Abstract
Overaccumulation of lipids in nonadipose tissues of obese rodents may lead to lipotoxic complications such as diabetes. To assess the pathogenic role of the lipogenic transcription factor, sterol regulatory element binding protein 1 (SREBP-1), we measured its mRNA in liver and islets of obese, leptin-unresponsive fa/fa Zucker diabetic fatty rats. Hepatic SREBP-1 mRNA was 2.4 times higher than in lean +/+ controls, primarily because of increased SREBP-1c expression. mRNA of lipogenic enzymes ranged from 2.4- to 4.6-fold higher than lean controls, and triacylglycerol (TG) content was 5.4 times higher. In pancreatic islets of fa/fa rats, SREBP-1c was 3.4 times higher than in lean +/+ Zucker diabetic fatty rats. The increase of SREBP-1 in liver and islets of untreated fa/fa rats was blocked by 6 weeks of troglitazone therapy, and the diabetic phenotype was prevented. Up-regulation of SREBP-1 also occurred in livers of Sprague–Dawley rats with diet-induced obesity. Hyperleptinemia, induced in lean +/+ rats by adenovirus gene transfer, lowered hepatic SREBP-1c by 74% and the lipogenic enzymes from 35 to 59%. In conclusion, overnutrition increases and adenovirus-induced hyperleptinemia decreases SREBP-1c expression in liver and islets. SREBP-1 overexpression, which is prevented by troglitazone, may play a role in the ectopic lipogenesis and lipotoxicity complicating obesity in Zucker diabetic fatty rats.
Insulin stimulates hepatic lipid synthesis by selectively up-regulating the expression of a lipogenic transcription factor, sterol regulatory element binding protein (SREBP)-1c (1). In insulin-deficient rats, SREBP-1c expression is low and is restored almost to normal within 6 h by insulin replacement (1). It is elevated in two insulin-resistant models of leptin deficiency, the ob/ob mouse and a transgenic mouse model of generalized lipodystrophy (2). Studies in isolated hepatocytes (1, 3, 4) and adipocytes (5) indicate that insulin induces SREBP-1c gene transcription. These reports raise the possibility that increased lipogenesis secondary to leptin unresponsiveness might also be the consequence of overexpression of SREBP-1 in nonadipose tissues. If so, its prevention might protect against lipotoxicity (6), i.e., the disease consequences of overaccumulation of unoxidized lipids in liver, skeletal muscle, pancreatic islets, and myocardium postulated to cause, respectively, insulin resistance (7, 8), noninsulin-dependent diabetes mellitus (9), and heart dysfunction (10).
In obese Zucker diabetic fatty (ZDF) rats, ectopic lipid overaccumulation and lipotoxicity occur early in life as the result of a loss-of-function mutation (fa) in their leptin receptors (11, 12). All of the foregoing disease consequences are evident by the age of 14 weeks, provided the rats' diet contains at least 6% fat. Prophylactic treatment with troglitazone (TGZ) of obese, prediabetic ZDF (fa/fa) rats prevents the increase in ectopic triacylglycerol (TG) deposition, the insulin resistance (13, 14), the noninsulin-dependent diabetes mellitus (14), and the myocardial heart dysfunction that otherwise occur (10).
In this study, we examine the relationships between the level of SREBP-1 expression in liver and islets and the level of ectopic lipid deposition in tissues. We find that SREBP-1c mRNA is high in overnourished rodents in which lipid deposition in tissues is increased and is reduced by maneuvers that lower tissue lipids. The results indicate that SREBP-1c overexpression may be the proximal molecular abnormality in a lipogenic cascade that leads to the noninsulin-dependent diabetes mellitus of obesity and that it may be a plausible target for pharmacologic manipulations to reduce its expression and/or activity.
Methods
Animals.
Obese homozygous (fa/fa) and lean wild-type (+/+) ZDF-drt male rats were bred in our laboratory from ZDF/drt-fa (F10) rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis). Sprague–Dawley rats were purchased from Charles River Breeding Laboratories. Animal experimentation was in accordance with institutional guidelines.
Treatment with TGZ in Prediabetic ZDF (fa/fa) Rats.
At 7 weeks of age, prediabetic (fa/fa) ZDF rats were matched for body weight and divided into two groups. One group was given a daily diet of powdered standard chow (Teklad FG rodent diet, Teklad, Madison, WI) containing 24% protein, 48% carbohydrate, and 6% fat (4.03 Kcal/g) in which TGZ had been mixed to provide 100–130 mg/day (Sankyo). A second group that served as controls was fed the same chow without TGZ. Body weight and food intake were measured weekly. Rats were studied at ≈13 weeks of age or 6 weeks after the start of the experiment.
