Abstract
The genome of Rhodobacter sphaeroides encodes the components of two distinct pathways for salvaging cobinamide (Cbi), a precursor of adenosylcobalamin (AdoCbl, coenzyme B12). One pathway, conserved among bacteria, depends on a bifunctional kinase/guanylyltransferase (CobP) enzyme to convert adenosylcobinamide (AdoCbi) to AdoCbi-phosphate (AdoCbi-P), an intermediate in de novo AdoCbl biosynthesis. The other pathway, of archaeal origin, depends on an AdoCbi amidohydrolase (CbiZ) enzyme to generate adenosylcobyric acid (AdoCby), which is converted to AdoCbi-P by the AdoCbi-P synthetase (CobD) enzyme. Here we report that R. sphaeroides strain 2.4.1 synthesizes AdoCbl de novo and that it salvages Cbi using both of the predicted Cbi salvaging pathways. AdoCbl produced by R. sphaeroides was identified and quantified by high-performance liquid chromatography and bioassay. The deletion of cobB (encoding an essential enzyme of the de novo corrin ring biosynthetic pathway) resulted in a strain of R. sphaeroides that would not grow on acetate in the absence of exogenous corrinoids. The results from a nutritional analysis showed that the presence of either CbiZ or CobP was necessary and sufficient for Cbi salvaging, that CbiZ-dependent Cbi salvaging depended on the presence of CobD, and that CobP-dependent Cbi salvaging occurred in a cbiZ+ strain. Possible reasons why R. sphaeroides maintains two distinct pathways for Cbi salvaging are discussed.
Cobamides, such as adenosylcobalamin (AdoCbl, coenzyme B12), are a group of complex cobalt-containing cyclic tetrapyrrole cofactors whose biosynthesis by bacteria and archaea requires substantial genetic information (>25 genes) (reviewed in references 25, 47, and 56). Two pathways for the de novo synthesis of the corrin ring have been described on the basis of the timing of cobalt insertion into the ring. The late cobalt insertion or aerobic pathway has been well studied in Pseudomonas denitrificans (9), while the early cobalt insertion or anaerobic pathway has been best studied in Salmonella enterica serovar Typhimurium LT2 (25). Many organisms, including those that synthesize AdoCbl de novo, salvage incomplete corrinoids (e.g., cobinamide [Cbi]) from their environments and use them as precursors for the synthesis of complete cobamide cofactors. Cbi is not an intermediate of the de novo AdoCbl biosynthesis pathway but can be converted into one by a process known as Cbi salvaging (Fig. 1) (24).
FIG. 1.
Abbreviated view of cobinamide salvaging pathways. Corrin ring-containing intermediates are in bold text. The letter A indicates the de novo corrin ring biosynthesis pathway. Abbreviations: Ado-, adenosyl-; AP, 1-amino-2-propanol; AP-P, 1-amino-2-propanol-phosphate; CobB, hydrogenobyrinic acid a,c-diamide synthase; CobD, adenosylcobinamide-phosphate synthetase; CobP, NTP:adenosylcobinamide kinase, GTP:adenosylcobinamide-phosphate guanylyltransferase; CobY, GTP:adenosylcobinamide-phosphate guanylyltransferase; CbiZ, adenosylcobinamide amidohydrolase. Functional groups are indicated as follows: Me, methyl; Ac, acetamide; and Pr, propionamide.
The first step of Cbi salvaging is adenosylation of the molecule to adenosylcobinamide (AdoCbi) (24). The adenosyltransferases which catalyze this reaction are broadly distributed throughout the three domains of life (13, 14, 20, 32, 38). Two distinct pathways for converting AdoCbi into an intermediate of the de novo AdoCbl biosynthesis pathway have been described for prokaryotes. One, which is to date found only in bacteria, relies on a bifunctional nucleoside triphosphate (NTP):AdoCbi kinase (EC 2.7.7.62), GTP:AdoCbi-phosphate (AdoCbi-P) guanylyltransferase (EC 2.7.1.156) enzyme (called CobP in P. denitrificans and CobU in S. Typhimurium), which phosphorylates AdoCbi to AdoCbi-P and converts AdoCbi-P to AdoCbi-GDP (10, 41, 55).
Previous work from our laboratory has shown that archaea lack the bifunctional NTP:AdoCbi kinase, GTP:AdoCbi-P guanylyltransferase enzyme and rely on a second pathway for Cbi salvaging (54, 62). In this pathway, AdoCbi is converted to adenosylcobyric acid (AdoCby) by an AdoCbi amidohydrolase (EC 3.5.1.90) known as CbiZ (58, 59, 62). The conversion of AdoCbi-P to AdoCbi-GDP for de novo AdoCbl biosynthesis in archaea is catalyzed by a monofunctional GTP:AdoCbi-P guanylyltransferase (EC 2.7.7.62) called CobY (54, 60), which has not been found in any bacterium.
We recently showed that a small percentage of bacterial genomes encode orthologs of both CobP-type and CbiZ-type Cbi salvaging enzymes, raising the question of why these organisms might contain two redundant Cbi salvaging systems (29). A phylogenetic analysis showed that CbiZ has its roots in the archaea and that the cbiZ gene was acquired by several bacterial lineages via horizontal gene transfer.
We previously showed that the CbiZ and CobP enzymes from the photosynthetic alphaproteobacterium Rhodobacter sphaeroides are functional in vitro and in vivo in a heterologous complementation system (29). However, the question of how the two Cbi salvaging systems might function in R. sphaeroides remained unresolved.
In this paper, we show that R. sphaeroides 2.4.1 synthesizes substantial amounts of cobalamin (Cbl) and that it salvages incomplete corrinoids from its environment. We present in vivo genetic evidence that both the bacterial-type CobP-dependent and archaeal-type CbiZ-dependent Cbi salvaging pathways are functional in this organism. This work represents the first in vivo genetic analysis of coenzyme B12 synthesis and salvaging in R. sphaeroides.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains and plasmids used in this study are listed in Table 1. All R. sphaeroides strains were derived from wild-type strain 2.4.1 (17). R. sphaeroides was grown at 30°C in Sistrom's medium A (52) lacking glutamate and aspartate and supplemented with 1 mg liter−1 CoCl2; hereafter, this modified Sistrom's medium is referred to as mSistrom's medium. Potassium succinate (30 mM) or potassium acetate (30 mM) was added as the sole carbon source. Escherichia coli was grown at 37°C in lysogenic broth (Difco) (7, 8). S. Typhimurium strains were derived from strain TR6583 (metE205 ara-9). S. Typhimurium was grown at 37°C in nutrient broth (NB) (Difco) or no-carbon E minimal medium (NCE) (6), containing MgSO4 (1 mM), glycerol (22 mM), and trace minerals (4).
TABLE 1.
