Abstract
In recent years, diseases of corals caused by opportunistic pathogens have become widespread. How opportunistic pathogens establish on coral surfaces, interact with native microbiota, and cause disease is not yet clear. This study compared the utilization of coral mucus by coral-associated commensal bacteria (“Photobacterium mandapamensis” and Halomonas meridiana) and by opportunistic Serratia marcescens pathogens. S. marcescens PDL100 (a pathogen associated with white pox disease of Acroporid corals) grew to higher population densities on components of mucus from the host coral. In an in vitro coculture on mucus from Acropora palmata, S. marcescens PDL100 isolates outgrew coral isolates. The white pox pathogen did not differ from other bacteria in growth on mucus from a nonhost coral, Montastraea faveolata. The ability of S. marcescens to cause disease in acroporid corals may be due, at least in part, to the ability of strain PDL100 to build to higher population numbers within the mucus surface layer of its acroporid host. During growth on mucus from A. palmata, similar glycosidase activities were present in coral commensal bacteria, in S. marcescens PDL100, and in environmental and human isolates of S. marcescens. The temporal regulation of these activities during growth on mucus, however, was distinct in the isolates. During early stages of growth on mucus, enzymatic activities in S. marcescens PDL100 were most similar to those in coral commensals. After overnight incubation on mucus, enzymatic activities in a white pox pathogen were most similar to those in pathogenic Serratia strains isolated from human mucosal surfaces.
Serratia is a gammaproteobacterium frequently isolated from waters, plants, and animals (7). Some isolates of Serratia are well-characterized symbionts of invertebrates. Serratia marcescens and Serratia liquefaciens have been identified as vertically transmitted symbionts of the sugar beet maggot (9). Serratia colonizes male and female reproductive tracts of the maggots, eggs, and pharyngeal filter. There, the bacteria are hypothesized to aid in metamorphosis by digesting chitinous puparial walls (9). In the gut of another insect, the diamondback moth, strains of S. marcescens appear to live as commensals capable of modestly (5 to 8%) increasing growth rates of the host (8). Serratia strains have also been isolated from feces and cloacal swabs from clinically normal captive birds, but not from organs or carcasses of sick or diseased animals housed within the same facility (3, 20). Serratia spp. have also been linked to diseases of invertebrate animals and their larvae (for reviews, see references 7, 15, and 21). To cause diseases in nematodes and flies, S. marcescens first colonizes the intestines, degrades cells of the alimentary tract and then spreads to other organs (14, 21). There are, however, exceptions to this mode of infection. Serratia entomophila, the causal agent of amber disease in grubs, grows within the alimentary tract of the animal to >106 CFU. However, bacteria neither attach to nor colonize surfaces of the gut; rather, they adhere to gut contents (10) and cause the appearance of signs by producing the Sep toxin that inhibits accumulation of the insect's digestive serine proteases and disrupts the cytoskeletal network (6). It appears, therefore, that various isolates of Serratia are capable of entering into a full range of interactions (from mutualistic to commensal to pathogenic) with their animal hosts (for reviews, see references 7, 15, and 21).
A strain of S. marcescens, PDL100, was shown to be associated with white pox disease of the threatened Caribbean coral Acropora palmata (22, 27). White pox disease results in coral tissue necrosis, exposing carbonate skeleton at a rate of 2.5 cm2 day−1 (22). It is not yet clear how S. marcescens PDL100 colonizes and infects corals. It is likely that to cause disease, the pathogen first needs to colonize and establish within the coral surface mucus layer.
The coral surface mucus layer contains polymers of mixed origin. Coral mucus is made in the mucocytes of the polyp, where the photosynthate produced by the coral symbiotic dinoflagellate Symbiodinium spp. is converted into polymers that are excreted onto the coral surface (for a review, see reference 2). A glycoprotein is the major component of coral mucus from both hard and soft corals (16, 17, 19). The composition of the glycoprotein differs among coral species (4, 17). The mucus polymer of Acropora formosa, for example, contains 36 to 38% of neutral sugars, 18 to 22% of amino sugars, and 19 to 30% of amino acids; lipids make up 4.2% of the polymer (17). In the mucus of A. formosa, the oligosaccharide decorations (two to four sugar residues long) are attached to the polypeptide backbone by an O-glycosidic link to serine or threonine through the carbon 1 of mannose (16). The glycoproteins from A. formosa and Pseudopterogorgia americana corals contain terminal arabinose residues linked by a β1→2 or β1→3 bond. In the mucus of acroporid corals, arabinose, N-acetyl-glucosamine, mannose, glucose, galactose, N-acetyl-galactosamine, and fucose were the major sugars; serine and threonine were the major amino acids (4, 17). The elucidation of the chemical structure of coral mucus is complicated by the fact that the mucus contains excretions of coral mucocytes, extracellular substances produced by the associated microbiota as well as oligomers that may result from the degradation of these polymers (for reviews, see references 2 and 24).
In this study, we tested the hypothesis that S. marcescens PDL100 is capable of a more extensive utilization of A. palmata mucus than other environmental or pathogenic isolates of S. marcescens. This hypothesis is based on the recent discoveries that pathogenic and commensal host-associated bacteria differ in their patterns of carbon source utilization during growth on components of the mucus that lines host surfaces (5, 26). These different strategies of mucus utilization may allow pathogenic bacteria to outcompete native residents and establish within the host's mucosa (5, 13, 26). To test this hypothesis, growth of the strain PDL100 on coral mucus and enzymatic activities induced during growth on mucus were assayed and compared to those of pathogenic and environmental isolates of S. marcescens and three native coral-associated bacteria.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. Escherichia coli and Serratia marcescens isolates were grown in LB broth (Fisher Scientific, Pittsburgh, PA). Bacteria isolated from A. palmata mucus were routinely grown in GASW broth (356 mM NaCl, 40 mM MgSO4, 20 mM MgCl2·6H2O, 8 mM KCl, 60 μM K2HPO4, 33 μM Tris, and 7 μM FeSO4, with 0.05% peptone, 0.2% yeast extract, and 2.0% glycerol [pH 7.0]) or on GASW medium solidified with 1.5% agar (GASWA) (Fisher Scientific) (23). Unless otherwise stated, seawater used in the experiments was collected at Crescent Beach (29°45′56"N, 81°15′11"W) and sterilized through a 0.22-μm filter. As needed for experiments, seawater samples were buffered with 10 mM HEPES to pH 7.0.
