Summary
Oxidative stress serves as an important host/environmental signal that triggers a wide range of responses from the human pathogen Staphylococcus aureus. Among these, a thiol-based oxidation sensing pathway through a global regulator MgrA controls the virulence and antibiotic resistance of the bacterium. Herein, we report a new thiol-based oxidation sensing and regulation system that is mediated through a parallel global regulator SarZ. SarZ is a functional homologue of MgrA and is shown to affect the expression of ~87 genes in S. aureus. It uses a key Cys residue, Cys13, to sense oxidative stress and to coordinate the expression of genes involved in metabolic switching, antibiotic resistance, peroxide stress defense, virulence, and cell wall properties. The discovery of this SarZ-mediated regulation, mostly independent from the MgrA-based regulation, fills a missing gap of oxidation sensing and response in S. aureus.
Keywords: sarZ, MgrA homologue, Transcription profiling, Oxidative sensing, Virulence, Antibiotic resistance
Introduction
Staphylococcus aureus is a Gram-positive human pathogen responsible for most wound and hospital-acquired infections (Lowy, 1998; Boyce, 1997). The coordinated expression of the organism’s virulence factors is regulated by global regulators such as Agr, SarA, and MgrA (Novick, 2003; Bronner et al., 2004; Cheung et al., 2004). Elucidating the detailed mechanisms by which these proteins regulate gene expression and their regulatory pathways is crucial to gain a comprehensive picture of S. aureus pathogenesis and may contribute to new strategies for the therapeutic intervention of infections.
The SarA or MarR family proteins are a common class of regulatory proteins in S. aureus. The global regulators SarA and MgrA and their homologues belong to this family of proteins (Bronner et al., 2004; Cheung et al., 2004; Hong et al., 2005; Chen et al., 2006; Cheung et al., 2008). Both SarA and MgrA affect expression of hundreds of genes in S. aureus and play major roles that control various properties of the bacterium (Dunmun et al., 2001; Luong et al., 2006). While the exact molecular mechanism leading to SarA signaling is still unclear, we recently have demonstrated that thiol-based oxidation is responsible for MgrA signaling (Chen et al., 2006). A Cys residue located at the dimerization domain of the MgrA dimer is redox sensitive. Its oxidation leads to dissociation of MgrA from its promoter DNA. A similar redox-sensing mechanism has been shown for OhrR type proteins in other gram positive bacteria (Fuangthong and Helmann, 2002; Lee et al., 2007; Newberry et al., 2007). However, MgrA is the first example of utilizing this mechanism to regulate antibiotic resistance and expression of virulence factors in a pathogen. MgrA contributes to the regulation of over 300 genes (Luong et al., 2006) including genes encoding virulence factors (for example: capsular polysaccharide, nuclease, α-toxin, coagulase, protease, and protein A), genes involved in autolysis (for example: lytM and lytN), other global regulatory genes (for example: agr, lytRS, arlRS, sarS, and sarV), and genes encoding efflux pumps such as norA, norB, and tet38 that confer resistance to antibiotics (Ingavale et al., 2003; Luong et al., 2003; Truong-Bolduc et al., 2003; Ingavale et al., 2005; Kaatz et al., 2005; Truong-Bolduc et al., 2005).
A search for MgrA homologues in the whole genome of S. aureus identified MgrH1 (MgrA Homologue 1) which shares a high sequence identity with MgrA and OhrR proteins (Fig. 1). MgrH1 is also known as SarZ due to its homology to SarA (Cheung et al., 2004). Most noticeably, the lone Cys residue that is key to oxidative sensing in MgrA and OhrR is conserved in MgrH1. Meanwhile, residues that form a hydrogen bonding network around this Cys and a hydrophobic pocket near the Cys are conserved among MgrA, OhrR, and MgrH1. These observations prompted us to propose that MgrH1, a member of MarR family proteins in S. aureus, could serve as a functional homologue of the global regulator MgrA and employ a similar oxidative sensing mechanism to regulate gene activation. In fact, a recently published study by Sekimizu and colleagues (Kaito et al., 2006) showed that this protein plays multiple roles as a regulator of both virulence factors and hemolysis genes in S. aureus. In the present study, we will use the name SarZ instead of MgrH1. In this paper, we show that SarZ affects the expression of 87 genes. Most importantly, we demonstrate that SarZ is a thiol-based oxidation sensing protein. SarZ senses oxidative stress and exerts a global regulatory role on metabolism, antibiotic resistance, oxidation resistance, autolysis, and virulence in S. aureus.
Fig. 1.