Rats with Diet-Induced Obesity (DIO).
From 4 weeks of age, Sprague–Dawley rats were given either their usual diet of 50 g/day of standard chow (Teklad FG rodent diet) containing 24% protein, 51% carbohydrate, and 4% fat (3.94 Kcal/g) or a high-fat diet (Teklad FG rodent diet) containing 24.5% protein, 7.5% carbohydrate, and 60% fat (6.7 Kcal/g). The supplementary fat contained 29.8% (vol/vol) palmitic acid, 12.7% (vol/vol) stearic acid, 47.8% (vol/vol) oleic acid, and 3.1% (vol/vol) linoleic acid. Rats were killed at 10 weeks of age.
Adenovirus-Induced Hyperleptinemic Rats.
Recombinant adenovirus containing either the rat leptin cDNA (AdCMV-leptin) or, as a control, the bacterial β-galactosidase (β-gal) gene (AdCMV-β-gal) was prepared as described (15). A total of 1012 plaque-forming units of virus was infused into the jugular vein of 8-week-old ZDF lean (+/+) rats. Food intake and body weight were measured daily. Each day, “diet-matched” animals were provided with the amount of food ingested by AdCMV-leptin-infused animals on the previous day. On the third day after treatment, animals were killed to harvest tissues.
Plasma Measurements.
Plasma glucose was measured by the glucose oxidase method with the glucose analyzer II (Beckman Instruments, Brea, CA). Plasma TG levels were measured by the GPO-Trinder triglyceride kit (Sigma). Plasma leptin and insulin were assayed with the Linco leptin and insulin assay kits (Linco Research Immunoassay, St. Charles, MO).
Tissue Preparation and Total RNA Extraction.
Livers were resected, and pancreatic islets were isolated (16). Tissues were immediately frozen in liquid nitrogen and stored at −70°C until use. Total RNA was extracted by the TRIzol isolation method according to the manufacturer's protocol (Life Technologies, Gaithersburg, MD).
TG Content of Tissue.
Totals lipids were extracted from 100 mg of tissue by the method of Folch et al. (17) and dried under N2 gas. TG content of tissues was measured by the GPO-Trinder triglyceride kit (Sigma).
Northern Blot Analysis.
Total RNA (25 μg) was subjected to electrophoresis on 1.2% formaldehyde-agarose gel and transferred onto Hybond-N+ membrane (Amersham Pharmacia) by capillary blotting (18). Prehybridization and hybridization were carried out as described (19). cDNA probes for the rat acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and glycerol-3-phosphate acyltransferase (GPAT) were prepared by reverse transcriptase–PCR (RT-PCR) with the primers shown in Table 1. The probe for mouse ADD-1/SREBP-1 was kindly provided by the laboratory of M. S. Brown and J. L. Goldstein (University of Texas Southwestern Medical Center). Hybridization signals were analyzed by Molecular Imager GS-363 (Bio-Rad), and the values were normalized to the signal generated with a probe for 18S ribosomal RNA.
Table 1.
Primers employed
| Gene | Sense primer (5′–3′) | Antisense primer (5′–3′) | Predicted size, bp | Species | GenBank accession no. |
|---|---|---|---|---|---|
| RT-PCR primers employed for Northern probes | |||||
| ACC | ACTCCAGGACAGCACAGATC | TCTGCCAGTCCAATTCTAGC | 535 | Rat | J03808 |
| FAS | AGCCCAGAGGGATCTGGTGA | TACACTCACTCGAGGCTCAG | 1,158 | Rat | M76767 |
| GPAT | TGATCAGCCAGGAGCAGCTG | AGACAGTATGTGGCACTCTC | 504 | Rat | AF021348 |
| Primers for Multiplex RT-PCR | |||||
| SREBP-1a | ACACAGCGGTTTTGAACGACATC | ACGGACGGGTACATCTTTACAG | 268 | Rat, mouse | L16995 (see ref. 21) |
| SREBP-1c | GGAGCCATGGATTGCACATT | AGGAAGGCTTCCAGAGAGGA | 191 | Rat | L16995 |
| SREBP-2 | CACAATATCATTGAAAAGCGCTACC | TTTTTCTGATTGGCCAGCTTCAGCA | 200 | Mouse | (see ref. 21) |
| ACC | ACAGTGAAGGCTTACGTCTG | AGGATCCTTACAACCTCTGC | 242 | Rat | J03808 |
| FAS | TGCTGTGGACCTCATCACTA | TGGATGATGTTGATGATAGAC | 297 | Rat | M76767 |
| GPAT | CCTCTGAACTGGAGAAGTGA | AGACAGTATGTGGCACTCTC | 287 | Rat | AF021348 |
| TBP | ACCCTTCACCAATGACTCCTATG | TGACTGCAGCAAATCGCTTGG | 186 | Mouse | D01034 |
Multiplex RT-PCR (MPX-RT-PCR).
