Abstract
Background:
Liver transplantation and resection surgery involve a period of ischaemia and reperfusion to the liver which initiates an inflammatory cascade resulting in liver and remote organ injury. Bucillamine is a low-molecular-weight thiol antioxidant that is capable of rapidly entering cells.
Methods:
The effect of bucillamine was studied in a rat model of liver ischaemia–reperfusion injury with 45 min of partial (70%) liver ischaemia and at 3 and 24 h of reperfusion. Controls included ischaemia-reperfusion (I/R) only, sham and bucillamine alone (without ischaemia reperfusion). Liver injury was assessed by serum transaminases (AST and ALT). Sinusoidal blood flow and hepatocyte apoptosis were measured using intravital microscopy (IVM).
Results:
The hepatocellular injury of I/R produced a markedly elevated serum AST which was reduced with bucillamine (2072.5 ± 511.79 vs. 932 ± 200.8, P < 0.05) at 3 h reperfusion. Bucillamine treatment with I/R also increased parenchymal blood flow [red blood cell (RBC) velocity 242.66 ± 16.86 vs. 181.11 ± 17.59, at the end of 3 h of reperfusion) and reduced hepatocyte necrosis/apoptosis at 3 h as well as 24 h (P > 0.001).
Conclusion:
Bucillamine reduces the hepatocellular injury of liver ischaemia reperfusion and improves parenchymal perfusion.
Keywords: liver ischaemia-reperfusion injury, intravital microscopy, antioxidants, Bucillamine
Introduction
Liver transplantation and liver resection surgery have increased dramatically as a result of the excellent duration and quality of life they can offer selected patients with chronic liver disease and liver cancers. Both procedures involve a period of ischaemia and reperfusion to the liver which initiates an inflammatory cascade resulting in liver and remote organ injury. When severe these changes can be fatal.
Reactive oxygen species (ROS) have a central role to play in ischaemia-reperfusion injury (I/R).1 ROS activate cytokines, macrophages and other components of the inflammatory pathway.2–4 When generated in large amounts, they can also cause direct oxidative damage to the cells through iron-mediated reactions.5 Thiol donors are antioxidants which can interrupt the redox signalling pathway and thereby reduce cytokine and macrophage activation.6 In addition, thiol donors can protect against oxidative injury by replenishing intracellular glutathione and other endogenous thiol compounds.7
Bucillamine is a low molecular weight thiol donor that is capable of rapidly entering cells. As an oral formulation it is marketed in Japan and Korea for the treatment of rheumatoid arthritis.8
Similar to other cysteine derivatives such as N-acetylcysteine (NAC), bucillamine has the ability to replenish intracellular reduced glutathione (GSH).9 These compounds can directly scavenge peroxides, but less efficiently than the glutathione/glutathione peroxidase system. Bucillamine preserves a highconcentration of oxidized glutathione, which may be its primary action. Bucillamine has two donatable thiol groups and is fourfold more potent than NAC in preventing I/R injury in in vitro studies10 and 16-fold more potent in in vivo studies.11
Bucillamine has undergone some preliminary investigations in experimental studies of I/R. Amersi and colleagues9 studied Bucillamine and I/R in a rat model of liver transplantation. Bucillamine decreased liver I/R injury and increased survival after transplant, with increased levels of GSH in the liver and decreased levels of oxidized glutathione in both the liver and blood.
In cardiac I/R injury, Horwitz and Sherman10 demonstrated in isolated rat cardiac myocytes that bucillamine is a potent antioxidant. Bucillamine (125–500 microM) prevented lactate dehydrogenase (LDH) release in cardiac myocytes exposed to hydrogen peroxide or a xanthine/xanthine oxidase system. Further, in dogs subjected to 90 min of coronary artery occlusion and 48 h of reperfusion, bucillamine, administered during reperfusion, decreased myocardial infarct size by 41%.9
Liver I/R is known to cause microcirculatory perfusion failure, activate polymorphonuclear leukocytes and increase leukocyte-endothelial cell interaction which in turn contribute to hepatocellular damage and liver dysfunction.12–16
The effect of bucillamine in the treatment of liver warm I/R injury has not been investigated, furthermore its effect on liver microcirculation is not known. The aim of the present study was to use a well-described model of liver ischaemia reperfusion injury to determine the effect of bucillamine administration on liver function, liver microcirculation and hepatocyte apoptosis.
Materials and methods
Animals and surgical preparation
The study was conducted under a project license from the Home Office in accordance with the Animals (Scientific Procedures) Act 1986. Male Sprague–Dawley rats, weighing 270–330 g, were used. Animals were kept in a temperature-controlled environment with a 12 h light-dark cycle and allowed tap water as well as standard rat chow pellets ad libitum. Animals were anaesthetized with 4% isoflurane and maintained with 2.0% isoflurane (Abbott Laboratories Ltd, Kent, UK). They were allowed to breathe spontaneously through a concentric mask connected to an oxygen regulator and monitored with a pulse oximeter (Ohmeda biox 3740 pulse oximeter; Ohmeda, Louisville, KY, USA).