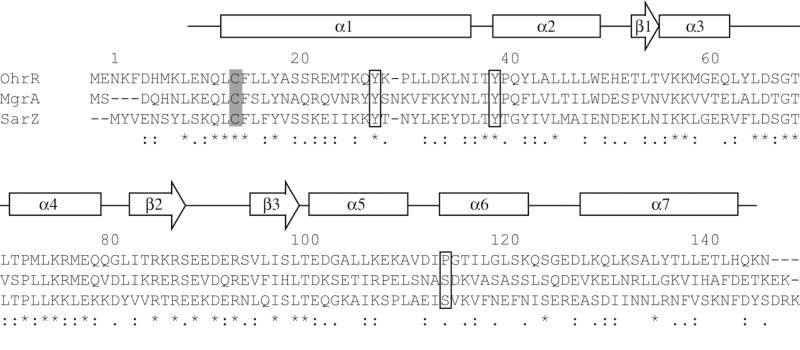
Sequence alignment of SarZ with its homologues MgrA and OhrR. Alignment of SarZ and MgrA sequences from S. aureus N315 and OhrR sequence from B. subtilis 168 was performed with CLUSTAL W ver. 1.83 program (Thompson et al., 1994). Asterisks, colons, and periods below the alignment indicate fully, strongly, and weakly conserved residues, respectively. The key Cys13 residues are shaded. Tyr26, Tyr38, Ser113 are denoted by squares. The secondary structure elements of MgrA are labeled as α and β to α helices and β sheet, respectively. Protein residue numbering based on the SarZ sequence.
Results and Discussion
Sequence similarity between SarZ and MgrA
A search for close homologues of MgrA in the GenBank database identified the SarZ protein. The sequence similarity between MgrA and SarZ is 71% with 34% identical residues (Fig. 1). The MgrA redox sensitive residue, Cys12, is conserved as the sole Cys residue, Cys13, in SarZ. Cys12 of MgrA is recognized by a network of hydrogen bonds involving Tyr26, Tyr38, and Ser113 from the other monomer (Chen et al., 2006). This network is also conserved in SarZ and provided by residues of Tyr27, Tyr38, and Ser113 (Fig. 1). In addition, residues that form a hydrophobic pocket in MgrA appear to be conserved in the sequence of SarZ. Collectively, these common features suggest that SarZ is a functional homologue of MgrA and uses its Cys13 residue to sense oxidative stress for gene regulation.
A recent report showed that sarZ regulates hemolysin production in S. aureus and affects expression of certain virulence factors (Kaito et al., 2006). However, the regulatory mechanism of SarZ and its effect on global gene activation in S. aureus have not been elucidated.
Identification of sarZ-regulated genes by microarray
First, transcription profiling of mRNAs was performed using Affymetrix GeneChips for the wild-type S. aureus Newman and the isogenic sarZ mutant (Bae et al., 2004) to identify genes controlled by sarZ. Transcription profiling of several other staphylococcal global regulators (agr, arlRS, mgrA, sarA, rot, and sigB) has already been used to evaluate their respective regulons (Dunman et al., 2001; Saïd-Salim et al., 2003; Bischoff et al., 2004; Liang et al., 2005; Luong et al., 2006). In addition, transcription profiling following perturbations such as heat, cold, stringent stress, SOS stress, mild acidic pH, H2O2, HOCl, NO, peracetic acid, and salicylate have been obtained and have revealed valuable information on stress responses of S. aureus (Weinrick, et al., 2004; Anderson et al., 2006; Chang et al., 2006a; Chang et al., 2006b; Richardson et al., 2006; Chang et al., 2007; Riordan et al., 2007).
In triplicate, S. aureus strain Newman and isogenic sarZ mutant cells were grown to both log (2 h, OD600=0.6) and early stationary (5 h, OD600=2.0) phases to take into account the possibility of growth phase-dependent regulation, then total RNA of each strain was isolated from each growth phase. Each RNA sample was subsequently labeled, hybridized to an S. aureus Affymetrix GeneChip and processed, according to manufacturer recommendations. Following normalization, replicates were averaged and the transcript titers of each mRNA species were compared across strains at each time point, as previously described (Beenken et al., 2004; Bischoff et al., 2004; Weinrick et al., 2004; Luong et al., 2006). Genes with decreased expression in the sarZ mutant as compared to wild-type were grouped as genes downregulated in the sarZ mutant (Table 1), while genes with increased expression were grouped as genes upregulated in the sarZ mutant. Real-time RT-PCR was used to verify GeneChip results for five genes from Table 1. As shown in Table 2, there was reasonable correlation between both measurements; although, the fold changes were much more profound in real-time RT-PCR.
Table 1.