TABLE 1.
Bacterial strains used in the study
| Strain | Description |
|---|---|
| Halomonas meridiana 33E7 | Isolated from Acropora palmata mucus (23); identity confirmed by 16S rRNA gene sequencing |
| Photobacterium mandapamensis 33C12 | Isolated from Acropora palmata mucus (23); identity confirmed by 16S rRNA gene sequencing |
| Photobacterium leiognathi 33G12 | Isolated from Acropora palmata mucus (23); identity confirmed by 16S rRNA gene sequencing |
| S. marcescens PDL100 | Isolated from a white pox lesion on Acropora palmata (22, 28) |
| S. marcescens MG1 | Isolated from a rotten cucumber |
| Serratia sp. strain ATCC 39006 | Cheesequake Salt Marsh (NJ) channel isolate, pigmented |
| S. marcescens ATCC 43422 | Human throat isolate, pigmented |
| S. marcescens ATCC 43820 | Human urine isolate |
| S. marcescens EL31 | Isolated from Florida Keys wastewater |
| S. marcescens EL34 | Isolated from Eden Pines (Florida Keys) canal water, pigmented |
| S. marcescens EL139 | Isolated from Florida Keys wastewater |
| S. marcescens EL202 | Isolated from Higgs Beach (Key West) |
| S. marcescens EL206 | Isolated from canal water by Mote Marine Tropical Research Laboratory (Summerland Key, FL) |
| S. marcescens EL266 | Isolated from Florida Keys wastewater |
| S. marcescens EL368 | Isolated from a seabird (Key Largo, FL) |
| S. marcescens EL402 | Isolated from a seabird (Florida Keys) |
Coral mucus samples were collected from different, apparently asymptomatic A. palmata colonies at Looe Key Reef (24°33′75"N, 81°24′05"W) in September 2006, winter of 2007, and February 2008 with a needleless syringe (23). Montastraea faveolata mucus samples were collected in a similar manner at the Dry Tortugas (24°37′43"N 82°52′24"W) in May 2008. Mucus samples were stored as aliquots at −80°C. As needed, aliquots were thawed and sterilized in polypropylene tubes by UV irradiation (254 nm) for 20 min and by filtration through a glass fiber (GFC) filter and then through 0.45-μm and 0.22-μm filters. Mucus samples were then separated into low- and high-molecular-mass fractions with VivaSpin-15 spin dialysis assemblies (VivaScience, Stonehouse, United Kingdom), following the manufacturer's instructions. The high-molecular-mass fraction was brought up to volume in artificial seawater (Instant Ocean, Mentor, OH) as described previously (13).
To evaluate growth on mucus components, overnight bacterial cultures (grown to an optical density at 600 nm of ∼1.9 in either LB or GASW broth) were pelleted, washed three times in phosphate-buffered saline (pH 7.0) and resuspended in phosphate-buffered saline. These inocula were mixed with seawater containing “total mucus,” its high- or low-molecular-mass mucus fractions resulting in the initial population densities of 102 to 103 CFU ml−1. Cells were prepared in a similar manner for coculture experiments; they were diluted to 102 CFU ml−1 and then mixed 1:1. All cultures were incubated at 30°C with shaking at 180 rpm. Growth was monitored by serially diluting cultures and plating them onto either LB agar or GASWA plates at the time intervals indicated below. To differentiate S. marcescens from coral commensals in cocultures, individual colonies were picked from LB and/or GASWA plates and patched onto xylose lysine deoxycholate agar (Oxoid). Differences in bacterial growth were compared using a one-way analysis of variance and Tukey's honestly significant difference (HSD) post hoc test with type I error significance at α = 0.05.
The extent to which the bacteria hydrolyzed polymers in mucus was examined using a 2,2′-bicinchoninic acid (BCA) assay (29). Briefly, spent cultures and controls were centrifuged, and equal volumes of cell-free supernatants were mixed with solution A (0.67 M Na2CO3·H2O, 0.32 M NaHCO3, 2.22 mM BCA) and solution B (5 mM CuSO4·5H2O, 12 mM l-serine). The reaction mixtures were heated to 100°C for 15 min, and A560 was measured (29). The reducing end equivalents were calculated using a regression equation from a standard curve using known concentrations of l-arabinose (y = 0.0073x + 0.0031; R2 = 0.9988).
Enzymatic assays.
Two overnight cultures of each isolate were grown in LB or GASW broth to an optical density at 600 nm of 2.0. Cells were pelleted, washed in filter-sterilized seawater, resuspended in the same volume of buffered seawater, and starved at 30°C with shaking for 3 days. Following starvation, 1 ml of cells was added to 2 ml of either 1× coral mucus or 10 mM HEPES-buffered seawater. Treatments of the negative control (coral mucus alone) and a blank (buffered seawater) were carried out in parallel. Enzymatic assays were conducted after 2 or 18 hours of incubation in mucus (at 30°C), by following established protocols (12, 18). Corresponding p-nitrophenyl enzymatic substrates were purchased from Sigma-Aldrich. All enzymatic reactions were conducted for exactly 24 h. Cellular debris and unused enzymatic substrate were pelleted at a relative centrifugal force of 16,000 × g. The supernatants were transferred to a clear polystyrene 96-well plate, and A405 was measured on a Victor3 plate reader (Perkin Elmer, Shelton, CT). Enzymatic activities in the mucus-only control were measured and subtracted from those of all other treatments; the activity was then calculated using modified Miller units (A405/A590).