Strains and plasmids used in this studya
| Strain or plasmid | Marker(s)b | Relevant genotype or characteristic | Reference(s) or source |
|---|---|---|---|
| R. sphaeroides strains | |||
| JE8777 | 2.4.1 wild type | T. Donohue | |
| JE10256 | ΔcbiZ | ||
| JE10476 | ΔcobP | ||
| JE10508 | ΔcbiZ ΔcobP | ||
| JE10754 | ΔcobB | ||
| JE10755 | ΔcobB ΔcbiZ | ||
| JE10756 | ΔcobB ΔcbiZ ΔcobP | ||
| JE10780 | Tcr | ΔcobB ΔcbiZ ΔcobP pRK404 | |
| JE10785 | Tcr | ΔcobB ΔcbiZ ΔcobP pCOBY47 | |
| JE10966 | ΔcobB ΔcobD | ||
| JE11296 | ΔcobB ΔcobP | ||
| JE11769 | Spr, Smr | ΔcobB ΔcbiZ cobD::aadA+ | |
| JE11843 | ΔcobB ΔcobP | ||
| JE11844 | ΔcobB ΔcobD ΔcobP | ||
| JE11902 | Tcr | ΔcobB ΔcobP pRK404 | |
| JE11903 | Tcr | ΔcobB ΔcobP pCOBY47 | |
| JE11906 | Tcr | ΔcobB ΔcobD ΔcobP pCOBY47 | |
| S. Typhimurium strains | |||
| TR6583 | metE205 ara-9 derivative of S. Typhimurium LT2 | K. Sanderson via J. R. Roth | |
| Derivatives of TR6583 | |||
| JE8185 | cbiB1309 | Lab collection | |
| JE8248 | cobS1313 | Lab collection | |
| JE10911 | Tcr | cbiB1309 pRsCOBD2 | |
| JE10912 | Tcr | cbiB1309 pRK404 | |
| E. coli strains | |||
| DH5α/F′ | F′ endA1 hsdR17(rK− mK+) glnV44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacIZYA-argF)U169 deoR φ80dlacΔ(lacZ)M15 | 45, 57 | |
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-kan+::Tn7 | 51 | |
| Plasmids | |||
| pGEM-T Easy | Apr | TA cloning vector | Promega |
| pK18mobsacB | Kmr, Sucs | Suicide vector for allelic exchange | 48 |
| pRK404 | Tcr | Shuttle vector with lac promoter | 19 |
| pSRA2 | Apr, Spr, Smr | aadA+ | 26 |
| pCOBY47 | Tcr | M. mazei cobY+ cloned into pRK404 | |
| pRsCBIZ4 | Kmr, Sucs | EcoRI-XbaI fragment for deletion of R. sphaeroides cbiZ | |
| pRsCBIZ5 | Kmr, Sucs | ΔcbiZ for R. sphaeroides | |
| pRsCOBB1 | Kmr, Sucs | EcoRI-BamHI fragment for deletion of R. sphaeroides cobB | |
| pRsCOBB2 | Kmr, Sucs | ΔcobB for R. sphaeroides | |
| pRsCOBD1 | Kmr, Sucs | XbaI-PstI fragment for deletion of R. sphaeroides cobD | |
| pRsCOBD2 | Tcr | R. sphaeroides cobD+ | |
| pRsCOBD3 | Kmr, Sucs | ΔcobD for R. sphaeroides | |
| pRsCOBD4 | Kmr, Sucs, Spr, Smr | cobD::aadA+ for R. sphaeroides | |
| pRsCOBP5 | Kmr, Sucs | EcoRI-XbaI fragment for deletion of R. sphaeroides cobP | |
| pRsCOBP7 | Kmr, Sucs | ΔcobP for R. sphaeroides |
Unless otherwise stated, all strains were constructed during the course of these studies.
Tcr, tetracycline resistance; Spr, spectinomycin resistance; Smr, streptomycin resistance; Apr, ampicillin resistance; Kmr, kanamycin resistance; Sucs, sucrose susceptibility.
For growth curves of R. sphaeroides, starter cultures were grown aerobically for 2 days in mSistrom's medium containing succinate and appropriate antibiotics. The cells were rinsed twice with sterile mSistrom's medium containing no carbon and were used to inoculate fresh medium (5% [vol/vol] inoculum). For the photoheterotrophic growth of R. sphaeroides, starter cultures were grown aerobically for 2 days in mSistrom's medium containing succinate and appropriate antibiotics. The cells were rinsed twice with sterile mSistrom's medium containing no carbon and were used to inoculate fresh medium (1% [vol/vol] inoculum) in airtight bottles with minimal headspace. The cultures were incubated for 3 days at 30°C with light with no shaking. For growth curves of S. Typhimurium, starter cultures were grown aerobically overnight in NB containing appropriate antibiotics and were used to inoculate fresh medium (0.5% or 5% [vol/vol] inoculum, as indicated in the legends of Fig. 2 and S2). Aerobic growth curves were obtained using a PowerWave XS microplate reader (Bio-Tek Instruments). When used, ampicillin was at 100 μg ml−1, kanamycin at 10 μg ml−1, tetracycline was at 12.5 μg ml−1 (for E. coli and S. Typhimurium) or 1 μg ml−1 (for R. sphaeroides), and spectinomycin was at 100 μg ml−1 (for E. coli) or 50 μg ml−1 (for R. sphaeroides). Dicyanocobyric acid [(CN)2Cby] was a gift from Paul Renz (Institut für Biologische Chemie und Ernährungswissenschaft, Universität-Hohenheim, Stuttgart, Germany). All other chemicals were purchased from Sigma.
FIG. 2.
The growth rate of an S. Typhimurium strain lacking adenosylcobalamin (5′-P) synthase is correlated with the concentration of cobamide in the medium. Cobamide-dependent, aerobic growth of S. Typhimurium. Strain JE8248 (metE205 ara-9 cob1313 [ΔcobS]) was grown in NCE medium (0.5% [vol/vol] inoculum) containing glycerol (22 mM), MgSO4 (1 mM), and trace minerals. CNCbl was added to the indicated concentrations. The OD at 37°C was monitored at 650 nm, and growth rates (ΔOD650 per h) were determined for the period from 6 to 8 h. Growth curves were obtained as described in the text. Each growth curve was performed eight times in triplicate, and error bars of one standard deviation are indicated. Equations of best-fit lines and correlation coefficients (R2 values) were calculated using Prism v4 (GraphPad Software).
Genetic and molecular techniques.
DNA manipulations were performed using previously described methods (3). Restriction and modification enzymes were purchased from Fermentas (Ontario, Canada) and were used according to the manufacturer's instructions. All DNA manipulations were performed with E. coli DH5α (45, 57). Plasmid DNA was isolated with the Wizard Plus SV plasmid miniprep kit (Promega). The PCR products were purified with the Wizard SV gel and PCR clean-up system kit (Promega). DNA sequencing reactions were performed using nonradioactive BigDye protocols (ABI Prism; Applied Biosystems) and resolved at the Biotechnology Center of the University of Wisconsin, Madison, WI. The plasmids derived from plasmids pRK404 (19) and pK18mobsacB (48) were conjugated into R. sphaeroides as described previously (37).
Sequence analysis.
Computer analyses of the DNA and protein sequences were done using the Integrated Microbial Genomes system (38) and tools available from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
Construction of the R. sphaeroides ΔcbiZ mutant.
The sequences of all the primers used in this work can be found in Table S1 (supplemental material). Primers Rs_ΔcbiZ_EcoRI_5′ and Rs_ΔcbiZ_XbaI_3′ were used to amplify a 1,569-bp fragment of R. sphaeroides chromosomal DNA containing 69 bp of the 5′ end of cbiZ (locus tag RSP_2406) and 1,500 bp of upstream sequence. This fragment was cloned into the EcoRI and XbaI sites of plasmid pK18mobsacB to yield plasmid pRsCBIZ4. Primers Rs_ΔcbiZ_XbaI_5′ and Rs_ΔcbiZ_SalI_3′ were used to amplify a 1,551-bp fragment of R. sphaeroides chromosomal DNA containing 48 bp of the 3′ end of cbiZ and 1,503 bp of downstream sequence. This fragment was cloned into the XbaI and SalI sites of pRsCBIZ4 to yield plasmid pRsCBIZ5, which contained an in-frame deletion of cbiZ that removed bp 70 to 610 and created a 6-bp XbaI restriction site. The hypothetical gene product encoded by this ΔcbiZ allele would be a 41-amino-acid (aa) peptide.
Plasmid pRsCBIZ5 was conjugated into wild-type R. sphaeroides, and the resulting mixed culture plated on mSistrom's agar containing cyanocobalamin (CNCbl, 1 μM) and kanamycin. A kanamycin-resistant transconjugant was picked, inoculated into 50 ml of mSistrom's broth containing potassium succinate (30 mM) and CNCbl (1 μM), and grown 3 days at 30°C with shaking. This culture was subcultured into 0.5 liter of mSistrom's broth containing potassium succinate (30 mM), CNCbl (1 μM), and sucrose (10%, wt/vol) and incubated 3 days at 30°C with light without shaking. This culture was diluted and plated on mSistrom's agar containing potassium succinate (30 mM) and CNCbl (1 μM) and incubated 3 days at 30°C. The resulting colonies were screened for kanamycin-sensitive variants on mSistrom's agar plates containing potassium succinate (30 mM) and CNCbl (1 μM). The presence of the in-frame deletion of cbiZ was confirmed after amplification and sequencing of the cbiZ region using primers Rs_cbiZ_seq_F and Rs_cbiZ_seq_R.