S. aureus genes affected by sarZ disruptiona
| ORF | Gene | Fold-change | H2O2 effectb | Description | |
|---|---|---|---|---|---|
| 2 h | 5 h | ||||
| Genes downregulated in the sarZ mutant | |||||
| SACOL1141 | isdC | -2.1 | Iron regulated Surface Determinant system | ||
| SACOL0860 | nuc | -2.5 | - | Thermonuclease precursor | |
| SACOL0459 | pbuX | -2.0 | Xanthine permiase | ||
| SACOL1210 | pyrR | -2.3 | Pyrimidine operon regulatory protein | ||
| SACOL2384 | sarZ | -3.4 | -11.2 | Staphylococcal accessory protein Z | |
| SA0166 | -2.1 | -2.4 | ABC transporter | ||
| SACOL1168 | efb | -2.9 | + | Fibrinogen-binding protein | |
| SACOL1169 | fib | -2.8 | Fibrinogen-binding protein precursor related protein | ||
| SACOL0784 | hisC | -2.0 | Histidinol-phosphate aminotransferase | ||
| SACOL1076 | purS | -2.5 | Phosphoribosylformylglycinamide synthetase | ||
| SACOL2352 | tcaA | -2.4 | Teicoplanin resistance associated protein | ||
| SACOL0157 | -3.0 | α-helical coiled-coil SrpF–like protein | |||
| SA0167 | -2.2 | SrpL–like membrane lipoprotein | |||
| SACOL0159 | -2.2 | ABC transporter | |||
| SACOL0185 | -2.5 | Peptide ABC transporter | |||
| SACOL0186 | -2.7 | Dipeptide ABC transporter, DppC–like protein | |||
| SACOL0218 | -2.0 | + | Conserved hypothetical protein | ||
| SACOL0219 | -2.5 | Conserved hypothetical protein | |||
| SACOL0430 | -2.6 | Cystathionine β-lyase family protein | |||
| SACOL0431 | -2.3 | Cystathionine γ-synthase family protein | |||
| SACOL0505 | -3.1 | ABC transporter | |||
| SACOL0507 | -2.3 | N-acetylmuramoyl-L-Ala amidase precursor | |||
| SACOL0602 | -2.3 | Haloacid dehydrogenase-like protein | |||
| SACOL0883 | -2.7 | ABC transporter | |||
| SACOL0910 | -3.0 | Hypothetical protein | |||
| SACOL1164 | -3.1 | Fibrinogen-binding related protein | |||
| SACOL1996 | -2.4 | ABC transporter | |||
| SACOL1997 | -2.7 | GntR family protein | |||
| SACOL2295 | -3.1 | Staphyloxanthine biosynthesis protein | |||
| SACOL2709 | -2.5 | Conserved hypothetical protein | |||
| SACOL2710 | -2.6 | Conserved hypothetical protein | |||
| Genes upregulated in the sarZ mutant | |||||
| SACOL1783 | acs | 2.2 | - | Acetyl-CoA synthetase | |
| SACOL0964 | argG | 8.2 | Arguininosuccinate synthetase | ||
| SACOL0963 | argH | 5.6 | Arguininosuccinate lyase | ||
| SACOL1429 | asd | 4.4 | Aspartate-semialdehyde dehyrogenase | ||
| SACOL0121 | deoD | 5.2 | + | Purine nucreoside phospholylase | |
| SACOL1872 | epiE | 3.0 | Epidermin immunity protein E | ||
| SACOL1873 | epiF | 2.9 | Epidermin immunity protein F | ||
| SACOL2482 | fabG | 4.1 | + | 3-oxoacyl-ACP reductase | |
| SACOL0655 | fruA | 5.2 | Fructose permiase | ||
| SACOL2525 | gntK | 6.5 | + | Gluconokinase | |
| SACOL2516 | gntR | 5.6 | + | Gluconate operon repressor | |
| SACOL2042 | ilvD | 3.2 | -2.3 | Dihydroxy-acid dehydrates | |
| SACOL2183 | lacD | 2.0 | Tagatose 1, 6-diphosphate aldrase | ||
| SACOL0248 | lrgB | 3.2 | Holin–like protein | ||
| SACOL1428 | lysC | 4.4 | Aspartokinase, α and β subunits | ||
| SACOL1551 | malA | 4.7 | α-glucosidase | ||
| SACOL0192 | msmX | 2.1 | Maltose ABC transporter | ||
| SACOL2397 | nirD | 3.0 | -2.2 | NAD (P) H Nitrite recuctase, small subunit | |
| SACOL1475 | norB | 5.7 | Drug transporter | ||
| SACOL2031 | nrgA | 4.7 | Ammonium transporter family protein | ||
| SACOL0872 | ohr | 2.7 | + | Organic hydroperoxide resistance protein | |
| SACOL1838 | pckA | 2.6 | Posphoenolpyruvate carboxykinase | ||
| SACOL0205 | pflA | 37.3 | + | Pyruvate formate lyase activating enzyme | |
| SACOL0204 | pflB | 18.8 | + | Pyruvate formate lyase | |
| SACOL2376 | scrA | 5.3 | PTS system, Sucrose-specific IIBC component | ||
| SACOL0387 | set11 | 9.1 | Exotoxin 11 | ||
| SACOL0122 | tet38 | 5.1 | + | Tetracycline resistance protein | |
| SACOL0516 | treP | 6.7 | PTS system, IIBC component | ||
| SACOL0517 | treC | 4.0 | α, α phosphotreharase | ||
| SACOL0012 | 2.8 | Homoserin O-acetyltransferase | |||
| SACOL0176 | 6.3 | + | Conserved hypothetical protein | ||
| SACOL0177 | 4.6 | Glucokinase-regulator-related protein | |||
| SACOL0183 | 3.4 | Conserved hypothetical protein | |||
| SACOL0469 | 2.9 | Exotoxin 1 | |||
| SACOL0504 | 6.3 | -3.0 | + | ABC transporter | |
| SACOL0708 | 2.8 | DAK2 domain protein | |||
| SACOL0709 | 3.0 | Conserved hypothetical protein | |||
| SACOL0882 | 2.9 | ABC transporter | |||
| SACOL0884 | 2.1 | -2.3 | ABC transporter | ||
| SACOL1476 | 13.8 | Amino acid permiase | |||
| SACOL1661 | 3.8 | - | Acetyl-CoA carboxylase, accC homologue | ||
| SACOL1847 | 3.8 | Conserved hypothetical protein | |||
| SACOL1849 | 2.3 | - | Conserved hypothetical protein | ||
| SACOL1915 | 4.1 | Glutamate ABC transporter | |||
| SACOL1916 | 3.8 | Amino acid ABC transporter | |||
| SACOL2461 | 3.7 | + | Conserved hypothetical protein | ||
| SACOL2579 | 3.6 | Phytone dehydrogenase | |||
| SACOL2585 | 4.0 | -2.0 | pfoS/R–like regulatory protein | ||
| SACOL2619 | 3.1 | -2.4 | Amino acid permiase | ||
| SACOL2704 | 3.0 | ATP phosphoribosyltransferase reguratory subunit | |||
| SAS0383 | 2.2 | Exotoxin | |||
| SAV0894 | 2.2 | ϕMu50B-like protein | |||
| SACOL0212 | 2.1 | 3-hydroxyacyl-CoA dehydrogenase | |||
The entire microarray data were deposited to the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO, http://www.ncbi.nlm.nih.gov/geo/) with a provisional entry number GSE13138. Three uncharacterized ORFs were omitted in this table.