To compare the totality of enzymatic activities induced during the growth of each isolate on A. palmata mucus, the activities were scaled to those of a starved control for each strain, and joining tree analysis was performed on the natural log of the resulting induction value. Hierarchical clustering was done separately at each time period, using Ward's method (30). The dendrograms were constructed using Euclidean distance metric, which serves to emphasize the similarities based on absolute (rather than relative) values of induction.
Nutrient utilization profiling with Biolog EcoPlates.
Isolates were grown in either LB or GASW broth overnight at 30°C with shaking. Cells were then pelleted and washed twice with 0.22-μm-filter-sterilized seawater. Cells were then resuspended in 2 volumes of filter-sterilized seawater and starved for 24 h at 30°C and then inoculated into EcoPlates (Biolog Inc., Hayward, CA). A590 was monitored using a Victor3 plate reader every 24 h for 72 h. For each substrate, the average well color development (AWCD) was calculated after the final reading and then converted into binary format. To evaluate the degree of similarity between S. marcescens PDL100 and each of the tested isolates, the Jaccard similarity coefficient and the odds ratio were used. Given the small sample size (contingency tables comprised of binary responses from 31 substrates and a control), both the odds ratio and the associated 95% confidence intervals were estimated after employing a modified Gart interval sample size correction (1). All statistical analyses were performed using Statistica version 8.0 (StatSoft, Inc., Tulsa, OK).
RESULTS AND DISCUSSION
Bacterial growth on mucus of A. palmata.
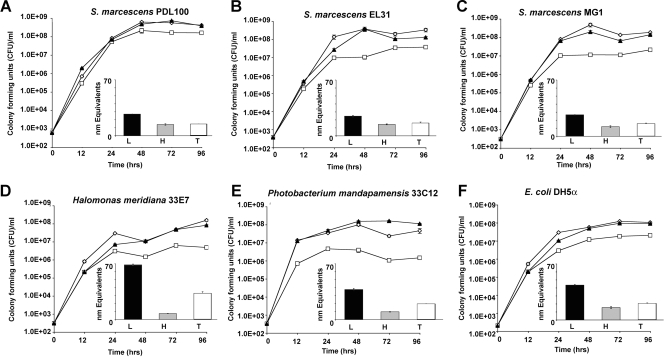
All tested strains utilized coral mucus as a growth substrate (Fig. 1). When grown on the crude preparation of “total mucus,” the white pox pathogen, Serratia marcescens PDL100, reached final population densities of 7 × 108 CFU ml−1, while other S. marcescens isolates reached somewhat lower final densities of 1.5 × 108 to 5 × 108 CFU ml−1 (Tukey's HSD P value of <0.0025). Two unrelated bacteria (“Photobacterium mandapamensis” 33C12 and Halomonas meridiana 33E7) that were previously characterized as dedicated A. palmata commensals (23) reached 1.6 × 108 CFU ml−1 and 8 × 107 CFU ml−1 (respectively). These observations are generally consistent with the report that crude preparations of coral mucus supported bacterial cultures with 108 to 109 CFU ml−1 (26). Escherichia coli DH5α, used as a negative control in this work, reached 9.4 × 107 CFU ml−1 when grown on crude A. palmata mucus (Fig. 1F). The observation that E. coli DH5α, not known for its ability to extensively utilize complex carbohydrate polymers, grew on mucus as well as coral-associated bacteria suggested either that the mucus sample contained a significant amount of monomers or that proteinaceous components of mucus were sufficient to support growth of various bacteria, including a laboratory strain of E. coli.
FIG. 1.
Bacterial growth on the mucus of Acropora palmata. Serratia marcescens, coral commensal bacteria, and E. coli DH5α were grown for 96 h in seawater containing approximately 60 mg ml−1 (wt/vol) total mucus (open diamonds) or its high-molecular-mass (>15 kDa; open squares) or low-molecular-mass (<15 kDa; closed triangles) fractions. (Insets) The extent to which the bacteria hydrolyzed substrates in mucus was examined using a BCA assay as described in Materials and Methods. Black bars indicate accumulation of reducing group equivalents in the low-molecular-mass fraction of mucus; gray bars indicate accumulation of reducing group equivalents in the high-molecular-mass fraction of mucus; and white bars indicate accumulation of reducing group equivalents in total mucus. (A) Growth of S. marcescens PDL100, a pathogen associated with white pox disease of A. palmata. PDL100 reached ∼2 × 108 CFU ml−1 on all mucus treatments, which was significantly higher than the densities of all of the other strains (Tukey's HSD P value of <0.0025). (B) Growth of S. marcescens EL31 isolated from wastewater in the Florida Keys. (C) Growth of S. marcescens MG1 isolated from a rotten cucumber. (D) Growth of H. meridiana 33E7 isolated from A. palmata mucus. (E) Growth of P. mandapamensis 33C12 isolated from A. palmata mucus. (F) Growth of E. coli DH5α on mucus and its components. Graphs show the results of a representative experiment; error bars indicate standard errors from three parallel independent cultures.
Bacterial growth on size-fractionated mucus of A. palmata.
To test whether the mucus sample contained a significant proportion of low-molecular-mass components that could support growth of generalists, it was dialyzed to separate low-molecular-mass (<15 kDa) and high-molecular-mass (>15 kDa) fractions. All tested strains of S. marcescens reached the final densities of 2 × 108 to 6 × 108 CFU ml−1 when grown on the low-molecular-mass fraction of A. palmata mucus (Fig. 1). H. meridiana, P. mandapamensis, and E. coli DH5α reached 1 × 108 to 1.5 × 108 CFU ml−1. This is consistent with the hypothesis that low-molecular-mass compounds in coral mucus are easily metabolizable by most bacteria.