Construction of the R. sphaeroides ΔcobB mutant.
Primers Rs_ΔcobB_EcoRI_5′ and Rs_ΔcobB_BamHI_3′ were used to amplify a 1,498-bp fragment of R. sphaeroides chromosomal DNA containing 9 bp of the 5′ end of cobB (locus tag RSP_3185) and 1,489 bp of upstream sequence. This fragment was cloned into the EcoRI and BamHI sites of plasmid pK18mobsacB to yield plasmid pRsCOBB1. Primers Rs_ΔcobB_BamHI_5′ and Rs_ΔcobB_XbaI_3′ were used to amplify a 1,517-bp fragment of R. sphaeroides chromosomal DNA containing 21 bp of the 3′ end of cobB and 1,496 bp of downstream sequence. This fragment was cloned into the BamHI and XbaI sites of pRsCOBB1 to yield plasmid pRsCOBB2, which contained an in-frame deletion that removed bp 10 to 1,302 and introduced a 6-bp BamHI restriction site. The hypothetical gene product encoded by this ΔcobB allele would be a 12-aa peptide.
Plasmid pRsCOBB2 was conjugated into the wild-type and ΔcbiZ R. sphaeroides strains, and the mutants were isolated as described above for the construction of ΔcbiZ. The presence of the in-frame deletion of cobB was confirmed by amplifying and sequencing the cobB region using primers Rs_cobB_seq_F and Rs_cobB_seq_R.
Construction of R. sphaeroides cobD strains.
Primers Rs_ΔcobD_XbaI_5′ and Rs_ΔcobB_PstI_3′ were used to amplify a 1,486-bp fragment of R. sphaeroides chromosomal DNA containing 9 bp of the 5′ end of cobD (locus tag RSP_0430) and 1,477 bp of upstream sequence. This fragment was cloned into the XbaI and PstI sites of plasmid pK18mobsacB to yield plasmid pRsCOBD1. Primers Rs_ΔcobD_PstI_5′ and Rs_ΔcobD_HindIII_3′ were used to amplify a 1,503-bp fragment of R. sphaeroides chromosomal DNA containing 21 bp of the 3′ end of cobD and 1,482 bp of downstream sequence. This fragment was cloned into the PstI and HindIII sites of pRsCOBD1 to yield plasmid pRsCOBD3, which contained an in-frame deletion of cobD that removed bp 10 to 912 and introduced a 6-bp PstI restriction site. The hypothetical gene product encoded by this ΔcobD allele would be a 12-aa peptide.
We replaced cobD with a spectinomycin resistance marker by cutting plasmid pSRA2 (26) with PstI to release the aadA+ gene encoding spectinomycin resistance. The DNA fragment containing the aad+ gene was gel purified using commercially available kits (Promega) and cloned into the PstI site of pRsCOBD3 to yield pRsCOBD4.
Plasmid pRsCOBD3 was conjugated into the R. sphaeroides ΔcobB strain, and mutants were isolated as described above for the construction of ΔcbiZ. Plasmid pRsCOBD4 was conjugated into R. sphaeroides ΔcobB ΔcbiZ, and the resulting strain was inoculated into 150 ml of mSistrom's medium containing potassium succinate (30 mM), CNCbl (50 nM), sucrose (10%, wt/vol), and spectinomycin and incubated 3 days at 30°C with light without shaking. After incubation, the cultures were diluted and plated on mSistrom's agar containing potassium succinate (30 mM), CNCbl (15 nM), and spectinomycin and incubated 3 days at 30°C in the dark. The colonies exposed to sucrose were screened for spectinomycin-resistant, kanamycin-sensitive variants on mSistrom's agar plates containing potassium succinate (30 mM) and CNCbl (1 μM). We confirmed the presence of mutant alleles of cobD in strains of interest by amplifying and sequencing the cobD region using primers Rs_cobD_seq_F and Rs_cobD_seq_R.
Construction of the R. sphaeroides ΔcobP mutant.
Primers Rs_ΔcobP_EcoRI_5′ and Rs_ΔcobP_XbaI_3′ were used to amplify a 1,502-bp fragment of R. sphaeroides chromosomal DNA containing 27 bp of the 5′ end of cobP (locus tag RSP_0602) and 1,475 bp of upstream sequence. This fragment was cloned into the EcoRI and XbaI sites of plasmid pK18mobsacB to yield plasmid pRsCOBP5. Primers Rs_ΔcobP_XbaI_5′ and Rs_ΔcobP_SalI_3′ were used to amplify a 1,545-bp fragment of R. sphaeroides chromosomal DNA containing 42 bp of the 3′ end of cobP and 1,543 bp of downstream sequence. This fragment was cloned into the XbaI and SalI sites of pRsCOBP5 to yield plasmid pRsCOBP7, which contained an in-frame deletion of cobP that removed bp 28 to 510 and introduced a 6-bp XbaI restriction site. The hypothetical gene product encoded by this ΔcobP allele would be a 25-aa peptide.
Plasmid pRsCOBP7 was conjugated into the wild-type, ΔcbiZ, ΔcobB, ΔcobB ΔcbiZ, and ΔcobB ΔcobD R. sphaeroides strains, and cobP derivatives of these strains were isolated as described above for the construction of the ΔcbiZ mutant. The presence of the in-frame deletion of cobP was confirmed by amplifying and sequencing the cobP region using primers Rs_cobP_seq_F and Rs_cobP_seq_R.
Construction of the cobY+ plasmid.
The Methanosarcina mazei cobY+ coding sequence was amplified using primers Mm_cobY_BamHI_5′ and Mm_cobY_BamHI_3′, and the resulting product was cloned into pGEM-T Easy (Promega), according to the manufacturer's instructions. The cobY+ coding sequence was excised from pGEM-T Easy with BamHI and was ligated into the BamHI site of plasmid pRK404 to yield plasmid pCOBY47. We confirmed that cobY+ was in the correct orientation by sequencing with primers pRK404_seq_F and pRK404_seq_R.
Construction of the cobD+ plasmid.
The R. sphaeroides cobD+ coding sequence plus 37 bp of the 5′ sequence was amplified using primers Rs_cobD_HindIII_5′ and Rs_cobD_PstI_3′, and the resulting product was cloned into the HindIII and PstI sites of plasmid pRK404 to yield plasmid pRsCOBD2. The identity of the insert was confirmed by sequencing with primers pRK404_seq_F and pRK404_seq_R.
Preparation of cyanopseudocobalamin (CNpseudoCbl).
When grown on 1,2-propanediol, S. Typhimurium does not synthesize Cbl but rather synthesizes the Cbl analog pseudoCbl, which contains an adenine moiety in place of the 5,6-dimethylbenzimidazole lower ligand of Cbl (2). S. Typhimurium strain TR6583 (metE205 ara-9) was grown overnight in 30 ml NB broth at 37°C with shaking. This culture was used to inoculate 6 liters of NCE minimal medium containing glycerol (10 mM), 1,2-propanediol (80 mM), MgSO4 (1 mM), dicyanocobinamide [(CN)2Cbi] (1 μM), and trace minerals (4) and incubated overnight at 37°C with shaking. Cells were harvested by centrifugation using a Beckman-Coulter Avanti J-25I centrifuge (15 min at 5,000 × g at 4°C). Corrinoids were extracted by resuspending the cell pellets in 2 volumes of methanol and shaking (200 rpm) for 2 h at 55°C (61). Cell debris was removed by centrifugation (2 h at 40,000 × g at 4°C), and the supernatants were dried under vacuum using a Savant concentrator (Thermo), resuspended in distilled H2O, and desalted with a 1-ml C18 Sep-Pak cartridge (Waters). The cartridge was washed with 40 ml distilled H2O, and corrinoids were eluted with methanol, dried under vacuum, and resuspended in 275 μl of potassium phosphate buffer (0.1 M, pH 6.5) containing KCN (10 mM). Cyano corrinoids were obtained after exposure to bright light for 30 min, followed by filtration using Corning 0.2-μm Spin-X centrifuge filters.