Previously described ORFs affected by exposure to hydrogen peroxide (Chang et al., 2006a).
+, Upregulation. -, Downregulation.
Table 2.
Relative quantification of expression levels of selected genes controlled by SarZ determined by real-time PCR with primers and SYBR green probes
| Gene | ORF | Real-time PCR | Primers |
|---|---|---|---|
| pflB | SACOL0204 | 146.0 ± 18.2 | 5’-AAAGCAGGCGTTATTACTGAAAGC-3’ 5’-CGTCAATACCTACACCACCGATAG-3’ |
| pflA | SACOL0205 | 56.0 ± 8.6 | 5’-TGACAAACATATTAGATTGACAGGAAAGC-3’ 5’-ATCATCAGAATAACCAGGCACAAGG-3’ |
| lrgB | SACOL0248 | 13.2 ± 2.7 | 5’-CTGTTATCCGTTATACCATTTTTC-3’ 5’-CCACCTATTTTGTAAGTCTTATAC-3’ |
| set11 | SACOL0387 | 13.5 ± 1.0 | 5’-CTATAACGGTTCTAACGTTGTAC-3’ 5’-CACCAACAGTAGATAGTCTAC-3’ |
| ohr | SACOL0872 | 4.0 ± 0.1 | 5’-GAGCATTAGATATTGATATCGTTC-3’ 5’-GTTAGTGTTACTTCTGGATGAG-3’ |
A total of 87 genes were found to be affected by sarZ at either 2 h and/or 5 h sampling times. Among them, 32 genes were downregulated and 55 genes were upregulated in the sarZ mutant. The number of genes downregulated in the sarZ mutant is much less at 2 h than at 5 h; conversely, many more genes are upregulated in the sarZ mutant at 2 h than at 5 h. Only two genes (sarZ and SA0166) were upregulated by sarZ mutation at both log and stationary phases. Thus, the sarZ mutation appears to primarily lead to upregulation of genes in the log-phase cells and downregulation of many genes in the post-exponential cells.
Like MgrA, SarZ appears to be a pleiotropic regulator. Three genes (pbuX, nuc, pyrR) that are downregulated in the sarZ mutant have been reported to be downregulated in the mgrA mutant as well (Luong et al., 2006). Only 6 genes (lacD, norB, scrA, SACOL1476, SACOL1847, SACOL1849) out of 55 that are upregulated in the sarZ mutant in the log phase are also upregulated in the mgrA mutant (Luong et al., 2006). Other than these overlaps, the genes appear to be regulated by sarZ in a manner independent of mgrA.
Genes involved in many metabolic processes are affected by sarZ. For example, genes for the intermediary (acs, pflAB, pckA, SACOL0177) and amino acid metabolisms (argGH, ilvD, lysC, hisC), genes for fatty acid synthesis (fabG, SACOL1661, SACOL0212), and genes for sugar metabolism (gntRK, lacD, malA, treC) show significant changes in the sarZ mutant. In particular, pflA (pyruvate formate lyase activating enzyme, Buis and Broderick 2005) and pflB (pyruvate formate lyase, Lindmark et al., 1969; Lehtio and Goldman 2004) showed the most dramatic changes with 37-fold and 19-fold increases, respectively, in the sarZ mutant compared to the wild-type at the 2 h sampling time. These genes encode two enzymes of the first committed steps of anaerobic respiration (Sawers and Bock 1988; Fuchs et al. 2007), indicating that sarZ may affect switching from oxygen-dependent to anaerobic metabolisms. The acs gene encodes acetyl-CoA synthetase that converts acetate to acetyl-CoA. All of acs, pflB, and pckA are involved in pyruvate metabolism. Acetic acid induces the expression of lrgAB (Rice et al., 2005). Pyruvate is a key metabolite for amino acid and fatty acid metabolism. Thus, sarZ might induce pleiotropic effects by affecting a key metabolite. The sarZ gene also affects 3 regulatory genes (pyrR, gntR, gntR-like gene) involved in regulating pyrimidine and gluconate metabolisms. Expression of 21 transporters for ammonium, purine, amino acids, and sugars were affected by sarZ disruption. In addition, two drug transporters (norB, tet38) showed an increased expression in the sarZ mutant. These transporters have been reported to be regulated by mgrA as well (Truong-Bolduc et al., 2005).