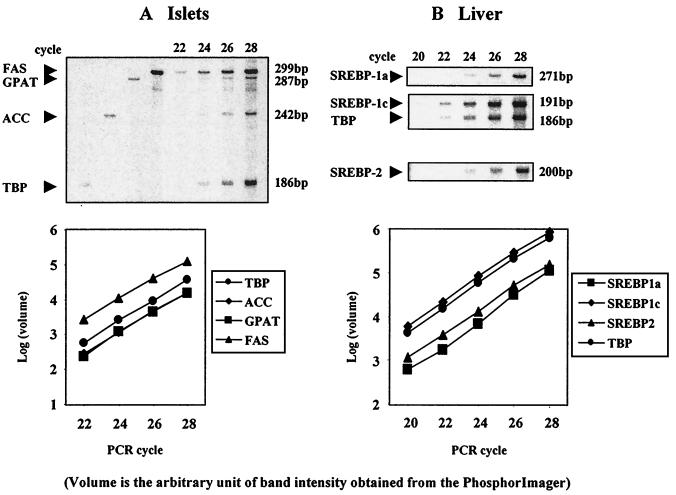
The procedure used was based on methods described by Jensen et al. (20) and O'Doherty et al. (21). Total RNA (1 μg) was treated with RNase-free DNase (Promega), and first-strand cDNA was generated with the oligo(dT) primer in the first-strand cDNA synthesis kit (CLONTECH). MPX-RT-PCR was carried out in 25-μl reactions with 1.5 μl of the diluted cDNA reaction as template mixed with 23.5 μl of PCR mix containing 1.25 units of Taq polymerase and buffer (Roche Molecular Biochemicals); 25 μM of dATP, dTTP, and dGTP; 2.5 μCi of 2,500 Ci/mmol [α33P]dCTP (Amersham Pharmacia; 1 Ci = 37 GBq); and 5 pmol each primer (Table 1). The standard thermal cycle profile was as follows for lipogenic gene mRNA (FAS, ACC, and GPAT): denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min for 24 cycles in liver and for 26 cycles in islets. For the SREBP isoforms, 26 cycles were used in liver, and 32 cycles were used in islets—all within the linear range of the PCR (Fig. 1).
Figure 1.
The validation of multiplexed amplification of several cDNAs. (A) Linear regression analysis of PCR amplification of the cDNAs of the lipogenic enzymes ACC, FAS, GPAT, and TATA binding protein (TBP) in rat islets. (B) Linear regression analysis of PCR amplification of members of the SREBP family in rat liver.
Reaction products were separated on 7 M urea, 1× TBE (0.1 M Tris base/83 mM boric acid/1 mM EDTA), and 6% polyacrylamide gels, dried, and PhosphorImager screens were scanned by a Molecular Imager System (GS-363). TATA box binding protein (TBP) mRNA was coamplified as an internal control, and data were expressed as ratios to its signal.
To avoid biased results caused by potential interference between individual amplicons, we analyzed the amplification kinetics of individual amplicons in reactions where several products were coamplified. Representative experiments, in which mRNA encoding lipogenic enzymes and TBP in pancreatic islets was simultaneously amplified (Fig. 1A), show the noncompetitive amplification of individual products and their almost identical rate of amplification, as indicated by the slopes within the exponential phase observed from a linear regression analysis (Fig. 1A). Similarly, no initial interference was detected with SREBP-1c and TBP in liver (Fig. 1B). However, when SREBP-1a or SREBP-2 primers were mixed, absolute product yield decreased, and unexpected products appeared (data not shown). Consequently, RT-PCRs were performed separately for each SREBP isoform in liver (Fig. 1B) and islets. Expression of SREBP-1c in liver was almost 10-fold higher than that of SREBP-1a (Fig. 1B), which is similar to the previously reported results with the RNase protection assay (22).
Statistical Analysis.
All results are expressed as the means ± SEM. The statistical significance of differences in mean values was assessed by Student's unpaired t test.
Results
SREBP-1 Expression in Liver of Obese ZDF (fa/fa) Rats.
