Abstract
Background and Purpose
Previous studies suggest that dynamic autoregulation in the posterior cerebral artery (PCA) is less efficient compared to the middle cerebral artery (MCA). We examined the role of cerebral vasodilation due to metabolic activation (i.e. visual stimulus) on autoregulatory characteristics in the two vascular territories.
Methods
Blood flow velocity (BFV) in the PCA and MCA and mean arterial pressure (MAP) were measured continuously in 45 healthy volunteers (62±3 years) while seated with eyes open. Additional 20 subjects (60±5 years) were examined with eyes closed and open. Autoregulation was assessed using transfer function gains in both the PCA and MCA territories in the low (0.03 – 0.07 Hz), high (0.07 – 0.15 Hz) and cardiac (~ 1 Hz) frequency ranges.
Results
With eyes open, gains were significantly higher in the PCA compared to the MCA in the low (PCA: 1.41±0.09 vs. MCA: 1.18±0.07, P=0.003) and high (PCA: 2.06±0.12 vs. MCA: 1.61±0.08, P=0.0001) frequencies. Opening eyes increased BFV and reduced cerebrovascular resistance index in the PCA but not MCA. This vasodilation in the PCA was associated with increased gain in the low (autoregulatory) frequency while MCA gain did not change (PCA: 0.89±0.14 vs. 1.31±0.17, MCA: 1.24±0.16 vs. 1.16±0.11, P=0.02).
Conclusions
Dilation of the PCA territory during visual cortex activation resulted in increased PCA transfer function gain without changing MCA gain. Thus, impaired autoregulation in the PCA reported in previous literature is likely the result of metabolic vasodilation and not an inherent difference in the autoregulatory characteristics of the posterior circulation.
Keywords: cerebral autoregulation, posterior cerebral artery, cerebral blood flow
INTRODUCTION
Under physiological conditions, cerebral autoregulation serves to maintain relatively constant flow to the brain in response to changes in cerebral perfusion pressure and metabolic demand.1, 2 Certain clinical entities such as reversible posterior leukoencephalopathy syndrome3 and eclampsia,4 conditions that predominantly affect white matter in the occipital-parietal region of the brain, suggest that the posterior circulation may be more vulnerable to changes in perfusion pressure as compared to the anterior circulation. Previous work using transcranial Doppler (TCD) ultrasound has demonstrated impaired autoregulation in the posterior cerebral artery (PCA) compared to the middle cerebral artery (MCA).5 Some have theorized that this may be due to differences in the sympathetic innervation of the vessel walls.6 However, there is no direct evidence linking the extent of sympathetic vascular innervation and autoregulatory response of the posterior circulation. An alternative hypothesis may be that autoregulation is different in the PCA and MCA territories because they are operating under different metabolic states.
Previous work has shown that dilated vascular beds have attenuated autoregulation compared with constricted beds.7 Since neuronal activity is closely related to cerebral blood flow, a phenomenon termed neurovascular coupling, vascular beds are more dilated when they are metabolically active. This applies to the visual cortices, which in the normal awake state are constantly activated.8–11 This would place the posterior circulation in a state of continuous vasodilation compared to the anterior circulation, which may explain why the posterior circulation may be more vulnerable to systemic pressure changes. However, the differences between cerebral autoregulation in the non-activated (eyes closed) and activated (eyes open) conditions in the PCA vs. MCA territories have not been previously examined.
We hypothesized that the metabolic state of the visual cortex would have a significant impact on autoregulation in the PCA territory of healthy individuals. That is, with the eyes open, autoregulation in the PCA territory would be less efficient due to vasodilation than in the MCA, but with the eyes closed, there would be no difference in the PCA and MCA autoregulation. We used TCD to simultaneously study dynamic cerebral autoregulation of the PCA and MCA territories in healthy volunteers using frequency-domain analysis and tested the effect of visual activation on autoregulation of the posterior cerebral circulation.