Many genes encoding cell surface proteins including isdC, epiEF, lrgB, efb, fib, tcaA, SACOL0507, SACOL1164, and SA0167 were affected by the sarZ mutation, as well as exonuclease nuc and 4 different exotoxins. A recent study showed that SarZ protein plays multiple roles as a regulator of both virulence factors and hemolysis genes in S. aureus (Kaito et al., 2006). Our microarray analysis did not find significant changes (at least 2-fold change) of hla, hlb, and RNAIII transcription levels in the sarZ mutant strain. Either the changes are small or, very likely, the difference is due to the use of strains (RN4220, NCTC8325-4) that carry the rsbU mutation which caused a reduction of sigB expression (Giachino et al., 2001; Horsburgh et al., 2002) in the previous study (Kaito et al., 2006).
Notably, 17 genes affected by sarZ mutation are also H2O2-responsive genes in S. aureus (Chang et al., 2006a). For instance, an ohr (organic hydroperoxide resistance) like gene (SACOL0872) shows a 2.7-fold increase in the sarZ mutant strain. The ohr gene was first isolated from Xanthomonas camperstris (Mongkolsuk et al., 1998) and studied extensively in B. subtilis (Volker et al., 1998; Fuangthong et al., 2001; Fuangthong and Helmann, 2002; Helmann et al., 2003, Hong et al., 2005; Lee et al., 2007). The organic hydroperoxide resistance gene ohrA in B. subtilis is located next to the cognate negative regulator ohrR that senses peroxide stress. The S. aureus ohr-like gene does not reside close to sarZ in the genome yet it appears to be regulated by SarZ. The pflA and pflB transcripts that were upregulated by sarZ disruption are also dramatically induced by H2O2 stress. These observations, together with the high sequence similarity (and conservation of the key Cys residue) between SarZ and MgrA/OhrR, strongly indicate that SarZ is a sensor and regulator for oxidative stress in S. aureus. To test this hypothesis we performed a series of biochemical and microbiological experiments.
Cys13 oxidation in SarZ
To evaluate the redox activity of Cys13 in SarZ, the protein was cloned (with an N-terminal His6 tag), overexpressed in E. coli, and purified over Ni-NTA column. The purified His6-tagged SarZ protein was reduced completely with DTT. The excess DTT was removed. Reduced SarZ was incubated with 4 equiv of cumene hydroperoxide (CHP) per SarZ monomer. The transient sulfenic acid intermediate (SarZ-Cys13-SOH) could be trapped by 4-chloro-7-nitrobenzo-2-oxa-1, 3-diazole (NBD-Cl) that reacts with sulfenic acid to give a product that absorbs at 347 nm (Fig. 2A), whereas the reduced thiol reacts with NBD-Cl to form a thiol-NBD conjugate that absorbs at 420 nm in a control with reduced SarZ. We further quantified Cys13 oxidation by measuring the free thiol content per SarZ monomer using 5,5-dithiobis (2-nitrobenzoic acid) (DTNB). Since there is only one Cys residue in the entire SarZ protein, we should observe 1 equiv of thiol per protein monomer in the absence of oxidation and no thiol after oxidation. As shown in Fig. 2B, both CHP and H2O2 (4 or 8 equiv per SarZ monomer) effectively oxidized Cys13 to give less than 0.2 equiv of free thiol per SarZ monomer after 10 min treatment.
Fig. 2.
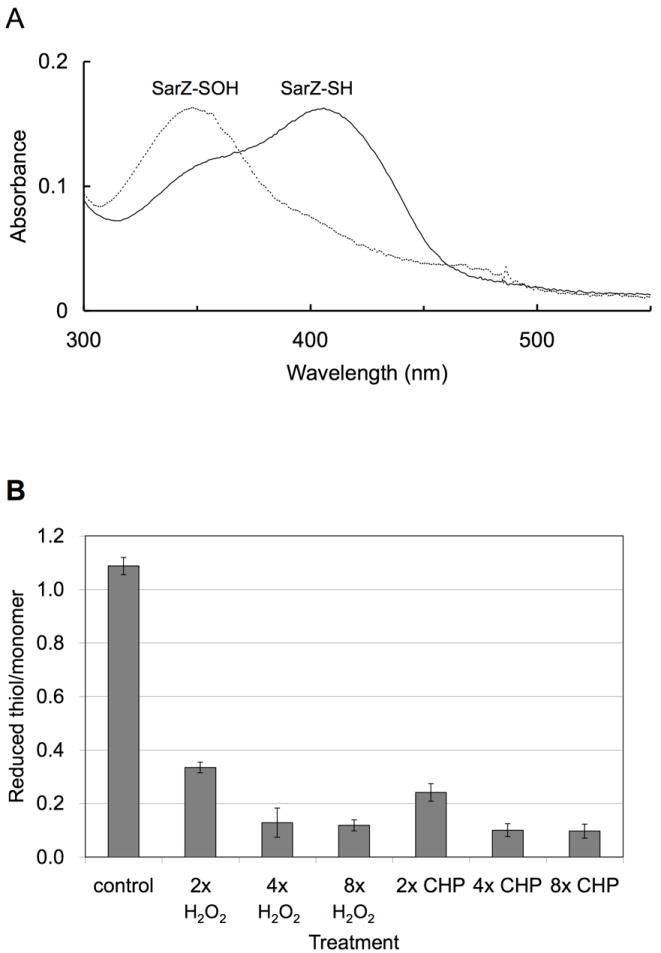
Monitoring oxidation of Cys13 in vitro. (A) Cysteine sulfenic acid formed in vitro from Cys13 oxidation by 4 equiv (per SarZ monomer) of CHP was trapped by the NBD-Cl assay. Reaction of the oxidized SarZ with NBD-Cl (solid line): Cys-S(O)-NBD absorbs at 347 nm; reaction of the reduced SarZ with NBD-Cl (dashed line): Cys-S-NBD absorbs at 420 nm. (B) Quantification of free thiol in SarZ upon oxidation and reduction by the DTNB assay. The reduced form of purified SarZ (control) contains one free thiol per monomer protein. This form of protein was treated with 2, 4 and 8 equiv (per SarZ monomer) of CHP or H2O2 for 10 min to generate the oxidized SarZ. Error bars are s.d.