Polyethylene catheters (Portex 2 Fr; Portex, Kent, UK) were inserted into the carotid artery (right or left) for monitoring of mean arterial blood pressure and the right jugular vein for administering normal saline to compensate for intra-operative fluid loss (1 ml/100 g body weight/hour).
Laparotomy was carried out through a midline incision. The ligamentous attachments of the liver were cut and the liver exposed. All animals were administered heparin 20 units/kg. Partial hepatic ischaemia of the left lateral and median lobes (70% of liver) was induced by clamping the corresponding vascular pedicle with an atraumatic microvascular clamp for 45 min. This model prevents splanchnic congestion by allowing flow through the remaining liver.17,18 Animals were randomly allocated to the following groups:
Experimental groups (n= 6 in each group)
Group 1 – Sham: These animals underwent laparotomy and liver mobilization under general anaesthesia but without clamping of the liver vascular pedicle.
Group 2 – IR: 45 min of partial hepatic ischemia followed by 3 h of reperfusion.
Group 3 – Bucillamine [15 mg/kg/h intravenously (i.v.)]+ IR.
Group 4 – SB: sham + bucillamine (15 mg/kg/h i.v.).
Group 5 – I/R: 24–45 min of ischaemia followed by 24 h of reperfusion.
Group6 – B: 24–45 min of ischaemia + bucillamine 15 mg/kg/h i.v. followed by 24 h of reperfusion.
The bucillamine group were administered a bucillamine infusion (15 mg/kg/h) over the operative period (For 10 min prior to ischaemia, during the period of ischaemia and for 3 h in the reperfusion period). Bucillamine was supplied by Santen pharmaceuticals, Osaka, Japan. Animals in the sham and I/R groups were given equivalent volumes of saline. Temperature of the animal was monitored and maintained at 36–38°C by means of a heating pad (Harvard Apparatus Ltd, Kent, UK).
Intravital microscopy
Intravital microscopy (IVM) was used to assess the perfusional changes associated with I/R and possible alterations with Bucillamine administration. The left lobe of the liver was gently mobilized and placed over a specially designed platform (Nikon microscope, Nikon, Tokyo, Japan). The surface of the liver was moistened with normal saline and visualized through a coverslip. Images (25 pictures/s) from the microscope were recorded using a camera (JVC video camera; JVC, Osaka, Japan) directly on to a computer for further analysis. Off-line microcirculatory analysis was performed from recorded images to measure red blood cell (RBC) velocity and sinusoidal diameter using Lucia G software (Laboratory Universal Computer Image Analysis; Nikon).
RBCs (from a previously bled rat) were labelled with fluorescein isothiocyanate (FITC) using a technique previously described19 and labelled RBCs (0.5 ml) were administered via the jugular vein at 30-min reperfusion. The hepatic microcirculation was evaluated after 30, 60, 120 and 180 min after reperfusion. Microcirculatory changes were studied in sinusoids (periportal, midzonal and pericentral sinusoids) within randomly selected acini. The following parameters were studied.
Mean RBC velocity
Mean RBC velocity was determined at 40× magnification. Ten sinusoids were randomly selected at each time point in each animal and RBC velocity was calculated using frame-by-frame analysis as previously described.20 Mean value was calculated for each time point.
Sinusoidal diameter
Sinusoidal diameter was measured in 10 randomly selected sinusoids at each time point in each animal and the mean was calculated.
Sinusoidal perfusion
Five randomly chosen non-overlapping rappaport acini were observed for a minute and recordings made from representative areas for 2 s with a JVC video camera (JVC TK-C1360B colour video camera; JVC) (25 frames per second) and stored on the computer for each time point. Perfusion was established by studying the ratio of perfused to total visible sinusoids after administration of FITC-labelled RBC. Sinusoidal perfusion was graded as continuously perfused (continuous perfusion for >1 min) (Nc) or interrupted (intermittent perfusion for 1-min period) (Ni) or non-perfused (Nn). The sinusoidal perfusion index was calculated as previously described21:
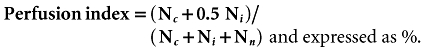 |
Sinusoidal blood flow
Sinusoidal blood flow was calculated as previously decribed.22
Leukocyte parameters
Leukocyte–endothelial interactions were studied by labelling leukocytes in vivo using administering rhodamine 6G (Sigma, Rodermark, Germany) 0.3 mg/kg intra-arterially22 at 150 min post-reperfusion. Ten randomly chosen post-sinusoidal venules were visualized for 20 s each under green filtered light. The number of adherent leukocytes were counted and expressed as cells per mm2 of endothelial surface (length of observed vessel length × diameter ×π= adherent cells per mm2) as previously described.22,23 Leukocytes adherence was measured as adherent leukocytes per mm2 of liver tissue.