METHODS
Subjects
Sixty-five healthy volunteers were recruited from laboratory personnel and members of the Harvard Cooperative Program on Aging subject registry. All subjects were carefully screened with a medical history, physical examination, and electrocardiogram to exclude any acute or chronic medical conditions. Subjects were asked to refrain from alcohol or nicotine use for at least 12-hours prior to the study. The study was approved by the Hebrew Rehabilitation Center for Aged and Brigham and Women’s Hospital institutional reviewboards, and followed institutional guidelines.
Experimental protocol
Instrumentation
Subjects reported to the cardiovascular laboratory at the Hebrew Rehabilitation Center (HRC) or Brigham and Women’s Hospital in the post-absorptive state, 2 hours after their last meal. Subjects were instrumented for heart rate (HR electrocardiogram) and beat-to-beat finger arterial pressure (Finapres, Ohmeda Monitoring Systems, Englewood, CO) as previously described.12 End-tidal CO2 was measured from nasal prongs, using a Vacumed CO2 Analyzer (Ventura, CA).
TCD ultrasonography (MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) was used to simultaneously measure changes in the right MCA and left PCA blood flow velocity (BFV). All the studies were performed by the same TCD technician to reduce variability. The MCA and PCA signals were identified according to the criteria of Aaslid et al13 and recorded at a depth of 50 to 60 mm for the MCA and 60 to 70 mm for the PCA. A Spencer probe fixation device was used to stabilize the Doppler probe for the duration of the study. The envelope of the velocity waveform, derived from a fast-Fourier analysis of the Doppler frequency signal, was digitized at 500 Hz, displayed simultaneously with the mean arterial pressure (MAP), ECG, and end-tidal CO2 signals, and stored for later off-line analysis.
Study Protocols
Eyes Open Sitting Protocol
Blood flow velocity in the MCA and PCA, MAP and end-tidal CO2 were simultaneously recorded for 5-minutes in 45 healthy volunteers in the eyes open sitting position.
Eyes Closed – Eyes Open Protocol
Twenty healthy volunteers initially established a steady state with eyes open after 5 minutes of rest in a supine position. The subjects then closed their eyes for 2 minutes, and the most stationary 1-minute data segment was obtained (Fig. 1). The subjects were then asked to open their eyes for 2 minutes, and again the most stationary 1-minute data segment was obtained for further analysis (Fig. 1). Blood flow velocity in the MCA and PCA, MAP and end-tidal CO2 were simultaneously recorded for 1 minute each in the eyes closed and eyes open states.
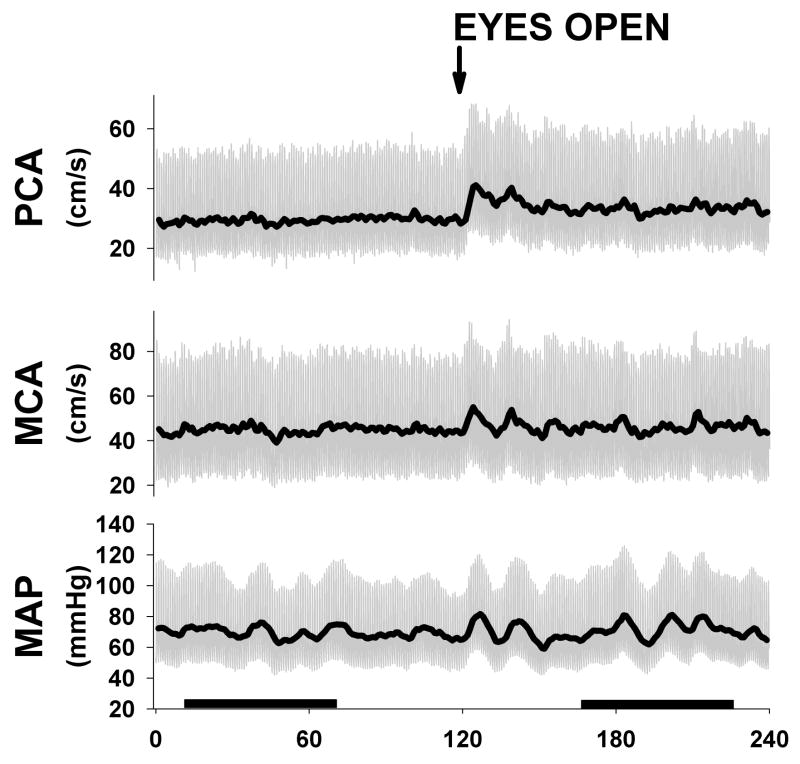
Figure 1.