To further support SarZ Cys13 oxidation in vivo, we employed a Cys alkylation based assay (Lee et al., 2004) with the use of a 600-Da alkylator (PEG-alk, Scheme S1) that efficiently alkylates Cys13 in SarZ in vitro and in vivo. Cell extracts containing His6-tagged SarZ expressed in the sarZ mutant were treated with PEG-alk. The protein extracts were separated by SDS-PAGE, transferred to membrane and the SarZ protein could be detected with an anti-His antibody. In the absence of peroxide stress, alkylation of SarZ led to a 600-Da molecular weight increase that was detected on SDS-PAGE gel. If S. aureus cells were first treated with 10 mM H2O2 for 20 min prior to the alkylation assay, the Cys13 residue of SarZ was modified by oxidation and could not be alkylated by the Cys alkyator as shown in Fig. S1. SarZ C13S mutant protein in H2O2 treated and untreated cells showed the same mobility as the unalkylated SarZ. Thus, these results show that Cys13 in SarZ is modified by exposure to H2O2 inside S. aureus.
Binding of SarZ to ohr promoter is affected by Cys13 oxidation
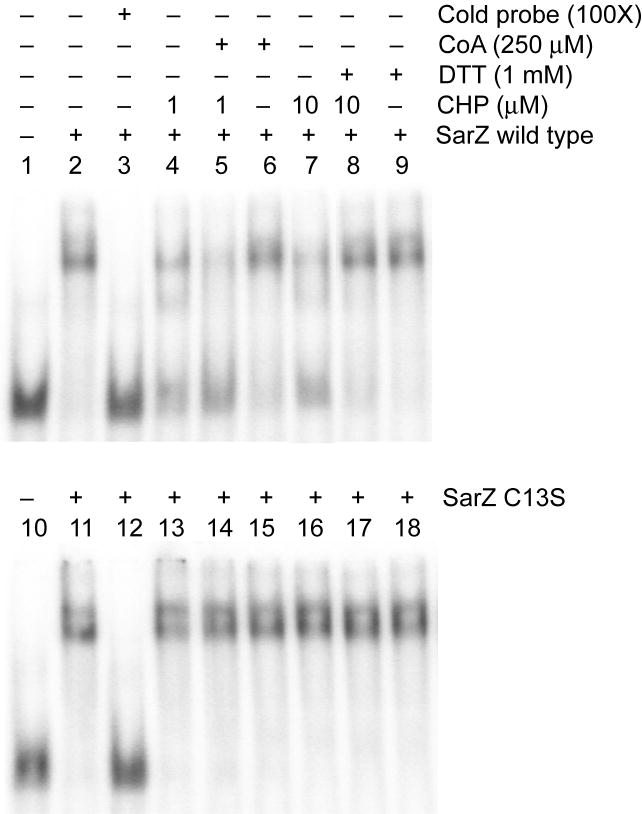
Next, we assessed whether oxidation of Cys13 affected SarZ’s ability to bind DNA. Since ohr is downregulated by sarZ, we examined binding of SarZ to the ohr promoter fragment with an electrophoretic mobility shift assay. A DNA fragment containing the ohr promoter was radioactively labeled, and mixed with purified SarZ. Binding complexes were separated on gel. Low mobility bands were observed with increasing concentrations of SarZ in the gel shift experiment. Approximately 8-fold excess of SarZ was required to completely shift 1 pmol of radioactively labeled promoter DNA (Fig. S2). The ohr promoter sequence has AT-rich palindromic sequences (See Experimental procedures), which is also observed in other many promoters of expression-changed genes in Table 1 (Data not shown).