We hypothesized that the high-molecular-mass fraction of mucus consists of polymers that are not readily available to all microbes. As shown in Fig. 1A, S. marcescens PDL100 reached the final density of 2 × 108 CFU ml−1, which is approximately seven times higher (Tukey's HSD P value of <0.0025) than the final population densities of the two other S. marcescens isolates (MG1, isolated from a rotten cucumber, and EL31, isolated from Florida Keys wastewater). Similarly to the S. marcescens strains MG1 and EL31, E. coli DH5α reached 3 × 107 CFU ml−1. H. meridiana 33E7 and P. mandapamensis 33C12 grew only to 1 × 106 to 6 × 106 CFU ml−1, approximately 50 to 100 times less than S. marcescens PDL100 (Fig. 1D and E). In general, the extent to which coral commensals utilized mucus is consistent with the studies of the degradation of the high-molecular-mass component (6 to 8 kDa) of coral mucus, which demonstrated that bacterial populations in situ reached ∼106 CFU ml−1 when grown on Acropora coral mucus in size exclusion cages (28).
Patterns of mucus utilization by bacteria.
BCA assays were carried out for each mucus fraction (low-molecular-mass, high-molecular-mass, and total mucus) after bacterial growth for 96 h. The BCA assay would determine both the protein content and the presence of reducing ends of sugars (29). As expected, the fractions of mucus differed in the numbers of the BCA-reactive species prior to incubation with bacteria; the number of BCA-reactive species was highest in the low-molecular-mass fraction of mucus (77 equivalents in low-molecular-mass fractions, 13 equivalents in high-molecular-mass fractions, and 44 equivalents in the crude preparation of “total mucus”). This supports the observations that in addition to the sulfated glycoprotein polymer, mucus samples contain a significant amount of low-molecular-mass compounds (16, 17).
Upon incubation with bacteria, the number of BCA-reactive species in mucus and its low-molecular-mass fraction decreased two- to threefold in the spent cultures of all S. marcescens, E. coli, and P. mandapamensis isolates. The number of BCA-reactive species also decreased (although to a more modest degree) after growth of H. meridiana. These results indicate that the components of the low-molecular-mass fraction were generally mineralized in similar fashions by the different bacteria used in this experiment. The reduction in the number of BCA-reactive species in the low-molecular-mass fraction may suggest that carbohydrate mono- and oligomers as well as short peptides that may be present in the low-molecular-mass fraction were mineralized.
As it relates to carbohydrate-degrading activities, an increase in the number of BCA-reactive species in the sample with the high-molecular-mass fraction of mucus could be indicative of an endoglycanase activity, while exoglycanases could be identified by no significant change in the number of reducing group equivalents (assuming that each cleaved monomer is then metabolized). The number of BCA-reactive species did not change significantly in the spent cultures of S. marcescens PDL100, MG1, or EL31 or E. coli DH5a grown on the high-molecular-mass fraction of mucus as the sole carbon source (Fig. 1). The number of BCA-reactive species was strongly reduced after the two coral isolates were grown on high-molecular-mass mucus. The results of BCA assays of the spent high-molecular-mass fraction suggest that Serratia isolates break down mucus through the action of exoglycanases, while the two native coral isolates may be preferentially utilizing the protein backbone of the mucus glycoprotein or lipids found in coral mucus (4, 17).
S. marcescens PDL100 dominates mucus microcosms in vitro.
To test whether the growth to higher final cell numbers on coral mucus corresponds with the ability to outcompete other bacteria, S. marcescens PDL100 was coinoculated with either H. meridiana 33E7 or P. mandapamensis 33C12 into mucus in seawater. In cocultures on two different batches of A. palmata mucus samples (both collected in the winter of 2007), after 96 h, S. marcescens PDL100 outgrew P. mandapamensis at a ratio of 8 to 2 (Fig. 2). In cocultures with H. meridiana, the coral commensal was not detectable after 96 h (Fig. 2). In monocultures on mucus, S. marcescens PDL100 reached 4.4 × 108 to 6.5 × 108 CFU ml−1, P. mandapamensis reached 1.8 × 107 to 2.4 × 107 CFU ml−1, and H. meridiana reached 3.5 × 107 to 4.4 × 107 CFU ml−1, generally consistent with the growth on another batch of mucus samples (Fig. 1). These results suggest that in the absence of other biotic factors, this opportunistic coral pathogen can outgrow representative coral microbiota.
FIG. 2.
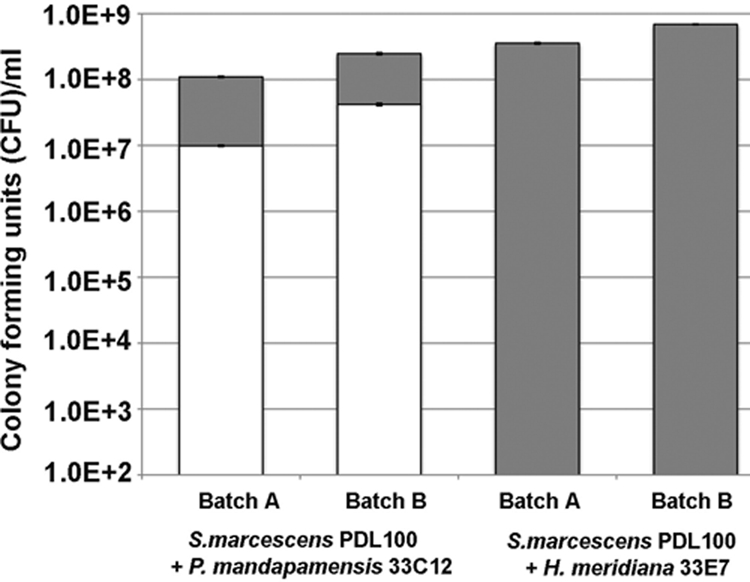
Growth of S. marcescens PDL100 and coral commensals in an in vitro coculture on mucus. Starved washed cultures of S. marcescens PDL100 were coinoculated into sterilized mucus with either P. mandapamensis 33C12 or H. meridiana 33E7. Two batches of mucus samples harvested from different A. palmata colonies in the winter of 2007 were used in these assays. Initial inocula were ∼100 CFU ml−1 for all bacteria. After 96 h and 7 days, aliquots were dilution plated onto GASW and LB agar and then patched onto xylose lysine deoxycholate agar (Oxoid) to distinguish between S. marcescens and coral commensals. Growth of the coral commensals is indicated by white bars; growth of S. marcescens PDL100 is indicated by gray bars. Error bars indicate standard errors of three technical replications.