Corrinoids were resolved using a Beckman Coulter System Gold 126 high-pressure liquid chromatography (HPLC) system equipped with a 150- by 4.6-mm Alltima HP C18 AQ column (Alltech). The column was developed with the slightly modified system I of Blanche et al. (11); corrinoids were detected with a photodiode array detector. The column was equilibrated at 1 ml min−1 with 85% solvent A (0.1 M potassium phosphate buffer [pH 6.5] plus 10 mM KCN) and 15% solvent B (50 mM potassium phosphate buffer [pH 8] plus 5 mM KCN, 50% acetonitrile). The column was developed, starting 2 min after injection, with a 48-min linear gradient to 40% solvent B. A second linear gradient developed the column to 100% solvent B over 16 min. A compound that eluted 27 min after injection was collected, dried under vacuum, resuspended in distilled H2O, and desalted with a 1-ml C18 Sep-Pak cartridge (Waters). The cartridge was washed with 40 ml distilled H2O, and CNpseudoCbl was eluted with methanol, dried under vacuum, and resuspended in 0.25 ml distilled H2O. The quantity of CNpseudoCbl purified was estimated at 420 nmol by comparison of A367 values to a standard curve of (CN)2Cbi (data not shown).
Corrinoid extraction and purification.
R. sphaeroides 2.4.1 was grown aerobically for 3 days in mSistrom's broth with 30 mM potassium succinate or potassium acetate. CFU were quantified by plating serial dilutions of cultures in triplicate on mSistrom's agar containing potassium succinate (30 mM). Cells were harvested and corrinoids were extracted as described above. Cell debris was removed by centrifugation (2 h at 40,000 × g at 4°C), and the supernatants were dried under vacuum and then resuspended in 1 ml of 0.1 M potassium phosphate buffer (pH 6.5) containing 10 mM KCN. Corrinoids were converted from their naturally occurring methyl-, hydroxy-, or adenosylated forms to their cyano forms by exposure to bright light for 30 min.
Corrinoids were separated by HPLC as described above. The column was equilibrated at 1 ml min−1 with 98% solvent A and 2% solvent B. The column was developed, starting 5 min after injection, with a 55-min linear gradient to 100% solvent B. Authentic (CN)2Cby, (CN)2Cbi, CNpseudoCbl, and CNCbl were used as standards and eluted at 21.6, 26.3, 25, and 28 to 32 min, respectively. The fractions were collected, dried under vacuum, and resuspended in 250 μl distilled H2O.
Corrinoid analysis.
The identity and quantity of the cobamides produced by R. sphaeroides were assessed by a bioassay using S. Typhimurium strain JE8248 (ΔcobS) as an indicator strain. In strain JE8248, the last step of Cbl synthesis is blocked, making growth dependent on the presence of exogenous cobamides (42). The growth of strain JE8248 indicated the presence of Cbl or another cobamide in the extract. We inoculated JE8248 (0.5%, vol/vol) into NCE minimal medium containing glycerol (11 mM), MgSO4 (1 mM), trace minerals (4), and concentrations of CNCbl between 25 and 250 pM. Growth kinetics under aerobic conditions at 37°C were monitored at 650 nm (data not shown). The slopes of the growth curves between 6 and 8 h (early exponential phase) were plotted against the concentrations of CNCbl in the medium, and a best-fit line was drawn (Fig. 2). JE8248 was grown in medium supplemented with fractions collected from HPLC separations, and the concentration of cobamides in these samples was calculated using the equation of the standard curve obtained with CNCbl. The limit of detection of the quantitative bioassay was 5 fmol of CNCbl.
RESULTS AND DISCUSSION
R. sphaeroides 2.4.1 synthesizes cobalamin via the aerobic pathway.
A bioinformatic analysis of the genome of R. sphaeroides 2.4.1 revealed the presence of genes encoding homologues of most of the known enzymes of the aerobic pathway of AdoCbl biosynthesis (56) (see Fig. S1 and Table S2 in the supplemental material). Therefore, in this paper, we use the P. denitrificans nomenclature for AdoCbl biosynthesis genes to refer to the cognate R. sphaeroides genes. Two of the predicted AdoCbl biosynthesis genes, putatively encoding 5,6-dimethylbenzimidazole (DMB) synthase (BluB) and hydrogenobyrinic acid a,c-diamide synthase (CobB), were located on chromosome 2, but the rest were found in nine loci on chromosome 1. Interestingly, the genome of R. sphaeroides 2.4.1 lacks a recognizable monooxygenase (such as CobG [49, 50] or CobZ [39]) of the type required for corrin ring contraction in other AdoCbl-synthesizing organisms.
Previous studies have reported that various strains of R. sphaeroides synthesized AdoCbl (16, 21, 30), but none of these studies looked specifically at corrinoid production by strain 2.4.1. We used a combined HPLC and quantitative bioassay procedure to determine the identity and quantity of cobamides produced by R. sphaeroides 2.4.1. (Fig. 2 and 3.)
FIG. 3.
Amount of cobalamin made by R. sphaeroides changes as a function of the carbon source. (A) Cobamide content of cells grown aerobically on succinate. Corrinoids extracted from R. sphaeroides 2.4.1 cells grown aerobically in mSistrom's medium containing succinate (30 mM) were separated by RP-HPLC. The cobamide contents of 2-min fractions were determined by quantitative bioassay and normalized to CFU. The error bars of one standard deviation are indicated. (B) Cobamide content of cells grown photoheterotrophically on succinate. Corrinoids extracted from R. sphaeroides 2.4.1 cells grown photoheterotrophically under oxygen-limited conditions in mSistrom's medium containing succinate (30 mM) were separated by RP-HPLC. The cobamide contents of 2-min fractions were determined by quantitative bioassay and normalized to CFU. The error bars of one standard deviation are indicated. (C) Cobamide content of cells grown photoheterotrophically on succinate. Zoomed-in view of panel B, showing the cobamide contents of early-eluting HPLC fractions. (D) Cobamide content of cells grown aerobically on acetate. Corrinoids extracted from R. sphaeroides 2.4.1 cells grown aerobically in mSistrom's medium containing acetate (30 mM) were separated by RP-HPLC. The cobamide contents of 2-min fractions were determined by quantitative bioassay and normalized to CFU. The error bars of one standard deviation are indicated. (E) Cobamide content of cells grown photoheterotrophically on acetate. Corrinoids extracted from R. sphaeroides 2.4.1 cells grown photoheterotrophically under oxygen-limited conditions in mSistrom's medium containing acetate (30 mM) were separated by RP-HPLC. The cobamide contents of 2-min fractions were determined by quantitative bioassay and normalized to CFU. The error bars of one standard deviation are indicated. (F.) Cobamide standards. Authentic CNCbl and CNpseudoCbl standards were separated by RP-HPLC. The cobamide contents of 2-min fractions were determined by quantitative bioassay and normalized to the percentage of the total corrinoids injected in the known sample. The error bars of one standard deviation are indicated.
R. sphaeroides 2.4.1 was grown aerobically or photoheterotrophically in mSistrom's medium containing succinate or acetate (30 mM each). Stationary-phase cultures of R. sphaeroides grown aerobically on succinate or acetate contained an average of 3.3 × 109 and 1.2 × 109 CFU per ml, respectively. Stationary-phase cultures of R. sphaeroides grown photoheterotrophically on succinate or acetate contained an average of 3.2 × 109 and 5.2 × 108 CFU per ml, respectively. Corrinoids were extracted from R. sphaeroides cultures, converted to their cyano forms, and resolved by reversed-phase (RP)-HPLC. Substantial amounts of pigments were present in the cell extracts. To ensure that none of these compounds prevented the detection of cobamides, fractions collected from the separated extracts were diluted and tested for the ability to allow the growth of S. Typhimurium strain JE8248 (ΔcobS), which lacks Cbl (5′-P) synthase, and is therefore a cobamide auxotroph (42). S. Typhimurium is known to be able to use cobamides with a variety of lower ligands, including DMB (34), adenine, 2-methyladenine (35), and benzimidazole (33), as well as a cobamide called norcobalamin, which differs from Cbl in that it lacks the methyl group at C176 in the loop connecting the lower ligand to the corrin ring (64). A cobamide auxotroph of the closely related bacterium E. coli grows in the presence of cobamides containing an even larger variety of lower ligands (15, 44), suggesting that the identity of the lower ligand is relatively unimportant to enteric bacteria. Therefore, while we were unable to rule out the possibility that R. sphaeroides synthesized a cobamide which could not be detected by our S. Typhimurium bioassay, we consider this unlikely. The growth rate of strain JE8248 was directly proportional to the concentration of cobamide in the medium (Fig. 2). We used the growth rate of strain JE8248 in medium supplemented with fractionated R. sphaeroides extracts to calculate the amount of cobamide present in each fraction. Growth rate was normalized to CFU to estimate the number of molecules of cobamide synthesized per cell under each growth condition tested.