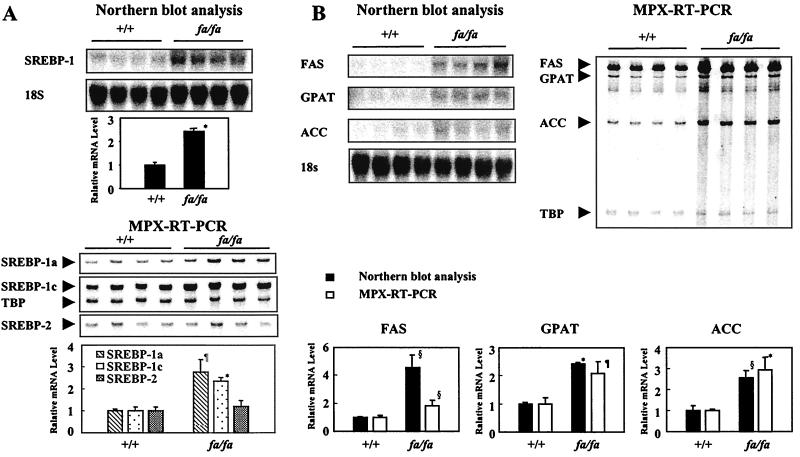
The clinical and laboratory data of obese fa/fa and lean ZDF +/+ rats are summarized in Table 2. The obese ZDF fa/fa rats developed diabetes even as plasma insulin levels increased to >250 microunits/ml (vs. 53.5 ± 8.6 microunits/ml in age-matched lean +/+ controls). Their TG content in the liver was 5.4 times that of the lean rats (P < 0.0005). To determine the relationship of the steatosis to the expression of SREBP-1, the mRNA of this transcription factor was measured by Northern blotting. It averaged 2.4 fold higher in the fat-laden livers of obese fa/fa rats than in the +/+ controls (P < 0.0005; Fig. 2A). RT-PCR was used to determine whether this elevation was the result of an increase in the SREBP-1a or -1c isoforms. The increase could be accounted for entirely by increased expression of SREBP-1c, the dominant lipogenic transcription factor of the liver (P < 0.0005; Fig. 2A). The lipogenic enzymes that are the targets of SREBP-1 transcriptional control, ACC, FAS, and GPAT, were 2.4- to 4.6-fold higher in the fa/fa rats (P < 0.005; Fig. 2B).
Table 2.
Comparison of clinical and laboratory phenotypes of prediabetic ZDF (fa/fa) rats treated with TGZ for 6 weeks vs. untreated ZDF (fa/fa) controls
| Parameter | Age, weeks | ZDF (+/+) rats (n = 5) | ZDF (fa/fa)
rats
|
|
|---|---|---|---|---|
| Untreated (n = 5) | TGZ-treated (n = 6) | |||
| Food intake, g/day | 7 | 36.0 ± 1.4 | 32.8 ± 1.2 | |
| 13 | 38.2 ± 3.0 | 40.3 ± 1.3 | ||
| Plasma glucose, mg/dl | 7 | 114.8 ± 5.6 | 117.0 ± 6.1 | |
| 13 | 90.8 ± 2.6 | 246.6 ± 26.1 | 102.5 ± 6.6* | |
| Plasma insulin, μU/ml | 7 | 151.0 ± 46.1 | 149.6 ± 36.1 | |
| 13 | 53.5 ± 8.6 | >250 | 141.3 ± 27.2* | |
| Plasma leptin, ng/ml | 7 | 24.8 ± 2.0 | 23.6 ± 2.6 | |
| 13 | 47.2 ± 2.1 | 41.7 ± 4.3 | ||
| Plasma triglyceride, mg/dl | 7 | 208.9 ± 26.3 | 208.5 ± 29.4 | |
| 13 | 63.4 ± 2.7 | 502.9 ± 57.3 | 103.3 ± 12.7* | |
| Plasma FFA, mM | 7 | 0.8 ± 0.1 | 1.88 ± 0.3 | 1.6 ± 0.1 |
| 13 | 1.2 ± 0.2 | 2.23 ± 0.2 | 1.6 ± 0.3* | |
| Liver triglyceride content, mg/g of wet weight | 13 | 2.5 ± 0.1 | 13.5 ± 1.6 | 5.1 ± 0.5* |
| Islet triglyceride content, ng per islet | 13 | 30.7 ± 2.8 | 406.0 ± 16.0 | 266.2 ± 4.8* |
| Body weight, g | 7 | 254.6 ± 15.4 | 250.3 ± 10.3 | |
| 13 | 278.0 ± 6.2 | 474.6 ± 17.9 | 520.0 ± 11.6§ | |
| Liver weight, g | 13 | 11.7 ± 0.5 | 22.9 ± 1.7 | 17.2 ± 1.1§ |
| Epididymal fat weight, g | 13 | 1.7 ± 0.1 | 10.8 ± 0.8 | 16.2 ± 1.3* |
*, P < 0.005;
, P < 0.05 between untreated ZDF (fa/fa) rats and ZDF rats treated with TGZ.
