Abstract
Reactive oxygen species such as hydrogen peroxide (H2O2) are involved in many cellular processes that positively and negatively regulate cell fate. H2O2, acting as an intracellular messenger, activates phosphatidylinositol-3 kinase (PI3K) and its downstream target Akt, and promotes cell survival. The aim of the current study was to understand the mechanism by which PI3K/Akt signaling promotes survival in SH-SY5Y neuroblastoma cells. We demonstrate that PI3K/Akt mediates phosphorylation of the pro-apoptotic Bcl-2 family member Bax. This phosphorylation suppresses apoptosis and promotes cell survival. Increased survival in the presence of H2O2 was blocked by LY294002, an inhibitor of PI3K activation. LY294002 prevented Bax phosphorylation and resulted in Bax translocation to the mitochondria, cytochrome c release, caspase-3 activation, and cell death. Collectively, these findings reveal a mechanism by which H2O2-induced activation of PI3K/Akt influences posttranslational modification of Bax and inactivate a key component of the cell death machinery.
Keywords: Reactive Oxygen Species (ROS), PI3/Akt, Bax, Mitochondria, Apoptosis
Introduction
Reactive oxygen species (ROS) are a natural byproduct of cellular metabolism. They are involved in various signaling pathways under normal physiological conditions [1]. The intracellular concentration of ROS is tightly regulated by cellular antioxidant defense mechanisms both enzymatically (antioxidant enzymes) and non-enzymatically (small antioxidant molecules, e.g. glutathione). An imbalance in the oxidant/antioxidant system, either due to excess ROS generation, impairment of antioxidant defense system, or both, leads to oxidative stress [2]. Chronic and sustained high toxic levels of ROS are associated with several pathological conditions including inflammatory diseases and the complications of diabetes [2–8]. In contrast, ROS are directly and indirectly involved in physiological signaling pathways [9, 10]. Low levels of ROS regulates cellular function in tumor cells [11], T cells [9], and macrophages [12].
We are interested in the underlying mechanisms by which ROS operate as second messengers. Mild increases in ROS act as second messengers in regulating survival signaling pathways [13, 14]. The pro-survival effects of low ROS levels suggest that they may be involved in neuronal preconditioning, such as hypoxic preconditioning against subsequent ischemic injury [15, 16]. This preconditioning model allows for the elucidation of subsequent survival pathways, which may serve as therapeutic targets for clinical intervention.
One of the most commonly used ROS for neuronal oxidative-stress preconditioning is H2O2 [17, 18]. H2O2 is formed by the dismutation of superoxide (O2•−) spontaneously or enzymatically in a reaction that is catalyzed by superoxide dismutase (SOD). In addition, many cell types produce H2O2 in response to growth factors such as VEGF, EGF, PDGF, and insulin that promote cell survival [19]. H2O2 is less reactive compared to other ROS, easily crosses membranes, and diffuses from its original site of production, all of which make H2O2 a candidate molecule for both inter- and intracellular signaling [20–23]. H2O2-mediated signaling alters the function of various proteins, including protein phosphatases, protein kinases, phospholipases, transcription factors, and ion channel proteins [24].
Exogenous H2O2 also activates Akt [25–27], which operates downstream of the PI3K cell survival pathway. Akt regulates downstream substrates such as Bcl-2 family proteins that mediate apoptosis. Bax is a pro-apoptotic member of the Bcl-2 family with three highly conserved BH domains (BH 1–3) and a hydrophobic C-terminal. The BH3 domain of the pro-apoptotic proteins is required for the translocation to and complex formation in, the mitochondrial membrane that leads to mitochondrial dysfunction and cell death [28–30]. Post-translational modifications (such as phosphorylation) of Bcl-2 members influences function and protein-protein interactions, and thus, play a major role in regulating cell fate. Indeed, phosphorylation abrogates Bax pro-apoptotic activity in human lung cancer [31] and in neutrophils [32].
In this study, we used SH-SY5Y human neuroblastoma cells as a model system for studying the molecular events regulating neuronal survival in response to H2O2. We tested the hypothesis that low levels of H2O2 regulate cell survival by altering the posttranslational modification of Bax through the PI3K/Akt pathway. We report that H2O2 protects SH-SY5Y cells from apoptosis and stimulates Akt phosphorylation in a PI3K-dependent manner. Preventing H2O2-induced PI3K activity with LY294002 results in Bax dephosphorylation and translocation to the mitochondria, which leads to cytochrome c release, caspase-3 activation, and apoptosis. These findings reveal a mechanism by which a H2O2- regulated signaling cascade promotes cell survival by inactivating a pro-apoptotic component of the cell death machinery.
Materials and Methods
Dulbecco’s modified Eagle’s medium (DMEM), Hank’s balanced salt solution (HBSS), trypsin-EDTA and calf serum (CS) were purchased from Gibco BRL (Gaithersburg, MD, USA). LY294002 was purchased from Calbiochem, Biosciences, Inc., (Santa Cruz, CA). Antibodies for Bax (Sc-7480, and Bax N-20) were purchased from Santa Cruz Biotechnology, Inc., CA; Akt, p-Akt (serine 473), and cleaved caspase-3 (Asp175) from Cell Signaling Technologies (Beverly, MA); phospho-serine (7F12) from Alexis Biochemical (San Diego, CA); Wortmannin and PP-2A/C were purchased from Calbiochem, cytochrome c from BD Pharmingen (San Diego, CA), and anti-oxphos complex IV, subunit I (anti-cytochrome oxidase, subunit I, Cox1), Alexa Flour 488, Alexa Flour 594, and DAPI were obtained from Invitrogen (Carlsbad, CA). Horseradish peroxidase-conjugated polyclonal goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from Santa Cruz Biotechnology, Inc, (Santa Cruz, CA). Enhanced chemiluminescence system using LumiGLO™ reagents were purchased from Cell Signaling, and molecular weight standards from Amersham (Arlington Heights, IL, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell Culture
SH-SY5Y human neuroblastoma cells were cultured in DMEM with 10% CS at 37°C in a humidified atmosphere containing 10% CO2 as described previously [33]. In all conditions, SH-SY5Y cells were seeded in 100 mm culture dishes at an initial density of 5 × 106 cells/cm2 and grown to approximately 80–90% confluency. Cells were serum starved for 4 h prior to treatments and then sub-cultured in serum-free DMEM for the specified times and experimental conditions. Previous work in our laboratory [34–37] and others [25] established that 20 μM LY294002 is not toxic to the cells and it effectively blocks the PI3K pathway in SH-SY5Y cells [34–37]. Cells were pre-treated with 20 μM LY294002, or Wortmannin (100 nM, and 1 μM) for 1 h then rinsed with fresh serum-free DMEM prior to treatment with a single bolus addition of 0.1 mM H2O2. Cells were collected at the end of 4 or 24 h treatments for subsequent immunoprecipitation, Western blotting, immunocytochemistry, or flow cytometry analyses.
