Abstract
The genome of Burkholderia thailandensis codes for several LuxR-LuxI quorum-sensing systems. We used B. thailandensis quorum-sensing deletion mutants and recombinant Escherichia coli to determine the nature of the signals produced by one of the systems, BtaR2-BtaI2, and to show that this system controls genes required for the synthesis of an antibiotic. BtaI2 is an acyl-homoserine lactone (acyl-HSL) synthase that produces two hydroxylated acyl-HSLs, N-3-hydroxy-decanoyl-HSL (3OHC10-HSL) and N-3-hydroxy-octanoyl-HSL (3OHC8-HSL). The btaI2 gene is positively regulated by BtaR2 in response to either 3OHC10-HSL or 3OHC8-HSL. The btaR2-btaI2 genes are located within clusters of genes with annotations that suggest they are involved in the synthesis of polyketide or peptide antibiotics. Stationary-phase cultures of wild-type B. thailandensis, but not a btaR2 mutant or a strain deficient in acyl-HSL synthesis, produced an antibiotic effective against gram-positive bacteria. Two of the putative antibiotic synthesis gene clusters require BtaR2 and either 3OHC10-HSL or 3OHC8-HSL for activation. This represents another example where antibiotic synthesis is controlled by quorum sensing, and it has implications for the evolutionary divergence of B. thailandensis and its close relatives Burkholderia pseudomallei and Burkholderia mallei.
Burkholderia thailandensis, Burkholderia pseudomallei, and Burkholderia mallei are closely related betaproteobacteria. These three species show extensive genome sequence identity (37, 72). B. mallei is an obligate mammalian pathogen that infects solipeds (horses, mules, and donkeys) and can also cause human infections (66, 69). B. pseudomallei is an emerging human pathogen found in soil and stagnant water, and it infects rice farmers in Southeast Asia and people of Northern Australia (8, 70). Once thought to be a nonpathogenic saprophyte endemic to the water and soil environments of central and northeastern Thailand (4), B. thailandensis has recently been found in the central United States and Australia (25, 34). Sufficiently high doses of B. thailandensis can cause mammalian infections (4, 11, 56).
We have been interested in acyl-homoserine lactone (acyl-HSL) quorum sensing among the B. mallei-B. pseudomallei-B. thailandensis group because quorum sensing has been implicated in the virulence of B. mallei and B. pseudomallei and because each member of this group has multiple quorum-sensing systems (60-62). Quorum sensing involves production of and response to a self-produced extracellular signal that allows individuals to monitor the group population density (3, 21, 67). Proteobacteria often use acyl-HSL signals for quorum sensing. The signals are produced by members of the LuxI family of acyl-HSL synthases. The proteins that respond to acyl-HSLs are members of the LuxR family of transcription factors (22). Dozens of species possess acyl-HSL quorum-sensing systems. The genes coding for LuxR and LuxI homologs are often adjacent on a chromosome and are considered cognate pairs. The nature of the acyl side chain varies depending on the specific LuxI homolog (22, 67, 68). A LuxR homolog responds best to the acyl-HSL produced by its cognate LuxI homolog. The genes regulated by acyl-HSL quorum sensing differ depending on the species under investigation (for a review, see reference 68).
Many species of Burkholderia possess one or multiple acyl-HSL quorum-sensing systems (16, 64). B. mallei, B. pseudomallei, and B. thailandensis each possess multiple luxR and luxI homologs. These are called bma genes in the case of B. mallei, bps genes in the case of B. pseudomallei, and bta genes in the case of B. thailandensis. The three species have highly conserved R1-I1 genes, R3-I3 genes, and two orphan luxR homologs (luxR homologs without adjacent luxI homologs) called R4 and R5. Both B. pseudomallei and B. thailandensis, the two species that have a known environmental lifestyle, have an additional quorum-sensing gene pair called R2-I2, which has been lost in B. mallei (45). There is a high level of interspecies amino acid sequence identity among the nonorphan R-I polypeptide pairs found in B. mallei, B. pseudomallei, and B. thailandensis. The cognate signal for the R1-I1 systems of each species is octanoyl-HSL (C8-HSL) (15, 57; J. R. Chandler and E. P. Greenberg, unpublished data). The R1-I1 system in B. pseudomallei has been reported to control siderophore production, phospholipase C production, and the oxidative stress response (41, 57). The R3-I3 pair in B. mallei, called BmaR3-BmaI3, produces and responds to N-3-hydroxy-octanoyl-HSL (3OHC8-HSL) (14), and B. thailandensis BtaI3 also produces 3OHC8-HSL (Chandler and Greenberg, unpublished). To date, the R2-I2 genes conserved in B. pseudomallei and B. thailandensis have received little attention.
Here, we report that the R2-I2 gene pairs of B. pseudomallei and B. thailandensis are embedded in large clusters of genes with annotations suggesting an involvement in antibiotic synthesis. We show that in B. thailandensis, BtaI2 primarily produces N-3-hydroxy-decanoyl-HSL (3OHC10-HSL) and lesser amounts of 3OHC8-HSL. BtaR2 is a receptor that responds to the BtaI2-produced acyl-HSLs 3OHC10-HSL and 3OHC8-HSL. We also show that B. thailandensis produces an antibiotic that is active against gram-positive bacteria and that the BtaR2-BtaI2 quorum-sensing system is required for the production of the antibiotic.
MATERIALS AND METHODS
Bacterial culture conditions.
The bacterial strains used in this study are listed in Table 1. B. thailandensis E264 and E264 derivatives were used throughout these investigations. Unless otherwise indicated, all bacteria except Streptococcus pyogenes were grown in Luria-Bertani (LB) broth. S. pyogenes was grown on Todd-Hewitt broth supplemented with 0.2% (wt/vol) yeast extract or on brain heart infusion medium. The following antibiotics were used at the indicated concentrations (per ml) for marker selection and for maintaining plasmids: 100 μg ampicillin, 15 μg gentamicin, 300 μg trimethoprim, and 25 μg kanamycin for E. coli and 100 μg trimethoprim and 150 μg kanamycin for B. thailandensis. To induce arabinose promoter-controlled gene expression in E. coli, we used 0.2% (wt/vol) l-arabinose. We used 0.2% (wt/vol) l-rhamnose to activate the rhamnose-responsive promoter in B. thailandensis. All bacteria were grown at 37°C.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant properties | Reference or source |
|---|---|---|
| B. thailandensis | ||
| E264 | Wild-type strain | 4 |
| JBT102 | btaI2 mutant of E264 | Chandler and Greenberg, unpublished |
| JBT105 | btaI1, btaI3 double mutant of E264 | Chandler and Greenberg, unpublished |
| JBT108 | btaR2 mutant of E264 | Chandler and Greenberg, unpublished |
| JBT112 | btaI1, btaI2, btaI3 triple mutant of E264 | Chandler and Greenberg, unpublished |
| BD909 | BTH_II1233::Km insertion mutant of E264 | This study |
| BD20 | BTH_II1233 mutant of E264 | This study |
| E. coli | ||
| DH5α | φ80lacZΔM15 Δ(lacZYA-argF)U169 hsdR17(rk−, mk−) recA1 endA1 supE44 thi-1 gyrA relA1 λ− | Invitrogen |
| MG4 | F− λ−ilvG rfb-50 rph-1 recA Δ(argF-lacIPOZYA)205 | 48 |
| SM10 | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Km λpir | 55 |
| B. subtilis | ||
| 168 | Wild-type strain; trpC2 | 5 |
| 3610 | Wild-type strain; ϕ3T | 9 |
| S. pyogenes | ||
| MGAS50055 | speA2; cerebrospinal fluid isolate | 58 |
| S. aureus | ||
| MN8 | tstH+; clinical isolate from nonmenstrual toxic shock syndrome case | 51 |
| COL | Methicillin-resistant laboratory strain | 24 |
| P. aeruginosa | ||
| PAO1 | Wild-type strain | 28 |
Plasmids and recombinant DNA procedures.
