Abstract
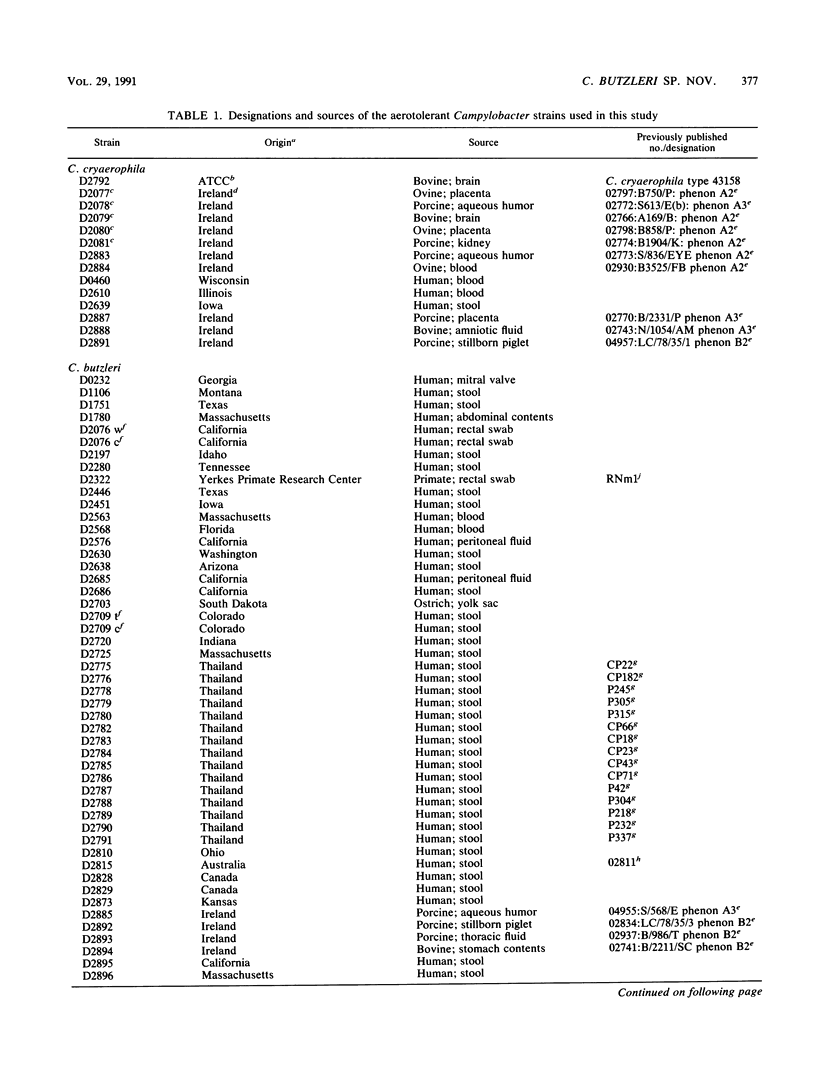
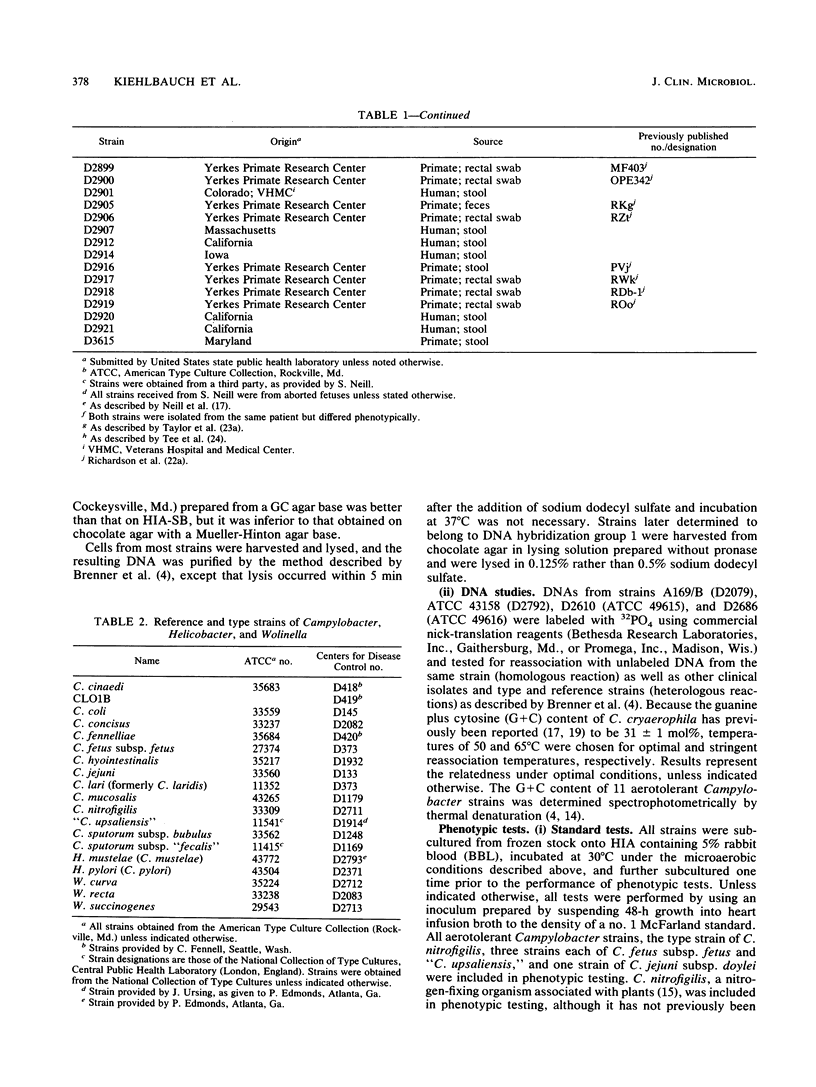
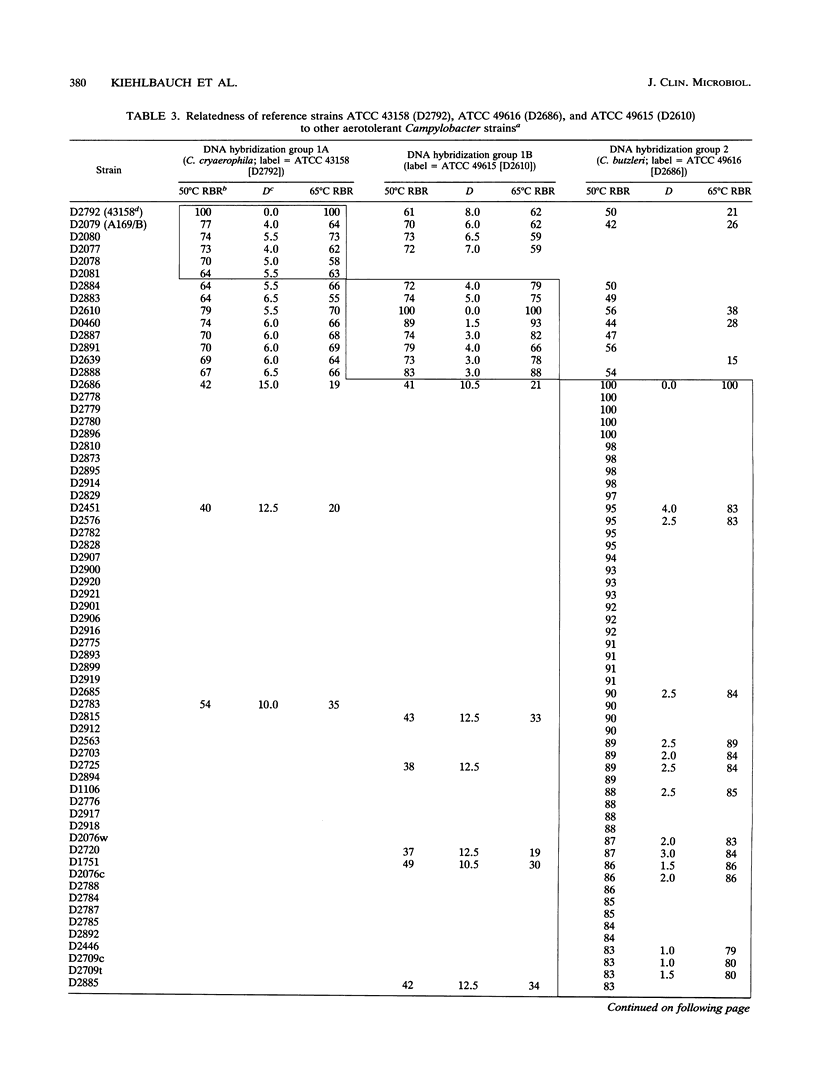
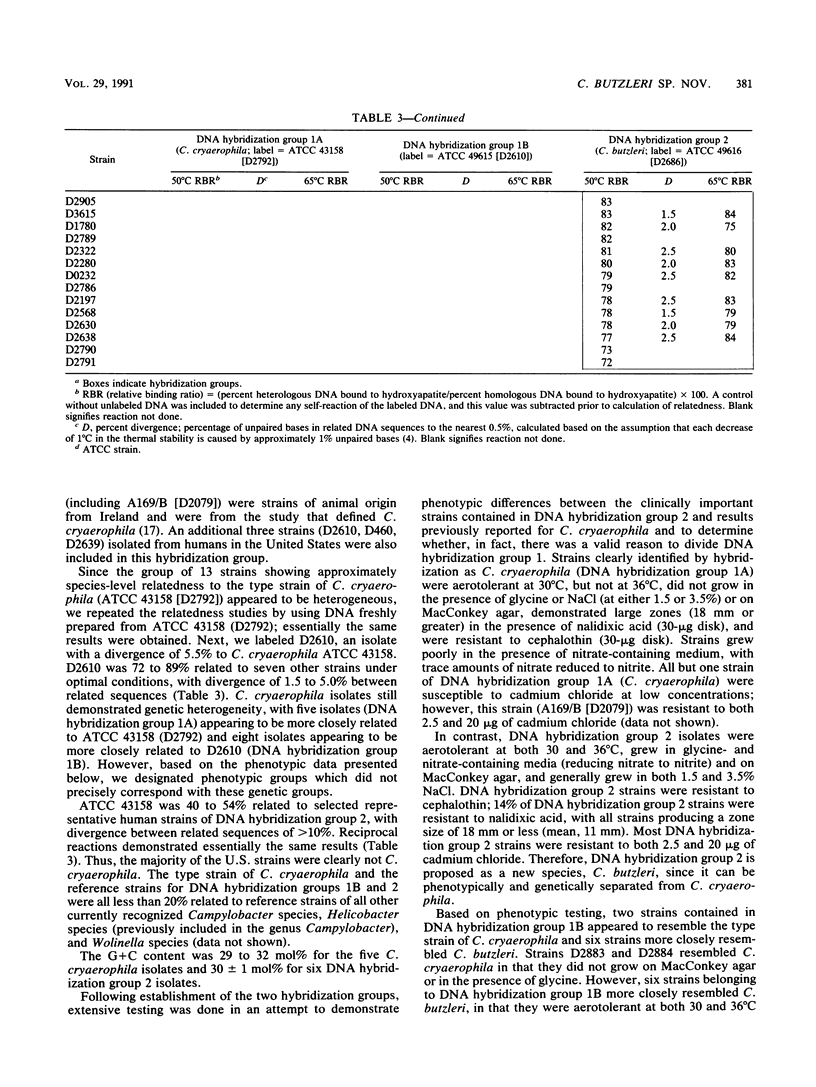
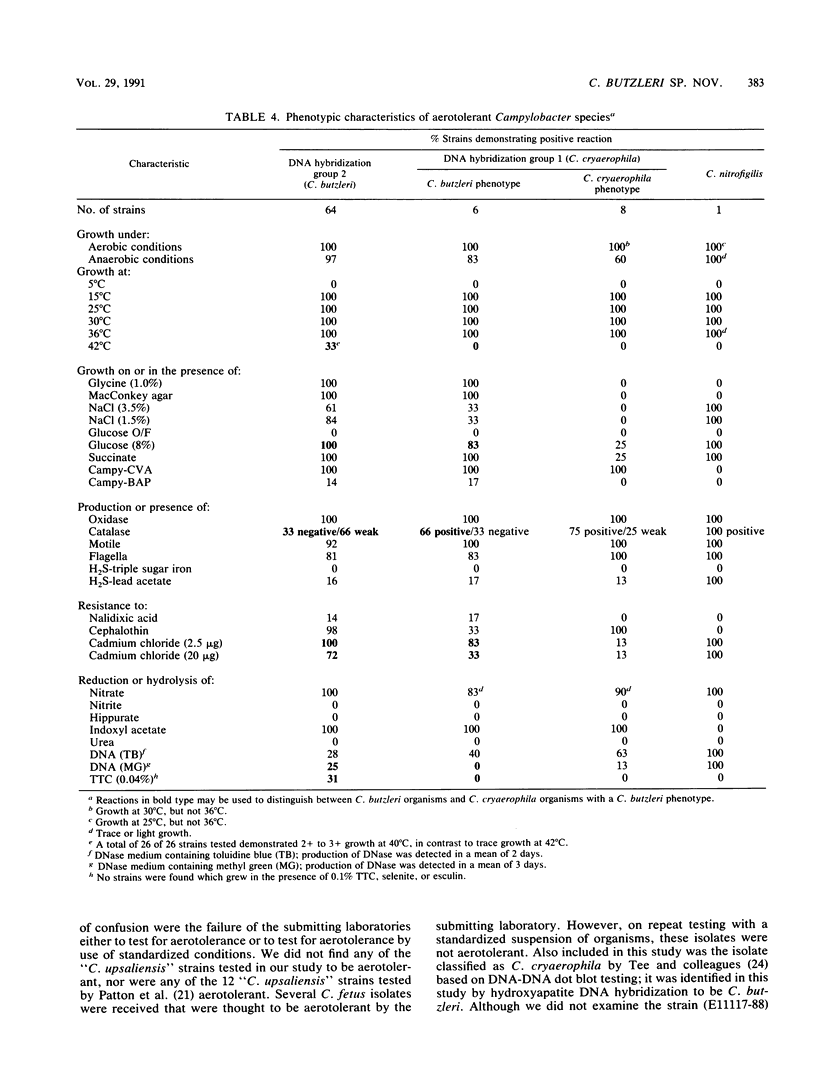
Seventy-eight aerotolerant Campylobacter isolates were characterized phenotypically and by DNA hybridization (hydroxyapatite method at 50 and 65 degrees C). Two DNA relatedness groups were found. (i) Sixty-four strains belonged to aerotolerant Campylobacter DNA hybridization group 2. These organisms were isolated from humans, primarily with diarrheal illness, and animals on several continents. Strains were aerotolerant at 30 and 36 degrees C and catalase negative or weakly catalase positive, grew in media containing glycine and on MacConkey agar, were susceptible to nalidixic acid, and were resistant to cephalothin. The name Campylobacter butzleri sp. nov. is proposed for this group. (ii) DNA hybridization group 1 consisted of the type strain of Campylobacter cryaerophila and 13 additional strains isolated from 10 animals outside the United States and from three humans within the United States. This group was genetically diverse; five strains were closely related to the type strain of C. cryaerophila (DNA hybridization group 1A), and eight strains were