Detection of hepatocellular death in vivo
Propidium iodide fluorescent dye stains the nuclei of cells that are lethally damaged.24 Propidium iodide (0.05 mg/kg) was injected after 3 h of reperfusion. The dead nuclei were identified as those stained with propidium iodide. These were counted in each high power field and an average value was taken for each subject after studying at least five high power fields (studying all regions of the acini). The area in each high power field was calculated using Lucia G software (Laboratory Universal Computer Image Analysis; Nikon) and results were expressed as number of dead cells per mm2.
At the end of the experiment animals were killed by exsanguinations. Serum and plasma samples were collected by spinning the blood at 400 g for 10 min and stored in aliquots of 0.2 ml at −80°C. A sample of liver tissue was taken from the left lobe and stored in formalin for histopathology.
In the recovery experiments (Group 5 and 6), the neck line was removed after the period of infusion (3 h reperfusion) and the neck wound closed using a 4-0 Vicryl continuous suture (Ethicon, Somerville, NJ, USA). The animals were recovered, administered adequate analgesia (Buprenorphine 0.15 mg/kg subcutaneously) and kept in a temperature controlled environment with a 12 h light-dark cycle and allowed tap water as well as standard rat chow pellets ad libitum. After 24 h, the animals were re-anaesthetized with 4% isoflurane and maintained with 2.0% isoflurane (Abbott Laboratories Ltd.). They were allowed to breathe spontaneously through a concentric mask connected to an oxygen regulator and monitored with a pulse oximeter (Ohmeda biox 3740 pulse oximeter; Ohmeda). The right carotid artery was cannulated with a polyethylene catheter (0.40-mm inner diameter; Portex) for administering fluorochromes. The abdomen was re-opened through the previous incision. The left lobe of liver was mobilized gently after dividing filmy adhesions that had formed after previous laparotomy and put under the IVM for visualization. It was difficult to mobilize the liver on account of adhesions and tissue oedema as compared with 3 h reperfusion groups and in vivo hepatocellular death was the only parameter studied. Experiments were terminated and samples collected as above.
Liver function
Blood was sampled at the end of the procedure from the IVC 3 h post-reperfusion and centrifuged at 400 g for 10 min. Serum was analysed on an autoanalyser (Hitachi 747; Hitachi, Tokyo, Japan) using commercially available kits (Boehringer Mannheim, Lewes, East Sussex, UK) for serum aspartate transaminase and serum alanine transaminase.
Histological investigations
At the end of the experiment, samples of liver were taken from the left lobe, fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin sections 4 µm thick were cut using a microtome and mounted on slides for haematoxylin and eosin staining. Assessment of liver injury was performed by a Consultant pathologist who was blinded to the study groups, using a scoring system devised by Suzuki et al.25 (Table 1).
Table 1.
Suzuki's criteria
| Numerical assessment | Congestion | Vacuolation | Necrosis |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Minimal | Minimal | Single cell |
| 2 | Mild | Mild | <30% |
| 3 | Moderate | Moderate | <60% |
| 4 | Severe | Severe | >60% |
Data collection and statistics
Data were continuously collected for oxygen saturation, blood pressure and mean arterial pressure (MAP). Averages for 1 min were calculated at 30, 60, 120 and 180 min post-reperfusion. Data are expressed as mean ± standard error of mean (SEM). Analysis of data was carried out using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Differences in data between groups were assessed using one-way anova with Bonferroni's post-hoc test. Data were considered statistically significant if P < 0.05.
Results
There were no procedure-related deaths in either group. The model was haemodynamically stable (Fig. 1a–b). There was a statistically significant transient fall in oxygen saturation immediately after reperfusion which was reduced using Bucillamine therapy (Fig. 1c).
Figure 1.
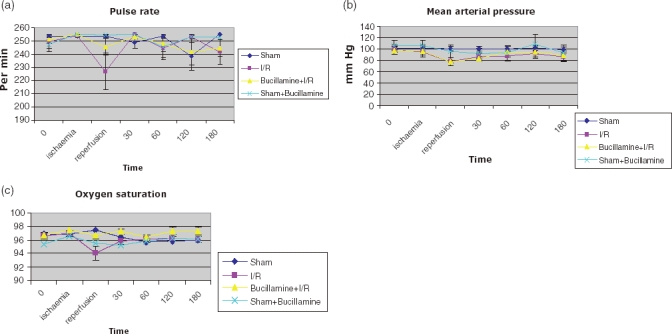
Haemodynamic parameters
Biochemistry
Transaminases were grossly elevated after I/R. The bucillamine I/R group had lower AST and ALT than the I/R group [(AST 932 ± 200.81 vs. 2072.5 ± 511.79, P < 0.05), (ALT 861.4 ± 262.63 vs. 2079.3 ± 322.33, P < 0.05)] (Fig. 2a–b). The AST as well as ALT were less raised in the B24 group as compared with the IR24 group, although this was statistically non-significant (AST, 3053 ± 1322.06 vs. 3379 ± 1501.51) (ALT, 1611 ± 588.12 vs. 1740.67 ± 723.11) (Fig. 2c–d).