Immunoprecipitation and Western Blot Analysis
SH-SY5Y cells were washed with HBSS and solubilized in ice-cold lysis buffer, containing 50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 1 mM EDTA, 150 mM NaCl, 1 mM phenylmethyl-sulfonyl fluoride (PMSF), 10 μg/ml aprotinin, 5 μg/ml leupeptin, and 1 mM sodium orthovanadate. Lysates were collected, sonicated briefly, centrifuged for 10 min at 4°C, and protein concentration determined using the Lowry method. Proteins were first immunoprecipitated, using an anti-Bax antibody (1:1000), or p-serine antibody (1:1000), as described previously [38], and then subjected to Western blot analysis. In the phosphatase experiment, PP-2A/C was added and incubated for 30 minutes. For Western blot analysis, samples were boiled in sample buffer (20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 20 mM DTT, 2% SDS, 20% glycerol, and 0.1% bromophenol blue), and loaded on 12.5% SDS-polyacrylamide gels (SDS-PAGE), followed by transfer to nitrocellulose membranes. Membranes were blocked in 5% non-fat milk in TBS-T (20 mM Tris, 0.16 M NaCl, and 0.10% Tween-20, pH 7.4) for more than 1 h at room temperature, and incubated overnight (at 4°C) with primary antibodies (Akt, p-Akt, Bax, and p-serine, 1:1000) in 5% non-fat milk in TBS-T. Membranes were then washed with TBS-T (3 times, 10 min each), and incubated with secondary goat anti- rabbit HRP (1:1000), or goat anti- mouse HRP antibody (1:2000) at room temperature for 1 h in 5% non-fat milk in TBS-T. An enhanced chemiluminescent (ECL) detection system was used according to the manufacturer’s protocol, and immunoblots were exposed to autoradiography film (Hyperfilm-ECL, Amersham Pharmacia Biotech). In some experiments, blots were stripped by incubation in buffer containing 2% SDS, 0.1 M Tris pH 6.8, 0.1 M DTT, and probed using a different antibody.
Immunocytochemistry
SH-SY5Y cells were seeded at a density of 5 × 106 cells/cm2 onto 25 mm glass coverslips arranged within the 100 mm culture dishes in order to maintain consistent culture conditions. Cells were serum starved for 4 h and exposed to experimental conditions as described previously. Cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. Coverslips were stained with DAPI and antibodies against Bax N-20 (1:100), Cox1 (1:50), cytochrome c (1:100), or cleaved caspase-3 (1:100) according to the experimental conditions described in the results. Samples were examined using a Nikon Diaphot 200 microscope with a 40 X objective lens. Digital images were captured with a Hamamatsu ORCA-ER CCD camera using Simple-PCI software (Compix Inc.).
Flow Cytometry
Analysis of DNA content was performed using flow cytometry as described previously [33]. After treatment, both floating and adherent (detached by trypsin-EDTA) cells were collected and rinsed in HBSS, fixed in ice cold 70% ethanol, and stored at 4°C prior to staining with 18 μg/ml propidium iodide and 40 μg/ml RNase A. DNA content of the cells was measured and separated into phases of the cell cycle based on the propidium iodide fluorescence. Apoptotic cells characteristically contain fragmented DNA, which is evident as a sub-Go peak on the cell cycle histogram. Analyses were performed by the University of Michigan Flow Cytometry Core Facility using an Epics flow cytometry system (Coulter Cytometry, Hialeah, FL). The data were analyzed using analysis of variance (ANOVA). All results are expressed as the mean percent cell death of 3 independent experiments ± the standard error of the mean (SEM).
RESULTS
Effect of Exogenous H2O2 on Akt Activation and Survival Signaling
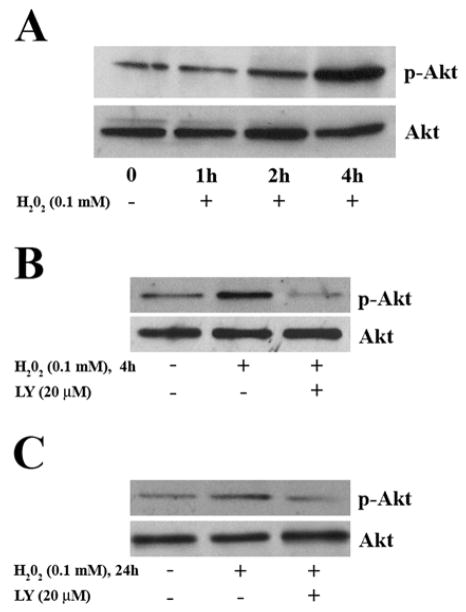
Exogenous H2O2 mimics the effect of endogenous receptor-induced H2O2, and activates multiple kinases [25–27, 39, 40]. To assess the effect of H2O2 on Akt activation, cells were exposed to 0.1 mM H2O2 for indicated times and lysates analyzed by Western blotting. Under our culture conditions, the intracellular concentration of H2O2 after a single bolus addition of 0.1 mM H2O2 is low [41]. We chose 0.1 mM H2O2, a condition that activates signaling but dose not lead to apoptosis. Treatment of SH-SY5Y cells with 0.1 mM H2O2 induced Akt phosphorylation (Ser 473) (Figure 1) with no change of Akt protein levels. Phosphorylation of Akt increased between 1 and 4 h of H2O2 exposure (Figure 1A). To assess the involvement of the PI3K pathway in H2O2–induced Akt activation, cells were pretreated with the PI3K inhibitor, LY294002. Pre-treatment of cells with LY294002 (20 μM) for 1 h prior to H2O2 treatment prevented H2O2-induced Akt phosphorylation at 4 h (Figure 1B) and 24 h (Figure 1C); data are representative of three independent experiments.