Figure 3A shows the profile of cobamides extracted from R. sphaeroides grown aerobically on 30 mM succinate. Most of the cyano derivatives of cobamides detected were associated with a broad peak eluting between 28 and 32 min. This elution profile was consistent with the one obtained with authentic CNCbl (Fig. 3F, black trace and bars). Figure 3B shows the profile of cobamides extracted from R. sphaeroides grown photoheterotrophically on 30 mM succinate. Figure 3C is an expanded view of Fig. 3B, showing the cobamides detected in fractions eluting between 20 and 26 min. Using the abovementioned bioassay, we calculated that succinate-grown R. sphaeroides 2.4.1 synthesized approximately 3,000 molecules of cobamide per cell under either aerobic or low-oxygen photosynthetic conditions. In contrast, acetate-grown R. sphaeroides 2.4.1 synthesized approximately 17,000 molecules of cobamide per cell under aerobic conditions (Fig. 3D) and approximately 7,000 molecules of cobamide per cell under low-oxygen photosynthetic conditions (Fig. 3E).
Previously reported work indicated that aerobically grown R. sphaeroides synthesized both Cbl and pseudoCbl (30). Cbl and pseudoCbl are cobamides that differ in the identity of the lower ligand; in Cbl, the lower ligand base is DMB, while in pseudoCbl it is adenine (46). Our bioassay was able to detect both Cbl and pseudoCbl and discriminated between them on the basis of their HPLC elution times (Fig. 3F). PseudoCbl eluted as a sharp peak, detectable by the growth of JE8248 on the fraction collected between 24 and 26 min. We did not detect the formation of pseudoCbl during the aerobic growth of R. sphaeroides 2.4.1 on succinate or during photosynthetic growth on acetate. Approximately 45 molecules per cell of cobamide consistent in elution time with pseudoCbl were detected during aerobic growth on acetate (Fig. 3D). Approximately 12 molecules per cell of cobamide consistent in elution time with pseudoCbl and 16 molecules per cell of unidentified earlier-eluting cobamides were detected during photosynthetic growth on succinate (Fig. 3C). No cobamides eluting between 20 and 24 min were detected under any other growth condition tested.
Notably, the genome of R. sphaeroides 2.4.1 encodes a homolog of the BluB DMB synthase known to catalyze the O2-dependent synthesis of DMB (see Fig. S1 and Table S2 in the supplemental material) (28, 53). This observation, together with the data shown in Fig. 3, led us to conclude that, under the conditions tested, the bulk of the cobamides synthesized by R. sphaeroides 2.4.1 are Cbl. Due to the conversion of cobamides to their cyano forms for analysis, we were unable to determine the identities of the upper ligands of the Cbl produced by R. sphaeroides, although the presence of orthologs of the CobO and PduO corrinoid adenosyltransferases (18, 32) in this organism (see Fig. S1 and Table S2 in the supplemental material) suggests that at least some was AdoCbl.
The photosynthetic growth conditions used, while oxygen limited, were not strictly anoxic, and we presume that the small amounts of oxygen present allowed the activity of the BluB DMB synthase, permitting Cbl synthesis under these conditions. The reduction of cobamide synthesis by acetate-grown cells under photosynthetic conditions relative to aerobic conditions (Fig. 3D and E) may therefore be due to oxygen limitation. Experiments are currently under way to test these ideas.
An R. sphaeroides strain that cannot synthesize the corrin ring cannot grow on acetate.
To test corrin-salvaging phenotypes in R. sphaeroides, we constructed strain JE10754, which harbored an in-frame deletion of the cobB gene, which putatively encodes hydrogenobyrinic acid a,c-diamide synthase, an enzyme required for de novo corrin ring biosynthesis (56). Recent work by others has shown that the growth of R. sphaeroides on acetate proceeds via a pathway that involves two AdoCbl-dependent enzymes, namely, ethylmalonyl-coenzyme A (CoA) mutase and methylmalonyl-CoA mutase (1, 22, 23, 63). Therefore, we predicted that a strain unable to synthesize AdoCbl would not grow on acetate.
Unlike the wild-type strain, JE10754 (ΔcobB), which could not synthesize the corrin ring de novo, failed to grow on acetate in the absence of exogenous corrinoids. Under the conditions used, the wild-type strain grew to a maximal optical density at 650 nm (OD650) of 0.59, with an exponential-phase growth rate of 0.04 ΔOD650 h−1. JE10754 grew as well as the wild-type if (CN)2Cby, (CN)2Cbi, or CNCbl (for structures see Fig. 1) was present in the medium, to maximal OD650 values of 0.65, 0.64, and 0.55, respectively, and with an exponential-phase growth rate of 0.04 ΔOD650 h−1 in all cases. These results demonstrated the ability of R. sphaeroides to salvage corrinoids from its environment and the need for Cbl during growth on acetate. The growth of JE10754 with succinate as a carbon source was comparable to that of the wild type on the first passage in corrinoid-free medium, but this strain was unable to grow on succinate after further passages into media lacking exogenous corrinoids (data not shown). This suggested that low levels of cobamides were required for the growth of R. sphaeroides on succinate and indicated that ethylmalonyl-CoA mutase and methylmalonyl-CoA mutase were not the only cobamide-requiring enzymes in R. sphaeroides.
A strain that lacks cobinamide amidohydrolase (AP forming, CbiZ) and cobinamide kinase/cobinamide-phosphate guanylyltransferase (CobP) activities cannot salvage cobinamide.
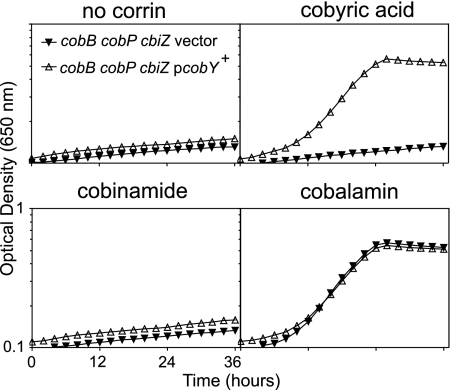
Before examining the roles of CbiZ- and CobP-dependent Cbi salvaging in R. sphaeroides, we needed to confirm that these were the only two pathways for Cbi salvaging in this organism. Therefore, we constructed a triple mutant containing in-frame deletions of both cobP and cbiZ in a ΔcobB strain. The resulting strain, JE10756 (ΔcobB ΔcbiZ ΔcobP), grew on acetate in the presence of CNCbl but not when (CN)2Cby or (CN)2Cbi substituted for CNCbl (Fig. 4, solid triangles). The inability of JE10756 to grow on (CN)2Cby was predicted to be due to the loss of CobP and its associated AdoCbi-P guanylyltransferase activity. Supporting this idea, growth on (CN)2Cby, but not on (CN)2Cbi, was restored by the ectopic expression of the Methanosarcina mazei cobY+ gene, which encodes AdoCbi-P guanylyltransferase (Fig. 4, open triangles) (54, 58). These results indicated that cobP and cbiZ encoded the only Cbi-salvaging functions in R. sphaeroides. The inability of strain JE10785 (ΔcobB ΔcobP ΔcbiZ pcobY+) to salvage Cbi indicated that CobP was the only enzyme with significant Cbi kinase activity in R. sphaeroides; no growth of strain JE10785 was observed even after 48 h of incubation (data not shown). The S. Typhimurium YcfN thiamine kinase has Cbi kinase activity (43), but a bioinformatic analysis of the R. sphaeroides 2.4.1 genome did not reveal homologs of YcfN.