Figure 2.
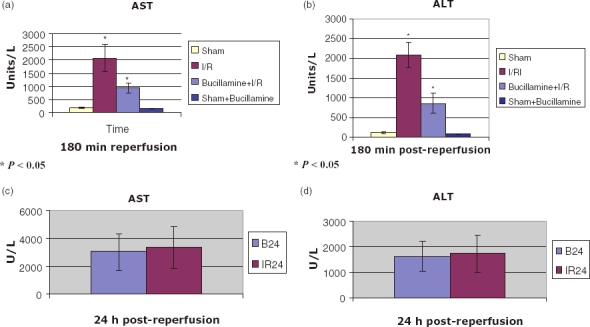
Liver enzymes
Intravital microscopy
We did not find any difference in the perfusion, leukocyte adhesion, in the different sinusoidal regions (periportal, midzonal and pericentral sinusoids) within the same group of animals. RBC velocity was taken as an average over the entire visible length of the sinusoid. Hepatocyte apoptosis/necrosis was more severe in the pericentral region and we studied all the regions to calculate the number of non-viable nuclei.
RBC velocity
RBC velocity remained stable in the Sham group over the duration of the experiment. I/R produced a gradual fall in RBC velocity from baseline which was significant vs. Sham-operated animals at 120 and 180 min (P < 0.001). In the bucillamine I/R group, the initial fall in the RBC velocity at 60 min post-reperfusion was similar to I/R (299.71 ± 18.15 vs. 244 ± 15.49, not significant) but after this the velocities remained steady for the subsequent 2 h duration. Bucillamine significantly increased the RBC velocity compared with the IR alone group as demonstrated in Fig. 3a. Velocities were lower in the SB group as compared with the sham group, but there was no change in the velocities over time (Fig. 3a).
Figure 3.
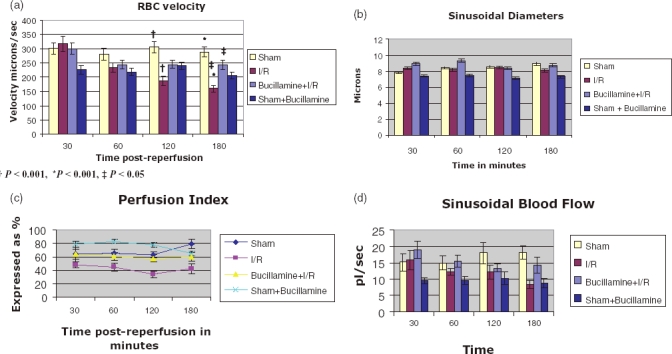
Parameters measured on intravital microscopy
Sinusoidal diameter
There was no statistically significant difference in the sinusoidal diameter in the I/R or bucillamine I/R groups (Fig. 3b).
Sinusoidal perfusion index
The sinusoidal perfusion index was lower in the I/R group as compared with SB and sham groups at all time points. Bucillamine therapy with I/R increased the sinusoidal perfusion although values were not statistically significant (Fig. 3c).
Sinusoidal blood flow
I/R reduced sinusoidal blood flow over the reperfusion period. Bucillamine with I/R maintained the sinusoidal blood flow, after an initial fall, although, none of these changes, however, were statistically significant (Fig. 3d).
Leukocyte adherence in venules and sinusoids
I/R injury was associated with adherence of leukocytes in the venules. Bucillamine with I/R reduced leukocyte adherence (385.66 ± 142.69) in the venules; however, this was not statistically reduced when compared with the I/R group (Fig. 4a). Bucillamine given with IR significantly reduced leukocyte adherence in sinusoids when compared with the IR alone group (29.97 ± 13.81 vs. 97.4 ± 7.49, P < 0.005) (Fig. 4b).
Figure 4.