Growth and utilization of mucus from a nonhost coral by the Serratia isolates.
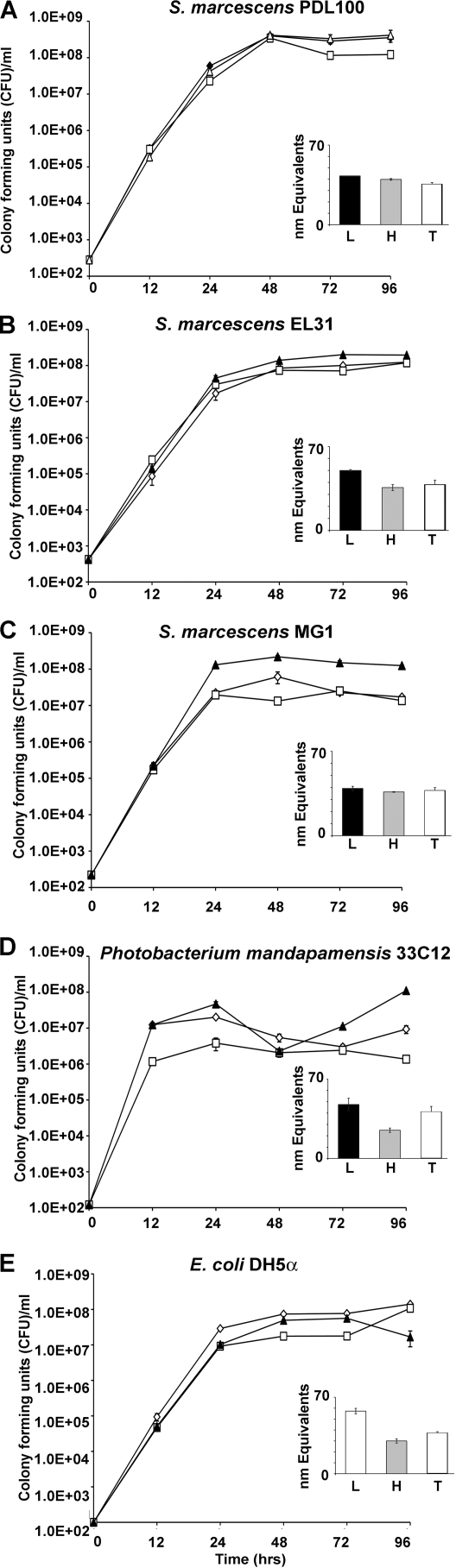
To test whether S. marcescens PDL100 would also grow better on mucus from a nonhost coral, Montastraea faveolata, experiments similar to those shown in Fig. 1 were carried out. All Serratia strains and a coral commensal tested reached 108 CFU ml−1 when grown on a “total mucus” sample of M. faveolata, significantly higher than the density of E. coli DH5α (Tukey's HSD P value of <0.0025). As shown in Fig. 3, on the high-molecular-mass fraction of the M. faveolata mucus, E. coli DH5α and S. marcescens PDL100 and EL31 reached the same final population density. Growth rates of both PDL100 and EL31 were similar on all mucus components, reaching 108 CFU ml−1, whereas MG1 reached densities that were 7- to 10-fold lower on the low- and high-molecular-mass fractions (Fig. 3). The A. palmata commensal, P. mandapamensis 33C12, reached densities approximately 10- to 100-fold lower than those of PDL100 on low- and high-molecular-mass M. faveolata mucus components.
FIG. 3.
Bacterial growth on mucus of Montastraea faveolata. Isolates of Serratia marcescens, P. mandapamensis 33C12, and E. coli DH5α were grown on mucus from a nonhost coral, M. faveolata. Bacteria were seeded into seawater containing 60 mg ml−1 (wt/vol) total mucus (closed triangles) or high-molecular-mass (>15 kDa; open squares) or low-molecular-mass (<15 kDa; open diamonds) fractions. Growth was monitored by dilution plating. (Insets) The extent to which the bacteria hydrolyzed substrates in mucus was examined using a BCA assay as described in Materials and Methods. White bars indicate accumulation of reducing group equivalents in the low-molecular-mass fraction of mucus; gray bars indicate accumulation of reducing group equivalents in the high-molecular-mass fraction of mucus; and black bars indicate accumulation of reducing group equivalents in total mucus. (A) Growth of S. marcescens PDL100. (B) Growth of S. marcescens EL31. (C) Growth of S. marcescens MG1. (D) Growth of P. mandapamensis 33C12. (E) Growth of E. coli DH5α. Graphs show the results from a representative experiment; error bars indicate standard errors from three parallel independent cultures.
BCA assays determined whether there were differences in the patterns of substrate degradation during growth on M. faveolata mucus. Similarly to results with A. palmata mucus, the amount of BCA-active species was large in the low-molecular-mass fraction of mucus (43 equivalents in low-molecular-mass, 22 equivalents in high-molecular-mass, and 48 equivalents in crude “total” mucus). Serratia isolates had similar abilities to generate the reducing group equivalents when grown on the low-molecular-mass fraction; however, the number of BCA-reactive species was not decreased as observed with A. palmata mucus, suggesting that this fraction contained oligomers that were degraded by exoglycanases. There was an increase in the number of BCA-reactive species for all bacteria grown on the high-molecular-mass fraction of M. faveolata mucus, which could be indicative of an endoglycanase activity.
S. marcescens PDL100 was similar to other Serratia strains and E. coli in its growth on mucus from a nonhost coral, M. faveolata. This observation suggests that the ability to cause disease in acroporid corals may be due, at least in part, to the ability of the strain PDL100 to build to higher population numbers within the mucus surface layer of its acroporid host.
Nutrient utilization profiling.