Example of one subject during eyes closed and open protocol. Subjects had eyes closed for 2 min and then were instructed to open eyes and were monitored for another 2 min. Note clear activation in PCA after eyes opening with no change in MCA velocities. Dark bars represent one min steady state sections used for transfer function analysis.
Data Processing
All data were displayed and digitized with commercially available data acquisition software (Windaq, Dataq Instruments). BFV, MAP and CO2 waveforms were re-sampled at 100 Hz using a custom MATLAB program. Beat-to-beat R-R interval was determined from the R wave of the ECG or from the peak blood pressure waveform. MAP and mean BFV were determined from the integrals of each waveform. A custom Matlab program was used to calculate the average mean systolic and diastolic BFV from peak and minimum TCD values within each beat cycle. Flow velocities from the Eyes Open Sitting Protocol were normalized to percentage of baseline blood flow with eyes open (n = 45). Flow velocities from the Eyes Closed – Eyes Open Protocol were normalized to percentage of baseline blood flow with eyes closed (n = 20). Individual responses were averaged across individuals to obtain mean responses for MAP and BFV to each trial. Cerebrovascular resistance index (CVRi) was calculated as the ratio of MAP to mean BFV in the PCA and MCA arterial territories. Coherence, gains and phases were calculated using the MAP and normalized BFV signal autospectra in the low (0.03 – 0.07 Hz), high (0.07 – 0.15 Hz) and cardiac (~ 1 Hz) frequency ranges. Detailed methods of mathematical calculations are described in our previous study.7
Gain in the low frequency range (0.03 – 0.07 Hz) is considered to be autoregulatory in nature since autoregulation is a relatively slow process and can take several seconds to engage.14 Increased transfer function gains indicate greater transmission of ABP fluctuation to cerebral blood flow, suggesting impaired autoregulation. Previous work has found decreased autoregulation during cardiovascular stress using this methodology.15 In addition, other groups have examined the phase shift between blood pressure and velocity to assess autoregulation. An angle approaching 0 degrees can be interpreted as absence of autoregulation since flow changes are occurring at exactly the same time as pressure changes, suggesting passive modulation of flow by pressure. In contrast, minus 90 degrees is considered optimal autoregulation since changes in flow are delayed presumably due to autoregulatory mechanisms.16, 17 Since gains in the cardiac frequency (i.e. ~1 Hz) are occurring too rapidly to be autoregulatory, they represent passive transmission of ABP to BFV and likely reflect the stiffness of vessels. For examination of autoregulation in the PCA and MCA territories while seated with eyes open, 5 minutes of data were analyzed. For comparison between eyes open and closed, steady state 1 minute data sets were examined. Previous work has used similar 1 minute data sets to assess the linear dynamic relationship between MAP and BFV via Fast Fourier Transform (FFT) based methodology.18–20 These short data sets are adequate to examine autoregulatory function since according to the Nyquist-Shannon theory, only twice the desired target frequency is needed for sampling, and thus a one minute-window is sufficient for our low frequency range (0.03 – 0.07 Hz, i.e. 33–14 sec). To validate the use of 1 minute transfer function, we took 5 min segments of data from the same subjects (n = 45) and divided them into five one minute segments and compared the low frequency gains derived from each one minute segment to the low frequency gains of the 5 minute segment. Gains in both the PCA and MCA were similar between 5 and 1 min data sets (PCA: 1.41±0.63 vs. 1.48±0.60; MCA: 1.17+/−0.49 vs. 1.27+/−0.47 respectively) and significantly correlated. In addition a factor analysis demonstrated a Cronbach’s alpha of 0.86 for the PCA and 0.78 for the MCA indicating that all values were measuring a single construct (i.e. autoregulation).