Figure 2.
mRNA expression profiles of SREBP family and lipogenic enzymes in liver. (A) Comparison of hepatic expression of SREBP-1, measured by Northern blotting (Upper), and that of SREBP-1a (▧), -1c (⊡), and SREBP-2 (░⃞), measured by RT-PCR in livers of lean (+/+) and fatty (fa/fa) ZDF rats. (B) Validation of MPX-RT-PCR (□) by comparison of mRNA expression profile of lipogenic enzyme expression with Northern blot analysis (■) in livers of lean (+/+) and obese (fa/fa) rats. Both techniques demonstrate higher expression of FAS, GPAT, and ACC in the obese group. Data were normalized in Northern blots by 18S ribosomal RNA and by TBP for RT-PCR. (*, P < 0.0005; ¶, P < 0.005; §, P < 0.05 lean +/+ vs. obese fa/fa.)
These findings are consistent with a role for increased SREBP-1c expression in the steatosis of overnutrition caused by leptin unresponsiveness.
SREBP-1 Expression in Pancreatic Islets of Obese ZDF (fa/fa) Rats.
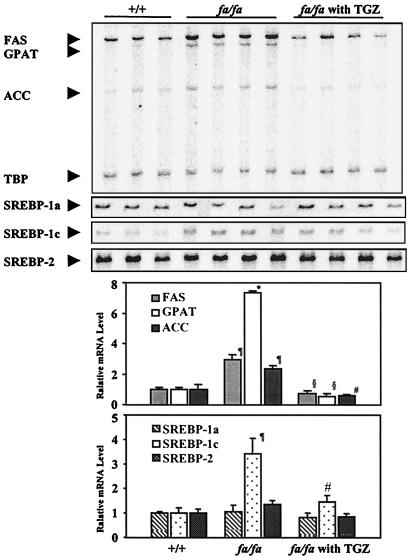
The TG content of nonadipose tissues other than liver is also markedly elevated in obese ZDF rats (23). In the pancreatic islets, for example, lipogenesis from both labeled glucose and labeled palmitate is increased as a consequence of enhanced expression of ACC, FAS, and GPAT (24, 25), and TG content may reach 50 times normal (24). To determine whether the enhanced lipogenic capacity of the pancreatic islets could be the result of an increase in the expression of SREBP-1c, the mRNA was semiquantified by MPX-RT-PCR. It was barely detectable in islets of normal +/+ rats, as would be expected in a tissue with low lipogenic activity. However, in islets of obese fa/fa rats, in which lipogenic activity is inappropriately high (24), SREBP-1c mRNA was 3.4 times that of lean +/+ controls (P < 0.05). This difference was accompanied by a 2.4- to 3.0-fold increase in the lipogenic enzymes ACC and FAS mRNA (P < 0.05) and a 7.3-fold increase in GPAT mRNA (P < 0.0005). This inappropriately high expression of the lipogenic transcription factor SREBP-1c and its target enzymes in islets could account for the abnormal lipogenic activity, the increased TG deposition (24), and the associated morphofunctional derangements of β-cells that culminate in diabetes (26).
Effect of an Antilipogenic Drug on Up-Regulated SREBP-1 Expression in Liver and Islets.
Treatment of prediabetic ZDF rats with TGZ prevents the increase in ectopic accumulation of TG in islets and other nonadipose tissues, while maintaining normal function of those tissues and preventing the diabetes that otherwise occurs (10, 23). We therefore treated a group of obese ZDF rats beginning at the age of 7 weeks until the age of 13 weeks with 200 mg/kg per day of TGZ mixed in the chow; they actually consumed 100–130 mg/day. The clinical effects of this treatment are indicated in Table 2. Food intake was not affected by the presence of TGZ in the chow. Body weight and weight of epididymal fat pads were higher in the treated rats than in untreated controls. After 6 weeks of treatment, plasma insulin and glucose levels remained at initial pretreatment values. Liver TG in TGZ-treated rats was only 38% (P < 0.005) and islet TG was 66% of untreated controls (P < 0.005; Table 2).