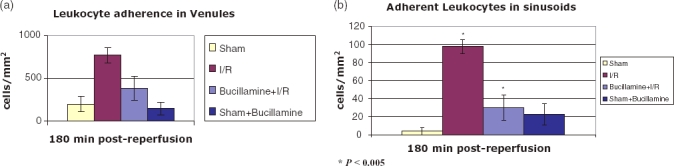
Adherent leukocytes seen on intravital microscopy after labelling with rhodamine in post sinusoidal venules and sinusoids
Hepatocellular cell death in vivo
The number of lethally damaged nuclei could be assessed in all of the animals in 3 h reperfusion groups. I/R produced significant hepatocellular death (1816 ± 293.09 cells/mm2) which was significantly reduced with bucillamine administration at 3-h reperfusion (258.48 ± 46.73 cells/mm2, P < 0.001) (Fig. 5a). IVM was not possible because of oedema and necrosis in one animal in the IR24 group and was only possible to get partial data in one animal in the B24 group. Complete data was obtained in five animals in both groups. There also was statistically significant difference in the non-viable nuclei between the B24 and IR24 groups (385.37 ± 49.37 vs. 923.98 ± 116.68, P < 0.005). (Fig. 5b)
Figure 5.
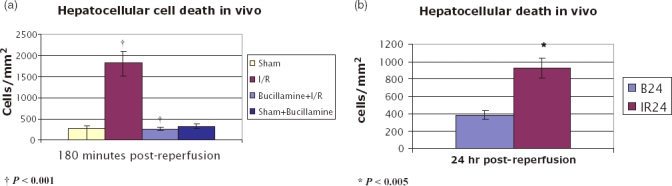
Nonviable cells seen on intravital microscopy after labelling with propidium iodide
Histology
I/R injury caused significant periportal congestion with severe necrosis in zones 2 and 3 (Fig. 6a). The bucillamine I/R group showed less damage. Portal as well as central venous congestion was seen but there was no significant spill out into the surrounding parenchyma. The only change appeared to be some degenerative changes in the perivenular hepatocytes (Fig. 6b). The SB group had well-preserved architecture and the sham group revealed minimal changes (Fig. 6c–d). In the IR24 group, severe damage with abundant ballooning degeneration and necrosis was seen which was reduced by bucillamine administration (Fig. 6e–f).
Figure 6.
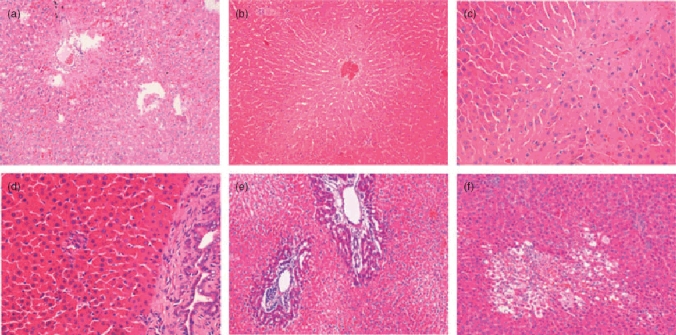
Histopathology: H and E Staining 20× magnification. (a) ischaemia-reperfusion (I/R) group; (b) bucillamine + I/R; (c) sham; (d) sham + bucillamine; (e) IR24 group; (f) B24
Discussion
This study has shown for the first time that bucillamine can reduce the effects of liver warm ischaemia–reperfusion injury. This is also the first study showing the effect of bucillamine on liver microcirculation in vivo. The protective effect of bucillamine was also seen during the late phase of reperfusion injury. The model of partial liver ischaemia and reperfusion injury used in this study is stable, reproducible and avoids splanchnic congestion found with total liver inflow occlusion.18 Technical manoeuvres such as performing the laparotomy, mobilization of the liver and performing IVM did not affect the heart rate, oxygen saturation or MAP as shown by stable parameters in the sham and the SB groups. There was a fall in mean arterial pressure after I/R injury in both the I/R and bucillamine I/R groups which was not statistically significant. Bucillamine administration without I/R has no effect on the vital parameters or haemodynamic stability of the animals suggesting that it has no direct effect on systemic or portal haemodynamics.
IVM has allowed novel insights into hepatic haemodynamics after I/R and the effect of bucillamine administration. Liver I/R is known to cause microcirculatory perfusion failure, activate polymorphonuclear leukocytes and increase leukocyte-endothelial cell interaction which in turn contribute to hepatocellular damage and liver dysfunction.12–16 Bucillamine administration with I/R was shown to maintain RBC velocity, sinusoidal blood flow and sinusoidal perfusion in the reperfusion period. It has also been shown to decrease leukocyte adhesions in venules and decrease hepatocyte apoptosis/ necrosis.