To test the hypothesis that differences in the abilities to grow on A. palmata coral mucus were due to the general metabolic capabilities of the strains, their abilities to utilize carbon and nitrogen sources were compared using Biolog EcoPlates. The EcoPlate contains N-acetyl-d-glucosamine, l-asparagine, l-serine, and l-threonine—all known to occur in the mucus of acroporid corals (4, 17). To broaden the range of tested strains, additional environmental isolates of S. marcescens collected from various locations in the Florida Keys and from the American Type Culture Collection and an additional coral commensal were also included in this test (Table 1; also see Table S2 in the supplemental material).
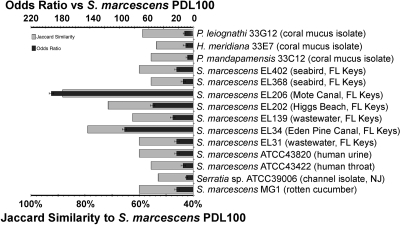
All tested isolates respired when incubated on N-acetyl-d-glucosamine and l-asparagine. All strains (with the exception of Serratia sp. strain ATCC 39006) respired when incubated on l-serine and l-threonine (see Table S2 in the supplemental material), the two amino acids that make up the protein backbone of the mucus glycoprotein in acroporids. Consistent with the earlier report (22), S. marcescens PDL100 utilized acetate, citrate, d-fructose, d-glucose, maltose, and d-sorbitol and was unable to ferment α-keto butyrate, cellobiose, and α-d-lactose. The substrate utilization profile of PDL100 corresponded most strongly with those of the two Florida Keys canal water isolates, EL206 and EL34, and a beach water isolate, EL202 (Fig. 4; also see Table S2 in the supplemental material). The profile of PDL100 correlated the least with those of the three coral commensals and a Cheesequake Salt Marsh (NJ) channel isolate, Serratia sp. strain ATCC 39006 (Fig. 4).
FIG. 4.
Metrics of correspondence between Biolog EcoPlate assays for S. marcescens PDL100, other S. marcescens isolates, and three coral commensals. The results of the Biolog assay were converted into binary data (see Table S2 in the supplemental material), and values for the strain PDL100 were compared with those for other S. marcescens strains, using the Jaccard similarity coefficient and the odds ratio. Isolates with similar metabolic capabilities have high Jaccard similarity and odds ratio. Statistical analyses were carried out using Statistica version 8.0 (StatSoft, Inc., Tulsa, OK) as described in Materials and Methods.
The rate of AWCD in Biolog Ecoplates (see Fig. S1 in the supplemental material) was used to test whether differences in the general metabolic rates could explain higher growth rates and higher population densities reached by S. marcescens PDL100 on the mucus of A. palmata. As shown in Fig. S1 in the supplemental material, the rate and the final AWCD were the highest in S. marcescens EL202. The rates and the final AWCDs were similar in the white pox isolate S. marcescens PDL100 and in MG1. These results further support the conclusion that the ability of S. marcescens PDL100 to more efficiently utilize mucus from its host coral cannot be simply explained by generally higher metabolic rates in this pathogen or by the general ability of this strain to utilize a more diverse set of carbon and nitrogen sources.
Enzyme induction in response to growth on coral mucus.
Because the ability of S. marcescens PDL100 to grow on A. palmata mucus to higher final population densities was not explained by either its metabolic rates or its general ability to grow on generic carbon and nitrogen sources, the subsequent experiments focused on identifying the potentially specific mechanisms underlying interactions of this pathogen with mucus from its coral host. An earlier study demonstrated that the in situ degradation of coral mucus involved activities of as-yet-uncharacterized proteases, esterases, and glycosidases (28). A hypothesis that S. marcescens PDL100 relies on a different suite of mucus-degrading enzymatic activities or that these activities are stronger than those of other bacteria was tested. This study focused on the glycosidases, because carbohydrates are a major part of mucus and account for approximately 60% of the mucus glycoprotein in acroporid corals (17).
Incubation with coral mucus induced the broadest range of enzymatic activities in S. marcescens MG1 and Serratia sp. strain ATCC 39006; their enzymatic activities were also the strongest (see Table S1 in the supplemental material). Even though only few activities in S. marcescens PDL100 were induced after incubation with coral mucus (see Table S1 in the supplemental material), this strain appeared to have strong constitutive α-d-glucopyranosidase and N-acetyl-β-d-galactosaminidase activities that were 2 to 10 times higher than in other isolates. In S. marcescens PDL100, a 2-hour incubation with coral mucus repressed either the accumulation or the function of α-d-galactopyranosidase, α-d-xylopyranosidase, β-d-fucopyranosidase, β-d-glucopyranosidase, and β-l-arabinopyranosidase. Some of the same enzymes were also downregulated in other isolates. We do not yet know whether the reduced activity of these enzymes was due to the absence of the potential substrates in mucus or due to the presence of substances that may inhibit induction, accumulation, or activities of these enzymes.
There were differences in temporal pattern of enzymatic induction; different enzymatic activities were induced after 2 and 18 hours of incubation on coral mucus. As shown in Table S1 in the supplemental material, after 2 hours of incubation on mucus, β-l-arabinofuranosidase was strongly induced in seven S. marcescens isolates, β-d-fucopyranosidase was upregulated in five S. marcescens isolates, and α-l-fucopyranosidase was induced in four S. marcescens isolates and in the coral commensal H. meridiana; xylopyranosidases were induced in four S. marcescens isolates and in the coral commensal P. mandapamensis. These same enzymatic activities were strongly decreased after 18 hours of incubation on mucus. β-d-Galactopyranosidases and β-d-glucopyranosidases were induced in three S. marcescens isolates after 18 h of incubation on mucus. This extended (18 h) incubation of coral commensals on mucus led to the induction of d-galactopyranosidases, β-d-glucopyranosidase, arabinofuranosidases, β-d-fucopyranosidase, β-d-xylopyranosidase, and N-acetyl-galactopyranosidase (see Table S1 in the supplemental material).