FIG. 4.
A strain lacking CbiZ and CobP cannot salvage cobinamide. Shown is the corrinoid-dependent aerobic growth of strain JE10708 (R. sphaeroides 2.4.1 ΔcobB ΔcobP ΔcbiZ pRK404 tet+; vector, solid triangles) and strain JE10785 (ΔcobB ΔcobP ΔcbiZ pCOBY47 M. mazei cobY+ tet+; pcobY+, open triangles) in mSistrom's medium containing acetate (30 mM) and tetracycline (1 μg μl−1). The OD650 was measured for 36 h at 30°C. Corrinoids were added at 15 nM. Growth curves were obtained as described in the text. Each growth curve was performed in triplicate, and error bars of one standard deviation are indicated. The scales of the axes for each panel are identical and are indicated for the lower left-hand panel only.
The CbiZ-dependent cobinamide salvaging system can function independently from CobP but requires AdoCbi-P synthetase (CobD) activity.
Knowing that the only pathways for Cbi salvaging in R. sphaeroides were either CbiZ or CobP dependent (Fig. 4), we next wanted to determine if these two systems could function independently in vivo. To test whether the archaeal-type CbiZ-dependent Cbi salvaging system of R. sphaeroides could function in the absence of CobP, we constructed strain JE11902, which harbored chromosomal deletions of cobB and cobP. As expected (Fig. 1), strain JE11902, which lacked AdoCbi-P guanylyltransferase activity, was unable to grow on acetate in the presence of either (CN)2Cby or (CN)2Cbi (Fig. 5, open squares). To overcome this difficulty, we constructed strain JE11903, which harbored chromosomal deletions of cobB and cobP, as well as plasmid pCOBY47 cobY+. Strain JE11903, which carried the wild-type allele of cbiZ, grew on acetate in the presence of (CN)2Cby or (CN)2Cbi (Fig. 5, solid triangles), suggesting that, similarly to Cbi salvaging in archaea, the entry point of Cbi into the pathway was AdoCbi-P synthetase (58, 62). To test this idea, we constructed strain JE11844, which harbored chromosomal deletions of cobB and cobP, the cobY+ plasmid pCOBY47, and an in-frame deletion of the cobD gene, which we predicted would encode the AdoCbi-P synthetase enzyme in R. sphaeroides. The identity of the R. sphaeroides cobD gene product as AdoCbi-P synthase was confirmed by the complementation of (CN)2Cby salvaging with this gene in an S. Typhimurium strain lacking AdoCbi-P synthase activity (see Fig. S2 in the supplemental material). As expected, the deletion of cobD abolished the ability of the ΔcobB ΔcobP pcobY+ strain to salvage both (CN)2Cby and (CN)2Cbi (Fig. 5, open circles). These results showed that R. sphaeroides could salvage Cbi in the absence of CobP by using CbiZ to cleave Cbi to Cby (Fig. 1).
FIG. 5.
The CbiZ-dependent cobinamide salvaging system of R. sphaeroides is functional and requires CobD activity. Shown is the corrinoid-dependent aerobic growth of strain JE11902 (R. sphaeroides 2.4.1 ΔcobB ΔcobP pRK404 tet+; vector, open squares), strain JE11903 (R. sphaeroides 2.4.1 ΔcobB ΔcobP pCOBY47 M. mazei cobY+ tet+; pcobY+, solid triangles), and strain JE11906 (ΔcobB ΔcobP ΔcobD pCOBY47 M. mazei cobY+ tet+; pcobY+, open circles) in mSistrom's medium containing acetate (30 mM) and tetracycline (1 μg μl−1). The OD650 was measured for 36 h at 30°C. Corrinoids were added at 15 nM. Growth curves were obtained as described in the text. Each growth curve was performed in triplicate, and the error bars of one standard deviation are indicated. The scales of the axes for each panel are identical and are indicated for the lower left-hand panel only.
The cobP-dependent cobinamide salvaging system does not require AdoCbi-P synthetase activity.
To test whether the bacterial-type cobP-dependent Cbi salvaging system of R. sphaeroides could function in the absence of cbiZ, we constructed strain JE10755, which contained in-frame deletions of both cobB and cbiZ. JE10755, which contained a wild-type allele of cobP, grew on acetate in the presence of (CN)2Cby, (CN)2Cbi, or CNCbl (Fig. 6, solid diamonds). To confirm that the observed growth on (CN)2Cbi was due to the Cbi kinase activity of CobP (10, 29), we constructed strain JE11769, which contained in-frame deletions of cobB and cbiZ and in which cobD was replaced with the aadA spectinomycin resistance gene. As expected (Fig. 1), the loss of cobD abolished the ability of the ΔcobB ΔcbiZ strain to salvage (CN)2Cby but did not affect (CN)2Cbi salvaging (Fig. 6, open circles). These results showed that R. sphaeroides could salvage Cbi in the absence of CbiZ by using the Cbi kinase activity of CobP.
FIG. 6.
The CobP-dependent cobinamide salvaging system of R. sphaeroides is functional and does not require CobD activity. Shown is the corrinoid-dependent aerobic growth of strain JE10755 (R. sphaeroides 2.4.1 ΔcobB ΔcbiZ; filled diamonds) and strain JE11769 (ΔcobB ΔcbiZ cobD::aadA+; open circles) in mSistrom's medium containing acetate (30 mM). The OD650 was measured for 36 h at 30°C. Corrinoids were added at 15 nM. Growth curves were obtained as described in the text. Each growth curve was performed in triplicate, and the error bars of one standard deviation are indicated. The scales of the axes for each panel are identical and are indicated for the lower left-hand panel only.
Cobinamide salvaging does not proceed obligately by the cbiZ-dependent pathway in a cbiZ+ strain.
In R. sphaeroides, the cbiZ gene is found in a locus with genes encoding predicted homologs of the BtuBFCD corrinoid-specific ABC transport system (see Fig. S1 in the supplemental material) (12, 31). It is possible that, in R. sphaeroides, the CbiZ enzyme associates with the BtuCD inner-membrane proteins to ensure the efficient cleavage of Cbi to Cby as it enters the cell. If this were the case, we predicted that Cbi salvaging might always proceed via the CbiZ-dependent pathway in a strain carrying a wild-type allele of cbiZ. To test this hypothesis, we constructed strain JE10966, which contained in-frame deletions of both cobB and cobD. As expected, JE10966 did not grow on acetate in the presence of Cby (data not shown). However, in the presence of (CN)2Cbi, JE10966 grew to a maximal OD650 of 0.61 at an exponential-phase growth rate of 0.04 ΔOD650 hour−1. The growth of JE10966 on (CN)2Cbi indicated that Cbi salvaging proceeded via the cobP-dependent pathway and that not all Cbi entering the cell was cleaved to Cby by CbiZ. Further experiments will be required to determine the relative flux of Cbi through each salvaging pathway.
What is the physiological role of CbiZ in R. sphaeroides?
CbiZ is required for Cbi salvaging in archaea (58, 62), but our results (Fig. 6) showed that CobP is sufficient for Cbi salvaging in R. sphaeroides. What, then, is the physiological role of CbiZ in this organism, and in other bacteria whose genomes contain homologues of both CobP and CbiZ (29)?
The CbiZ homologue of the archaeon Methanopyrus kandleri has significant Cbl amidohydrolase activity (59), demonstrating that CbiZ enzymes are not limited to removing the aminopropanol group of Cbi but can also remove the lower ligand from cobamides, converting them to Cby. Natural cobamides exist with a wide variety of lower ligands (46), and the activity of some cobamide-dependent enzymes depends on the identity of the lower ligand (5, 36). Our data show that Cbl is preferentially synthesized by R. sphaeroides (Fig. 3).
In vitro, the R. sphaeroides CbiZ enzyme uses Cbl as a substrate, albeit poorly (29), but it has not been tested with any other cobamide (e.g., pseudoCbl).
We speculate that one or more of the cobamide-dependent enzymes of R. sphaeroides may require a DMB lower ligand for activity and that the role of CbiZ in this organism may be to cleave the lower ligand from non-Cbl cobamides found in the environment, so they can be converted into Cbl.