Statistical Analysis
The effects of vascular territory (PCA vs. MCA) or visual activation (eyes-closed vs. eyes-open) on BFV, MAP, end-tidal CO2, CVRi, and transfer function coherence, gains, and phases were assessed by using a repeated-measures two-way ANOVA, respectively. Data are presented as means ± SE, and levels of p < 0.05 are considered statistically significant.
RESULTS
Subject Characteristics
Demographic and baseline data for all the subjects studied in Eyes Open Sitting (n = 45) and Eyes Closed – Eyes Open (n = 20) protocols are shown in Table 1. Subjects in the two groups were similar in age and hemodynamic variables.
Table 1.
Baseline subject characteristics
| EO sitting | EC supine | EO supine | |
|---|---|---|---|
| n | 45 | 20 | |
| Gender (male:female) | 25:20 | 10:10 | |
| Age, yr | 62 ± 3 | 60 ± 5 | |
| Systolic BP, mmHg | 129 ± 3 | 128 ± 6 | 125 ± 6 |
| Diastolic BP, mmHg | 63 ± 2 | 64 ± 4 | 63 ± 4 |
| Mean BP, mmHg | 84 ± 2 | 87 ± 4 | 85 ± 4 |
| Heart rate, beats/min | 66 ± 2 | 65 ± 3 | 65 ± 3 |
| End tidal CO2, Torr | 36 ± 1 | 40 ± 1 | 40 ± 1 |
Baseline subject characteristics in eyes open (EO) sitting, eyes closed (EC) and EO supine position. Values are presented as means ± SE; n, no. of subjects; BP, blood pressure.
Hemodynamics and Cerebral Autoregulation: Eyes Open, Sitting
Table 2 summarizes the cerebral hemodynamics and autoregulation in the eyes open sitting state in 45 healthy subjects. BFV was significantly lower in the PCA as compared to the MCA territory. Transfer function gains were much higher in the PCA as compared to the MCA territory in low (autoregulatory) and high frequency ranges, but the differences were not significant in the cardiac frequency. There were no significant differences in transfer function phases between the two vascular territories.
Table 2.
Blood Flow Velocity and Autoregulation in the PCA and MCA Territories While Sitting
| PCA | MCA | P value | |
|---|---|---|---|
| Systolic BFV, cm/s | 58 ± 3 | 88 ± 5 | <0.0001 |
| Diastolic BFV, cm/s | 20 ± 2 | 32 ± 2 | <0.0001 |
| Mean BFV, cm/s | 35 ± 2 | 55 ± 3 | <0.0001 |
| CVRi, mmHg·s·cm-1 | 2.74 ± 0.20 | 1.87 ± 0.19 | 0.002 |
| Gain | |||
| Low | 1.41±0.09 | 1.18±0.07 | 0.003 |
| High | 2.06±0.12 | 1.61±0.08 | 0.0001 |
| Cardiac | 1.96±0.08 | 1.86±0.07 | 0.07 |
| Coherence | |||
| Low | 0.56±0.03 | 0.61±0.03 | 0.08 |
| High | 0.63±0.03 | 0.62±0.03 | 0.56 |
| Cardiac | 0.99±0.00 | 0.99±0.00 | 0.04 |
| Phase | |||
| Low | −40.79±4.56 | −45.91±4.39 | 0.08 |
| High | −37.07±4.98 | −42.75±5.40 | 0.32 |
| Cardiac | −23.45±5.52 | −24.11±4.44 | 0.90 |
Comparison of the PCA and the MCA in EO sitting position (n = 45) are shown. BFV, blood flow velocity; CVRi, cerebrovascular resistance index. Transfer function coherence, gain, and phase relating fluctuations in arterial blood pressure and cerebral blood flow velocity in the low-frequency range (Low: 0.03 – 0.07 Hz), high-frequency (High: 0.07 – 0.15 Hz), and cardiac-frequency range (Cardiac: within beat). Phase shift angle in degrees. All values are given as mean ± SE.