The plasmids used in this study are listed in Table 2. Oligonucleotides were purchased from Integrated DNA Technologies, Coralville, IA (the sequences are available upon request). B. thailandensis genomic DNA was purified by using a Gentra Puregene Cell Plus isolation kit (Qiagen, Valencia, CA). PCR fragments were amplified with the Failsafe PCR System and buffer J or K premix solution (Epicentre Biotechnologies, Madison, WI) or with an Expand Long Template kit (Roche Diagnostics, Pleasanton, CA). B. thailandensis genomic DNA was used as the template for all PCRs. Plasmid DNA was isolated by using a Qiaprep mini-spin kit, and the PCR fragments used for cloning were purified with a Qiaquick PCR gel extraction kit (Qiagen, Valencia, CA).
TABLE 2.
Plasmids
| Plasmids | Relevant propertiesa | Reference or source |
|---|---|---|
| pJN105 | araC-ParaBAD cloned into pBBR1MCS-5; Gmr | 44 |
| pQF50 | Broad-host-range lacZ fusion vector; Apr | 18 |
| pSCrhaB2 | rhaRS-PrhaBAD, dhfr cloned into pBBR1MCS; Tpr | 6 |
| pJRC115 | Mobilizable suicide vector; Tpr | Chandler and Greenberg, unpublished |
| pGEM-T Easy;amrRAB-oprA;;FRT-Km | pGEM-T Easy with Δ(amrRAB-oprA)::FRT-PEM7-kam-FRT fragment; Apr Kmr | Mima and Schweizer, unpublished |
| pI2P50 | pQF50 containing a 244-bp fragment of the btaI2 promoter extending from position +6 with respect to the translation start site to −238; Apr | This study |
| pJNR2 | pJN105 containing the btaR2 gene; Gmr | This study |
| pSCR2 | pSCrhaB2 containing the btaR2 gene; Tpr | This study |
| pQF1233 | pQF50 containing a 422-bp fragment of the BTH_II1233 promoter extending from position +13 with respect to the translation start site to −409; Apr | This study |
| pLARA1233 | pJRC115 with ΔBTH_II1233 extending from +13 with respect to the translational start site to + 060; Tpr | This study |
| pKM1233 | FRT-PEM7-kam-FRT fragment from pGEM-T Easy;amrRAB-oprA;;FRT-Km cloned into pLARA1233; Apr Kmr | This study |
| pINF1233 | pJRC115 with ΔBTH_II1233 extending from +13 with respect to the translational start site to +4313; Tpr | This study |
ParaBAD, PrhaBAD, PEM7, and dhfr are the arabinose-inducible promoter, the rhamnose-inducible promoter, the synthetic bacterial EM7 promoter, and the dihydrofolate reductase gene.
To create the BtaR2 expression vector pJNR2, the open reading frame of btaR2 (BTH_II1231) from bp −18 to +705 relative to the predicted translational start site was PCR amplified. This procedure introduced PstI and SacI restriction sites at the ends of the btaR2 fragment. The btaR2 PCR product was ligated to PstI-SacI-digested pJN105, which carries the l-arabinose-inducible promoter (PBAD) (44). To generate the lacZ transcriptional fusion constructs pI2P50 and pQF1233, B. thailandensis DNA upstream of btaI2 (BTH_II1227) and BTH_II1233 was PCR amplified by using primer pairs that incorporated NcoI and HindIII restriction sites at the ends of the PCR products. These products were cloned into NcoI-HindIII-digested pQF50 (18). The btaI2-lacZ fusion extends from +6 to −238 in relation to the predicted translational start site. BTH_II1233-lacZ extends from +13 to −409 in reference to the predicted translational start site.
To regulate expression of BtaR2 in B. thailandensis, it was necessary to place btaR2 under the control of an inducible promoter other than the l-arabinose promoter. This is because B. thailandensis is known to metabolize l-arabinose (71). We chose the rhamnose-inducible promoter because of its utility in other Burkholderia species (6). We constructed the BtaR2 expression vector pSCR2 by incorporating a PCR-amplified btaR2-containing DNA fragment into NcoI-HindIII-digested pSCrhaB2 (6).
The suicide vector pJRC115 was used to deliver modified genes into the B. thailandensis genome by allelic replacement. pJRC115 and all other constructs used to create the B. thailandensis mutant strains JBT102, JBT105, JBT108, and JBT112 will be described elsewhere (Chandler and Greenberg, unpublished). The method used for mutant construction in B. thailandensis is similar to that described recently (2). To create pLARA1233, we used two-step PCR. The first step involved synthesizing DNA fragments of approximately 1 kb that were homologous to sequences flanking the intended site of deletion in BTH_II1233. These products were mixed, and a second-step PCR was done to anneal and amplify the DNA adjacent to the intended site of deletion in BTH_II1233, creating a 2-kb amplicon for subsequent site-specific recombination. Amplification created an internal XbaI restriction site in the product. The resulting PCR product was digested with XmaI and HindIII and ligated to XmaI-HindIII-digested pJRC115 to yield pLARA1233. pLARA1233 was digested with XbaI and ligated to a 1.2-kb DNA fragment from XbaI-digested pGEM-T Easy;amrRAB-oprA;;FRT-Km Δ(amrRAB-oprA)::FRT-PEM7-kam-FRT (T. Mima and H. Schweizer, unpublished data), which contains a kanamycin resistance cassette, creating the marked suicide vector pKM1233. pINF1233 was made by ligating a 2-kb DNA fragment generated by two-step PCR, and this product was digested and ligated to XbaI-HindIII-digested pJRC115. To construct the BTH_II1233 kanamycin insertion mutant strain BD909 and the unmarked BTH_II1233 deletion strain BD20, pKM1233 and pINF1233 were mobilized from E. coli SM10 into B. thailandensis. Trimethoprim-resistant transconjugants were identified and then counterselected by a method similar to that described elsewhere (2). PCR, followed by DNA sequencing, was used to confirm the mutations. The nucleic acid sequence coordinates of insertion and replacement during the construction of BD909 and BD20 can be found in Table 2.
LC/MS/MS identification of acyl-HSLs.
Acyl-HSLs were isolated from 10-ml cultures (optical density at 600 nm [OD600], 4.0) of wild-type B. thailandensis and the indicated mutant strains. Cells were removed from the culture fluid by centrifugation, and the culture fluid was sterilized by using a 0.2-μm filter (Millipore, Billerica, MA). For liquid chromatography-electrospray ionization-tandem mass spectrometry (LC/MS/MS), 0.4 nmol of deuterated C6-HSL (D3-C6-HSL) and deuterated C12-HSL (D3-C12-HSL) was added to the filtered culture fluid, which was then extracted with two equal volumes of acidified ethyl acetate (0.1 ml/liter glacial acetic acid) and dried to completion under a constant stream of nitrogen gas. This was followed by a solid-phase extraction and suspension of the material in 50 μl of 5% MeOH. Ten microliters of each sample was analyzed by LC/MS/MS as described elsewhere (26). The relative abundance of acyl-HSLs in each sample was determined by integrating the area under the analyte peaks and comparing these values to those of D3-C6-HSL and D3-C12-HSL standard curves (26). Relative abundance is a measurement of the ratio of the areas of the analyte and internal standard peaks to the ratio of the amount of analyte and internal standard in each sample.