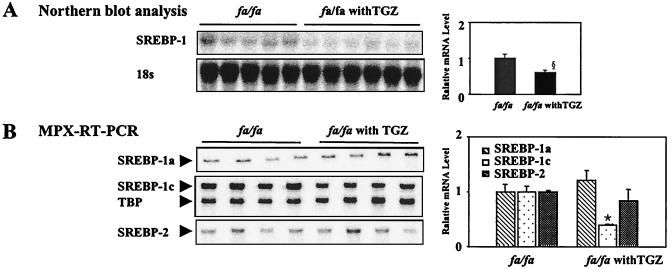
SREBP-1 mRNA in the livers of TGZ-treated rats was 40% below that of untreated controls (P < 0.05; see Fig. 4A). This difference was the result of a 61% reduction in SREBP-1c (P < 0.001). There was no change in SREBP-1a (see Fig. 4B). Similarly, TGZ treatment, which prevented TG accumulation in pancreatic islets and preserved their functional, morphologic, and biochemical integrity (23), also prevented the increase in SREBP-1c mRNA. The level was 57% below untreated ZDF rats (P < 0.05) and did not differ significantly from the expression level in islets of lean +/+ rats (Fig. 3).
Figure 4.
The effect of TGZ treatment on hepatic expression of the SREBPs. (A) Northern analysis of SREBP-1. (B) MPX-RT-PCR analysis of SREBP-1a (▧), -1c (⊡), and -2 (░⃞). (*, P < 0.001; §, P < 0.05 obese fa/fa vs. obese fa/fa with TGZ.)
Figure 3.
Comparison of mRNA expression profiles of lipogenic enzymes [FAS (░⃞), GPAT (□), ACC (░⃞), and SREBP-1a (▧), SREBP-1c (⊡), and SREBP-2 (░⃞)] in islets of lean (+/+) ZDF rats, untreated obese, prediabetic (fa/fa) ZDF rats, or obese, prediabetic (fa/fa) ZDF rats treated with TGZ. (*, P < 0.0005; ¶, P < 0.05 lean +/+ vs. obese fa/fa; §, P < 0.005; #, P < 0.05 obese fa/fa vs. obese fa/fa with TGZ.)
Diet-Induced Hyperinsulinemia and SREBP-1 Expression.
Because human obesity is usually induced environmentally, we determined whether DIO in normal rats is associated with up-regulation of SREBP-1. Sprague–Dawley 4-week-old rats were fed a diet containing 60% fat for 6 weeks. Their clinical and laboratory data appear in Table 3. Compared with rats on a 4% diet, insulin levels were 2.3-fold higher, and leptin rose more than 7-fold (P < 0.005). Plasma TG levels increased by 137%, and hepatic TG content rose by 191% (P < 0.005).
Table 3.
Comparison of Sprague–Dawley rats after 4% or 60% dietary fat content treatment for 6 weeks
| Parameter | Sprague–Dawley rats
|
|
|---|---|---|
| Control (n = 4) | High-fat diet (n = 3) | |
| Phenotype | ||
| Food intake, Kcal/day | 119.6 ± 3.1 | 137.2 ± 3.1* |
| Plasma glucose, mg/dl | 77.2 ± 4.3 | 151.9 ± 3.2* |
| Plasma insulin, microunits/ml | 37.5 ± 10.0 | 87.5 ± 10.0* |
| Plasma leptin, ng/ml | 1.6 ± 0.3 | 11.5 ± 1.7* |
| Plasma triglyceride, mg/dl | 78.8 ± 4.4 | 186.7 ± 12.4* |
| Liver triglyceride content, mg/g wet weight | 2.3 ± 0.5 | 6.7 ± 1.0* |
| Body weight, g | 284 ± 10.9 | 444.3 ± 13.5* |
| Lipogenic expression in liver | ||
| SREBP-1 | 1.00 ± 0.10 | 2.88 ± 0.19* |
| ACC | 1.00 ± 0.23 | 2.21 ± 0.21† |
| FAS | 1.00 ± 0.15 | 0.64 ± 0.08 |
*, P < 0.005;
, P < 0.01 as compared to 4% chow-treated Sprague–Dawley rats.
Hepatic SREBP-1 expression was 2.9-fold higher after 6 weeks of overnutrition (P < 0.005), in association with a 2.2-fold increase in expression of the lipogenic enzyme ACC (P < 0.01; Table 3). FAS expression was not altered significantly. Thus, DIO is also accompanied by augmented SREBP-1 expression comparable to the fa/fa rats, consistent with a role in the accompanying hyperlipidemia and hepatic steatosis. In contrast to the tissues of obese fa/fa, which are congenitally leptin-resistant and therefore naïve to leptin action, tissues of DIO are chronically exposed to hyperleptinemia. Perhaps the down-regulatory action of leptin on expression of lipogenic enzymes (27) accounted for the less intense steatosis that resulted (6.7 mg of TG/g of wet weight in DIO rats vs. 13.5 mg/g in fa/fa rats).
Effect of Adenovirus-Induced Hyperleptinemic on SREBP-1 Expression in Normal Rats.
