Abstract
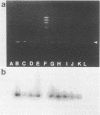
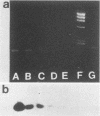
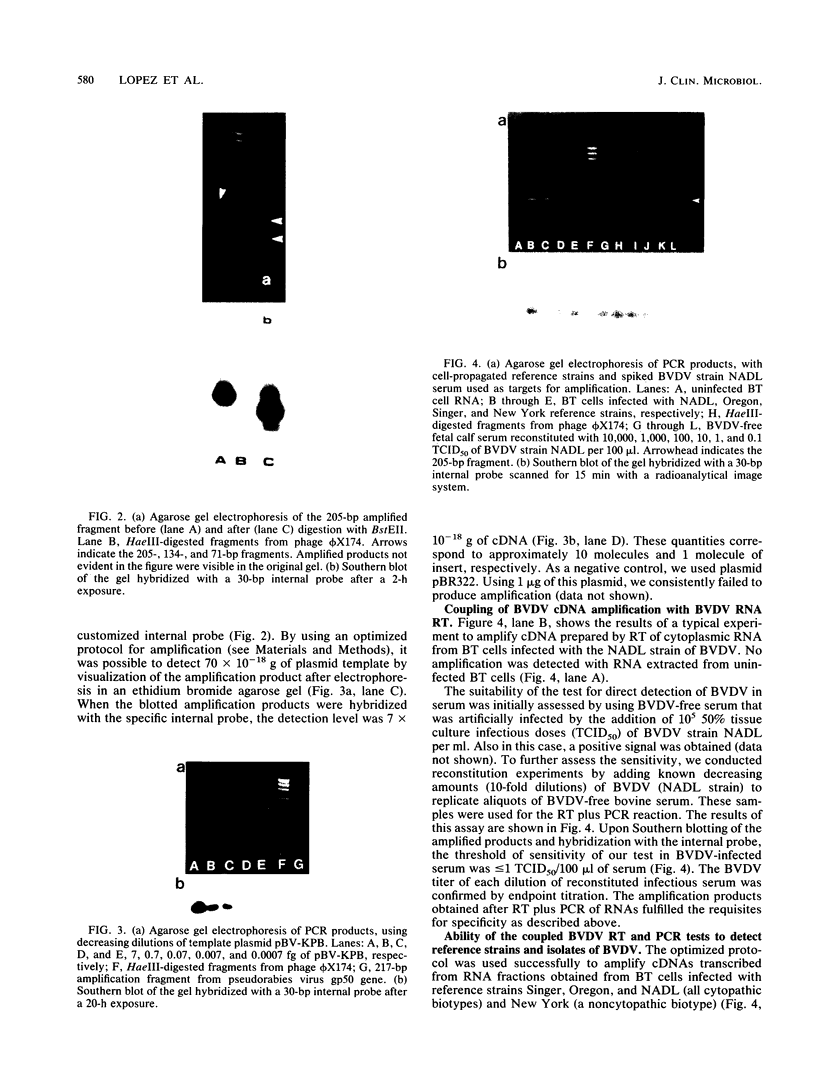
The polymerase chain reaction was used to detect genomic sequences of the positive-stranded RNA of bovine viral diarrhea virus (BVDV), a member of the family Togaviridae. Using a set of 20-bp primers located within the conserved 3' region of the BVDV genome, we were able to consistently amplify a 205-bp target sequence from BVDV cDNA. BVDV RNAs from cell culture-propagated BVDV reference strains, diverse unrelated cytopathic and noncytopathic field isolates, and clinical serum samples were transcribed to cDNA by using avian myeloblastosis virus reverse transcriptase and further specifically amplified by using the polymerase chain reaction assay. The amplification assay was sensitive enough to detect one molecule of cloned BVDV cDNA. Reconstitution experiments conducted by adding decreasing amounts of BVDV (NADL strain) to BVDV-free serum indicated that the threshold of sensitivity of the assay was less than or equal to 1 50% tissue culture infective dose. These results show that the polymerase chain reaction may be used for the rapid detection of diverse strains of BVDV in cell cultures, biological products, and clinical specimens from cattle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolin S. R., McClurkin A. W., Coria M. F. Frequency of persistent bovine viral diarrhea virus infection in selected cattle herds. Am J Vet Res. 1985 Nov;46(11):2385–2387. [PubMed] [Google Scholar]
- Brownlie J., Clarke M. C., Howard C. J. Experimental production of fatal mucosal disease in cattle. Vet Rec. 1984 Jun 2;114(22):535–536. doi: 10.1136/vr.114.22.535. [DOI] [PubMed] [Google Scholar]
- Brownlie J., Clarke M. C., Howard C. J., Pocock D. H. Pathogenesis and epidemiology of bovine virus diarrhoea virus infection of cattle. Ann Rech Vet. 1987;18(2):157–166. [PubMed] [Google Scholar]
- Carman W. F., Williamson C., Cunliffe B. A., Kidd A. H. Reverse transcription and subsequent DNA amplification of rubella virus RNA. J Virol Methods. 1989 Jul;25(1):21–29. doi: 10.1016/0166-0934(89)90097-9. [DOI] [PubMed] [Google Scholar]
- Colett M. S., Larson R., Gold C., Strick D., Anderson D. K., Purchio A. F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988 Jul;165(1):191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- Fernelius A. L. Noncytopathogenic Bovine Viral Diarrhea Viruses Detected and Titrated by Immunofluorescence. Can J Comp Med Vet Sci. 1964 May;28(5):121–126. [PMC free article] [PubMed] [Google Scholar]
- Gama R. E., Horsnell P. R., Hughes P. J., North C., Bruce C. B., al-Nakib W., Stanway G. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989 Jun;28(2):73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- Harkness J. W. The control of bovine viral diarrhoea virus infection. Ann Rech Vet. 1987;18(2):167–174. [PubMed] [Google Scholar]
- Hart C., Schochetman G., Spira T., Lifson A., Moore J., Galphin J., Sninsky J., Ou C. Y. Direct detection of HIV RNA expression in seropositive subjects. Lancet. 1988 Sep 10;2(8611):596–599. doi: 10.1016/s0140-6736(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Rossi C. R., Bridgman C. R., Kiesel G. K. Viral contamination of bovine fetal lung cultures and bovine fetal serum. Am J Vet Res. 1980 Oct;41(10):1680–1681. [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K. Microtiter tests for detecting antibody in bovine serum to parainfluenza 3 virus, infectious bovine rhinotracheitis virus, and bovine virus diarrhea virus. Appl Microbiol. 1971 Jul;22(1):32–36. doi: 10.1128/am.22.1.32-36.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sommer R., Tautz D. Minimal homology requirements for PCR primers. Nucleic Acids Res. 1989 Aug 25;17(16):6749–6749. doi: 10.1093/nar/17.16.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERDAHL N. R., GRACE O. D., HOERLEIN A. B. Cultivation in tissue-culture of cytopathogenic agent from bovine mucosal disease. Proc Soc Exp Biol Med. 1957 Apr;94(4):795–797. doi: 10.3181/00379727-94-23091. [DOI] [PubMed] [Google Scholar]
- Zignego A. L., Deny P., Feray C., Ponzetto A., Gentilini P., Tiollais P., Bréchot C. Amplification of hepatitis delta virus RNA sequences by polymerase chain reaction: a tool for viral detection and cloning. Mol Cell Probes. 1990 Feb;4(1):43–51. doi: 10.1016/0890-8508(90)90038-2. [DOI] [PubMed] [Google Scholar]