Fig. 1.
H2O2 stimulates phosphorylation of Akt in SH-SY5Y Cells. (A) Serum starved SH-SY5Y cells were treated with 0.1 mM H2O2 for the indicated times. Whole cell lysates were analyzed by Western blotting, using an anti-phospho-Akt antibody (1:1000, upper panel). Blots were then stripped and blotted for Akt protein (1:1000, lower panel). Cells were pre-treated with or without 20 μM LY294002 for 1 h, followed by H2O2 treatment for 4 h (B) or for 24 h (C) and immunoblotted for phospho-Akt as indicated (upper panels). Blots were then stripped and blotted for Akt protein (lower panels). Data are from one of three representative experiments.
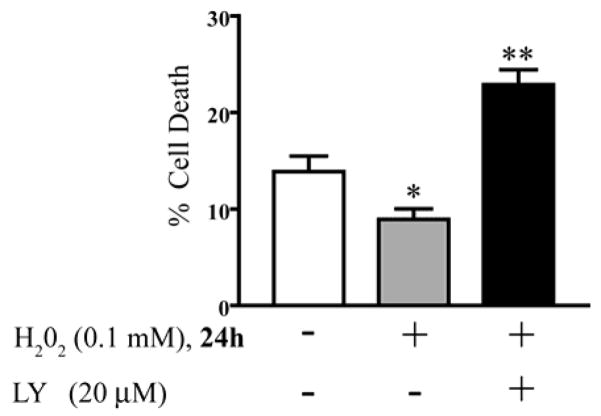
Previous work in our laboratory has shown that the PI3K/Akt pathway is critical for cellular protection against apoptosis [42, 43]. To assess the effect of H2O2 –mediated PI3K/Akt-activity on survival, serum-starved cells with no treatment (control) were compared with serum-starved cells treated with H2O2 in the presence or absence of the PI3K pathway inhibitor LY294002. We acknowledge that H2O2 is not present at the end point of our experiments (24 h), however, we intended to show that a low dose of H2O2 protected cell death even after 24 h. Flow cytometry was used to quantitate the percentage of cells undergoing apoptosis as previously described [34, 44–47]. Treatment of cells with LY alone had no effect on the percentage of cell death when compared to untreated control cells (data not shown). H2O2 significantly protected SH-SY5Y cells from serum-deprivation-induced apoptosis measured by flow cytometry (Figure 2, p < 0.05). However, the PI3K inhibitor LY294002 prevented the H2O2 induced protection (Figure 2, p < 0.01). Our findings show that blocking basal levels of PI3K is not sufficient to block cell death induced by serum starvation (as shown in the control condition), however, H2O2 suppresses apoptosis via the PI3K pathway, confirmed by the fact that LY blocked the effects of H2O2.
Fig. 2.
Effect of H2O2 on cell death. Serum deprived SH-SY5Y cells with no treatment for 24 h (control), and treated with 0.1 mM H2O2 for 24 h without LY pre-treatment (H2O2) or with pre-treatment with 20 μM LY294002 (LY+H2O2). DNA was stained with propidium iodide and DNA content was measured by flow cytometry. Percent cell death is shown as mean ± S.E.M. for four separate experiments. Treatment of the serum-starved cells with H2O2 alone was significantly different from control (*, p < 0.05). Serum-starved cells with pre-treatment with LY and then H2O2 addition (LY+H2O2) were significantly different from both control and from H2O2 alone (*, p < 0.01).
H2O2-induced Bax Phosphorylation Depends on PI3K
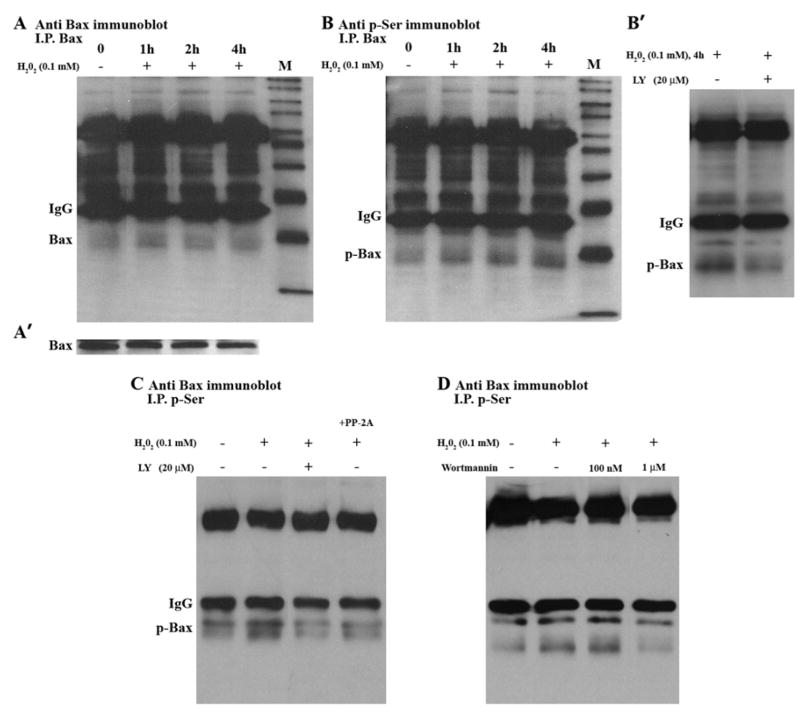
Previous reports suggest that Bax is inactivated through serine phosphorylation [31, 32]. Therefore, we examined how PI3K/Akt signaling alters Bax phosphorylation in cells exposed to a low level of H2O2. SH-SY5Y cells were treated with 0.1 mM H2O2 for various times, and Bax protein was detected by Western blotting. As shown in Figure 3A′, total Bax protein levels did not change during the time course of H2O2 treatment. Bax is immunoprecipitated, and an equal amount of sample was used for immunoblotting. Bax protein level was determined by immunoblotting for Bax (Fig. 3A) and Bax phosphorylation was determined by phosphoserine immunoblotting (Fig. 3B). Figure 3A shows that Bax protein level was not changed, while Bax phosphorylation increased (Fig. 3B). Additionally, increased Bax phosphorylation was confirmed by immunoprecipitation with an antibody to phosphoserine and subsequent immunoblotting for Bax (data not shown). Fig. 3B′ shows that inhibition of PI3K by LY blocked H2O2-induced Bax phosphorylation at 4 h. Inhibition of the PI3K pathway also blocked the H2O2 induced phosphorylation at 24h as shown in Figure 3C, and Bax protein level did not change (not shown). To further confirm Bax serine phosphorylation, we used protein phosphatase 2A (PP2A), which has recently being shown to dephosphosphorylate Bcl-2 proteins [48]. PP-2A decreased H2O2-induced Bax phosphorylation, as is shown in Fig. 3C (last lane). To further confirm the involvement of PI3K pathway, Wortmannin, another inhibitor of PI3K/Akt signaling pathway, was also examined. There was a dose dependent decrease in H2O2–induced Bax phosphorylation by Wortmannin. Wortmannin at 1 μM concentration decreased H2O2–induced Bax phosphorylation, while low dose Wortmannin(100 nM) had no effect (Fig. 3D). These results show that Bax phosphorylation is dependent upon PI3K activity in SH-SY5Y cells. For immunoprecipitation control, IP experiments were done without cell lysates, and no cross reactivity with the precipitating antibody was observed. IP efficiency was also confirmed by running supernatant, which collected after beads precipitation, and no bands were detected on the blots.