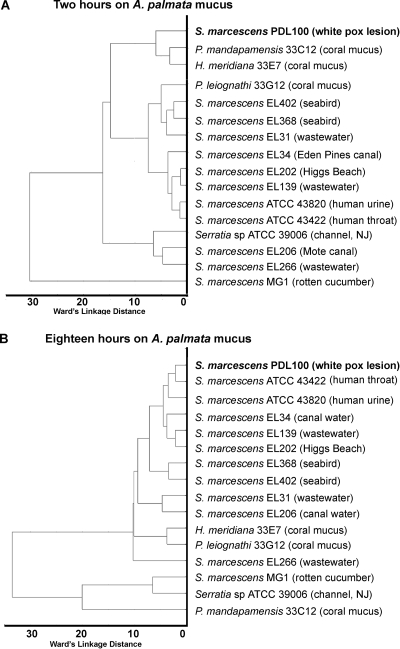
To further compare patterns of enzymatic induction during growth on coral mucus, the data in Table S1 in the supplemental material were analyzed using Ward's linkage as the joining rule and a Euclidean distance metric, as described in Materials and Methods. As shown in Fig. 5, during the initial 2-h incubation on the mucus of A. palmata, S. marcescens PDL100 was most similar to the two other A. palmata isolates, P. mandapamensis 33C12 and H. meridiana 33E7 (Fig. 5A), with the rotten-cucumber isolate (S. marcescens MG1) being an extreme outlier. After 18 h of incubation on coral mucus, the pattern of enzymatic induction in S. marcescens PDL100 was most similar to those of the human pathogens S. marcescens ATCC 43422 and ATCC 43820 (Fig. 5B). In its interactions with coral mucus, S. marcescens PDL100 was distinct from other S. marcescens isolates isolated from throughout the Florida Keys from wastewaters, canals, beaches, or seabirds.
FIG. 5.
Clustering of strains based on enzymatic activities induced during growth on coral mucus. The hierarchical tree plot was constructed using Ward's linkage as the joining rule and Euclidean distance metric. (A) The hierarchical tree plot of enzymatic activities induced in S. marcescens isolates and coral commensals after 2 hours of incubation on the total mucus of A. palmata. (B) The hierarchical tree plot of enzymatic activities induced in S. marcescens isolates and coral commensals after 18 hours of incubation on the mucus of A. palmata. Statistical analyses were carried out using Statistica version 8.0 (StatSoft, Inc., Tulsa, OK) as described in Materials and Methods.
Conclusions. (i) Differences in mucus utilization reveal an ecophysiological basis of white pox.
Of the strains tested in this study, S. marcescens PDL100 grew to the highest final population densities on the mucus of A. palmata and on its low- and high-molecular-mass components (Fig. 1). The pattern of mucus-induced enzymatic activities in PDL100 was distinct from those of other environmental Serratia isolates (Fig. 5). A comparison of the totality of mucus-induced enzymes in S. marcescens PDL100 with those induced in other bacteria suggests that during early stages of mucus colonization, the white pox pathogen was most similar to coral commensals. These differences in utilization of the available carbon sources between the pathogens and commensals occupying the same ecological niche have also been observed in pathogenic and commensal E. coli isolates (5).
(ii) Patterns of mucus utilization by bacteria shed light on mucus composition.
The compositions of mucus differ among coral species (4, 17, 19), although they appear to be consistent in corals of the same species harvested in different locations and at different depths (11). The structures of the polymers from A. palmata mucus are not yet known. In the glycoproteins of A. formosa and the soft coral P. americana, β-arabinose was the terminal residue. If the composition of A. palmata mucus is similar, it is reasonable to expect arabinosidases to be strongly induced during the initial stages of growth on mucus. Consistent with this hypothesis, β-arabinosidases were induced in 9 out of 12 Serratia isolates during the first 2 hours of exposure to mucus. In the coral commensal bacteria, arabinosidases were induced after 18 h of incubation on mucus (see Table S1 in the supplemental material).
It is becoming increasingly clear that nutrients and signals found in the coral surface mucopolysaccharide layer dictate, at least in part, the structure and the composition of the associated microbiota (23, 25, 26). Elucidation of the differences in the strategies for coral commensals and opportunistic pathogens will help define the mechanisms of coral disease and may lead to research on defining potential approaches for managing or treating coral diseases.
Supplementary Material
Acknowledgments
This research was supported by the Florida Sea Grant program (grant no. NA060AR4170014 to M.T. and K.B.R.), funding from the Protect Our Reefs Florida specialty license plate program (to K.B.R., M.T., and C.J.K.), a Charles A. and Anne Morrow Lindbergh Foundation grant (to K.B.R. and M.T.), National Geographic Society grant no. 8207-07 (to M.T., K.B.R., and C.J.K.), Florida Department of Environmental Protection grant no. SP626 (to K.P.S. and E.K.L.), and a seed grant from the University of Florida School of Natural Resources and Environment (UF SNRE). C.J.K. is a UF SNRE Alumni Fellow. This research is a part of CRIS project FLA-SWS-04591 of the Florida Agricultural Experiment Station.
We are grateful to W. Dietz Bauer for critical comments on the manuscript.