Acyl-HSL bioassays.
To measure the BtaR2 response to acyl-HSLs, we used recombinant E. coli with a btaI2-lacZ fusion or a BTH_II1233-lacZ fusion. Bioassays were performed as described elsewhere (15). Briefly, an overnight culture of E. coli MG4 (48) containing pJNR2 and pI2P50 was used to inoculate fresh LB broth containing arabinose (starting OD600, 0.05). When the OD600 reached 0.7, 0.5 ml of culture was added to tubes containing dried C8-HSL, C10-HSL, C12-HSL, 3OHC6-HSL, 3OHC8-HSL, or 3OHC10-HSL as indicated. After 3 h with shaking at 37°C, 50 μl of chloroform was added to each tube and β-galactosidase activity was measured using a Tropix Galacto-Light Plus kit according to the manufacturer's recommendations (Applied Biosystems, Foster City, CA). E. coli MG4 carrying pJNR2 and pQF1233 was used to measure the regulation of the BTH_II1233 promoter by BtaR2. The protocol was identical to that described for E. coli MG4 containing pJNR2 and pI2P50, except that only 3OHC8-HSL and 3OHC10-HSL were tested as signals.
Real-time PCR.
Wild-type B. thailandensis and the btaR2 mutant JBT108 were grown in 25 ml of LB broth supplemented with 2% glycerol and 25 mM MOPS (morpholinepropanesulfonic acid), pH 7.0. At an OD600 of 2.7, 4 ml of culture was treated with RNA Protect (Qiagen, Valencia, CA), and after centrifugation, the resulting cell pellet was suspended in 200 μl of Tris-EDTA (10 mM Tris base and 1 mM EDTA, pH 8) containing 10 mg/ml lysozyme. Total RNA was purified with an RNeasy spin purification kit (Qiagen, Valencia, CA). For quantitative real-time PCR (qRT-PCR), cDNA was generated from 2 μg of RNA by using a TaqMan kit (Applied Biosystems, Foster City, CA). Primers were designed to amplify 100- to 200-bp targets for use in qRT-PCRs. The qRT-PCRs used 2× SYBR green master mix (Applied Biosystems, Foster City, CA) with 40 cycles of 15 s at 95°C followed by 60 s at 62°C. The qRT-PCR program ended with a dissociation curve that was used to verify that a single product was amplified in each reaction and that primer dimers did not form. Threshold cycle (CT) values were obtained with a manual threshold setting of 0.2. Values were generated by the calculation 2(35−CT). The results are reported as the calculated transcript amount of a given gene per calculated rpoB transcript. The reported values show the averages and ranges of biological replicates assayed in triplicate.
Antimicrobial susceptibility testing.
To assess antimicrobial activity in B. thailandensis culture fluid, we routinely used a diffusion disc assay (29). B. thailandensis cultures were grown in LB broth containing MOPS (50 mM; pH 7.0) at 37°C. Colonies grown overnight on LB agar plates were used as the starting inoculum. Unless otherwise indicated, when B. thailandensis cultures reached late stationary phase (OD600, 9 to 10) the cells were removed by centrifugation and the culture fluid was filtered through a 0.45-μm membrane. Antibiotic assay discs (13 mm; Whatman, Florham Park, NJ) were saturated with sterile culture fluid and deposited onto LB agar plates overlaid with 100 μl of a 1:10 dilution of an overnight culture of B. subtilis 168 (5) or other bacterial species as indicated. The plates were incubated at 37°C overnight.
To measure antimicrobial activity more precisely and to assess whether the activity was bactericidal or bacteriostatic, we used the following assay. Fluid from 24-h B. thailandensis cultures grown in LB containing 50 mM MOPS (pH 7.0) at 37°C was collected and filter sterilized (0.45-μm-pore-size membrane). Early log-phase B. subtilis 3610 (9) cells were inoculated into the filtered B. thailandensis culture fluid diluted with fresh LB-MOPS broth (the initial B. subtilis density was approximately 1 × 106 cells per ml). After 24 h with shaking at 37°C, the B. subtilis cell density was determined by plate counting on LB agar.
RESULTS
The btaI2 gene product catalyzes the synthesis of 3OHC8-HSL and 3OHC10-HSL.
Acyl-HSL detection often involves bioassays or bioassays coupled to thin-layer-chromatographic separation of acyl-HSLs (50, 54). These methods rely on a specific LuxR homolog for acyl-HSL detection, and they are biased for specific acyl-HSLs. It is cumbersome to conduct a comprehensive quantitative analysis of acyl-HSLs using bioassays. Thus, we employed LC/MS/MS, which detects acyl-HSLs in a complex mixture with high sensitivity irrespective of acyl side chain length or substitution and directly measures the relative abundance of acyl-HSLs (26), to analyze acyl-HSLs produced by B. thailandensis. We analyzed acyl-HSLs in ethyl acetate extracts of stationary-phase (OD600, 4) culture fluid from wild-type B. thailandensis and quorum-sensing mutants lacking btaI2 (JBT102) or both btaI1 and btaI3 (JBT105). Wild-type B. thailandensis produced C8-HSL, 3OHC8-HSL, 3OHC10-HSL, and a small amount of N-3-hydroxy-dodecanoyl-HSL (3OHC12-HSL) (Table 3). The btaI2 mutant produced C8-HSL and 3OHC8-HSL, but it did not produce detectable levels of 3OHC10-HSL (Table 3). This analysis suggests that BtaI2 is responsible for the synthesis of 3OHC10-HSL. The btaI1-btaI3 double mutant produced both 3OHC8-HSL and 3OHC10-HSL. Taken together, the data indicate that in B. thailandensis, BtaI2 produces 3OHC8-HSL and 3OHC10-HSL. C8-HSL appears to be produced by either BtaI1 or BtaI3. BtaI1, BtaI3, or both are also capable of producing significant amounts of 3OHC8-HSL. Consistent with this interpretation, the closely related B. mallei and B. pseudomallei I1 synthases produce C8-HSL (15, 57), and the B. mallei BmaI3 synthesizes 3OHC8-HSL (14). The work of Chandler and Greenberg (unpublished data) supports the notion that BtaI1 produces C8-HSL and BtaI3 makes 3OHC8-HSL in B. thailandensis.
TABLE 3.
Relative amounts of acyl-HSLs produced by wild-type and quorum-sensing mutant strains of B. thailandensis
| Acyl-HSL | Relative abundancea
|
||
|---|---|---|---|
| Wild type | ΔbtaI2 | ΔbtaI1, ΔbtaI3 | |
| C8-HSL | 27.5 (0.2) | 54.6 (0.6) | NDb |
| 3OHC8-HSL | 11.0 (0.06) | 45.4 (0.6) | 12.6 (1.8) |
| 3OHC10-HSL | 57.6 (0.5) | ND | 87.4 (1.8) |
| 3OHC12-HSL | 3.9 (0.2) | ND | ND |
The values are the average relative percentages of acy-HSLs from two independent cultures; the range is indicated in parentheses.