Fig. 3.
Bax phosphorylation in SH-SY5Y Cells. (A) Cells were treated with H2O2 for the indicated times. Cell lysates were immunoprecipitated with anti-Bax antibody (I.P.Bax) and then immunoblotted with Bax antibody. (A′) Whole cell lysates were analyzed by Western blotting, using Bax antibody. (B) Cells were treated with H2O2 for the indicated times. Cell lysates were immunoprecipitated with anti-Bax antibody (I.P.Bax) and then immunoblotted with anti-phosphoserine antibody. (B′) Cells were pre-treated with or without LY, and then treated with H2O2 for 4 h. Cell lysates were immunoprecipitated with Bax antibody and then immunoblotted with anti- phosphoserine antibody. (C) 24 h time course; Cell lysates from control (no treatment), H2O2 alone, LY + H2O2, and H2O2 + PP-2A/C were immunoprecipitated with anti- phosphoserine antibody, and then immunoblotted with Bax antibody. (D) Cells were pre-treated with or without Wortmannin (W), and then treated with H2O2 for24 h. Cell lysates were immunoprecipitated with anti- phosphoserine antibody and then immunoblotted with Bax antibody.
Inhibition of PI3K Causes Bax Activation and Translocation to the Mitochondria
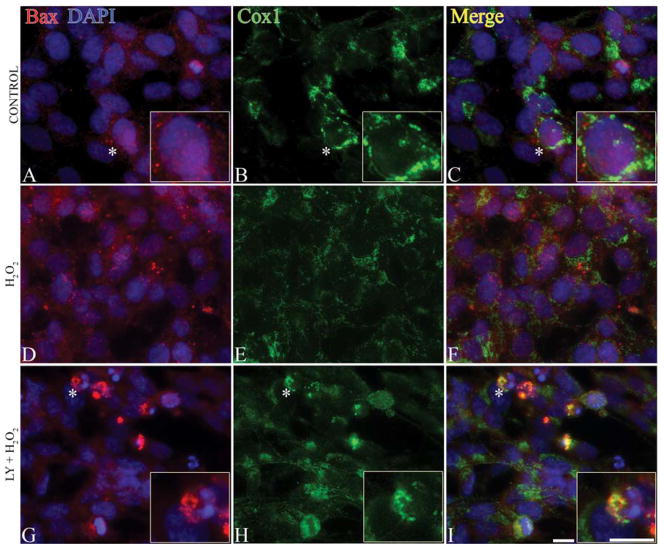
Bax activation and localization are involved in apoptosis [49, 50]. Upon stimulation, Bax undergoes conformational rearrangement, resulting in activation and translocation to the mitochondria [51]. Therefore, we examined Bax intracellular localization following H2O2 treatment (Figure 4). SH-SY5Y cells were pretreated + LY294002 for 1 h and then treated with 0.1 mM H2O2 for 24 h, followed by immunostaining with N-20 to detect active Bax, Cox1 to identify mitochondria, and DAPI to label nuclei (Figure 4). Bax was diffusely expressed in the cytoplasm of the healthy SH-SY5Y cells and showed punctate staining in apoptotic cells. Bax remained cytoplasmic following treatment with 0.1 mM H2O2 for 4 h (data not shown) or 24 h (Figure 4D–4F) and DAPI staining confirmed intact nuclei (Figure 4D). Inhibition of PI3K by pretreatment of cells with 20 μM LY294002 for 1 h resulted in Bax translocation from the cytoplasm to the mitochondria (Figure 4G–4I).
Fig. 4.
Bax translocation to the mitochondria following PI3K inhibition. (A–C) Control SH-SY5Y cells; (D–F) cells treated with 0.1 mM H2O2 for 24 h; (G–I) cells pre-treated with PI3K inhibitor (20 μM of LY294002, LY) and then treated with 0.1 mM H2O2 for 24 h (LY + H2O2). Cells were double immunostained with Bax (N-20, red), Cox1 (green) and nuclei were visualized by DAPI (blue). Asterisks indicate cells in inserts. Normal healthy control cells are shown in A and C. In H2O2 only treatments (D–F), cells are healthy with intact nuclei and diffuse Bax staining in the cytoplasm. PI3K inhibition resulted in punctate Bax staining (G) and Bax translocation to mitochondria (H) as is evident by yellow signal in the merged image (I). Bars = 10 μm.