Hemodynamics and Cerebral Autoregulation: Comparing Eyes Closed to Eyes Open States
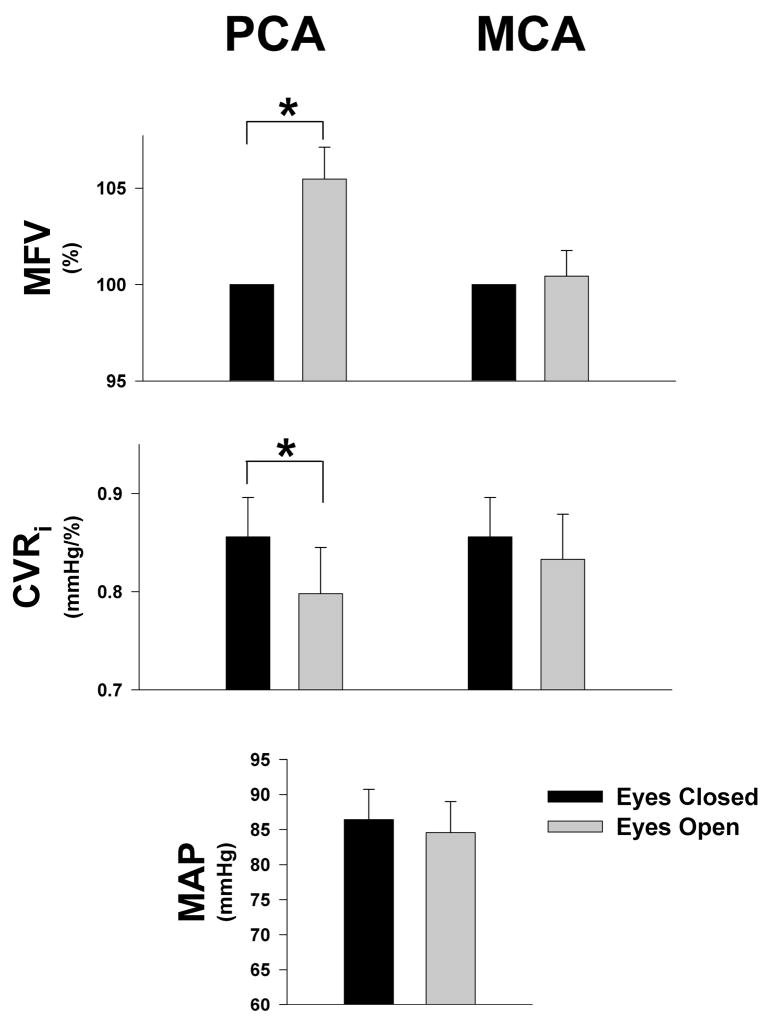
The effects of visual activation on cerebral hemodynamics and autoregulation were studied during 1-minute each of eyes-closed (EC) and eyes-open (EO) recordings in the supine position. We selected 1-minute data sets that were in steady state without significant difference in the values across the sixty seconds (Fig. 1). Despite there being no difference in mean arterial blood pressure or end-tidal CO2 in the two states, PCA flow increased significantly with eyes open while CVRi significantly decreased, suggesting a metabolic vasodilation unrelated to pressure or pCO2 changes. In contrast, the MCA territory did not change (Fig. 2).
Figure 2.