Oxidation of SarZ with CHP (1 and 10 μM) or H2O2 led to weakened binding of the protein to DNA (lanes 4 and 7, Fig. 3 and Fig. S2); a recent study did indicate that the sulfenic acid form of OhrR retains DNA-binding activity and it is the further modified OhrR that loses DNA affinity (Lee et al., 2007). The sulfenic acid or other Cys13 oxidized form of SarZ generated in this case may be stable and resist further oxidation. Addition of excess reducing agent (DTT) restored the binding of SarZ to promoter DNA to its original level (lanes 8, Fig. 3). Control experiments showed that the C13S mutant SarZ, with Cys13 mutated to Ser, binds the promoter DNA with a similar affinity as the wild-type SarZ (Fig. 3); however, this binding was not affected by treatment with oxidants (Lane 13 and 15, Fig. 3) which confirms that Cys13 serves as the redox sensing residue. In addition, SarZ treated with a low concentration of CHP (1 μM) was sensitive to Coenzyme A (CoA) on DNA binding (Lane 5, Fig. 3). CoA is the major low molecular weight thiol in S. aureus (delCardayré et al., 1998). The SarZ-SOH intermediate may form a mixed disulfide with CoA which induces its dissociation from DNA as reported for OhrR (Lee et al., 2007). Overall, the electrophoretic mobility shift assay results confirmed that oxidation of Cys13 regulates binding of SarZ to DNA. The exact form of Cys13 oxidation that leads to weakened binding of SarZ to DNA is still unclear and will be studied in the future.
Fig. 3.
Electrophoretic mobility shift assay showing the effect of oxidation on the DNA binding of purified SarZ. Purified wild-type SarZ or SarZ C13S (32 pmol) was incubated with a radiolabeled nucleotide containing ohr promoter region (1 pmol, See Experimental procedures) in 20 μl reaction mixture. CHP was added to the reaction assay and incubated for 30 min at room temperature. When indicated, 1 mM DTT or 250 μM of CoA was added after 30 min incubation with oxidants for another 30 min at room temperature before samples were separated on the gel. All experiments were performed in the presence of 10 μg ml-1 Salmon sperm DNA.
Gene expression induced by H2O2 in Newman and sarZ mutant strains
To support the biochemical observation that SarZ is an oxidation sensor, we evaluated expression levels of genes regulated by SarZ under normal and H2O2-challenged growth conditions. Expression of ohr was monitored in wild-type, sarZ and mgrA mutant strains treated with and without 10 mM H2O2 using real-time RT-PCR (Table 3). As expected, ohr was induced by addition of H2O2 to cultures of strain Newman. This gene is constitutively activated in sarZ mutant but not in mgrA mutant. It is known that ohr genes are negatively regulated by OhrR in B. subtilis and related bacteria, and confer resistance to peroxides (Fuangthong et al., 2001; Sukchawalit et al., 2001; Chuchue et al., 2006; Oh et al., 2007). Our result indicated that this gene is also activated in S. aureus upon peroxide challenge. It appears to be regulated by SarZ instead of MgrA.
Table 3.
Relative quantification of ohr expression by real-time PCR with primers and SYBR green probe
| Real-time PCR (fold) | ||
|---|---|---|
| Strain | − | + H2O2 |
| Newman | 1.00 ± 0.33 | 2.18 ± 0.82 |
| Newman mgrA | 0.68 ± 0.05 | 4.71 ± 2.44 |
| Newman sarZ | 3.41 ± 0.33 | 4.71 ± 0.18 |
Peroxide and antibiotic resistance assay
Plate assays were employed to test the potential resistance of the sarZ mutant strain towards various peroxides and antibiotics. As shown in Fig. 4, sarZ mutant exhibited higher resistance towards CHP as compared to the wild-type. This phenotype could be complemented by expressing SarZ (from a plasmid) in the sarZ mutant strain. We also observed a slight resistance of sarZ mutant towards H2O2, and again the phenotype could be complemented by expressing SarZ and SarZ C13S mutant in the sarZ mutant strain. In addition, we found that the sarZ mutant strain is more resistant to some antibiotics such as vancomycin and chloramphenicol (Fig. 4). Although no specific resistance gene was found to be regulated by sarZ, this protein does affect expression of many cell-wall related proteins. We speculate that this resistance phenotype is due to changes of bacterial cell wall properties, which may render staphylococci more resistant to cell wall lysis induced by antibiotic or reduced permeability of the cell wall. These phenotypes could be complemented by expressing SarZ in the sarZ mutant strain. When SarZ C13S was expressed in the sarZ mutant strain, a slightly decreased resistance to vancomycin was observed as compared to the complementary experiment with the wild-type SarZ. This suggests involvement of Cys13 in the regulation mechanism.
Fig. 4.
