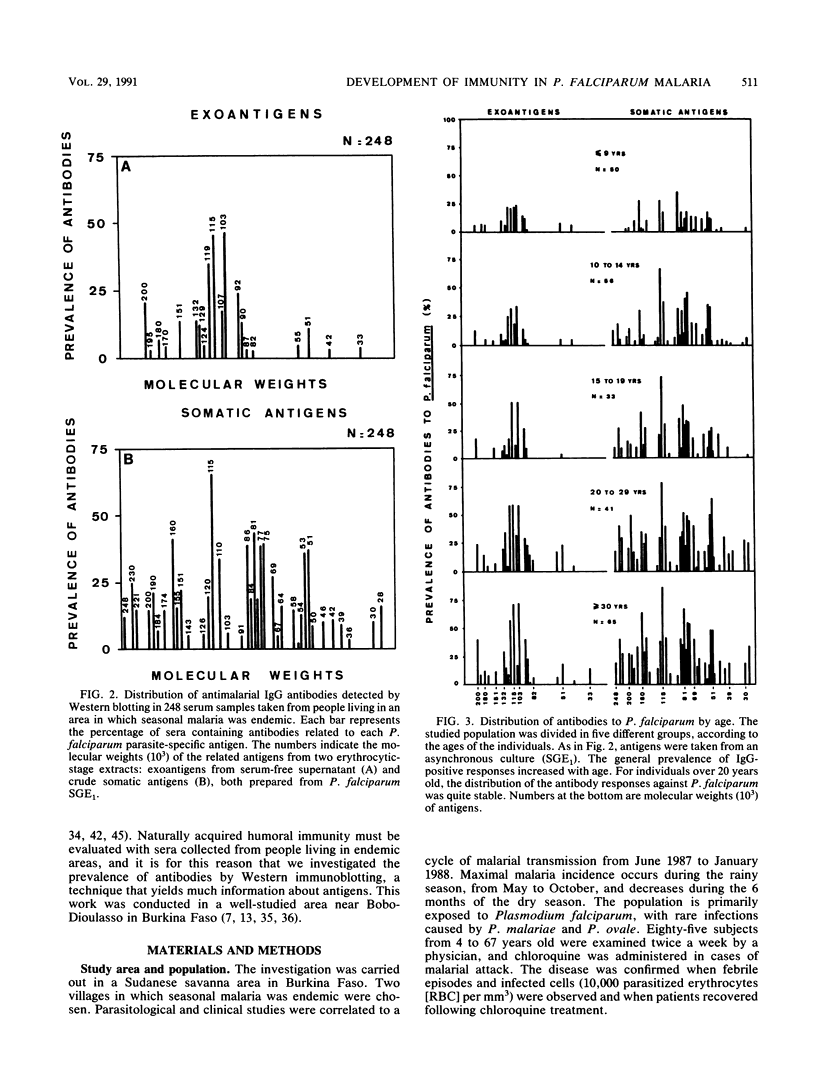
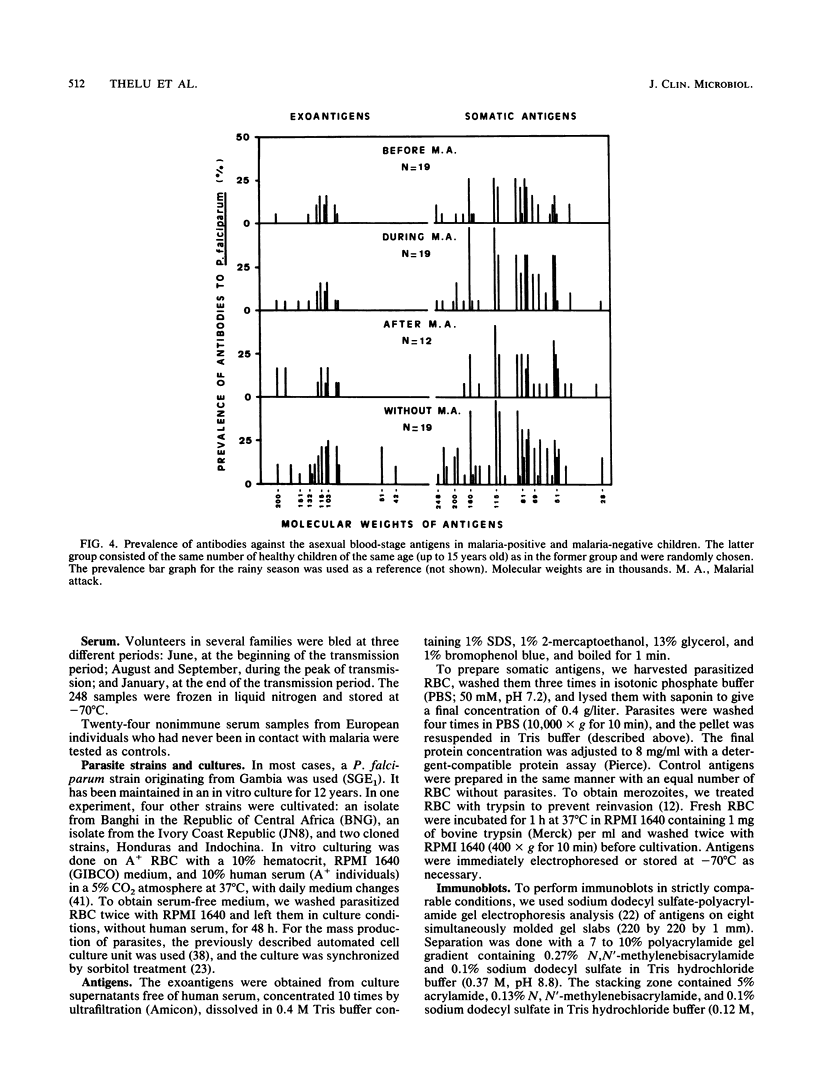
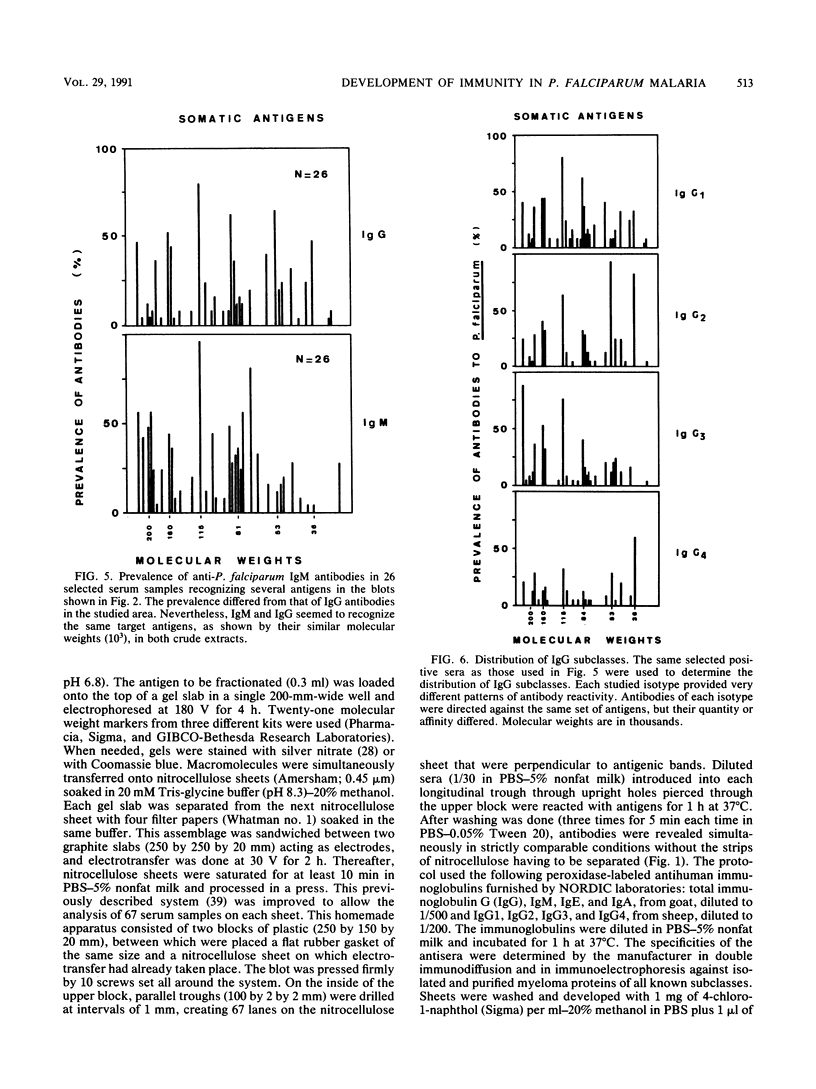
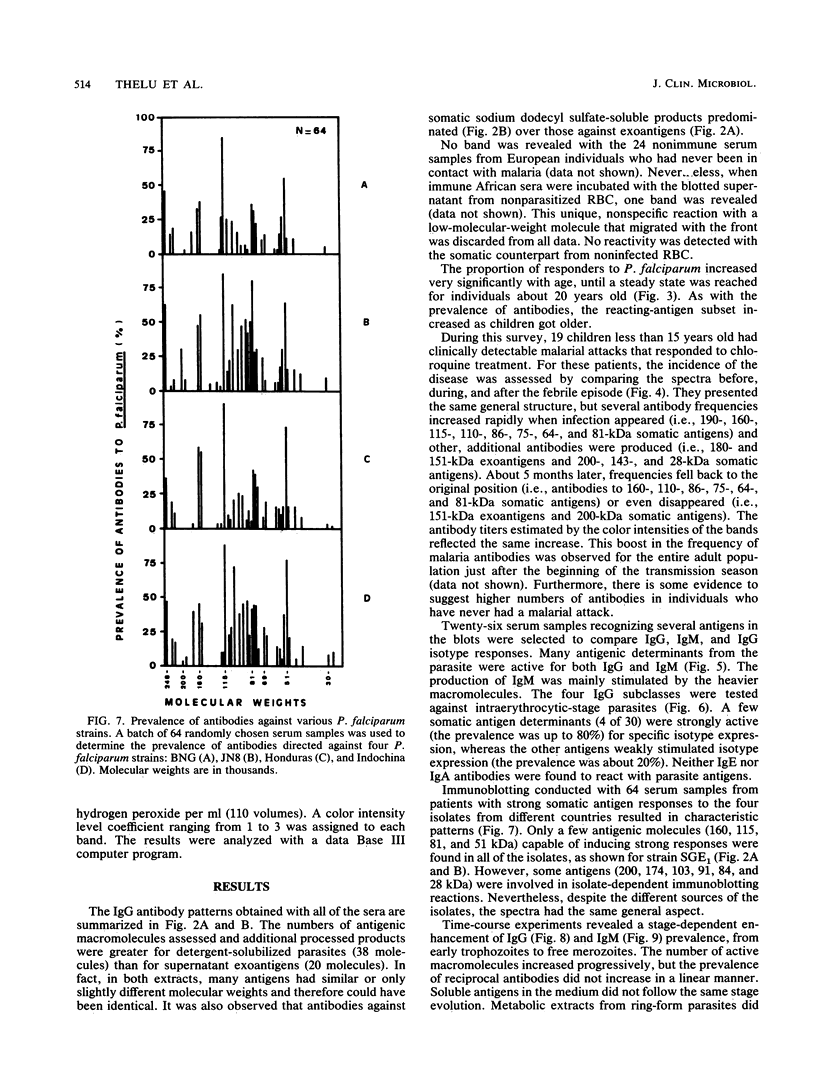
Abstract
A longitudinal study was carried out in Burkina Faso to investigate the natural development of the immune response to Plasmodium falciparum malaria. Three bleedings were carried out before, during, and after the seasonal peak of transmission. Detailed antigen mapping and antibody prevalence of the 248 collected serum samples were established by immunoblotting on the basis of several epidemiological and biological parameters. An improved Western immunoblotting system was used to analyze up to 67 serum samples on each nitrocellulose sheet. This system allowed us to perform the entire study with strictly comparable conditions. Two different blood-stage antigens (exoantigens and somatic antigens) were used to analyze the distribution of different classes and subclasses of immunoglobulins according to the age of the individuals, the presence or absence of a malarial attack, the transmission period, the origin of parasite isolates, and the response to intraerythrocytic stages. Although this analysis emphasizes strong individual variations, reactions with two major antigens of 115 and 103 kDa were especially noted. These antigens induced high antibody levels and prevalences but were probably not involved in protection. The prevalence of immunoglobulin G (IgG) antibodies differed by isotype. Most of antigens stimulating IgG production were also responsible for the IgM antibody response. The role played by these antibodies in the development of natural immunity against malaria is discussed.