Hemodynamic responses to eye opening in healthy (n = 20) subjects. Bars represent the values in MFV (mean blood flow velocity), CVRi (cerebrovascular resistance index) and MAP (mean arterial pressure) during the eyes open (EO) and eyes closed (EC) trials in the middle (MCA) and posterior cerebral arteries (PCA). MFV was normalized to eyes closed state. Values are means ± SE. *Significant difference between EO and EC states, P < 0.001
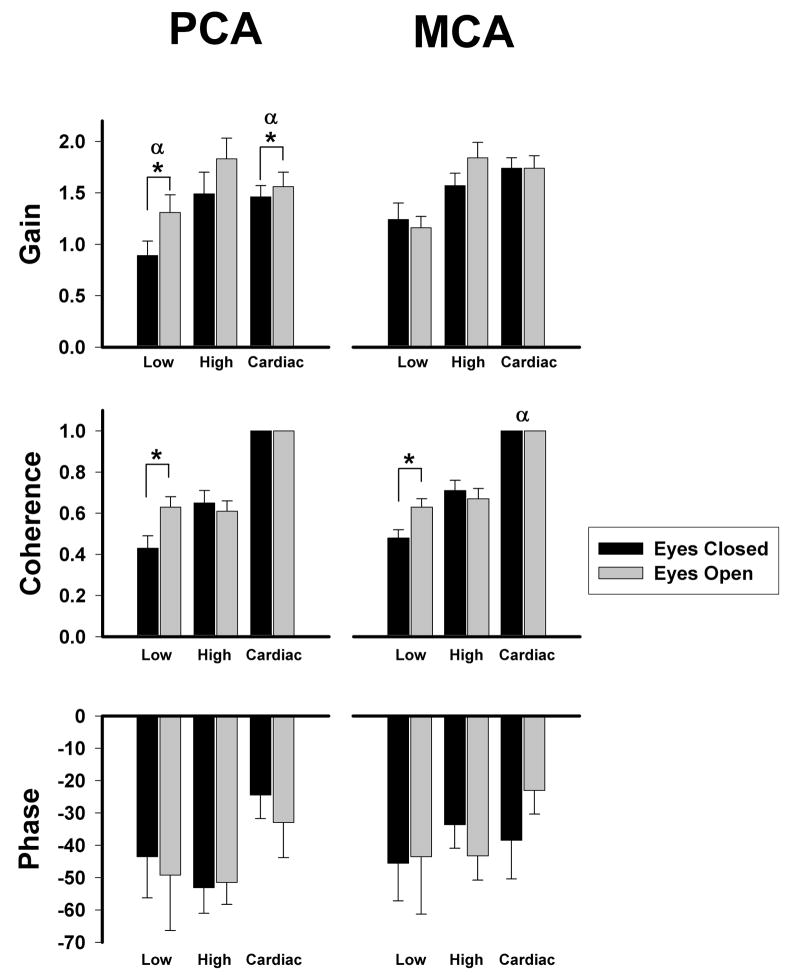
Consistent with flow changes, low frequency transfer function gains increased in the PCA with eyes open (p = 0.02) but did not change in the MCA (Fig. 3). Thus as the PCA territory dilated when eyes were opened, gains increased, suggesting impaired autoregulation. In the cardiac frequency range, again only the PCA showed significant increases in transfer function gains with eyes open (p = 0.012, Fig. 3), while MCA remained unchanged. In the eyes closed conditions the PCA gains were lower than MCA gains in low (PCA: 0.89 ± 0.14 vs. MCA: 1.24 ± 0.16; p = 0.04) and cardiac frequencies (PCA: 1.46 ± 0.11 vs. MCA: 1.74 ± 0.10; p = 0.02). In contrast, gain values were similar in the high frequency range in both vascular beds between eyes open and closed. After eye opening, both arteries showed significant increase in the low frequency coherence (p = 0.008, Fig. 3) suggesting more linear relationship between MAP and BFV, although there was no significant difference between the two arteries. In addition, cardiac frequency coherence was significantly lower during both eyes closed and open in the PCA (0.996±0.001) vs. the MCA (0.997±0.001, P=0.021), however this slight difference likely has little clinical relevance. Transfer function phases at all three frequency ranges were not significantly different between the PCA and MCA in response to visual activation.
Figure 3.
Transfer function coherence, gain, and phase in the low-frequency range (Low: 0.03 – 0.07 Hz), high-frequency range (High: 0.07 – 0.15 Hz), and cardiac-frequency range (Cardiac: within beat) at eyes-closed (EC) and eyes-open (EO) states in healthy (n = 20) subjects. Values are means ± SE. *Significant difference between eyes open and closed, P < 0.05. α, Significant difference between the two arteries, P < 0.05.