There are several reported or predicted cobamide-dependent enzymes in R. sphaeroides, including the ethylmalonyl-CoA and methylmalonyl-CoA mutases required for growth on acetate (MeaA, locus tag RSP_0961; McmA, locus tag RSP_2192, respectively) (1, 22, 23), methionine synthase (MetH, locus tag RSP_3346) (16), glutamate mutase (40) (the genome of R. sphaeroides 2.4.1 does not encode a homolog of known forms of this enzyme), class II ribonucleotide reductase (NrdJ; locus tag RSP_2495), and Mg-protoporphyrin monomethyl ester cyclase (BchE, locus tag RSP_0281) (27). One or more of these enzymes may require Cbl. If this hypothesis of the physiological role of CbiZ were correct, the R. sphaeroides CbiZ might be significantly more active with pseudoCbl than Cbl. Experiments are under way to test this idea.
Supplementary Material
Acknowledgments
This work was supported by PHS grant R01-GM40313 from the National Institute of General Medical Sciences (to J.C.E.-S.). M.J.G. was supported in part by the College of Agricultural and Life Sciences Louis and Elsa Thomsen Wisconsin Distinguished Graduate Fellowship.
We thank Tim Donohue (UW—Madison) for providing R. sphaeroides 2.4.1 and plasmid pSRA2 and Jörn Kalinowski (Universität Bielefeld) for providing plasmid pK18mobsacB. We also thank Yann Dufour, Roger Greenwell, Rachelle Lemke, and Eva Ziegelhoffer (UW—Madison) for valuable advice about working with R. sphaeroides.
Footnotes
Published ahead of print on 17 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alber, B. E., R. Spanheimer, C. Ebenau-Jehle, and G. Fuchs. 2006. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol. Microbiol. 61297-309. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P. J., J. Lango, C. Carkeet, A. Britten, B. Kräutler, B. D. Hammock, and J. R. Roth. 2008. One pathway can incorporate either adenine or dimethylbenzimidazole as an alpha-axial ligand of B12 cofactors in Salmonella enterica. J. Bacteriol. 1901160-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. Greene Publishing Associates & Wiley Interscience, New York, NY.
- 4.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker, H. A., R. D. Smyth, H. Weissbach, J. I. Toohey, J. N. Ladd, and B. E. Volcani. 1960. Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole. J. Biol. Chem. 235480-488. [PubMed] [Google Scholar]
- 6.Berkowitz, D., J. M. Hushon, H. J. Whitfield, Jr., J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertani, G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanche, F., B. Cameron, J. Crouzet, L. Debussche, D. Thibaut, M. Vuilhorgne, F. J. Leeper, and A. R. Battersby. 1995. Vitamin B12: how the problem of its biosynthesis was solved. Angew. Chem. Int. Ed. Engl. 34383-411. [Google Scholar]
- 10.Blanche, F., L. Debussche, A. Famechon, D. Thibaut, B. Cameron, and J. Crouzet. 1991. A bifunctional protein from Pseudomonas denitrificans carries cobinamide kinase and cobinamide phosphate guanylyltransferase activities. J. Bacteriol. 1736052-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanche, F., D. Thibaut, M. Couder, and J. C. Muller. 1990. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal. Biochem. 18924-29. [DOI] [PubMed] [Google Scholar]
- 12.Borths, E. L., B. Poolman, R. N. Hvorup, K. P. Locher, and D. C. Rees. 2005. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake. Biochemistry 4416301-16319. [DOI] [PubMed] [Google Scholar]
- 13.Buan, N. R., K. Rehfeld, and J. C. Escalante-Semerena. 2006. Studies of the CobA-type ATP:Co(I)rrinoid adenosyltransferase enzyme of Methanosarcina mazei strain Gö1. J. Bacteriol. 1883543-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buan, N. R., S. J. Suh, and J. C. Escalante-Semerena. 2004. The eutT gene of Salmonella enterica encodes an oxygen-labile, metal-containing ATP:corrinoid adenosyltransferase enzyme. J. Bacteriol. 1865708-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkholder, P. R. 1951. Determination of vitamin B12 with a mutant strain of Escherichia coli. Science 114459-460. [DOI] [PubMed] [Google Scholar]
- 16.Cauthen, S. E., J. R. Pattison, and J. Lascelles. 1967. Vitamin B(12) in photosynthetic bacteria and methionine synthesis by Rhodopseudomonas spheroides. Biochem. J. 102774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhary, M., X. Zanhua, Y. X. Fu, and S. Kaplan. 2007. Genome analyses of three strains of Rhodobacter sphaeroides: evidence of rapid evolution of chromosome II. J. Bacteriol. 1891914-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debussche, L., M. Couder, D. Thibaut, B. Cameron, J. Crouzet, and F. Blanche. 1991. Purification and partial characterization of Cob(I)alamin adenosyltransferase from Pseudomonas denitrificans. J. Bacteriol. 1736300-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditta, G., T. Schmidhauser, E. Yakobson, P. Lu, X. W. Liang, D. R. Finlay, D. Guiney, and D. R. Helinski. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13149-153. [DOI] [PubMed] [Google Scholar]
- 20.Dobson, C. M., T. Wai, D. Leclerc, H. Kadir, M. Narang, J. P. Lerner-Ellis, T. J. Hudson, D. S. Rosenblatt, and R. A. Gravel. 2002. Identification of the gene responsible for the cblB complementation group of vitamin B12-dependent methylmalonic aciduria. Hum. Mol. Genet. 113361-3369. [DOI] [PubMed] [Google Scholar]
- 21.Dresow, B., G. Schlingmann, L. Ernst, and V. Koppenhagen. 1980. Extracellular metal-free corrinoids from Rhodopseudomonas sphaeroides. J. Biol. Chem. 2557637-7644. [PubMed] [Google Scholar]
- 22.Erb, T. J., I. A. Berg, V. Brecht, M. Muller, G. Fuchs, and B. E. Alber. 2007. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA 10410631-10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erb, T. J., J. Retey, G. Fuchs, and B. E. Alber. 2008. Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclade of coenzyme B12-dependent acyl-CoA mutases. J. Biol. Chem. 28332283-32293. [DOI] [PubMed] [Google Scholar]
- 24.Escalante-Semerena, J. C. 2007. Conversion of cobinamide into adenosylcobamide in bacteria and archaea. J. Bacteriol. 1894555-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escalante-Semerena, J. C., and M. J. Warren. 5 August 2008, posting date. Chapter 3.6.3.8, Biosynthesis and use of cobalamin (B12). In A. Böck, R. Curtiss III, J. B. Kaper, P. D. Karp, F. C. Neidhardt, T. Nyström, J. M. Slauch, and C. L. Squires (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org.