PI3K Inhibition Induces Cytochrome c Release into the Cytoplasm and Caspase-3 Activation
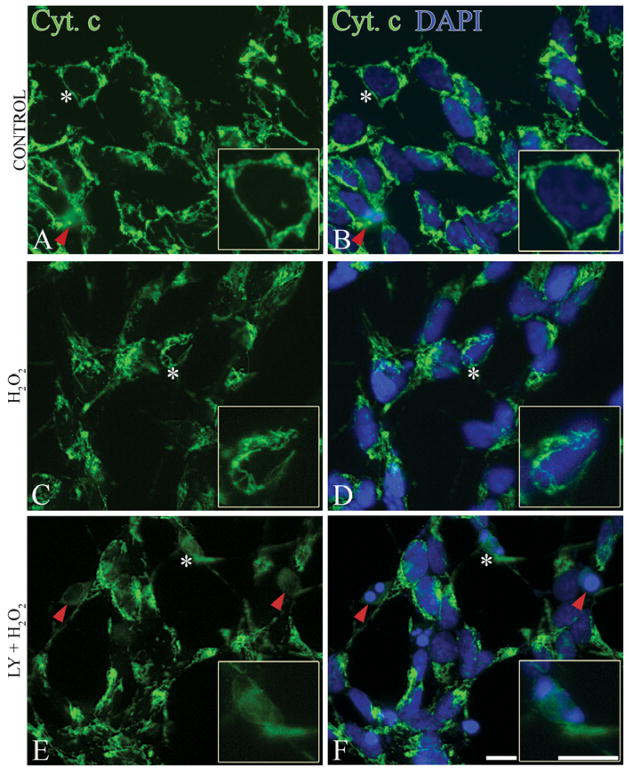
Mitochondrial Bax triggers cytochrome c release from mitochondria, resulting in apoptosome formation, caspase activation, and apoptosis [52, 53]. Therefore, we next investigated cytochrome c release (Figure 5) in SH-SY5Y cells following H2O2 treatment with or without pre-treatment of LY294002. In healthy cells, cytochrome c staining was punctate (Figure 5A) and cells contained intact nuclei (Figure 5B). Treatment with 0.1 mM H2O2 alone for 24 h did not change cytochrome c localization; healthy cells are shown with punctuate cytochrome c (Figure 5C) and intact nuclei (Figure 5D). However, pre-treatment of cells with LY294002 for 1 h resulted in cytochrome c release into the cytoplasm (Figure 5E) and condensed nuclei typical of apoptosis (Figure 5F).
Fig. 5.
Cytochrome c release into the cytoplasm following PI3K inhibition. (A, B) Control cells; (C, D) cells treated with 0.1 mM H2O2 for 24 h; (E, F) cells pre-treated with 20 μM of LY294002 (LY), followed by 0.1 mM H2O2 treatment for 24 h (LY + H2O2). Cells were immunostained with cytochrome c (green) and nuclei were visualized by DAPI staining (blue). Asterisks indicate cells in inserts. Healthy cells (A, B and C, D) show punctate cytochrome c staining. Apoptotic cells with diffused cytochrome c (E), and condensed nuclei (F) are shown by red arrowheads. Bars = 10μm.
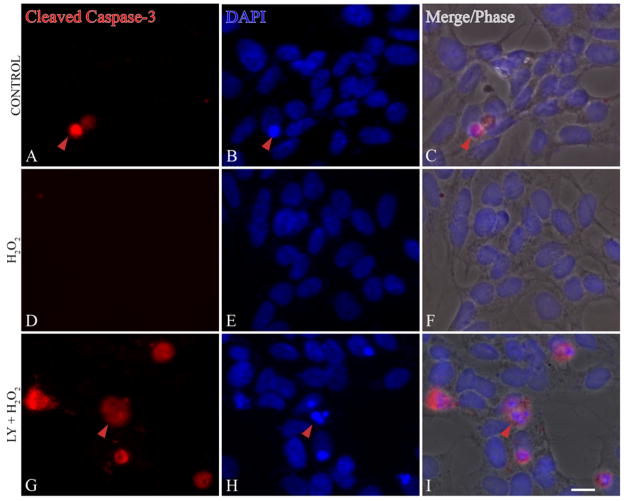
To investigate whether cytochrome c release results in caspase-3 activation, control and H2O2 treated (± LY294002) SH-SY5Y cells were stained with an antibody to detect cleaved caspase-3 fragments. Caspase-3 was not activated by H2O2 (0.1 mM) treatment alone and DAPI staining confirmed that the nuclei are intact and healthy (Figure 6D–6F). However, pre-treatment with LY294002 (20 μM, 1 h) resulted in caspase-3 cleavage (Figure 6G) and condensed nuclei (Figure 6H–I).
Fig. 6.
Inhibition of PI3K pathway lead to caspase-3 activation. (A–C) Control; (D–F) SH-SY5Y cells treated with 0.1 mM H2O2 for 24 h; (G–I) 20 μM of LY294002 (LY) followed by 0.1 mM H2O2 for 24 h (LY + H2O2). Cells were immunostained with cleaved caspase-3 (red), and nuclei were visualized by DAPI staining (blue). Caspase-3 activation is shown by the presence of red staining in G. Red arrowheads indicate cleaved caspase-3 staining in cells with condensed nuclei (I). Merged fluorescence signals are overlaid onto phase images (C, F, I) Bars = 10μm.
DISCUSSION
The current study demonstrates that H2O2–mediated Akt activation, downstream of PI3K, results in Bax phosphorylation and inactivation. Phosphorylation of Bax regulates cell survival by preventing Bax translocation to the mitochondria. Inhibition of the PI3K pathway by LY294002 results in Bax dephosphorylation and activation, followed by Bax translocation to the mitochondria, leading to cytochrome c release, caspase-3 activation and cell death.
Exogenous H2O2 increases Akt phosphorylation via the PI3K pathway in neuroblastoma cells. Involvement of PI3K in Akt phosphorylation was confirmed when the PI3K inhibitor LY294002 blocked Akt phosphorylation. Increasing evidence suggests that ROS, H2O2 in particular, mediate protein phosphorylation [13, 19, 25, 54–56], and H2O2–mediated Akt phosphorylation is reported in vascular smooth muscle cells [26], and in different cell types such as HeLa cells, epithelial cancer cells, and fibroblasts [25]. Our findings support the concept that protein phosphorylation is redox sensitive and is altered by ROS generation [1, 57–59]. Since the PI3K/Akt pathway is known to be important for cell survival in multiple systems [25, 60–62], we further examined cell survival in our system.