DISCUSSION
Our data show that autoregulation in the PCA territory is altered by metabolic activation by eye opening in healthy older adult subjects. As we hypothesized, during eyes open, the PCA vascular bed is vasodilated and BFV is increased to meet the increased neuronal metabolic demand of the visual cortex. In this state, PCA is more vulnerable to blood pressure fluctuations as compared to the MCA territory, as reflected by the higher PCA transfer functions gains in the low frequency (autoregulatory) range. However, when the eyes are closed and the visual cortex is in a metabolically quiescent state, the PCA autoregulation may even be more effective than the MCA, as shown by the lower gains in the low and cardiac frequency range.
Higher PCA transfer function gains have been previously reported by Haubrich et al. who studied 30 older adults (mean age 65 ± 10 years) without cerebrovascular disease or dysautonomia and showed higher gains in the PCA compared to the MCA.5 However, these subjects were studied with eyes open in an illuminated room (personal communication, Haubrich). Our group has also studied autoregulation in the PCA and MCA territories in elderly healthy volunteers using a sit-to-stand protocol.12 In the eyes open state, transitioning from a sitting to a standing position was associated with a significantly greater decline in the PCA as compared with the MCA territory BFV. However, this response was not tested in the eyes closed state. To our knowledge, the present study is the first to assess PCA autoregulation in two different metabolic states of the visual cortex. Our findings are consistent with previous assessments of dynamic autoregulation in the MCA territory during hypercapnia, which showed an increase in transfer function gain and BFV, suggesting that vasodilated vascular beds have an impaired autoregulatory response.23
Contrary to the current literature suggesting that autoregulation of the PCA is impaired compared to that of the MCA,5 our study demonstrates that transmission of the blood pressure oscillations to cerebral blood flow (low frequency gain) in the PCA appears to be attenuated, suggesting improved autoregulation, during a quiescent eyes-closed state when the vessels are less vasodilated. These findings demonstrate that the PCA autoregulation is just as effective as the MCA autoregulation when the eyes are closed.
These data highlight the importance of metabolic state on cerebral autoregulatory function. Since our study did not involve direct imaging modalities assessing cerebral metabolic rate of oxygen (CMRO2) or cerebral perfusion, we could not show any direct evidence that eye opening resulted in regional increase in blood flow to the visual cortex. However, prior studies using blood oxygen level dependent (BOLD) MRI have shown focal increases in blood flow to the visual cortex with eye opening 21, 22, suggesting that the observed changes seen in the PCA territory in this study resulted from neurovascular coupling. None of the prior studies that addressed the differences between autoregulation in the PCA and MCA territories were done in the eyes closed state.5, 12 We speculate that the constant relative vasodilation of the posterior circulation seen with awake individuals may explain why the posterior circulation has been observed to have more vulnerable autoregulation compared to the anterior circulation in clinical practice. This finding is consistent with our previous work in the MCA in which transfer function gain significantly increased as CVRi decreased.7
In summary, we have shown that autoregulation in the PCA territory is likely altered by neurovascular coupling and the metabolic state of the visual cortex. While the transfer function gains in the PCA territory were much higher compared to the MCA territory in the eyes open state, this difference in autoregulation vanished in the eyes closed state.
STUDY LIMITATION
Our study only included healthy older adults without evidence of overt chronic diseases, and thus our results can only be generalized to this population. The possibility that dynamic autoregulatory property could differ in a younger population or adults with chronic vascular diseases warrant further study. Also, our study included two protocols, which differed in the subject position (sitting vs. supine) and the length of TCD recordings (5 min vs. 1 min), and thus comparisons between protocols must be examined cautiously.
Acknowledgments
This work was supported by grants AG004390, AG08812, and AG05134 from the National Institute of Aging, Bethesda, MD. Dr. Sorond is the recipient of Beeson K23 Award AG30967. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine. Dr. Serrador is the recipient of the SFI Walton Visiting Professorship at the National University of Ireland – Galway.