Full text
PDF
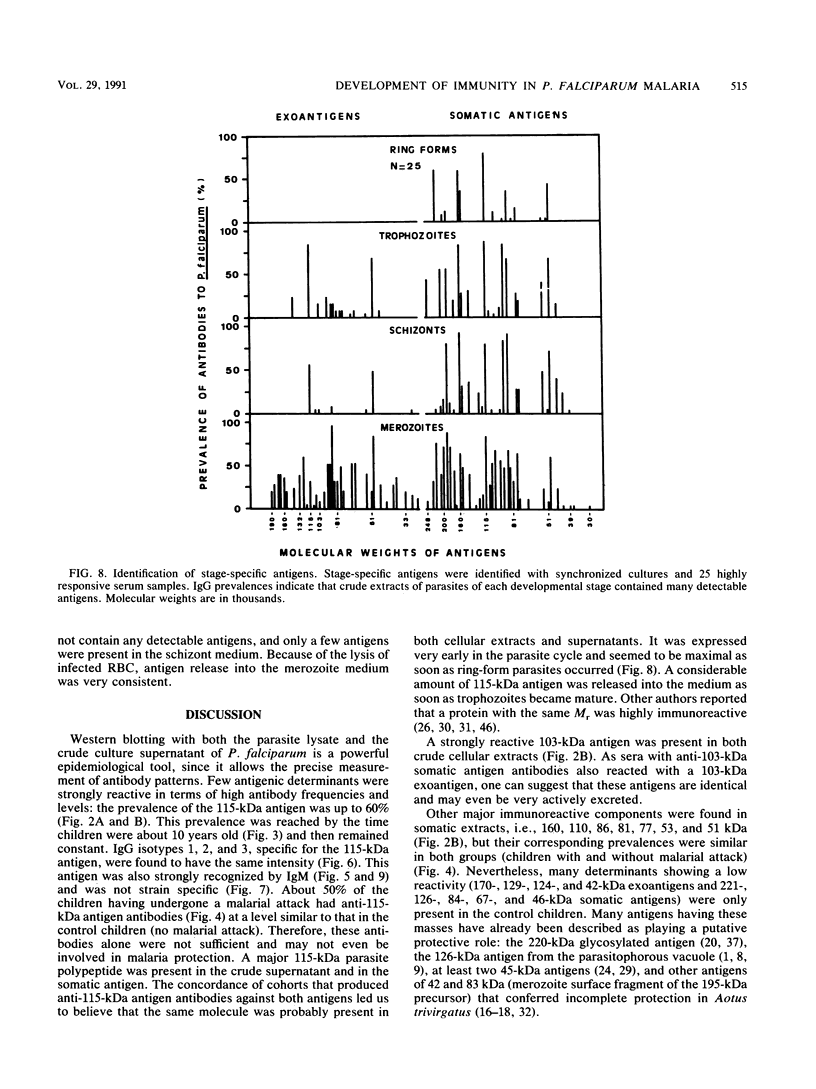
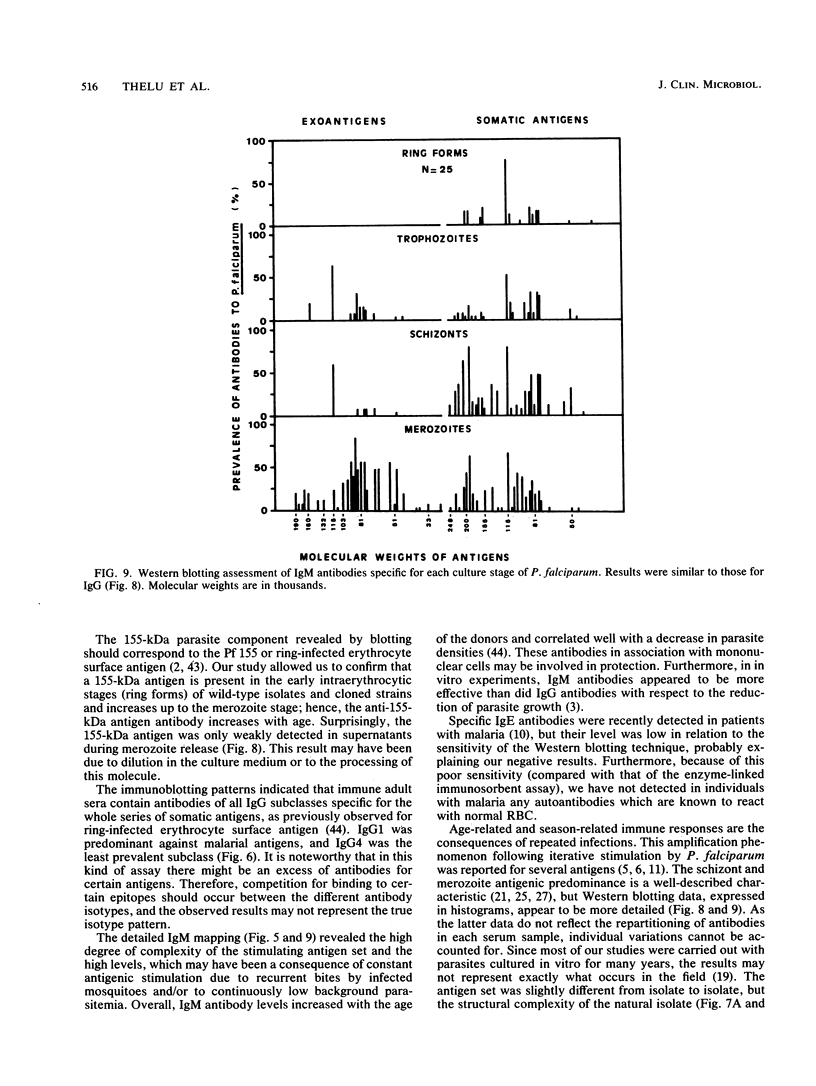


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatia A., Delplace P., Fortier B., Dubremetz J. F., Vernes A. Immunochemical analysis of a major antigen of Plasmodium falciparum (P126) among ten geographic isolates. Am J Trop Med Hyg. 1987 Jan;36(1):15–19. doi: 10.4269/ajtmh.1987.36.15. [DOI] [PubMed] [Google Scholar]
- Brown G. V., Culvenor J. G., Crewther P. E., Bianco A. E., Coppel R. L., Saint R. B., Stahl H. D., Kemp D. J., Anders R. F. Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med. 1985 Aug 1;162(2):774–779. doi: 10.1084/jem.162.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Greenwood B. M., Terry R. J. Cellular mechanisms involved in recovery from acute malaria in Gambian children. Parasite Immunol. 1986 Nov;8(6):551–564. doi: 10.1111/j.1365-3024.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L. J. The challenge of malaria vaccine: trials and tribulations. Lancet. 1987 Feb 14;1(8529):371–373. doi: 10.1016/s0140-6736(87)91738-7. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Wirtz R. A., Brabin B. J., Battistutta D., Cattani J. A., Maizels R. M., Alpers M. P. Differential antibody responses to Plasmodium falciparum and P. vivax circumsporozoite proteins in a human population. J Clin Microbiol. 1989 Jun;27(6):1346–1351. doi: 10.1128/jcm.27.6.1346-1351.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. H., Collins F. H., Brandling-Bennett A. D., Schwartz I. K., Roberts J. M. Age-specific prevalence of antibody to a synthetic peptide of the circumsporozoite protein of Plasmodium falciparum in children from three villages in Kenya. Am J Trop Med Hyg. 1987 Sep;37(2):220–224. doi: 10.4269/ajtmh.1987.37.220. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Crewther P. E., Culvenor J. G., Perrin L. H., Brown G. V., Kemp D. J., Anders R. F. Variation in p126, a parasitophorous vacuole antigen of Plasmodium falciparum. Mol Biol Med. 1988 Dec;5(3):155–166. [PubMed] [Google Scholar]