We show that mild oxidation induced by low levels of H2O2 promotes cell survival, and correlates with Akt activation. However, inhibition of the PI3K pathway and Akt down-regulation enhances cell death in SH-SY5Y cells. These findings are in agreement with other reports that Akt activation inhibits the apoptosis induced by serum withdrawal in lymphoid cells, fibroblasts, and neurons [63–67]. To characterize the mechanism of cell survival in our system, we examined downstream events of Akt activation. It is established that Bcl-2 family proteins, downstream of PI3K/Akt, are the key regulators of cell fate. In this regard we further investigated the effect of H2O2–mediated Akt upregulation on the proapoptotic protein Bax. Specifically, we examined whether Bax phosphorylation and activation is regulated by Akt.
The novel finding reported in this study is that H2O2 regulates Bax phosphorylation downstream of the PI3K/Akt pathway and contributes to cell survival. This association between H2O2-induced Akt activation and Bax phosphorylation, and suppression of apoptosis has not previously been reported. We show that H2O2-induced Bax phosphorylation is blocked by PI3K inhibitor LY294002, confirming that PI3K/Akt signaling regulates Bax phosphorylation. Recent studies indicate that Bcl-2 family proteins, downstream of PI3K/Akt, play a vital role in regulating cellular survival/death at the mitochondrial level [44, 50, 68–72]. Nechushtan and colleagues reported that structural re-arrangement in the C-terminal region of Bax is responsible for Bax activation [73]. It has also been shown that the phosphorylation state of Bax is a critical regulator of survival in human lung cancer cells [31] and neutrophils [32]. Our findings highlight a critical role for the PI3K/Akt pathway in regulating Bax activity and promoting cell survival following mild oxidation by H2O2. Taken together, these findings support our previous work regarding PI3K/Akt regulation of cell survival in neuronal cells [36, 62] and demonstrate that Bax plays a significant role in regulating neuronal death [50].
In this report, we demonstrate the involvement of PI3K signaling in Bax regulation and suppression of apoptosis. Following treatment with low levels of H2O2, Bax remains in the cytoplasm, however, inhibition of the PI3K pathway by LY294002 results in Bax activation and translocation to the mitochondria. Dephosphorylation of Bax alters its structure and allows its insertion into the mitochondria. Our study supports previous findings of our laboratory [50] and others [51] that activated Bax translocates to the mitochondria. Bax is a key regulator of mitochondrial integrity and alters mitochondrial membrane stability [74]. Some studies suggested that mitochondrial translocation of Bax creates pores in the outer membrane of mitochondria that allow cytochrome c release into the cytosol [52]. Therefore, we examined whether Bax translocation to the mitochondria results in cytochrome c release in SH-SY5Y cells.
In our study, cytochrome c is retained in the mitochondria after exposure of the cells to a low level of H2O2. However, PI3K inhibition resulted in diffused cytochrome c staining, an indication of cytochrome c release from the mitochondria into the cytoplasm. Cytochrome c is a peripheral protein of the mitochondrial inner membrane, which is released into the cytosol after mitochondrial damage. Our findings support the concept that activated Bax is involved in a multi-step apoptotic pathway [49, 75] that results in the loss of mitochondrial membrane integrity and the release of apoptotic molecules such as cytochrome c [52, 76]. We further characterized the apoptotic pathway by examining caspase-3 activation.
In SH-SY5Y cells Bax translocation to the mitochondria and cytochrome c release was followed by caspase-3 activation. Our study confirms other findings that Bax activation and translocation induces apoptosis [49, 50]. Mitochondrial Bax triggers cytochrome c release from mitochondria, resulting in apoptosome formation, caspase activation, and apoptosis [52, 53, 77]. This is the final commitment to cell death in many neuronal and non-neuronal systems [78]. Activated caspase-3, is detected both in vitro, and in animal models of Alzheimer’s, Huntington’s and Parkinson’s disease [79–82]. Together, our findings support a model where translocation of activated Bax to the mitochondria is followed by cytochrome c release, caspase-3 activation, and apoptosis (Figure 7).
Fig. 7.
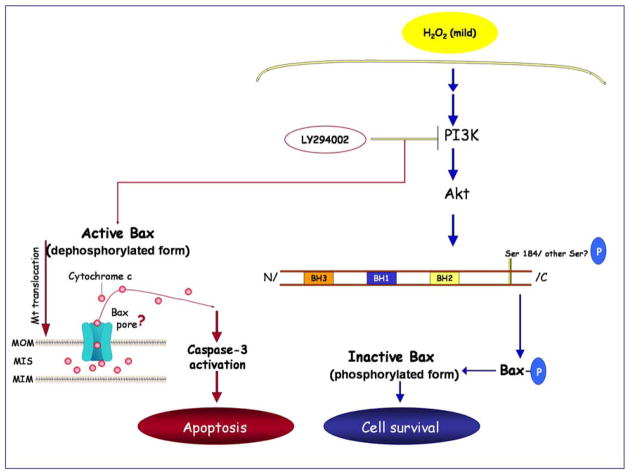
Activation of Akt by H2O2 regulates the activity of the pro-apoptotic Protein Bax. H2O2, that produces a mild oxidative environment, activates the PI3K/Akt survival pathway (blue arrows). When Akt is activated by H2O2, Bax is located in the cytoplasm in its phosphorylated (inactive) form and the cell survives (blue arrows). Inhibition of the PI3K pathway results in dephosphorylation of Bax and its translocation to the mitochondria (red arrows). Insertion of Bax into the mitochondria leads to cytochrome c release (possibly through Bax pore formation), caspase-3 activation, and apoptosis (red arrows). Abbreviations: MOM, mitochondrial outer membrane; MIM: mitochondrial inner membrane; MIS, mitochondrial intermembrane space.