RBC velocity
In our experiments, there was a gradual drop in the RBC velocity in the I/R group, whereas the RBC velocity remained stable after an initial drop in the bucillamine group. This would be consistent with the scavenging of oxygen free radicals by bucillamine. RBC mechanical properties play a key role in tissue perfusion.26,27 RBC velocity can be affected by changes in both aggregability and deformability of the RBCs, and oxygen free radicals which form as a result of I/R affect RBC aggregation and deformability.28 Decreased RBC deformability is associated with oxygen free radical damage during sepsis and is linked to multiorgan failure.29 Pre-treatment with an oxygen free radical scavenger prevents such adverse changes in deformability.30 RBC aggregation also has an impact on blood flow mainly in low shear regions.31 In experimental settings, externally generated oxygen free radicals (i.e. outside RBCs) increase aggregation of RBCs whereas internally generated oxygen free radicals affect the deformability.32 By scavenging oxygen free radicals, bucillamine might be preventing RBC aggregation and deformability thus maintaining RBC velocity. After absorption, bucillamine enters the RBCs rapidly and is carried within the erythrocytes.33–35 This might be of importance in the beneficial effect of bucillamine.
Sinusoidal perfusion
I/R injury decreased the sinusoidal perfusion which is a known consequence of severe liver I/R injury12 and results in significant compromise of hepatic tissue oxygenation and damage and functional impairment of parenchymal and non-parenchymal cells.12,14,15 The severity of sinusoidal perfusion failure is proportional to the ischaemia time.12 Bucillamine was shown to reduce the perfusion abnormality of I/R with a increased sinusoidal perfusion. This could be related to its effect on (i RBCs (decreased aggregability or better maintenance of deformability) and/or (ii) white blood cell (WBC) adhesions. Sinusoidal perfusion was also better maintained in the SB group, which although, as mentioned earlier had lower RBC velocity, had normal liver function, indicating that a combination of better perfusion and maintenance of RBC velocity would decrease abnormality in liver function.
Sinusoidal diameter
Changes in sinusoidal diameter by constriction of hepatic stellate cells mediated by endothelin-1 are known to influence the hepatic perfusion in endotoxaemia.23,33,36 Our results do not suggest such a role in liver I/R injury. The increase in sinusoidal diameter in the Sham group from 30 min to 180 min post-reperfusion could be attributed to the effect of anaesthesia. This would suggest that there might be a relative decrease in the sinusoidal diameters in the I/R and bucillamine groups. The sinusoidal dilatation in the bucillamine group at 30 min could explain the better perfusion and cytoprotection. Our results also do not suggest primary vasodilator function for bucillamine as the sinusoidal diameter did not increase in the SB group.
Sinusoidal blood flow
There was a progressive decrease in sinusoidal blood flow after I/R injury. Sinusoidal blood flow was maintained after bucillamine administration. Sinusoidal blood flow is essentially related to the velocity of flow and diameter and our data on sinusoidal flow are similar to that for RBC velocity as there was no significant change in the diameter. The sinusoidal blood flow values were lower (although not significant as compared with sham) in the SB group demonstrating that the maintained blood flow in the group who had I/R with administration of bucillamine was not related to a direct effect of bucillamine but related to its effect on the inflammatory cascade of I/R.
Leukocyte–endothelial interactions
The I/R group showed leukocyte adherence in the sinusoids and post-sinusoidal venules. Jaeschke et al. first showed that leukocyte infiltration into liver parenchyma is associated with the development of liver I/R injury.23,37 Hepatic I/R induces accumulation, adherence and extravasation of leukocytes in both sinusoids and post-sinusoidal venules.13 The increased number of adherent leukocytes in venules, but not in sinusoids, is known to correlate with the extent of liver dysfunction.13 Leukocyte adherence in venules is mainly mediated by an increased expression of ICAM-1.14,23,38 Our study showed a decreased leukocyte adherence in venules with bucillamine after I/R. Bucillamine is known to scavenge ROS thus decreasing Kupffer cell and leukocyte activation. Leukocyte adhesion in sinusoids may decrease perfusion of sinusoids.13 However, although we could show significantly increased adherent sinusoids in the I/R group the number of adherent leukocytes were very few, which would suggest that the adherent leukocytes are not the cause of reduced parenchymal perfusion. They might, however, be contributing to the hepatocellular injury and dysfunction by release of cytokines and generation of ROS.
Hepatocellular injury/apoptosis
The propidium iodide staining confirmed that there was significant hepatocellular necrosis associated with I/R injury in this model. Bucillamine administration reduced the number of non-viable nuclei by more than 80%. Liver warm I/R is associated with necrosis/apoptosis of hepatocytes.39 In liver I/R, oncotic necrosis and apoptosis share features and mechanisms.39,40 It is suggested that the ability of a necrotic process to be converted to an apoptotic one and vice versa illustrates that the pathways in the two processes could be shared, a phenomenon called necroapoptosis.41,42 Irreversible injury in anoxic hepatocytes is precipitated by an abrupt increase in plasma membrane permeability which results in uptake of propidium iodide, labelling the non-viable nuclei.24 This technique has been used to demonstrate non-viable hepatocytes.24,43,44 This study has shown that with better perfusion and decreased leukocyte adherence, bucillamine reduces the hepatocyte damage, which could be related to its effect on scavenging of ROS.