ND, none detected.
BtaR2 regulates btaI2 transcription in response to 3OHC8-HSL or 3OHC10-HSL.
It is common for luxI homologs to be positively autoregulated by the acyl-HSL signal(s) produced by the proteins they encode and the transcription factor encoded by the adjacent luxR homolog (10, 12, 15, 23, 32, 35, 39, 52, 53). The btaR2 and btaI2 gene organization is unusual in that there is approximately 3.3 kb of DNA between these two genes, and this intervening DNA contains three open reading frames. We hypothesized that btaR2 and btaI2 may represent a cognate pair of quorum-sensing genes and that btaI2 transcription would be activated by BtaR2 in response to 3OHC8-HSL, 3OHC10-HSL, or both signals. To test our hypothesis, we created a plasmid with a 244-bp DNA fragment containing the putative promoter region of btaI2 (from −238 to +6 of the predicted translational start site) fused to a promoterless lacZ. This plasmid was introduced into E. coli with or without an arabinose-inducible btaR2. Activation of btaI2-lacZ transcription required BtaR2 and either 3OHC8-HSL or 3OHC10-HSL (Fig. 1A). There was also a response to N-decanoyl-HSL (C10-HSL) or N-dodecanoyl-HSL (C12-HSL), neither of which was detected in B. thailandensis culture fluid. There was no detectable response to C8-HSL or several other acyl-HSLs tested.
FIG. 1.

Transcriptional activation of genes in the btaI2 operon requires 3OHC10-HSL or 3OHC8-HSL and BtaR2. (A) Acyl-HSL dose responses of the btaI2 promoter in E. coli containing a BtaR2 expression vector (pJNR2) and a btaI2-lacZ fusion vector (pI2P50). The following acyl-HSLs were tested: 3OHC10-HSL (▪), 3OHC8-HSL (•), 3OHC6-HSL (□), C12-HSL (▾), C10-HSL (○), and C8-HSL (▴). The open diamonds indicate the 3OHC10-HSL response in the absence of BtaR2. The error bars represent the range of three independent experiments. β-Galactosidase activity is given as millions of relative light units. (B) Relative transcript levels of btaI2 and the downstream gene BTH_II1224 from wild-type B. thailandensis (gray bars) and the btaR2 mutant strain JBT108 (white bars). The error bars represent the range of two independent experiments assayed in triplicate.
To verify that btaI2 transcription is regulated by BtaR2 in B. thailandensis, we measured the abundance of btaI2 mRNA in the wild type and the btaR2 mutant strain JBT108 by using qRT-PCR. The btaI2 gene is the first of five genes in a putative operon residing on chromosome II. The qRT-PCR analysis showed that transcript levels of btaI2 and a downstream gene (1224) were substantially higher in the wild type than they were in the btaR2 mutant (Fig. 1B).
The btaR2 and btaI2 genes reside among a cluster of putative antimicrobial biosynthetic genes conserved in B. pseudomallei but absent in B. mallei.
Three of the genes in the putative btaI2 operon are predicted to code for enzymes involved in the production of secondary metabolites (Fig. 2). The second gene codes for a protein with similarity to nonribosomal peptide synthetases (NRPSs). NRPSs are involved in the production of amino acid-derived secondary metabolites (30). The third gene's product contains a domain similar to those found in NRPS enzymes, and the fourth gene codes for a protein with identity to a Pseudomonas syringae halogenase involved in the synthesis of the phytotoxin coronatine (59, 63). There is a cluster of genes (1233 to 1241) on chromosome II, upstream of btaR2, that codes for several additional NRPS polypeptides and several modular polyketide synthases (PKSs). PKSs function by incorporating fatty acids into secondary metabolites (30). Many secondary metabolites produced by NRPSs, PKSs, and hybrid NRPS-PKS enzymes are medically important antimicrobials, immunosuppressants, or anticancer molecules (19, 20). Furthermore, the cluster distal to btaR2 also harbors genes that may play a role in the immunity to and transport of antibiotics (36, 40, 65). We hypothesize that B. thailandensis uses BtaR2-BtaI2 quorum sensing to control antibiotic production.
FIG. 2.
Organization of the B. thailandensis btaR2-btaI2 genomic region. Shown is a map of the genes in the immediate vicinity of btaR2 and btaI2. The btaR2 and btaI2 genes are separated by three open reading frames encompassing about 3.3 kb of DNA. btaI2 resides in a predicted five-gene operon that contains open reading frames annotated to function in antibiotic synthesis. btaR2 is 3 kb upstream of a cluster containing putative antibiotic biosynthesis genes. The blue arrows labeled R2 and I2 represent btaR2 and btaI2, respectively. The red arrows are annotated as NRPS genes, green arrows indicate PKS genes, pink arrows are potential accessory antibiotic synthesis genes, orange arrows are putative transport genes, the brown arrow is a metallopeptidase, and gray indicates genes of unknown function. The black lines between coding regions represent intergenic DNA. The purple bars represent the btaR2-btaI2 genomic region and the flanking DNA, which is conserved in B. pseudomallei and mostly absent from the B. mallei chromosome. The genomic sequences were obtained from the publicly available genome sequences of B. thailandensis strain E264, B. pseudomallei strain K96243, and B. mallei strain ATCC 23344. The alignments were generated using the nucleotide BLAST algorithm (73). The B. thailandensis E264 genomic sequence was used as the reference sequence. A solid purple bar indicates congruence in nucleic acid sequence; the amino acid sequences within these regions share >90% identity. Vertical black bars represent nucleic acid sequence with dissimilarity, and gaps between purple bars are missing sequences. The arrows representing btaR2, btaI2, and their surrounding genes are drawn to scale.
The btaI2 and btaR2 genes reside amid a large cluster of genes, many of which we suspect are involved in production of secondary metabolites (Fig. 2). Does B. pseudomallei, which possesses btaI2 and btaR2 homologs, have a similar cluster of genes, and does B. mallei, which does not have btaI2 and btaR2 homologs, have this cluster? The alignment in Fig. 2 shows that there is extensive identity over an approximately 120-kb R2-I2 region of the B. thailandensis and B. pseudomallei chromosome II. This 120-kb region is completely absent in B. mallei. The genes flanking this region are conserved among all three species.
B. thailandensis produces an antibiotic.
Because btaI2 and btaR2 are embedded in a chromosomal region with many genes annotated as coding for functions known to be involved in antibiotic synthesis, transport, and immunity in other bacteria, we hypothesized that B. thailandensis produces an antibiotic and that antibiotic production may require BtaR2 and an acyl-HSL produced by BtaI2. To test the hypothesis, we first screened fluid taken from B. thailandensis cultures at various cell densities (Fig. 3A). To obtain the fluid, cells were removed by centrifugation and the supernatant fluid was subjected to microfiltration. The filtered culture fluid was used to saturate paper diffusion discs, which were placed on agar plates containing a growing lawn of Bacillus subtilis. After overnight incubation at 37°C, a zone of B. subtilis growth inhibition was observed around discs which had been saturated with fluid from stationary-phase cultures but not around discs saturated with fluid from logarithmic-phase cultures (Fig. 3B). Thus, B. thailandensis produces a secondary metabolite with antimicrobial activity.
FIG. 3.