To explore further a possible role of leptin in suppressing SREBP-1 expression, we induced hyperleptinemia in wild-type +/+ ZDF rats by infusing AdCMV-leptin. AdCMV-β-gal was infused as a control. Previously, we had observed that adenovirus-induced hyperleptinemia in normal rats causes profound depletion of TG from adipocytes and nonadipocytes alike, as well as a generalized down-regulation of lipogenic enzymes (27).
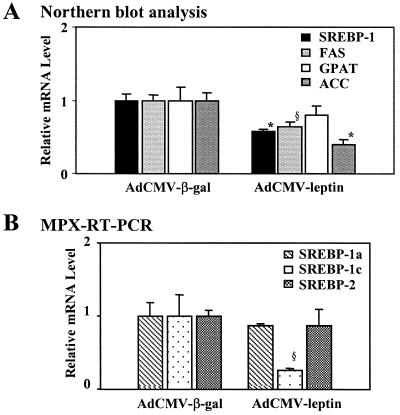
Hyperleptinemia averaged 43 ± 9 ng/ml 3 days after the treatment with AdCMV-leptin. Food intake declined by 10 ± 5 g/day and body weight was 30 g below AdCMV-β-gal-infused controls; plasma insulin was reduced by 11 microunits/ml or about 66%. Hepatic SREBP-1 mRNA declined by 42% (P < 0.005), and SREBP-1c declined by 74% (P < 0.05; Fig. 5). There was a 59% and 35% reduction in ACC and FAS mRNA, respectively (P < 0.005; P < 0.05; Fig. 5A). GPAT mRNA was unchanged. Although these hyperleptinemic rats were hypoinsulinemic, they were not hyperglycemic (15), which suggests enhanced sensitivity to insulin. Therefore, the reduction in SREBP-1 expression might reflect an overriding down-regulatory influence of leptin on SREBP-1c expression.
Figure 5.
(A) Effect of adenoviral induction of hyperleptinemia on hepatic expression of SREBP-1 (■) and the lipogenic enzymes FAS (░⃞), GPAT (□), and ACC (░⃞). Data were normalized by 18S RNA. (B) Semiquantification of SREBP-1a (▧), -1c (⊡), and -2 (░⃞) by RT-PCR. (*, P < 0.005; §, P < 0.05 AdCMV-β-gal vs. AdCMV-leptin.)
Discussion
The discovery of SREBP-1 (28, 29), a lipogenic transcription factor up-regulated by insulin, has raised the possibility that it may be involved in hyperinsulinemic syndromes of overnutrition, in which lipogenesis is increased in nonadipose tissues, producing so-called “lipotoxicity” (9). We therefore studied two rat models of overnutrition and hyperinsulinemia.
In congenitally leptin-unresponsive obese ZDF fa/fa rats, obesity develops rapidly, and nonoxidative products of lipid metabolism overaccumulate in nonadipose tissues such as the pancreatic islets, liver, and heart, causing diabetes (23), steatosis, and myocardial dysfunction (10). In the more gradual and less severe DIO of otherwise normal Sprague–Dawley rats, the TG accumulation and hyperglycemia are more moderate. Expression of hepatic SREBP-1c, the isoform most implicated in hepatic lipogenesis, was increased in the liver of both models. In the ZDF fa/fa rats, the increase is associated with a rise in mRNA of the lipogenic enzymes ACC, FAS, and GPAT. In DIO, only ACC is increased significantly, perhaps accounting for the more modest steatosis. A profound difference between the two obesity models is that tissues of fa/fa rats have never experienced leptin action, whereas those of DIO rats have fully functional leptin receptors and endogenous hyperleptinemia. However, the increase in SREBP-1 expression was high in DIO; therefore, if leptin actually attenuated the lipogenic activity of SREBP-1, perhaps it did so by reducing cleavage to the active moiety (31).
TGZ treatment prevented the increase in lipogenic enzymes and TG content in ZDF fa/fa rats and also prevented the increased expression of SREBP-1c. The mechanism by which TGZ exerts this effect is unknown. Nevertheless, because TGZ treatment completely blocked the morphofunctional disruption of β-cells that causes diabetes (23), prevention or reversal of inappropriate expression of SREBP-1c by other interventions might also be an effective antidiabetic strategy. Virus-induced hyperleptinemia in normal +/+ rats was also associated with a decline in hepatic SREBP-1c to subnormal levels, presumably via a different pathway than that of TGZ. To our knowledge, this study is the first demonstration of such a role for leptin.