- 26.Frigaard, N. U., H. Li, K. J. Milks, and D. A. Bryant. 2004. Nine mutants of Chlorobium tepidum each unable to synthesize a different chlorosome protein still assemble functional chlorosomes. J. Bacteriol. 186646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gough, S. P., B. O. Petersen, and J. O. Duus. 2000. Anaerobic chlorophyll isocyclic ring formation in Rhodobacter capsulatus requires a cobalamin cofactor. Proc. Natl. Acad. Sci. USA 976908-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray, M. J., and J. C. Escalante-Semerena. 2007. Single-enzyme conversion of FMNH2 to 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc. Natl. Acad. Sci. USA 1042921-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray, M. J., N. K. Tavares, and J. C. Escalante-Semerena. 2008. The genome of Rhodobacter sphaeroides strain 2.4.1 encodes functional cobinamide salvaging systems of archaeal and bacterial origins. Mol. Microbiol. 70824-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi, M., and T. Kamikubo. 1970. Isolation of 5,6-dimethylbenzimidazolyl cobamide coenzyme from Rhodopseudomonas spheroides. FEBS Lett. 10249-252. [DOI] [PubMed] [Google Scholar]
- 31.Hvorup, R. N., B. A. Goetz, M. Niederer, K. Hollenstein, E. Perozo, and K. P. Locher. 2007. Asymmetry in the structure of the ABC transporter binding protein complex BtuCD-BtuF. Science 3171387-1390. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, C. L., E. Pechonick, S. D. Park, G. D. Havemann, N. A. Leal, and T. A. Bobik. 2001. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J. Bacteriol. 1831577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, M. G., and J. C. Escalante-Semerena. 1992. Identification of 5,6-dimethylbenzimidazole as the Coα ligand of the cobamide synthesized by Salmonella typhimurium. Nutritional characterization of mutants defective in biosynthesis of the imidazole ring. J. Biol. Chem. 26713302-13305. [PubMed] [Google Scholar]
- 34.Keck, B., M. Munder, and P. Renz. 1998. Biosynthesis of cobalamin in Salmonella typhimurium: transformation of riboflavin into the 5,6-dimethylbenzimidazole moiety. Arch. Microbiol. 17166-68. [DOI] [PubMed] [Google Scholar]
- 35.Keck, B., and P. Renz. 2000. Salmonella typhimurium forms adenylcobamide and 2-methyladenylcobamide, but no detectable cobalamin during strictly anaerobic growth. Arch. Microbiol. 17376-77. [DOI] [PubMed] [Google Scholar]
- 36.Lengyel, P., R. Mazumder, and S. Ochoa. 1960. Mammalian methylmalonyl isomerase and vitamin B(12) coenzymes. Proc. Natl. Acad. Sci. USA 461312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang, J. H., G. M. Nielsen, D. P. Lies, R. H. Burris, G. P. Roberts, and P. W. Ludden. 1991. Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J. Bacteriol. 1736903-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowitz, V. M., F. Korzeniewski, K. Palaniappan, E. Szeto, G. Werner, A. Padki, X. Zhao, I. Dubchak, P. Hugenholtz, I. Anderson, A. Lykidis, K. Mavromatis, N. Ivanova, and N. C. Kyrpides. 2006. The integrated microbial genomes (IMG) system. Nucleic Acids Res. 34D344-D348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGoldrick, H. M., C. A. Roessner, E. Raux, A. D. Lawrence, K. J. McLean, A. W. Munro, S. Santabarbara, S. E. Rigby, P. Heathcote, A. I. Scott, and M. J. Warren. 2005. Identification and characterization of a novel vitamin B12 (cobalamin) biosynthetic enzyme (CobZ) from Rhodobacter capsulatus, containing flavin, heme, and Fe-S cofactors. J. Biol. Chem. 2801086-1094. [DOI] [PubMed] [Google Scholar]
- 40.Ohmori, H., H. Ishitani, K. Sato, S. Shimizu, and S. Fukui. 1974. Metabolism of glutamate in purple nonsulfur bacteria: participation of vitamin B12. Agric. Biol. Chem. 38359-365. [Google Scholar]
- 41.O'Toole, G. A., and J. C. Escalante-Semerena. 1995. Purification and characterization of the bifunctional CobU enzyme of Salmonella typhimurium LT2. Evidence for a CobU-GMP intermediate. J. Biol. Chem. 27023560-23569. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G. A., M. R. Rondon, and J. C. Escalante-Semerena. 1993. Analysis of mutants of Salmonella typhimurium defective in the synthesis of the nucleotide loop of cobalamin. J. Bacteriol. 1753317-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otte, M. M., J. D. Woodson, and J. C. Escalante-Semerena. 2007. The thiamine kinase (YcfN) enzyme plays a minor but significant role in cobinamide salvaging in Salmonella enterica. J. Bacteriol. 1897310-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlman, D., and J. M. Barrett. 1958. Biosynthesis of cobalamin analogues by Propionibacterium arabinosum. Can. J. Microbiol. 49-15. [DOI] [PubMed] [Google Scholar]
- 45.Raleigh, E. A., K. Lech, and R. Brent. 1989. Selected topics from classical bacterial genetics, p. 1.4. In F. A. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. Wiley Interscience, New York, NY. [DOI] [PubMed] [Google Scholar]
- 46.Renz, P. 1999. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of other bases found in natural corrinoids, p. 557-575. In R. Banerjee (ed.), Chemistry and biochemistry of B12. John Wiley & Sons, Inc., New York, NY.
- 47.Roessner, C. A., and A. I. Scott. 2006. Fine-tuning our knowledge of the anaerobic route to cobalamin (vitamin B12). J. Bacteriol. 1887331-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 14569-73. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder, S., A. D. Lawrence, R. Biedendieck, R. S. Rose, E. Deery, R. M. Graham, K. J. McLean, A. W. Munro, S. E. Rigby, and M. J. Warren. 2009. Demonstration that CobG, the monooxygenase associated with the ring contraction process of the aerobic cobalamin (vitamin B12) biosynthetic pathway, contains a Fe-S center and a mononuclear non-heme iron center. J. Biol. Chem. 2844796-4805. [DOI] [PubMed] [Google Scholar]
- 50.Scott, A. I., C. A. Roessner, N. J. Stolowich, J. B. Spencer, C. Min, and S. I. Ozaki. 1993. Biosynthesis of vitamin B12. Discovery of the enzymes for oxidative ring contraction and insertion of the fourth methyl group. FEBS Lett. 331105-108. [DOI] [PubMed] [Google Scholar]
- 51.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 52.Sistrom, W. R. 1960. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J. Gen. Microbiol. 22778-785. [DOI] [PubMed] [Google Scholar]
- 53.Taga, M. E., N. A. Larsen, A. R. Howard-Jones, C. T. Walsh, and G. C. Walker. 2007. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature 446449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas, M. G., and J. C. Escalante-Semerena. 2000. Identification of an alternative nucleoside triphosphate: 5′-deoxyadenosylcobinamide phosphate nucleotidyltransferase in Methanobacterium thermoautotrophicum ΔH. J. Bacteriol. 1824227-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas, M. G., T. B. Thompson, I. Rayment, and J. C. Escalante-Semerena. 2000. Analysis of the adenosylcobinamide kinase/adenosylcobinamide phosphate guanylyltransferase (CobU) enzyme of Salmonella typhimurium LT2. Identification of residue H46 as the site of guanylylation. J. Biol. Chem. 27527376-27386. [DOI] [PubMed] [Google Scholar]
- 56.Warren, M. J., E. Raux, H. L. Schubert, and J. C. Escalante-Semerena. 2002. The biosynthesis of adenosylcobalamin (vitamin B12). Nat. Prod. Rep. 19390-412. [DOI] [PubMed] [Google Scholar]
- 57.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. De Cruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 173469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodson, J. D., and J. C. Escalante-Semerena. 2004. CbiZ, an amidohydrolase enzyme required for salvaging the coenzyme B12 precursor cobinamide in archaea. Proc. Natl. Acad. Sci. USA 1013591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodson, J. D., and J. C. Escalante-Semerena. 2006. The cbiS gene of the archaeon Methanopyrus kandleri AV19 encodes a bifunctional enzyme with adenosylcobinamide amidohydrolase and α-ribazole-phosphate phosphatase activities. J. Bacteriol. 1884227-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodson, J. D., R. F. Peck, M. P. Krebs, and J. C. Escalante-Semerena. 2003. The cobY gene of the archaeon Halobacterium sp. strain NRC-1 is required for de novo cobamide synthesis. J. Bacteriol. 185311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodson, J. D., A. A. Reynolds, and J. C. Escalante-Semerena. 2005. ABC transporter for corrinoids in Halobacterium sp. strain NRC-1. J. Bacteriol. 1875901-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodson, J. D., C. L. Zayas, and J. C. Escalante-Semerena. 2003. A new pathway for salvaging the coenzyme B12 precursor cobinamide in archaea requires cobinamide-phosphate synthase (CbiB) enzyme activity. J. Bacteriol. 1857193-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zarzycki, J., A. Schlichting, N. Strychalsky, M. Muller, B. E. Alber, and G. Fuchs. 2008. Mesaconyl-coenzyme A hydratase, a new enzyme of two central carbon metabolic pathways in bacteria. J. Bacteriol. 1901366-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zayas, C. L., K. Claas, and J. C. Escalante-Semerena. 2007. The CbiB protein of Salmonella enterica is an integral membrane protein involved in the last step of the de novo corrin ring biosynthetic pathway. J. Bacteriol. 1897697-7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.