In summary, we demonstrated that the cell survival/death pathway is redox sensitive and confirmed a stepwise mechanism by which H2O2-induced stimulation of Akt promotes survival through posttranslational modification of the proapoptotic protein Bax. Our findings show that a low level of H2O2 activates Akt via the PI3K pathway and induces Bax phosphorylation, and as a result Bax remains in the cytoplasm in its inactive conformation. However, inhibition of the PI3K pathway results in dephosphorylation of its downstream proteins Akt and Bax, resulting in Bax translocation to the mitochondria, cytochrome c release, and caspase-3 activation (as illustrated in Figure 7). Further studies will address whether post-translational modification of Bax alters its protein-protein interactions with other Bcl-2 family members, or mitochondrial proteins. These interactions will impact cell survival through regulation of mitochondrial function. The strong association between cellular redox state and the regulation of apoptosis is an exciting new area of investigation. Insight into these mechanisms could yield novel therapeutic strategies to regulate cell survival for many disorders that involve the dysregulation of apoptosis, including cancer and neurodegenerative diseases.
Acknowledgments
This work was supported by the National Institutes of Health (DK 076160), the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, and the Program for Neurology Research & Discovery and The A. Alfred Taubman Medical Research Institute at the University of Michigan. This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases and the UM-BRCF Core Flow Cytometry facility.
The authors would like to thank Drs. Kelli Sullivan, and Andrea Vincent for editing this manuscript. This work was supported by the National Institutes of Health (NS36778, NS38849), the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, and the Program for Neurology Research & Discovery at the University of Michigan. This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases and the UM-BRCF Core Flow Cytometry facility.
Abbreviations
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DTT
dithiothreitol
- EDTA
ethylene diamine tetra acetic acid
- H2O2
hydrogen peroxide
- PI-3K
phosphatidylinositol 3-kinase
- ROS
reactive oxygen species
- SDS- PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- S.E.M
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular Regulation by Hydrogen Peroxide. J Am Soc Nephrol. 2003;14:S211–215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep. 1978;2:113–128. doi: 10.1016/0309-1651(78)90032-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson I, Adinolfi C, Doctrow S, Huffman K, Joy KA, Malfroy B, Soden P, Rupniak HT, Barnes JC. Oxidative signalling and inflammatory pathways in Alzheimer’s disease. Biochem Soc Symp. 2001:141–149. doi: 10.1042/bss0670141. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi S, Wood NW. Molecular pathogenesis of Parkinson’s disease. Hum Mol Genet. 2005;14:2749–2755. doi: 10.1093/hmg/ddi308. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 7.Feldman EL, Stevens MJ, Greene DA. Pathogenesis of diabetic neuropathy. Clin Neurosci. 1997;4:365–370. [PubMed] [Google Scholar]
- 8.Halliwell B. Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson’s disease, Alzheimer’s disease, traumatic injury or stroke? Acta Neurol Scand Suppl. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 9.Hehner SP, Breitkreutz R, Shubinsky G, Unsoeld H, Schulze-Osthoff K, Schmitz ML, Droge W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- 10.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 12.Theus SA, Tabor DR, Soderberg LS, Barnett JB. Macrophage tumoricidal mechanisms are selectively altered by prenatal chlordane exposure. Agents Actions. 1992;37:140–146. doi: 10.1007/BF01987903. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro HP, Stern A. Redox modulation of tyrosine phosphorylation-dependent signal transduction pathways. Free Radical Biology and Medicine. 1996;21:323–333. doi: 10.1016/0891-5849(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 14.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA. Free radical biology and medicine: it’s a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 15.Das DK. Thioredoxin Regulation of Ischemic Preconditioning. Antioxidants & Redox Signaling. 2004;6:405–412. doi: 10.1089/152308604322899477. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama T, Engelman RM, Maulik N, Das DK. Role of Akt Signaling in Mitochondrial Survival Pathway Triggered by Hypoxic Preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 17.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 18.Hyslop PA, Hinshaw DB, Scraufstatter IU, Cochrane CG, Kunz S, Vosbeck K. Hydrogen peroxide as a potent bacteriostatic antibiotic: Implications for host defense. Free Radical Biology and Medicine. 1995;19:31–37. doi: 10.1016/0891-5849(95)00005-i. [DOI] [PubMed] [Google Scholar]
- 19.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for Generation of H(2)O(2) for Platelet-Derived Growth Factor Signal Tran sduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. The Biochemical Journal. 1998;333(Pt 2):291. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annual Review Of Pharmacology And Toxicology. 1999;39:67. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 22.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. The EMBO Journal. 1991;10:2247. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griendling KK, Harrison DG. Dual role of reactive oxygen species in vascular growth. Circulation Research. 1999;85:562. doi: 10.1161/01.res.85.6.562. [DOI] [PubMed] [Google Scholar]
- 24.Fruehauf JP, Meyskens FL., Jr Reactive Oxygen Species: A Breath of Life or Death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal Growth Factor Receptor-dependent Akt Activation by Oxidative Stress Enhances Cell Survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive Oxygen Species Mediate the Activation of Akt/Protein Kinase B by Angiotensin II in Vascular Smooth Muscle Cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 27.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda Si, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Letters. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 28.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Current Opinion in Cell Biology. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 Domain of BAX Identifies Residues Critical for Dimerization and Killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosulich SC, Worrall V, Hedge PJ, Green S, Clarke PR. Regulation of apoptosis by BH3 domains in a cell-free system. Curr Biol. 1997;7:913–920. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 31.Xin M, Deng X. Nicotine Inactivation of the Proapoptotic Function of Bax through Phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 32.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. xPhosphorylation of Bax Ser184 by Akt Regulates Its Activity and Apoptosis in Neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 33.Cynthia M, Van Golen ELF. Insulin-like growth factor I is the key growth factor in serum that protects neuroblastoma cells from hyperosmotic-induced apoptosis. Journal of Cellular Physiology. 2000;182:24–32. doi: 10.1002/(SICI)1097-4652(200001)182:1<24::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Kim B, Feldman EL. Insulin-like growth factor I prevents mannitol-induced degradation of focal adhesion kinase and Akt. J Biol Chem. 2002;277:27393–27400. doi: 10.1074/jbc.M201963200. [DOI] [PubMed] [Google Scholar]