Sensitivity of B. subtilis to a substance in B. thailandensis stationary-phase culture fluid. (A) Growth curve of the wild-type B. thailandensis strain E264. The arrowheads marked a to f indicate points where culture fluid was taken for the analysis shown in panel B. The open arrowheads indicate points in growth where antibiotic was produced. (B) Antibiotic sensitivity assays. Paper diffusion discs were saturated with fluid from a B. thailandensis E264 culture at the indicated points (a to f in panel A) and placed on lawns of B. subtilis. A zone of clearing around a diffusion disc indicates the region where B. subtilis growth was inhibited. (C) Antibiotic activity of B. thailandensis culture fluid against S. aureus COL, S. pyogenes MGAS5005, E. coli DH5α, and P. aeruginosa PAO1.
We tested the specificity of the antibacterial component from stationary-phase culture fluid (OD600, 9 to 10) by using the diffusion disc assay to assess whether the component inhibited the growth of other bacterial species. Growth of the gram-positive bacteria Staphylococcus aureus (including methicillin-resistant S. aureus) and Streptococcus pyogenes was inhibited by the antibiotic. Neither of the two gram-negative bacteria we tested, E. coli and Pseudomonas aeruginosa, showed growth inhibition by B. thailandensis culture fluid under the growth conditions we tested (Fig. 3C). The compound may not be active against gram-negative bacteria, or it may be that there were insufficient amounts of the antibiotic for inhibition of gram-negative bacteria in the preparations we tested.
The btaR2-btaI2 quorum-sensing system is required for antibiotic production.
To determine whether stationary-phase antibiotic production required the btaR2-btaI2 quorum-sensing system, we first asked whether synthesis of the factor required acyl-HSL signaling as follows. We tested cell-free stationary-phase culture fluid of a B. thailandensis strain with mutations in all three of the acyl-HSL synthase genes, btaI1, btaI2, and btaI3 (Chandler and Greenberg, unpublished), for anti-B. subtilis activity as described above (Fig. 4). Fluid from cultures of the triple acyl-HSL synthase mutant, JBT112, did not block B. subtilis growth unless the cultures were grown in the presence of added 3OHC8-HSL or 3OHC10-HSL. Addition of C8-HSL was unable to restore antibiotic production to JBT112. As a control, we determined that 3OHC10-HSL alone does not have antibiotic activity (Fig. 4). From this experiment, we conclude that a BtaI2-produced acyl-HSL is required for production of the antibacterial factor.
FIG. 4.
A B. thailandensis acyl-HSL synthesis mutant requires exogenous 3OHC8-HSL or 3OHC10-HSL for antibiotic production. Diffusion disc assays with fluid from a stationary-phase culture of the btaI1, btaI2, btaI3 triple mutant JBT112 grown without added signal or with 2 μM 3OHC8-HSL or 3OHC10-HSL, as indicated, are shown. Growth of B. subtilis is inhibited by culture fluid from 3OHC10-HSL- or 3OHC8-HSL-grown B. thailandensis JBT112, but not by JBT112 grown without an added acyl-HSL or in the presence of 2 μM C8-HSL. The bottom panel shows a diffusion disc that had been soaked in sterile medium containing 2 μM 3OHC10-HSL. This control shows that 3OHC10-HSL itself is not an antimicrobial molecule.
To investigate whether the acyl-HSL-dependent production of antibacterial activity required BtaR2, we tested the ability of the btaR2 mutant, JBT108, to produce an antibacterial factor. As shown in Fig. 5A, fluid from a stationary-phase JBT108 culture does not inhibit growth of B. subtilis, and the antibiotic synthesis phenotype was complemented by a plasmid-borne copy of btaR2. Another way to assess antimicrobial activity is to incubate B. subtilis in broth containing fluid from stationary-phase B. thailandensis cultures and assess bacterial growth as changes in CFU (Fig. 5B). When we incubated B. subtilis in medium containing 50% (vol/vol) fluid from a wild-type B. thailandensis stationary-phase culture, the B. subtilis cell density did not change from that of the inoculum. In the presence of 50% (vol/vol) fluid from a stationary-phase culture of the btaR2 mutant, JBT108, the B. subtilis cell density increased to that seen in the absence of B. thailandensis culture fluid. This difference in CFU was also observed when we used 10% (vol/vol) B. thailandensis culture fluid from either the wild type or JBT108 (Fig. 5B). This experiment supports the conclusion that BtaR2-dependent quorum sensing is required for production of the antibacterial factor. It also suggests that the factor exerts bacteriostatic activity rather than bactericidal activity.
FIG. 5.
B. thailandensis BtaR2 is required for antibiotic production. (A) A diffusion disc experiment showing antibiotic activity of fluid from a B. thailandensis E264 (wild-type) stationary-phase culture, a JBT108 (btaR2 mutant) stationary-phase culture, and JBT108 complemented with the BtaR2 expression plasmid pSCR2. The control is strain JBT108 carrying the empty vector pSCrhaB2. B. subtilis was used as the indicator strain. (B) Influence of B. thailandensis culture fluid (50% or 10% [vol/vol] as indicated) on growth of B. subtilis in broth assessed by colony counting. The white bars represent culture fluid from the parent B. thailandensis E264, the gray bars indicate fluid from the btaR2 mutant JBT108, and the hatched bar is a control B. subtilis culture with no added B. thailandensis culture fluid. The error bars indicate standard deviations.
A putative nonribosomal peptide synthetase is required for quorum-sensing-regulated antibiotic production in B. thailandensis.
As discussed above, the btaI2 gene cluster and a cluster of genes located upstream of btaR2 encode proteins annotated as NRPS and PKS enzymes, which we predict are responsible for the production of the BtaR2-BtaI2-controlled antibiotic. The cluster of genes distal to btaR2 (1233 to 1241) is predicted to constitute an operon. We first asked whether the promoter upstream of the first gene in the cluster, 1233, was quorum sensing controlled by constructing a transcriptional fusion of −409 to +13 (with respect to the predicted translational start site) of the putative 1233 promoter to lacZ. We introduced a 1233 promoter-lacZ fusion plasmid into E. coli containing a BtaR2 expression vector and tested whether either 3OHC8-HSL or 3OHC10-HSL activated the lacZ reporter (Fig. 6A). Either 3OHC8-HSL or 3OHC10-HSL was sufficient for BtaR2-dependent induction of lacZ.
FIG. 6.