Footnotes
Conflict of interest: none
References
- 1.Diehl RR, Linden D, Lucke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res. 1998;8:7–12. doi: 10.1007/BF02267598. [DOI] [PubMed] [Google Scholar]
- 2.Strandgaard S, Paulson OB. Cerebral autoregulation. Stroke. 1984;15:413–416. doi: 10.1161/01.str.15.3.413. [DOI] [PubMed] [Google Scholar]
- 3.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 4.Digre KB, Varner MW, Osborn AG, Crawford S. Cranial magnetic resonance imaging in severe preeclampsia vs eclampsia. Arch Neurol. 1993;50:399–406. doi: 10.1001/archneur.1993.00540040055015. [DOI] [PubMed] [Google Scholar]
- 5.Haubrich C, Wendt A, Diehl RR, Klotzsch C. Dynamic autoregulation testing in the posterior cerebral artery. Stroke. 2004;35:848–852. doi: 10.1161/01.STR.0000120729.99039.B6. [DOI] [PubMed] [Google Scholar]
- 6.Edvinsson L, Owman C, Siesjo B. Physiological role of cerebrovascular sympathetic nerves in the autoregulation of cerebral blood flow. Brain Res. 1976;117:519–523. doi: 10.1016/0006-8993(76)90760-5. [DOI] [PubMed] [Google Scholar]
- 7.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: Transfer gain in different frequency domains. J Appl Physiol. 2005;98:151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- 8.Vafaee MS, Meyer E, Marrett S, Paus T, Evans AC, Gjedde A. Frequency-dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J Cereb Blood Flow Metab. 1999;19:272–277. doi: 10.1097/00004647-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Aaslid R. Visually evoked dynamic blood flow response of the human cerebral circulation. Stroke. 1987;18:771–775. doi: 10.1161/01.str.18.4.771. [DOI] [PubMed] [Google Scholar]
- 10.Uzuner N, Ozkan S, Gucuyener D, Ozdemir G. Cerebral blood flow velocity changes to visual stimuli in patients with multiple sclerosis. Mult Scler. 2002;8:217–221. doi: 10.1191/1352458502ms798oa. [DOI] [PubMed] [Google Scholar]
- 11.Vafaee MS, Marrett S, Meyer E, Evans AC, Gjedde A. Increased oxygen consumption in human visual cortex: Response to visual stimulation. Acta Neurol Scand. 1998;98:85–89. doi: 10.1111/j.1600-0404.1998.tb01724.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorond FA, Khavari R, Serrador JM, Lipsitz LA. Regional cerebral autoregulation during orthostatic stress: Age-related differences. J Gerontol A Biol Sci Med Sci. 2005;60:1484–1487. doi: 10.1093/gerona/60.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 14.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: Insights from the frequency domain. J Appl Physiol. 1998;85:1113–1122. doi: 10.1152/jappl.1998.85.3.1113. [DOI] [PubMed] [Google Scholar]
- 16.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995;26:1801–1804. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 18.Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation. 2001;104:898–902. doi: 10.1161/hc3301.094908. [DOI] [PubMed] [Google Scholar]
- 19.Panerai RB, Dawson SL, Eames PJ, Potter JF. Cerebral blood flow velocity response to induced and spontaneous sudden changes in arterial blood pressure. Am J Physiol Heart Circ Physiol. 2001;280:H2162–2174. doi: 10.1152/ajpheart.2001.280.5.H2162. [DOI] [PubMed] [Google Scholar]
- 20.Carey BJ, Panerai RB, Potter JF. Effect of aging on dynamic cerebral autoregulation during head-up tilt. Stroke. 2003;34:1871–1875. doi: 10.1161/01.STR.0000081981.99908.F3. [DOI] [PubMed] [Google Scholar]
- 21.Marx E, Deutschlander A, Stephan T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. Neuroimage. 2004;21:1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Henning S, Merboldt KD, Frahm J. Task- and eeg-correlated analyses of bold mri responses to eyes opening and closing. Brain Res. 2006:1073–1074. 359–364. doi: 10.1016/j.brainres.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 23.Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of co2 on dynamic cerebral autoregulation measurement. Physiol Meas. 1999;20:265–275. doi: 10.1088/0967-3334/20/3/304. [DOI] [PubMed] [Google Scholar]