- Delplace P., Dubremetz J. F., Fortier B., Vernes A. A 50 kilodalton exoantigen specific to the merozoite release-reinvasion stage of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Nov;17(2):239–251. doi: 10.1016/0166-6851(85)90021-0. [DOI] [PubMed] [Google Scholar]
- Desowitz R. S. Plasmodium-specific immunoglobulin E in sera from an area of holoendemic malaria. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):478–479. doi: 10.1016/0035-9203(89)90254-x. [DOI] [PubMed] [Google Scholar]
- Esposito F., Lombardi S., Modiano D., Zavala F., Reeme J., Lamizana L., Coluzzi M., Nussenzweig R. S. Prevalence and levels of antibodies to the circumsporozoite protein of Plasmodium falciparum in an endemic area and their relationship to resistance against malaria infection. Trans R Soc Trop Med Hyg. 1988;82(6):827–832. doi: 10.1016/0035-9203(88)90007-7. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazin P., Robert V., Cot M., Carnevale P. Plasmodium falciparum incidence and patency in a high seasonal transmission area of Burkina Faso. Trans R Soc Trop Med Hyg. 1988;82(1):50–55. [PubMed] [Google Scholar]
- Good M. F., Miller L. H., Kumar S., Quakyi I. A., Keister D., Adams J. H., Moss B., Berzofsky J. A., Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988 Oct 28;242(4878):574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K. Immunology of human malaria. A cellular perspective. Parasite Immunol. 1989 Mar;11(2):105–116. doi: 10.1111/j.1365-3024.1989.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Nicholls S. C. Immunization against Plasmodium falciparum with recombinant polypeptides produced in Escherichia coli. Parasite Immunol. 1988 Nov;10(6):607–617. doi: 10.1111/j.1365-3024.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Panton L. J., Marsh K., Ling I. T., Winchell E. J., Wilson R. J. Antigenic diversity and size diversity of Plasmodium falciparum antigens in isolates from Gambian patients. I. S-antigens. Parasite Immunol. 1986 Jan;8(1):39–55. doi: 10.1111/j.1365-3024.1986.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Theander T. G., Jensen J. B., Mølbak K., Jepsen S. Soluble Plasmodium falciparum antigens contain carbohydrate moieties important for immune reactivity. J Clin Microbiol. 