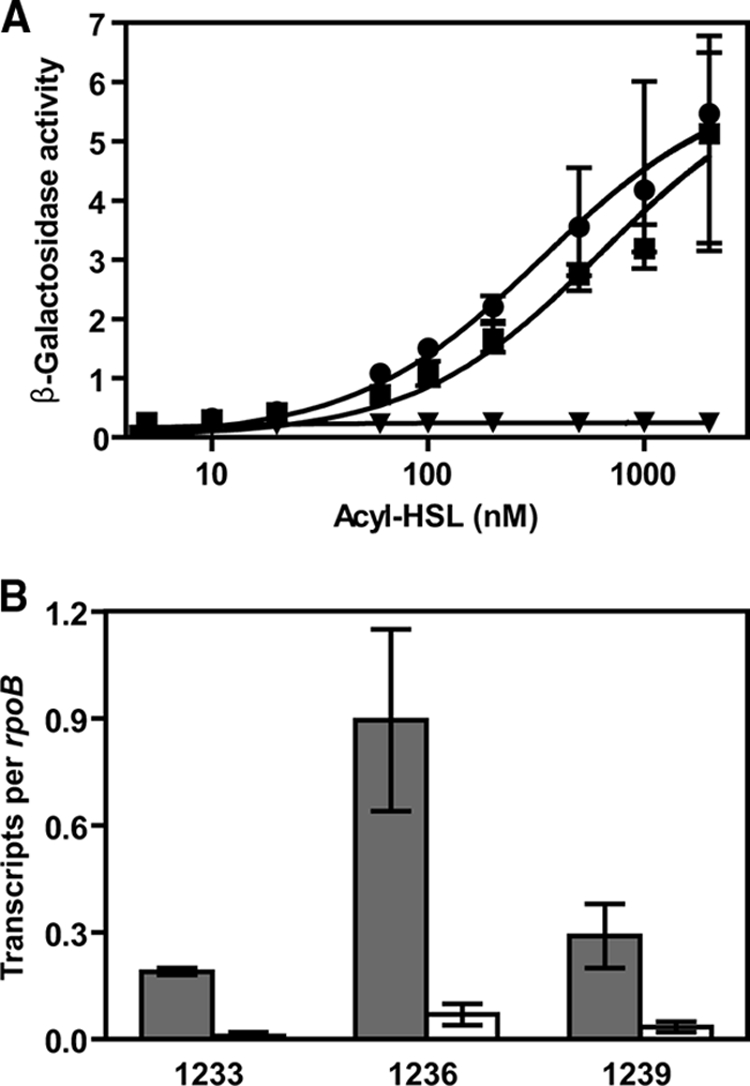
Dependence of BTH_II1233, -1236, and -1239 transcription on the BtaI2-BtaR2 quorum-sensing system. (A) Acyl-HSL dose responses of the BTH_II1233-lacZ fusion on pQF1233 and btaR2 on pJNR2 in E. coli; 3OHC10-HSL, ▪; 3OHC8-HSL, •. The BTH_II1233-lacZ response to 3OHC10-HSL in E. coli without a BtaR2 expression vector is also shown (▾). The error bars indicate the range of three independent experiments. β-Galactosidase activity is given as millions of relative light units. (B) Relative transcript levels of BTH_II1233 and the downstream genes BTH_II1236 and BTH_II1239 from wild-type B. thailandensis E264 (gray bars) and the btaR2 mutant strain JBT102 (white bars). The values represent the range of two independent experiments assayed in triplicate.
To obtain further data about quorum-sensing control of the 1233 to 1241 genes, we compared 1233, 1236, and 1239 transcript levels in the wild type and the btaR2 JBT108 mutant by qRT-PCR. The wild type showed about 10-fold more transcript for these genes than the mutant (Fig. 6B). Therefore, we conclude that BtaR2 and either 3OHC8-HSL or 3OHC10-HSL regulate a cluster of putative secondary-metabolite genes, including 1233, 1236, and 1239.
To determine whether gene 1233 itself is required for antibiotic production, we constructed a 1233 insertion mutant strain and a 1233 in-frame deletion strain of B. thailandensis and tested stationary-phase culture fluid from both of these 1233 mutants for antibiotic activity. Neither strain made detectable levels of the antibiotic (Fig. 7). Thus, gene 1233 on chromosome II is required for antibiotic production. We have not tested any of the other genes in the 1233-to-1241 cluster, but we suspect that all or several are required for antibiotic production.
FIG. 7.

BTH_II1233 mutants do not produce antibiotic. The influence of B. thailandensis culture fluid (10% vol/vol) on growth of B. subtilis in broth was assessed by colony counting. The B. thailandensis wild-type strain E264 is indicated by the white bar. The BTH_II1233 kanamycin insertion mutant, BD909, and the in-frame deletion mutant, BD20, are indicated by the gray and hatched bars, respectively. For reference, a control culture with no added B. thailandensis culture fluid is shown (black bar). The error bars indicate standard deviations.
DISCUSSION
B. thailandensis, B. pseudomallei, and B. mallei are closely related species, and it has been proposed that the limited genomic differences between these species reflect adaptations defining their specific niches. B. mallei is the only one of the three species that is an obligate animal pathogen (66, 69). Both B. thailandensis and B. pseudomallei exist in tropical soils (8, 70). All three species have two quorum-sensing systems in common, the so-called systems 1 and 3, which have been investigated previously (14, 15, 41, 57). The B. thailandensis btaR2 and btaI2 genes encoding quorum-sensing system 2 have conserved counterparts in B. pseudomallei, but not in B. mallei, and prior to this study, little was known about this quorum-sensing system.
The BtaR2-BtaI2 quorum-sensing system of B. thailandensis E264 is embedded in an approximately 120-kb DNA element, conserved in B. thailandensis and B. pseudomallei but absent in B. mallei. Evidence indicates that the obligate animal pathogen B. mallei has evolved from an ancestor common to B. thailandensis and B. pseudomallei in large part by undergoing a number of genome size reductions by deletion of regions not required for virulence (31, 45). This appears to be the case for the 120-kb R2-I2-containing DNA element. Other investigators have shown that both of the B. mallei quorum-sensing systems contribute to virulence (61). It is logical to assume that system 2 in B. pseudomallei is not critical for virulence. Rather, system 2 of both B. pseudomallei and B. thailandensis might be important for survival in the soil environment. System 2 is present in the two species that exhibit a saprophytic soil lifestyle but not in the species that does not occur as a soil saprophyte.
We have presented evidence that the B. thailandensis BtaR2-BtaI2 quorum-sensing system generates 3OHC8-HSL and 3OHC10-HSL and responds to either compound by regulating the synthesis of an antibiotic that is active against a variety of gram-positive bacteria. Our evidence indicates that BtaI2 is the only one of the three LuxI homologs in B. thailandensis that produces significant amounts of 3OHC10-HSL but that both BtaI2 and BtaI3 produce 3OHC8-HSL (this study and Chandler and Greenberg, unpublished). BtaR2 responds to either 3OHC8-HSL or 3OHC10-HSL about equally well. It is interesting that BtaR2 responds to the signals produced by its cognate acyl-HSL synthase, BtaI2, and that one of these signals is also produced by a noncognate acyl-HSL synthase, BtaI3. It is possible that 3OHC8-HSL production via BtaI2 may also jump-start the BtaR3-BtaI3 system. If true, this would be an aspect of network complexity that has not been observed in other bacteria with multiple acyl-HSL quorum-sensing systems. At this time, we do not know about the timing of btaR2-btaI2 and btaR3-btaI3 expression and whether the BtaI2-dependent production of 3OHC8-HSL provides an advantage to these systems or whether it is of no particular consequence.
The BtaR2-BtaI2 quorum-sensing system controls at least two gene clusters that reside on the 120-kb element that is also present in B. pseudomallei but absent from B. mallei. This 120-kb element can be considered a quorum-sensing island. We have shown that BtaR2-BtaI2 controls the production of a secondary metabolite with antibiotic activity against a variety of gram-positive bacteria. It is not a coincidence that we focused our initial assays for antimicrobial activity on B. subtilis, which like B. thailandensis is a soil bacterium. A classical view of the benefit of antibiotic production is that it can give microbes in their environment a competitive advantage over other antibiotic-sensitive microbes in the same habitat (33, 38, 49). An emerging view is that antibiotics actually serve intercellular signaling functions rather than as weapons against competitors (13, 17, 27). Our findings are consistent with the classical view and at odds with the emerging view.