more closely related to one another (DNA hybridization group 1B). Strains in DNA hybridization group 1B were phenotypically diverse, with two of eight strains resembling C. cryaerophila. The seven strains from DNA hybridization groups 1A and 1B which resembled C. cryaerophila and the C. cryaerophila type strain were aerotolerant only at 30 degrees C and catalase positive, did not grow in glycine or on MacConkey agar, were generally susceptible to nalidixic acid, and were resistant to cephalothin. The remaining six strains of DNA hybridization group 1B phenotypically resembled C. butzleri; however, they were generally catalase positive and susceptible to nalidixic acid and cephalothin. DNA hybridization group 1B is not designated as a separate species at this time since it cannot, with certainty, be separated genetically from C. cryaerophila or phenotypically from C. butzleri.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W. A., Neill S. D., O'Brien J. J., Ferguson H. W., Hanna J. Isolation of Spirillum/Vibrio-like organisms from bovine fetuses. Vet Rec. 1977 May 21;100(21):451–452. doi: 10.1136/vr.100.21.451. [DOI] [PubMed] [Google Scholar]
- Ellis W. A., Neill S. D., O'Brien J. J., Hanna J. Isolation of spirillum-like organisms from pig fetuses. Vet Rec. 1978 Feb 4;102(5):106–106. doi: 10.1136/vr.102.5.106-a. [DOI] [PubMed] [Google Scholar]