The maintenance of normal fatty acid homeostasis in nonadipocytes may require a leptin-mediated restraint on SREBP-1c-mediated lipogenesis, in keeping with the putative regulatory role of this hormone in fatty acid homeostasis (6). If leptin action is absent, but insulin action is present or increased, SREBP-1c expression will be inappropriately high, in which case, dietary excess will lead to the ectopic overaccumulation of lipids that cause damage to certain nonadipocytes such as the pancreatic β-cells (30). In this case, pharmacologic reduction of SREBP-1 expression and/or tissue-specific inhibition of its activating protease (31) might prevent the disease consequences of chronic overnutrition.
Note Added in Proof.
Similar reduction of SREBP-1 by leptin has been reported recently by Soukas et al. (32).
Acknowledgments
The authors thank Susan Kennedy for outstanding secretarial work and Per B. Jensen and Kay McCorkle for excellent technical assistance. We acknowledge the support of the Department of Veterans Affairs, National Institutes of Health Grants DK02700-37 and HL-20948, the National Institutes of Health/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research Program, and Novo-Nordisk Corporation.
Abbreviations
- ZDF
Zucker diabetic fatty
- TG
triacylglycerol
- DIO
diet-induced obesity
- β-gal
β-galactosidase
- AdCMV
adenovirus cytomegalovirus
- ACC
acetyl-CoA carboxylase
- FAS
fatty acid synthase
- GPAT
glycerol-3-phosphate acyltransferase
- RT-PCR
reverse transcriptase–PCR
- MPX-RT-PCR
multiplex RT-PCR
- TGZ
troglitazone
References
- 1.Shimomura I, Bashmakov Y, Ikemoto S, Horton J D, Brown M S, Goldstein J L. Proc Natl Acad Sci USA. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimomura I, Bashmakov Y, Horton J D. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 3.Foretz M, Guichard C, Ferre P, Foufelle F. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Liepvre X, Berthelier-Lubrano C, Spiegelman B, Kim J B, Ferre P, et al. Mol Cell Biol. 1999;19:3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J B, Sarraf P, Wright M, Yao K M, Mueller E, Solanes G, Lowell B B, Spiegelman B M. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger R H, Zhou Y-T, Orci L. Proc Natl Acad Sci USA. 1999;96:2327–2332. doi: 10.1073/pnas.96.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randle P J. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.McGarry J D. J Cell Biochem. 1994;55:29–38. doi: 10.1002/jcb.240550005. [DOI] [PubMed] [Google Scholar]
- 9.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y-T, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger R H. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips M S, Liu Q, Hammond H A, Dugan V, Hey P J, Caskey C J, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 12.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- 14.Nolan J J, Ludvik B, Beerdsen P, Joyce M, Olefsky J. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Koyama K, Yuan X, Lee Y, Zhou Y-T, O'Doherty R, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naber S P, McDonald J M, Jarett L, McDaniel M L, Ludvigsen C W, Lacy P E. Diabetologia. 1980;19:439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- 17.Folch J M, Lees M, Stanley G H S. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Ingelbrecht I L, Mandelbaum C I, Mirkos T E. BioTechniques. 1998;25:420–425. doi: 10.2144/98253st03. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. p. 752. [Google Scholar]
- 20.Jensen J, Serup P, Karlsen C, Nielsen T F, Madsen O D. J Biol Chem. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- 21.O'Doherty R M, Jensen P B, Anderson P, Jones J G, Berman H K, Kearney D, Newgard C B. J Clin Invest. 2000;105:479–488. doi: 10.1172/JCI8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimomura I, Shimano H, Horton J D, Goldstein J L, Brown M S. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higa M, Zhou Y-T, Ravazzola M, Baetens D, Orci L, Unger R H. Proc Natl Acad Sci USA. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Hirose H, Zhou Y-T, Esser V, McGarry J D, Unger R H. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y-T, Shimabukuro M, Lee Y, Koyama K, Higa M, Ferguson T, Unger R H. Diabetes. 1998;47:1904–1908. doi: 10.2337/diabetes.47.12.1904. [DOI] [PubMed] [Google Scholar]
- 26.Orci L, Ravazzola M, Baetens D, Inman L, Amherdt M, Peterson R G, Newgard C B, Johnson J H, Unger R H. Proc Natl Acad Sci USA. 1990;87:9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y-T, Wang Z-W, Higa M, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1999;96:2391–2395. doi: 10.1073/pnas.96.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tontonoz P, Kim J B, Graves R A, Spiegelman B M. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokoyama C, Wang X, Briggs M R, Admon A, Wu J, Hua X, Goldstein J L, Brown M S. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 30.Shimabukuro M, Zhou Y-T, Levi M, Unger R H. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown M S, Goldstein J L. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 32.Soukas A, Cohen P, Socci N D, Friedman J M. Genes Dev. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]