B. thailandensis joins a list of bacteria that control antibiotic synthesis by an acyl-HSL quorum-sensing system. The list includes Erwinia carotovora (1, 43), Pseudomonas chloroaphis (47), and Burkholderia vietnamiensis (46), which is in the B. cepacia complex rather than the B. mallei-B. pseudomallei-B. thailandensis complex (7, 42). It is possible that quorum control of antibiotic synthesis in B. thailandensis allows microcolonies in soil environments to resist invasion by competing species and thus provides a selective advantage to the cells in the microcolony. The hypothesis that quorum-sensing control of antibiotic synthesis provides B. thailandensis an advantage in soil environments or in microcolonies can be tested experimentally, and such tests may shed light on the evolution of acyl-HSL quorum sensing.
Acknowledgments
This work was funded by an NIAID award for the Northwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (U54AI057141) to E.P.G., Defense Threat Reduction Agency award HDTRA1-06-C-0038 to W.C.N., and a grant from the National Science Foundation (0821220) to M.E.A.C. B.A.D. was supported in part by the National Institute of General Medical Sciences (NRSA T32 GM07270), J.R.C. by the National Institutes of Health (NRSA 1 F32 AI073027-01A2), and S.B.P by a postdoctoral fellowship from the Cystic Fibrosis Foundation.
We thank Brad Borlee, Karine Gibbs, Jason Hickman, and Amy Schaefer for insightful discussions concerning the manuscript.
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Bainton, N. J., P. Stead, S. R. Chhabra, B. W. Bycroft, G. P. Salmond, G. S. Stewart, and P. Williams. 1992. N-(3-oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem. J. 288997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, A. R., Y. Kang, K. S. Inamasu, M. S. Son, J. M. Vukovich, and T. T. Hoang. 2008. Genetic tools for allelic replacement in Burkholderia species. Appl. Environ. Microbiol. 744498-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109421-424. [DOI] [PubMed] [Google Scholar]
- 4.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48317-320. [DOI] [PubMed] [Google Scholar]
- 5.Burkholder, P. R., and N. H. Giles. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 34345-348. [PubMed] [Google Scholar]
- 6.Cardona, S. T., and M. A. Valvano. 2005. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54219-228. [DOI] [PubMed] [Google Scholar]
- 7.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5719-729. [DOI] [PubMed] [Google Scholar]
- 8.Dance, D. A. 2000. Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74159-168. [DOI] [PubMed] [Google Scholar]
- 9.Dean, D. H., J. C. Orrego, K. W. Hutchison, and H. O. Halvorson. 1976. New temperate bacteriophage for Bacillus subtilis, rho 11. J. Virol. 20509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Kievit, T. R., Y. Kakai, J. K. Register, E. C. Pesci, and B. H. Iglewski. 2002. Role of the Pseudomonas aeruginosa las and rhl quorum-sensing systems in rhlI regulation. FEMS Microbiol. Lett. 212101-106. [DOI] [PubMed] [Google Scholar]
- 11.Deshazer, D. 2007. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol. Lett. 27764-69. [DOI] [PubMed] [Google Scholar]
- 12.Devine, J. H., G. S. Shadel, and T. O. Baldwin. 1989. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc. Natl. Acad. Sci. USA 865688-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich, L. E., T. K. Teal, A. Price-Whelan, and D. K. Newman. 2008. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 3211203-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duerkop, B. A., J. P. Herman, R. L. Ulrich, M. E. Churchill, and E. P. Greenberg. 2008. The Burkholderia mallei BmaR3-BmaI3 quorum-sensing system produces and responds to N-3-hydroxy-octanoyl homoserine lactone. J. Bacteriol. 1905137-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duerkop, B. A., R. L. Ulrich, and E. P. Greenberg. 2007. Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J. Bacteriol. 1895034-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberl, L. 2006. Quorum sensing in the genus Burkholderia. Int. J. Med. Microbiol. 296103-110. [DOI] [PubMed] [Google Scholar]
- 17.Fajardo, A., and J. L. Martinez. 2008. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 11161-167. [DOI] [PubMed] [Google Scholar]
- 18.Farinha, M. A., and A. M. Kropinski. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 1723496-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felnagle, E. A., E. E. Jackson, Y. A. Chan, A. M. Podevels, A. D. Berti, M. D. McMahon, and M. G. Thomas. 2008. Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol. Pharm. 5191-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischbach, M. A., and C. T. Walsh. 2006. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 1063468-3496. [DOI] [PubMed] [Google Scholar]
- 21.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35439-468. [DOI] [PubMed] [Google Scholar]
- 22.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50727-751. [DOI] [PubMed] [Google Scholar]
- 23.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 1762796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 1872426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass, M. B., J. E. Gee, A. G. Steigerwalt, D. Cavuoti, T. Barton, R. D. Hardy, D. Godoy, B. G. Spratt, T. A. Clark, and P. P. Wilkins. 2006. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J. Clin. Microbiol. 444601-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould, T. A., J. Herman, J. Krank, R. C. Murphy, and M. E. Churchill. 2006. Specificity of acyl-homoserine lactone synthases examined by mass spectrometry. J. Bacteriol. 188773-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 4361171-1175. [DOI] [PubMed] [Google Scholar]
- 28.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 4373-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgensen, J. H., J. D. Turnidge, and J. A. Washington. 1999. Antibacterial susceptibility tests: dilution and disc diffusion methods, p. 1526-1543. In P. R. Murray, E. J. Barron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken, ed., Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 30.Keating, T. A., and C. T. Walsh. 1999. Initiation, elongation, and termination strategies in polyketide and polypeptide antibiotic biosynthesis. Curr. Opin. Chem. Biol. 3598-606. [DOI] [PubMed] [Google Scholar]
- 31.Kim, H. S., M. A. Schell, Y. Yu, R. L. Ulrich, S. H. Sarria, W. C. Nierman, and D. DeShazer. 2005. Bacterial genome adaptation to niches: divergence of the potential virulence genes in three Burkholderia species of different survival strategies. BMC Genom. 6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 211137-1146. [DOI] [PubMed] [Google Scholar]
- 33.Laville, J., C. Voisard, C. Keel, M. Maurhofer, G. Defago, and D. Haas. 1992. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc. Natl. Acad. Sci. USA 891562-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy, A., A. J. Merritt, M. Aravena-Roman, M. M. Hodge, and T. J. Inglis. 2008. Expanded range of Burkholderia species in Australia. Am. J. Trop. Med. Hyg. 78599-604. [PubMed] [Google Scholar]
- 35.Lewenza, S., and P. A. Sokol. 2001. Regulation of ornibactin biosynthesis and N-acyl-l-homoserine lactone production by CepR in Burkholderia cepacia. J. Bacteriol. 1832212-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewinson, O., J. Adler, N. Sigal, and E. Bibi. 2006. Promiscuity in multidrug recognition and transport: the bacterial MFS Mdr transporters. Mol. Microbiol. 61277-284. [DOI] [PubMed] [Google Scholar]
