskey CH, Chelly JE, McKenzie R, Kovac AL, Diemunsch P, et al. Pooled analysis of three large clinical trials to determine the optimal dose of dolasetron mesylate needed to prevent postoperative nausea and vomiting. The Dolasetron Prophylaxis Study Group. J Clin Anesth. 2000;12(1):1–8. doi: 10.1016/s0952-8180(99)00123-3. [DOI] [PubMed] [Google Scholar]
- 110.Mikawa K, Takao Y, Nishina K, Shiga M, Maekawa N, Obara H. Optimal dose of granisetron for prophylaxis against postoperative emesis after gynecological surgery. Anesth Analg. 1997;85(3):652–6. doi: 10.1097/00000539-199709000-00030. [DOI] [PubMed] [Google Scholar]
- 111.Chan MT, Chui PT, Ho WS, King WW. Single-dose tropisetron for preventing postoperative nausea and vomiting after breast surgery. Anesth Analg. 1998;87(4):931–5. doi: 10.1097/00000539-199810000-00035. [DOI] [PubMed] [Google Scholar]
- 112.Kazemi-Kjellberg F, Henzi I, Tramer MR. Treatment of established postoperative nausea and vomiting: a quantitative systematic review. BMC Anesthesiol. 2001;1(1):2. doi: 10.1186/1471-2253-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kovac AL. Prophylaxis of postoperative nausea and vomiting: controversies in the use of serotonin 5-hydroxytryptamine subtype 3 receptor antagonists. J Clin Anesth. 2006;18(4):304–18. doi: 10.1016/j.jclinane.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 114.Hesketh P, Navari R, Grote T, Gralla R, Hainsworth J, Kris M, et al. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy-induced Emesis Prevention Group. J Clin Oncol. 1996;14(8):2242–9. doi: 10.1200/JCO.1996.14.8.2242. [DOI] [PubMed] [Google Scholar]
- 115.Leslie JB, Gan TJ. Meta-analysis of the safety of 5-HT3 antagonists with dexamethasone or droperidol for prevention of PONV. Ann Pharmacother. 2006;40(5):856–72. doi: 10.1345/aph.1G381. [DOI] [PubMed] [Google Scholar]
- 116.Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjects. J Clin Pharmacol. 2004;44(5):520–31. doi: 10.1177/0091270004264641. [DOI] [PubMed] [Google Scholar]
- 117.Stoltz R, Parisi S, Shah A, Macciocchi A. Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm Drug Dispos. 2004;25(8):329–37. doi: 10.1002/bdd.410. [DOI] [PubMed] [Google Scholar]
- 118.Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. 2004;15(2):330–7. doi: 10.1093/annonc/mdh047. [DOI] [PubMed] [Google Scholar]
- 119.MGI Pharma 2008 ALOXI (Palonosetron HCl) prescribing information [online]. Accessed 6 August 2008. URL: http://www.aloxi.com/common/downloads/pi.pdf.
- 120.Aapro MS, Macciocchi A, Gridelli C. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting in elderly patients. J Support Oncol. 2005;3(5):369–74. [PubMed] [Google Scholar]
- 121.Sepulveda-Vildosola AC, Betanzos-Cabrera Y, Lastiri GG, Rivera-Marquez H, Villasis-Keever MA, Del Angel VW, et al. Palonosetron Hydrochloride Is an Effective and Safe Option to Prevent Chemotherapy-induced Nausea and Vomiting in Children. Arch Med Res. 2008;39(6):601–6. doi: 10.1016/j.arcmed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Rojas C, Grunberg S, Rosti G. Creating real benefit for patients at risk of nausea and vomiting: palonosetron-from bench to bedside. Clin Adv Hematol Oncol. 2007;5(12 Suppl 19):1–20. [PubMed] [Google Scholar]
- 123.Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107(2):469–78. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- 124.Bissoli F, McGuiggan M, Bertazzoli M. Post-marketing experience of palonosetron confirms a favourable benefit/risk profile. Support Care Cancer. 2005;13(413):A04–17. [Google Scholar]
- 125.Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14(10):1570–7. doi: 10.1093/annonc/mdg417. [DOI] [PubMed] [Google Scholar]
- 126.Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98(11):2473–82. doi: 10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 127.Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17(9):1441–9. doi: 10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 128.Vernalis Pharmaceuticals 2006 Apokyn (apomorphine hydrochloride injection) prescribing information [online]. Accessed 28 August 2008. URL: http://www.apokyn.com/pdf/APOKYN_PI.pdf.