These findings suggest that this agent may prove to be a useful target in liver protection against I/R injury and could be of clinical benefit in the field of liver transplantation or liver resection surgery. In phase I human studies in normal volunteers, bucillamine at doses up to 25 mg/kg/h i.v. for 3 h elicited no serious drug-related adverse effects33 and would merit a future clinical study.
Acknowledgments
The authors would like to thank Santen Pharmaceutical Company, Osaka, Japan for kindly providing bucillamine. The late Dr Wen Xuang Yang for helping set up the project and Mr Bernie Cousins for his contribution in setting up intravital microscopy.
Conflicts of interest
None declared.
References
- 1.Waxman K. Shock: ischemia, reperfusion, and inflammation. New Horiz. 1996;4:153–160. [PubMed] [Google Scholar]
- 2.Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, et al. Inflammation in the course of early myocardial ischemia. FASEB J. 1991;5:2529–2537. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 4.Le MO, Louis H, Stordeur P, Collet JM, Goldman M, Deviere J. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology. 1997;113:1701–1706. doi: 10.1053/gast.1997.v113.pm9352875. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz LD, Sherman NA, Kong Y, Pike AW, Gobin J, Fennessey PV, et al. Lipophilic siderophores of Mycobacterium tuberculosis prevent cardiac reperfusion injury. Proc Natl Acad Sci USA. 1998;95:5263–5268. doi: 10.1073/pnas.95.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, et al. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- 7.Ceconi C, Curello S, Albertini A, Ferrari R. Effect of lipid peroxidation on heart mitochondria oxygen consuming and calcium transporting capacities. Mol Cell Biochem. 1988;81:131–135. doi: 10.1007/BF00219315. [DOI] [PubMed] [Google Scholar]
- 8.Matsuno H, Sugiyama E, Muraguchi A, Nezuka T, Kubo T, Matsuura K, et al. Pharmacological effects of SA96 (bucillamine) and its metabolites as immunomodulating drugs – the disulfide structure of SA-96 metabolites plays a critical role in the pharmacological action of the drug. Int J Immunopharmacol. 1998;20:295–304. doi: 10.1016/s0192-0561(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 9.Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, et al. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA. 2002;99:8915–8920. doi: 10.1073/pnas.132026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz LD, Sherman NA. Bucillamine prevents myocardial reperfusion injury. J Cardiovasc Pharmacol. 2001;38:859–867. doi: 10.1097/00005344-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, et al. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 12.Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–1431. [PMC free article] [PubMed] [Google Scholar]
- 13.Vollmar B, Menger MD, Glasz J, Leiderer R, Messmer K. Impact of leukocyte-endothelial cell interaction in hepatic ischemia-reperfusion injury. Am J Physiol. 1994;267:G786–G793. doi: 10.1152/ajpgi.1994.267.5.G786. [DOI] [PubMed] [Google Scholar]
- 14.Vollmar B, Glasz J, Menger MD, Messmer K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery. 1995;117:195–200. doi: 10.1016/s0039-6060(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 15.Vollmar B, Glasz J, Post S, Menger MD. Role of microcirculatory derangements in manifestation of portal triad cross-clamping-induced hepatic reperfusion injury. J Surg Res. 1996;60:49–54. doi: 10.1006/jsre.1996.0009. [DOI] [PubMed] [Google Scholar]
- 16.Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology. 1999;46(Suppl. 2):1452–1457. [PubMed] [Google Scholar]
- 17.Koo A, Komatsu H, Tao G, Inoue M, Guth PH, Kaplowitz N. Contribution of no-reflow phenomenon to hepatic injury after ischemia-reperfusion: evidence for a role for superoxide anion. Hepatology. 1992;15:507–514. doi: 10.1002/hep.1840150325. [DOI] [PubMed] [Google Scholar]
- 18.Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J. 2005;19:1155–1157. doi: 10.1096/fj.04-3220fje. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerhackl B, Parekh N, Brinkhus H, Steinhausen M. The use of fluorescent labeled erythrocytes for intravital investigation of flow and local hematocrit in glomerular capillaries in the rat. Int J Microcirc Clin Exp. 1983;2:119–129. [PubMed] [Google Scholar]
- 20.Kelly D, Piasecki C, Anthony A, Dhillon AP, Pounder RE, Wakefield AJ. Reversal and protection against indomethacin-induced blood stasis and mucosal damage in the rat jejunum by a beta3-adrenoceptor agonist. Aliment Pharmacol Ther. 1998;12:1121–1129. doi: 10.1046/j.1365-2036.1998.00400.x. [DOI] [PubMed] [Google Scholar]