Footnotes
Published ahead of print on 24 April 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agresti, A. 1999. On logit confidence intervals for the odds ratio with small samples. Biometrics 55:597-602. [DOI] [PubMed] [Google Scholar]
- 2.Brown, B. E., and J. C. Bythell. 2005. Perspectives on mucus secretion in reef corals. Mar. Ecol. Prog. Ser. 296:291-309. [Google Scholar]
- 3.D'Aloia, M. A., T. A. Bailey, J. H. Samour, J. Naldo, and J. C. Howlett. 1996. Bacterial flora of captive houbara (Chlamydotis undulate), kori (Ardeotis kori) and rufous-crested (Eupodotis ruficrista) bustards. Avian Pathol. 3:459-468. [DOI] [PubMed] [Google Scholar]
- 4.Ducklow, H. W., and R. Mitchell. 1979. Composition of mucus released by coral reef coelenterates. Limnol. Oceanogr. 21:706-714. [Google Scholar]
- 5.Fabich, A. J., S. A. Jones, F. Z. Chowdhury, A. Cernosek, A. Anderson, D. Smalley, J. W. McHargue, G. A. Hightower, J. T. Smith, S. M. Autieri, M. P. Leatham, J. J. Lins, R. L. Allen, D. C. Laux, P. S. Cohen, and T. Conway. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatehouse, H. S., S. D. Marshall, R. M. Simpson, L. N. Gatehouse, T. A. Jackson, and J. T. Christeller. 2008. Serratia entomophila inoculation causes a defect in exocytosis in Costelytra zealandica larvae. Insect Mol. Biol. 17:375-385. [DOI] [PubMed] [Google Scholar]
- 7.Grimont, F., and P. A. D. Grimont. 2006. The genus Serratia, p. 219-244. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrant (ed.), The prokaryotes, vol. 6. Springer, New York, NY. [Google Scholar]
- 8.Indiragandhi, P., R. Anandham, M. Madhaiyan, S. Poonguzhali, G. H. Kim, V. S. Saravanan, and T. Sa. 2007. Cultivable bacteria associated with larval gut of prothiofos-resistant, prothiofos-susceptible and field-caught populations of diamondback moth, Plutella xylostella, and their potential for antagonism towards entomopathogenic fungi and host insect nutrition. J. Appl. Microbiol. 103:2664-2675. [DOI] [PubMed] [Google Scholar]
- 9.Iverson, K. L., M. C. Bromel, A. W. Anderson, and T. P. Freeman. 1984. Bacterial symbionts in the sugar beet root maggot, Tetanops myopaeformis (von Röder). Appl. Environ. Microbiol. 47:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, T. A., D. G. Boucias, and J. O. Thaler. 2001. Pathobiology of amber disease, caused by Serratia spp., in the New Zealand grass grub, Costelytra zealandica. J. Invertebr. Pathol. 78:232-243. [DOI] [PubMed] [Google Scholar]
- 11.Klaus, J. S., I. Janse, J. M. Heikoop, R. A. Sanford, and B. W. Fouke. 2007. Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environ. Microbiol. 9:1291-1305. [DOI] [PubMed] [Google Scholar]
- 12.Knee, E. M., F. C. Gong, M. Gao, M. Teplitski, A. R. Jones, A. Foxworthy, A. J. Mort, and W. D. Bauer. 2001. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol. Plant-Microbe Interact. 14:775-784. [DOI] [PubMed] [Google Scholar]
- 13.Krediet, C., K. B. Ritchie, and M. Teplitski. 5 May 2009. Catabolite control of coral mucus degradation. Dis. Aquat. Org. doi: 10.3354/dao02084. [DOI] [PubMed]
- 14.Kurz, C. L., S. Chauvet, E. Andres, M. Aurouze, I. Vallet, G. P. Michel, M. Uh, J. Celli, A. Filloux, S. De Bentzmann, I. Steinmetz, J. A. Hoffmann, B. B. Finlay, J. P. Gorvel, D. Ferrandon, and J. J. Ewbank. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurz, C. L., and J. J. Ewbank. 2000. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 8:142-144. [DOI] [PubMed] [Google Scholar]
- 16.Meikle, P., G. N. Richards, and D. Yellowlees. 1987. Structural determination of the oligosaccharide side chains from a glycoprotein isolated from the mucus of the coral Acropora formosa. J. Biol. Chem. 262:16941-16947. [PubMed] [Google Scholar]
- 17.Meikle, P., G. N. Richards, and D. Yellowlees. 1988. Structural investigations on the mucus from six species of coral. Mar. Biol. 99:187-193. [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 19.Molchanova, V. I., R. G. Ovodova, Y. S. Ovodov, Y. N. Elkin, and V. Fernandezsantana. 1985. Studies of the polysaccharide moiety of corallan, a glycoprotein from Pseudopterogorgia americana. Carbohydr. Res. 141:289-293. [Google Scholar]
- 20.Naldo, J. L., C. D. Silvanose, J. H. Samour, and T. A. Bailey. 1998. Developmental intestinal aerobic microflora in the kori bustard (Ardeotis kori). Avian Pathol. 27:359-365. [DOI] [PubMed] [Google Scholar]
- 21.Nehme, N. T., S. Liegeois, B. Kele, P. Giammarinaro, E. Pradel, J. A. Hoffmann, J. J. Ewbank, and D. Ferrandon. 2007. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson, K. L., J. W. Porter, K. B. Ritchie, S. W. Polson, E. Mueller, E. C. Peters, D. L. Santavy, and G. W. Smith. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl. Acad. Sci. USA 99:8725-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie, K. 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 322:1-14. [Google Scholar]
- 24.Ritchie, K. B., and G. W. Smith. 2004. Microbial communities of coral surface mucopolysaccharide layers, p. 259-264. In E. Rosenberg and Y. Loya (ed.), Coral health and diseases. Springer-Verlag, New York, NY.
- 25.Rosenberg, E., O. Koren, L. Reshef, R. Efrony, and I. Zilber-Rosenberg. 2007. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5:355-362. [DOI] [PubMed] [Google Scholar]
- 26.Sharon, G., and E. Rosenberg. 2008. Bacterial growth on coral mucus. Curr. Microbiol. 56:481-488. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland, K. P., and K. B. Ritchie. 2004. White pox disease of the Caribbean elkhorn coral, Acropora palmata, p. 289-300. In E. Rosenberg and Y. Loya (ed.), Coral health and diseases. Springer-Verlag, New York, NY.
- 28.Vacelet, E., and B. A. Thomassin. 1991. Microbial utilization of coral mucus in long-term in situ incubation over a coral reef. Hydrobiologia 211:19-32. [Google Scholar]
- 29.Waffenschmidt, S., and L. Jaenicke. 1987. Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal. Biochem. 165:337-340. [DOI] [PubMed] [Google Scholar]
- 30.Ward, J. H., Jr. 1963. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58:236-244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.