- Gill K. P. Aerotolerant campylobacter strain isolated from a bovine preputial sheath washing. Vet Rec. 1983 May 7;112(19):459–459. doi: 10.1136/vr.112.19.459-a. [DOI] [PubMed] [Google Scholar]
- Kazmi S. U., Roberson B. S., Stern N. J. Cadmium chloride susceptibility, a characteristic of Campylobacter spp. J Clin Microbiol. 1985 May;21(5):708–710. doi: 10.1128/jcm.21.5.708-710.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Patton C. M., Barrett T. J., Moss C. W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987 Apr;25(4):706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Smibert R. M., Krieg N. R. Effect of incubation temperature, ageing, and bisulfite content of unsupplemented Brucella agar on aerotolerance of Campylobacter jejuni. Can J Microbiol. 1988 Sep;34(9):1069–1074. doi: 10.1139/m88-188. [DOI] [PubMed] [Google Scholar]
- Lior H., Patel A. Improved toluidine blue-DNA agar for detection of DNA hydrolysis by campylobacters. J Clin Microbiol. 1987 Oct;25(10):2030–2031. doi: 10.1128/jcm.25.10.2030-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan E. F., Neill S. D., Mackie D. P. Mastitis in dairy cows associated with an aerotolerant campylobacter. Vet Rec. 1982 Mar 6;110(10):229–230. doi: 10.1136/vr.110.10.229. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Mills C. K., Gherna R. L. Hydrolysis of indoxyl acetate by Campylobacter species. J Clin Microbiol. 1987 Aug;25(8):1560–1561. doi: 10.1128/jcm.25.8.1560-1561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S. D., Ellis W. A., O'Brien J. J. Designation of aerotolerant Campylobacter-like organisms from porcine and bovine abortions to the genus Campylobacter. Res Vet Sci. 1979 Sep;27(2):180–186. [PubMed] [Google Scholar]
- Neill S. D., Ellis W. A., O'Brien J. J. The biochemical characteristics of Campylobacter-like organisms from cattle and pigs. Res Vet Sci. 1978 Nov;25(3):368–372. [PubMed] [Google Scholar]
- Neill S. D., O'Brien J. J., Ellis W. A. The isolation of aerotolerant Campylobacter. Vet Rec. 1980 Feb 16;106(7):152–153. doi: 10.1136/vr.106.7.152. [DOI] [PubMed] [Google Scholar]
- Patton C. M., Shaffer N., Edmonds P., Barrett T. J., Lambert M. A., Baker C., Perlman D. M., Brenner D. J. Human disease associated with "Campylobacter upsaliensis" (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989 Jan;27(1):66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic-Uroic T., Patton C. M., Nicholson M. A., Kiehlbauch J. A. Evaluation of the indoxyl acetate hydrolysis test for rapid differentiation of Campylobacter, Helicobacter, and Wolinella species. J Clin Microbiol. 1990 Oct;28(10):2335–2339. doi: 10.1128/jcm.28.10.2335-2339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K., Mueller L. Isolation and characterization of catalase-negative and catalase-weak strains of Campylobacter species, including "Campylobacter upsaliensis," from humans with gastroenteritis. J Clin Microbiol. 1989 Sep;27(9):2042–2045. doi: 10.1128/jcm.27.9.2042-2045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Baird R., Dyall-Smith M., Dwyer B. Campylobacter cryaerophila isolated from a human. J Clin Microbiol. 1988 Dec;26(12):2469–2473. doi: 10.1128/jcm.26.12.2469-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Swenson J. M., Baker C. N., McDougal L. K., Stocker S. A., Hill B. C. Methods for determining susceptibility of fastidious and unusual pathogens to selected antimicrobial agents. Diagn Microbiol Infect Dis. 1988 Mar;9(3):139–153. doi: 10.1016/0732-8893(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Vandamme P., Falsen E., Rossau R., Hoste B., Segers P., Tytgat R., De Ley J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991 Jan;41(1):88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]