- 21.Post S, Palma P, Rentsch M, Gonzalez AP, Menger MD. Differential impact of Carolina rinse and University of Wisconsin solutions on microcirculation, leukocyte adhesion, Kupffer cell activity and biliary excretion after liver transplantation. Hepatology. 1993;18:1490–1497. [PubMed] [Google Scholar]
- 22.Wunder C, Brock RW, McCarter SD, Bihari A, Harris K, Eichelbronner O, et al. Inhibition of haem oxygenase activity increases leukocyte accumulation in the liver following limb ischaemia-reperfusion in mice. J Physiol. 2002;540:1013–1021. doi: 10.1113/jphysiol.2001.015446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croner RS, Hoerer E, Kulu Y, Hackert T, Gebhard MM, Herfarth C, et al. Hepatic platelet and leukocyte adherence during endotoxemia. Crit Care. 2006;10:R15. doi: 10.1186/cc3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman B, Nieminen AL, Gores GJ, Lemasters JJ. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988;2:146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Toledo-Pereyra LH. Monoclonal antibody to intercellular adhesion molecule 1 as an effective protection for liver ischemia and reperfusion injury. Transplant Proc. 1993;25:3325–3327. [PubMed] [Google Scholar]
- 26.Schmid- Schonbein H. Fluid dynamics and hemorheology in vivo: the interactions of hemodynamic parameters and hemorheological ‘properties’ in determining the flow behavior of blood in microvascular networks. In: Lowe GDO, editor. Clinical Blood Rheology. Boca Raton, FL: CRC Press; 1988. pp. 129–219. [Google Scholar]
- 27.Shiga T, Maeda N, Kon K. Erythrocyte rheology. Crit Rev Oncol Hematol. 1990;10:9–48. doi: 10.1016/1040-8428(90)90020-s. [DOI] [PubMed] [Google Scholar]
- 28.Lowe GDO, Barbanel JC. Plasma and blood viscocity. In: Lowe GDO, editor. Clinical Blood Rheology. Boca Raton, FL: CRC Press; 1988. pp. 1–10. [Google Scholar]
- 29.Machiedo GW, Powell RJ, Rush BF, Jr, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 30.Powell RJ, Machiedo GW, Rush BF, Jr, Dikdan G. Effect of alpha-tocopherol on red cell deformability and survival in sepsis. Curr Surg. 1989;46:380–382. [PubMed] [Google Scholar]
- 31.Cabel M, Meiselman HJ, Popel AS, Johnson PC. Contribution of red blood cell aggregation to venous vascular resistance in skeletal muscle. Am J Physiol. 1997;272:H1020–H1032. doi: 10.1152/ajpheart.1997.272.2.H1020. [DOI] [PubMed] [Google Scholar]
- 32.Baskurt OK, Temiz A, Meiselman HJ. Effect of superoxide anions on red blood cell rheologic properties. Free Radic Biol Med. 1998;24:102–110. doi: 10.1016/s0891-5849(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 33.Horwitz LD. Bucillamine: a potent thiol donor with multiple clinical applications. Cardiovasc Drug Rev. 2003;21:77–90. doi: 10.1111/j.1527-3466.2003.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara S, Ishigama M, Kageyama T. Phase I study of N-(Mercapt-2-methlpropionyl)-L-cysteine (SA96). (I) Single administration study. Rinsho Yakuri. 1985;16:611–620. [Google Scholar]
- 35.Sugawara S, Ishigama M, Kageyama T. Phase I study of N-(Mercapt-2-methlpropionyl)-L-cysteine (SA96). (II) Continuous 6-day administration study. Rinsho Yakuri. 1985;16:621–630. [Google Scholar]
- 36.Ring A, Stremmel W. The hepatic microvascular responses to sepsis. Semin Thromb Hemost. 2000;26:589–594. doi: 10.1055/s-2000-13215. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 38.Iwata K, Shimazu M, Wakabayashi G, Ohshima A, Yoshida M, Kitajima M. Intraportal perfusion of prostaglandin E1 attenuates hepatic postischaemic microcirculatory impairments in rats. J Gastroenterol Hepatol. 1999;14:634–641. doi: 10.1046/j.1440-1746.1999.01929.x. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 40.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 41.Lemasters JJ. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276:G1–G6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 42.Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Brock RW, Lawlor DK, Harris KA, Potter RF. Initiation of remote hepatic injury in the rat: interactions between Kupffer cells, tumor necrosis factor-alpha, and microvascular perfusion. Hepatology. 1999;30:137–142. doi: 10.1002/hep.510300132. [DOI] [PubMed] [Google Scholar]
- 44.Zhang JX, Jones DV, Clemens MG. Effect of activation on neutrophil-induced hepatic microvascular injury in isolated rat liver. Shock. 1994;1:273–278. doi: 10.1097/00024382-199404000-00005. [DOI] [PubMed] [Google Scholar]