- 129.Grunberg SM, Van den Burgt JA, Berry S, Rubenstein EB, Berry D.Prevention of delayed nausea and vomiting (D-CINV): carryover effect analysis of pooled data from 2 phase III studies of palonosetron (PALO) Proc Am Soc Clin Oncol 200423738Abstract 8051. [Google Scholar]
- 130.Hajdenberg J, Grote T, Yee L, Arevalo-Araujo R, Latimer LA. Infusion of palonosetron plus dexamethasone for the prevention of chemotherapy-induced nausea and vomiting. J Support Oncol. 2006;4(9):467–71. [PubMed] [Google Scholar]
- 131.Grote T, Hajdenberg J, Cartmell A, Ferguson S, Ginkel A, Charu V. Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol. 2006;4(8):403–8. [PubMed] [Google Scholar]
- 132.Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer. 2008;112(9):2080–7. doi: 10.1002/cncr.23364. [DOI] [PubMed] [Google Scholar]
- 133.Grunberg SM, Dugan M, Muss HB, Wood M, Burdette-Radoux S, Weisberg T. Efficacy of a 1-day 3-drug antiemetic regimen for prevention of acute and delayed nausea and vomiting induced by moderately emetogenic chemotherapy. J Clin Oncol. 2007;25(18S):520s. [Google Scholar]
- 134.Einhorn LH, Brames MJ, Dreicer R, Nichols CR, Cullen MT, Jr, Bubalo J. Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer. 2007;15(11):1293–300. doi: 10.1007/s00520-007-0255-6. [DOI] [PubMed] [Google Scholar]
- 135.Hunt TL, Gallagher SC, Cullen MT, Jr, Shah AK. Evaluation of safety and pharmacokinetics of consecutive multiple-day dosing of palonosetron in healthy subjects. J Clin Pharmacol. 2005;45(5):589–96. doi: 10.1177/0091270005275061. [DOI] [PubMed] [Google Scholar]
- 136.White PF, Scuderi PE. Prevention of postoperative nausea and vomiting (PONV): A dose-ranging study involving palonosetron, a potent 5-HT3 receptor antagonist. Anesthesiology. 2005;103:A703. [Google Scholar]
- 137.Kovac AL, Candiotti KA, Melson TI, Gan TJ. Efficacy of palonosetron in preventing PONV and PDNV: results of a phase 3 multicenter, randomized, double-blind, dose-ranging study. Presented at SAMBA; 3–6; San Diego, CA. May, 2007. [Google Scholar]
- 138.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107(2):439–44. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 139.Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. Anesth Analg. 2008;107(2):445–51. doi: 10.1213/ane.0b013e31817b5ebb. [DOI] [PubMed] [Google Scholar]
- 140.Lichtor JL, Glass PS. We’re tired of waiting. Anesth Analg. 2008;107(2):353–5. doi: 10.1213/ane.0b013e31817b604e. [DOI] [PubMed] [Google Scholar]
- 141.White PF, Sacan O, Nuangchamnong N, Sun T, Eng MR. The relationship between patient risk factors and early versus late postoperative emetic symptoms. Anesth Analg. 2008;107(2):459–63. doi: 10.1213/ane.0b013e31817aa6e4. [DOI] [PubMed] [Google Scholar]
- 142.Fisher DM. The “big little problem” of postoperative nausea and vomiting: do we know the answer yet. Anesthesiology. 1997;87:1271–3. doi: 10.1097/00000542-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 143.Tarricone R, Girolami F. Economic Evaluation of a New Antiemetic Drug – Palonosetron versus Ondansetron: Assessment of the Drug Price Ratio in Five European Countries. Clin Drug Investig. 2005;25(9):597–608. doi: 10.2165/00044011-200525090-00005. [DOI] [PubMed] [Google Scholar]