- 35.Meyer GE, Shelden E, Kim B, Feldman EL. Insulin-like growth factor I stimulates motility in human neuroblastoma cells. Oncogene. 2001;20:7542–7550. doi: 10.1038/sj.onc.1204927. [DOI] [PubMed] [Google Scholar]
- 36.Kim B, van Golen CM, Feldman EL. Insulin-like growth factor I induces preferential degradation of insulin receptor substrate-2 through the phosphatidylinositol 3-kinase pathway in human neuroblastoma cells. Endocrinology. 2005;146:5350–5357. doi: 10.1210/en.2005-0356. [DOI] [PubMed] [Google Scholar]
- 37.Schwab TS, Madison BB, Grauman AR, Feldman EL. Insulin-like growth factor-I induces the phosphorylation and nuclear exclusion of forkhead transcription factors in human neuroblastoma cells. Apoptosis. 2005;10:831–840. doi: 10.1007/s10495-005-0429-y. [DOI] [PubMed] [Google Scholar]
- 38.Leventhal PS, Randolph AE, Vesbit TE, Schenone A, Windebank AJ, Feldman EL. Insulin-like Growth Factor-II as a Paracrine Growth Factor in Human Neuroblastoma Cells. Experimental Cell Research. 1995;221:179–186. doi: 10.1006/excr.1995.1365. [DOI] [PubMed] [Google Scholar]
- 39.Yoshizumi M, Abe J-i, Haendeler J, Huang Q, Berk BC. Src and Cas Mediate JNK Activation but Not ERK1/2 and p38 Kinases by Reactive Oxygen Species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between protein kinase C delta and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J Biol Chem. 2000;275:7470–7473. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- 41.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 42.Cheng HL, Steinway M, Delaney CL, Franke TF, Feldman EL. IGF-I promotes Schwann cell motility and survival via activation of Akt. Mol Cell Endocrinol. 2000;170:211–215. doi: 10.1016/s0303-7207(00)00324-5. [DOI] [PubMed] [Google Scholar]
- 43.Kim B, Feldman EL. Differential Regulation of Focal Adhesion Kinase and Mitogen-Activated Protein Kinase Tyrosine Phosphorylation During Insulin-Like Growth Factor-I-Mediated Cytoskeletal Reorganization. Journal of Neurochemistry. 1998;71:1333–1336. doi: 10.1046/j.1471-4159.1998.71031333.x. [DOI] [PubMed] [Google Scholar]
- 44.Singleton JR, Dixit VM, Feldman EL. Type I Insulin-like Growth Factor Receptor Activation Regulates Apoptotic Proteins. J Biol Chem. 1996;271:31791–31794. doi: 10.1074/jbc.271.50.31791. [DOI] [PubMed] [Google Scholar]
- 45.Sgonc R, Wick G. Methods for the detection of apoptosis. Int Arch Allergy Immunol. 1994;105:327–332. doi: 10.1159/000236777. [DOI] [PubMed] [Google Scholar]
- 46.Kokileva L. Multi-step chromatin degradation in apoptosis. DNA breakdown in apoptosis. Int Arch Allergy Immunol. 1994;105:339–343. doi: 10.1159/000236779. [DOI] [PubMed] [Google Scholar]
- 47.van Golen CM, Soules ME, Grauman AR, Feldman EL. N-Myc overexpression leads to decreased beta1 integrin expression and increased apoptosis in human neuroblastoma cells. Oncogene. 2003;22:2664–2673. doi: 10.1038/sj.onc.1206362. [DOI] [PubMed] [Google Scholar]
- 48.Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Molecular and cellular biology. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of Dimer Formation and Intracellular Localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 50.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis. 2006;23:11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C. Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. PNAS. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell JW, Golovoy D, Vincent AM, Mahendru PIA, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 54.Nakashima I, Kato M, Akhand AA, Suzuki H, Takeda K, Hossain K, Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- 55.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic Signaling Mediated by Oxidants in Ras-Transformed Fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 56.Zick Y, Sagi-Eisenberg R. A combination of H2O2 and vanadate concomitantly stimulates protein tyrosine phosphorylation and polyphosphoinositide breakdown in different cell lines. Biochemistry. 1990;29:10240–10245. doi: 10.1021/bi00496a013. [DOI] [PubMed] [Google Scholar]
- 57.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 58.Kevil CG, Okayama N, Alexander JS. H2O2-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol. 2001;281:C1940–1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- 59.Chiarugi P, Taddei ML, Ramponi G. Oxidation and tyrosine phosphorylation: synergistic or antagonistic cues in protein tyrosine phosphatase. Cell Mol Life Sci. 2005;62:931–936. doi: 10.1007/s00018-004-4448-1. [DOI] [PubMed] [Google Scholar]
- 60.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends in Biochemical Sciences. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 61.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 62.Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004:04–1581fje. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- 63.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of Neuronal Survival by the Serine-Threonine Protein Kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 67.McGinnis KM, Gnegy ME, Wang KK. Endogenous bax translocation in SH-SY5Y human neuroblastoma cells and cerebellar granule neurons undergoing apoptosis. J Neurochem. 1999;72:1899–1906. doi: 10.1046/j.1471-4159.1999.0721899.x. [DOI] [PubMed] [Google Scholar]
- 68.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 69.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 70.van Golen CM, Castle VP, Feldman EL. IGF-I receptor activation and BCL-2 overexpression prevent early apoptotic events in human neuroblastoma. Cell Death Differ. 2000;7:654–665. doi: 10.1038/sj.cdd.4400693. [DOI] [PubMed] [Google Scholar]
- 71.Peso Ld, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-Induced Phosphorylation of BAD Through the Protein Kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 72.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. Embo J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu YT, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. PNAS. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax Is Present as a High Molecular Weight Oligomer/Complex in the Mitochondrial Membrane of Apoptotic Cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 76.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated Targeting of BAX to Mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 79.Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from Parkinsonian brain. Journal of Neural Transmission. 2000;107:335–341. doi: 10.1007/s007020050028. [DOI] [PubMed] [Google Scholar]
- 80.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. PNAS. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is Induced by {beta}-Amyloid in Cultured Central Nervous System Neurons. PNAS. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]