1987 Nov;25(11):2075–2079. doi: 10.1128/jcm.25.11.2075-2079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Stage-specific proteins and glycoproteins of plasmodium falciparum: identification of antigens unique to schizonts and merozoites. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3695–3699. doi: 10.1073/pnas.77.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lyon J. A., Geller R. H., Haynes J. D., Chulay J. D., Weber J. L. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2989–2993. doi: 10.1073/pnas.83.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- McBride J. S., Walliker D., Morgan G. Antigenic diversity in the human malaria parasite Plasmodium falciparum. Science. 1982 Jul 16;217(4556):254–257. doi: 10.1126/science.6178159. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Miettinen-Baumann A., Strych W., McBride J., Heidrich H. G. A 46,000 dalton Plasmodium falciparum merozoite surface glycoprotein not related to the 185,000-195,000 dalton schizont precursor molecule: isolation and characterization. Parasitol Res. 1988;74(4):317–323. doi: 10.1007/BF00539452. [DOI] [PubMed] [Google Scholar]
- Nkuo T. K., Deas J. Sera from Cameroon recognize proteins of Plasmodium falciparum isolates from geographically diverse areas of the world. Trans R Soc Trop Med Hyg. 1987;81(6):891–895. doi: 10.1016/0035-9203(87)90340-3. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R., Rieder H. Characterization of antigens from erythrocytic stages of Plasmodium falciparum reacting with human immune sera. Trans R Soc Trop Med Hyg. 1981;75(1):163–165. doi: 10.1016/0035-9203(81)90055-9. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., McIntyre P., Langford C. J., Woodrow G., Brown G. V., Anders R. F., Kemp D. J. Variation in the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):291–301. doi: 10.1016/0166-6851(88)90049-7. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Otoo L. N., Pombo D., Sugars L. Y., Menon A., De Groot A. S., Johnson A., Alling D., Miller L. H., Good M. F. Differential non-responsiveness in humans of candidate Plasmodium falciparum vaccine antigens. Am J Trop Med Hyg. 1989 Aug;41(2):125–134. [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Robert V., Carnevale P., Ouedraogo V., Petrarca V., Coluzzi M. La transmission du paludisme humain dans un village de savane du sud-ouest du Burkina Faso. Ann Soc Belg Med Trop. 1988 Jun;68(2):107–121. [PubMed] [Google Scholar]
- Robert V., Gazin P., Boudin C., Molez J. F., Ouedraogo V., Carnevale P. La transmission du paludisme en zone de savane arborée et en zone rizicole des environs de Bobo Dioulasso (Burkina Faso). Ann Soc Belg Med Trop. 1985;65 (Suppl 2):201–214. [PubMed] [Google Scholar]
- Saul A., Myler P., Schofield L., Kidson C. A high molecular weight antigen in Plasmodium falciparum recognized by inhibitory monoclonal antibodies. Parasite Immunol. 1984 Jan;6(1):39–50. doi: 10.1111/j.1365-3024.1984.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Thelu J., Ambroise-Thomas P., Chumpitazi B., Kupka P. Purification and immunochemical study of Plasmodium falciparum exoantigens. J Parasitol. 1985 Oct;71(5):542–546. [PubMed] [Google Scholar]
- Thelu J., Ambroise-Thomas P. Perspectives ouvertes à l'étude épidémiologique et immunologique du paludisme par la technique d'immuno-transfert. C R Acad Sci III. 1988;306(1):11–16. [PubMed] [Google Scholar]
- Thélu J., Ambroise-Thomas P. A septate polycarbonate cell culture unit used for Plasmodium falciparum and hybridomas. Trans R Soc Trop Med Hyg. 1988;82(3):360–362. doi: 10.1016/0035-9203(88)90117-4. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- Wahlgren M., Björkman A., Perlmann H., Berzins K., Perlmann P. Anti-Plasmodium falciparum antibodies acquired by residents in a holoendemic area of Liberia during development of clinical immunity. Am J Trop Med Hyg. 1986 Jan;35(1):22–29. doi: 10.4269/ajtmh.1986.35.22. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]
- Winchell E. J., Ling I. T., Wilson R. J. Stage-specific production of S-antigens of Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1985;79(2):187–191. doi: 10.1016/0035-9203(85)90332-3. [DOI] [PubMed] [Google Scholar]