- 37.Lin, C. H., G. Bourque, and P. Tan. 2008. A comparative synteny map of Burkholderia species links large-scale genome rearrangements to fine-scale nucleotide variation in prokaryotes. Mol. Biol. Evol. 25549-558. [DOI] [PubMed] [Google Scholar]
- 38.Little, A. E., C. J. Robinson, S. B. Peterson, K. F. Raffa, and J. Handelsman. 2008. Rules of engagement: interspecies interactions that regulate microbial communities. Annu. Rev. Microbiol. 62375-401. [DOI] [PubMed] [Google Scholar]
- 39.Llamas, I., N. Keshavan, and J. E. Gonzalez. 2004. Use of Sinorhizobium meliloti as an indicator for specific detection of long-chain N-acyl homoserine lactones. Appl. Environ. Microbiol. 703715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llano-Sotelo, B., E. F. Azucena, Jr., L. P. Kotra, S. Mobashery, and C. S. Chow. 2002. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem. Biol. 9455-463. [DOI] [PubMed] [Google Scholar]
- 41.Lumjiaktase, P., S. P. Diggle, S. Loprasert, S. Tungpradabkul, M. Daykin, M. Camara, P. Williams, and M. Kunakorn. 2006. Quorum sensing regulates dpsA and the oxidative stress response in Burkholderia pseudomallei. Microbiology 1523651-3659. [DOI] [PubMed] [Google Scholar]
- 42.Mahenthiralingam, E., T. A. Urban, and J. B. Goldberg. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3144-156. [DOI] [PubMed] [Google Scholar]
- 43.McGowan, S., M. Sebaihia, S. Jones, B. Yu, N. Bainton, P. F. Chan, B. Bycroft, G. S. Stewart, P. Williams, and G. P. Salmond. 1995. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional activator. Microbiology 141541-550. [DOI] [PubMed] [Google Scholar]
- 44.Newman, J. R., and C. Fuqua. 1999. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227197-203. [DOI] [PubMed] [Google Scholar]
- 45.Nierman, W. C., D. DeShazer, H. S. Kim, H. Tettelin, K. E. Nelson, T. Feldblyum, R. L. Ulrich, C. M. Ronning, L. M. Brinkac, S. C. Daugherty, T. D. Davidsen, R. T. Deboy, G. Dimitrov, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, H. Khouri, J. F. Kolonay, R. Madupu, Y. Mohammoud, W. C. Nelson, D. Radune, C. M. Romero, S. Sarria, J. Selengut, C. Shamblin, S. A. Sullivan, O. White, Y. Yu, N. Zafar, L. Zhou, and C. M. Fraser. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 10114246-14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, J. H., I. Hwang, J. W. Kim, S. O. Lee, B. Conway, E. P. Greenberg, and K. Lee. 2001. Characterization of quorum-sensing signaling molecules produced by Burkholderia cepacia G4. J. Microbiol. Biotechnol. 11804-811. [Google Scholar]
- 47.Pierson, L. S., III, V. D. Keppenne, and D. W. Wood. 1994. Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J. Bacteriol. 1763966-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralling, G., S. Bodrug, and T. Linn. 1985. Growth rate-dependent regulation of RNA polymerase synthesis in Escherichia coli. Mol. Gen. Genet. 201379-386. [DOI] [PubMed] [Google Scholar]
- 49.Robleto, E. A., J. Borneman, and E. W. Triplett. 1998. Effects of bacterial antibiotic production on rhizosphere microbial communities from a culture-independent perspective. Appl. Environ. Microbiol. 645020-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305288-301. [DOI] [PubMed] [Google Scholar]
- 51.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147236-242. [DOI] [PubMed] [Google Scholar]
- 52.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shadel, G. S., and T. O. Baldwin. 1991. The Vibrio fischeri LuxR protein is capable of bidirectional stimulation of transcription and both positive and negative regulation of the luxR gene. J. Bacteriol. 173568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 946036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilizable system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1784-791. [Google Scholar]
- 56.Smith, M. D., B. J. Angus, V. Wuthiekanun, and N. J. White. 1997. Arabinose assimilation defines a nonvirulent biotype of Burkholderia pseudomallei. Infect. Immun. 654319-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song, Y., C. Xie, Y. M. Ong, Y. H. Gan, and K. L. Chua. 2005. The BpsIR quorum-sensing system of Burkholderia pseudomallei. J. Bacteriol. 187785-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumby, P., S. F. Porcella, A. G. Madrigal, K. D. Barbian, K. Virtaneva, S. M. Ricklefs, D. E. Sturdevant, M. R. Graham, J. Vuopio-Varkila, N. P. Hoe, and J. M. Musser. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J. Infect. Dis. 192771-782. [DOI] [PubMed] [Google Scholar]
- 59.Ullrich, M., and C. L. Bender. 1994. The biosynthetic gene cluster for coronamic acid, an ethylcyclopropyl amino acid, contains genes homologous to amino acid-activating enzymes and thioesterases. J. Bacteriol. 1767574-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulrich, R. L., D. Deshazer, E. E. Brueggemann, H. B. Hines, P. C. Oyston, and J. A. Jeddeloh. 2004. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J. Med. Microbiol. 531053-1064. [DOI] [PubMed] [Google Scholar]
- 61.Ulrich, R. L., D. Deshazer, H. B. Hines, and J. A. Jeddeloh. 2004. Quorum sensing: a transcriptional regulatory system involved in the pathogenicity of Burkholderia mallei. Infect. Immun. 726589-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ulrich, R. L., H. B. Hines, N. Parthasarathy, and J. A. Jeddeloh. 2004. Mutational analysis and biochemical characterization of the Burkholderia thailandensis DW503 quorum-sensing network. J. Bacteriol. 1864350-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaillancourt, F. H., E. Yeh, D. A. Vosburg, S. E. O'Connor, and C. T. Walsh. 2005. Cryptic chlorination by a non-haem iron enzyme during cyclopropyl amino acid biosynthesis. Nature 436:1191-1194. [DOI] [PubMed] [Google Scholar]
- 64.Venturi, V., A. Friscina, I. Bertani, G. Devescovi, and C. Aguilar. 2004. Quorum sensing in the Burkholderia cepacia complex. Res. Microbiol. 155238-244. [DOI] [PubMed] [Google Scholar]
- 65.Vetting, M. W., L. P. S. de Carvalho, M. Yu, S. S. Hegde, S. Magnet, S. L. Roderick, and J. S. Blanchard. 2005. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 433212-226. [DOI] [PubMed] [Google Scholar]
- 66.Waag, D., and D. DeShazer. 2005. Glanders: new insights into an old disease, p. 209-237. In L. E. Lindler, F. J. Lebeda, and G. W. Korch (ed.), Biological weapons defense: infectious diseases and counterbioterrorism. Humana Press, Totowa, NJ.
- 67.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21319-346. [DOI] [PubMed] [Google Scholar]
- 68.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25365-404. [DOI] [PubMed] [Google Scholar]
- 69.Whitlock, G. C., D. M. Estes, and A. G. Torres. 2007. Glanders: off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 277115-122. [DOI] [PubMed] [Google Scholar]
- 70.Wiersinga, W. J., T. van der Poll, N. J. White, N. P. Day, and S. J. Peacock. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4272-282. [DOI] [PubMed] [Google Scholar]
- 71.Wuthiekanun, V., M. D. Smith, D. A. Dance, A. L. Walsh, T. L. Pitt, and N. J. White. 1996. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J. Med. Microbiol. 45408-412. [DOI] [PubMed] [Google Scholar]
- 72.Yu, Y., H. S. Kim, H. H. Chua, C. H. Lin, S. H. Sim, D. Lin, A. Derr, R. Engels, D. DeShazer, B. Birren, W. C. Nierman, and P. Tan. 2006. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, Z., S. Schwartz, L. Wagner, and W. Miller. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7203-214. [DOI] [PubMed] [Google Scholar]