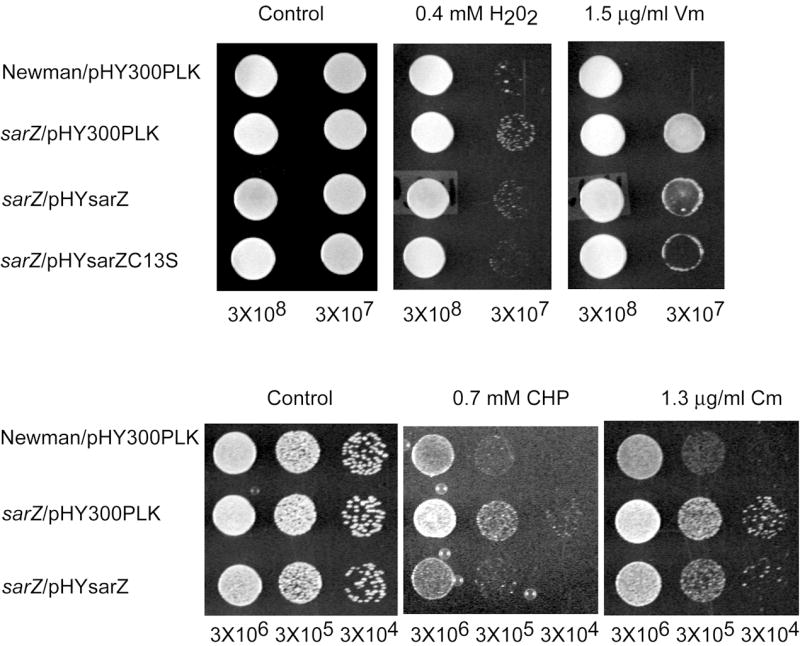
Plate sensitivity assay. Susceptibility of S. aureus strains toward antibiotics and oxidants were monitored using plate assay. Potential resistance of sarZ mutant strains to vancomycin (Vm, 1.5 μg ml-1), chloramphenicol (Cm, 1.6 μg ml-1), cumene hydroperoxide (CHP, 0.7 mM) or H2O2 (0.4 mM) were assayed as described in Methods, and compared with that of wild-type Newman. All plates were incubated at 37 °C for 24 h.
Regulation of autolysis by sarZ
To test whether the sarZ mutation changes the bacterial cell wall properties towards autolysis, we performed autolysis assay induced by Triton X-100, a common reagent used to promote autolysis. Four stains were assayed, including wild-type Newman, sarZ mutant, sarZ mutant complemented with a vector carrying the sarZ gene, and sarZ mutant carrying the empty vector as a control. As shown in Fig. 5, upon Triton X-100 treatment the sarZ mutant strain showed reduced autolysis rate compared to wild-type Newman. Expression of SarZ in the mutant restored its susceptibility towards Triton X-100 to levels similar to wild-type Newman. The empty vector control gave the same phenotype as the mutant. Thus, sarZ mutation does affect the bacterial cell wall properties, which may account for the observed resistance towards certain antibiotics.
Fig. 5.
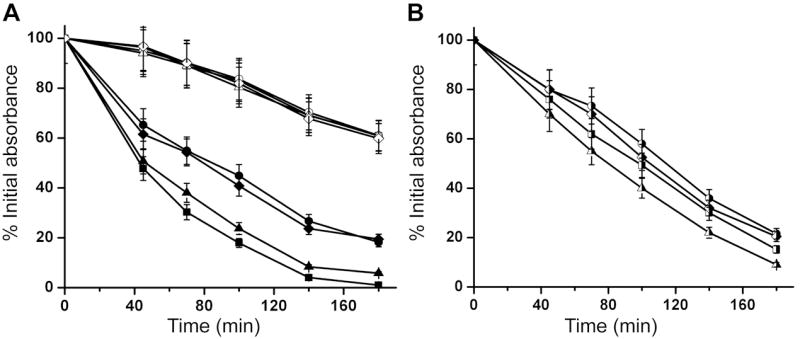
Autolysis Assay. Analysis of sarZ controlled autolytic activity in S. aureus strain Newman. (A) Effect of the sarZ mutation on Triton X-100 induced autolysis. □ and ■, wild-type S. aureus strain Newman; ● and ○, sarZ mutant strain; Δ and ▲, sarZ mutant strain supplemented with plasmid pYJ335 containing sarZ gene; ◊ and ◆, sarZ mutant strain supplemented with empty vector pYJ335. The lysis activity with (filled symbol) and without (open symbol) 0.05% Triton X-100 was monitored by measuring OD600 versus time. (B) Effect of oxidative stress on the SarZ-regulated autolysis in S. aureus strain Newman. Triton X-100 (0.05%) induced autolysis in S. aureus strains pre-treated with 400 μM H2O2 was monitored by recording the decline of OD600 in a period of three hours. The same bacteria strains from (A) are assayed and shown with the same symbol (half filler symbol). Results are mean ± s.d. from three independent experiments.
The autolysis rates of all four strains against Triton X-100 were retarded after oxidative challenge with 400 μM H2O2 as shown in Fig. 5B. Wild-type Newman and sarZ complementary strains showed a significant decrease of autolysis activity to a similar level as sarZ mutant strain. Inactivation of SarZ leads to upregulation of autolysis regulators such as lrgB (Table 1), which is a holin-like protein negatively controling autolytic genes in S. aureus (Groicher et al., 2000). The real-time PCR analysis of lrgB also showed that the lrgB gene was induced by addition of 10 mM H2O2 to cultures of strain Newman (7.55 ± 2.06 fold) and constitutively expressed in sarZ mutants like ohr gene (4.81 ± 0.49 fold). This effect may yield a decreased autolysis rate. The autolysis activities of sarZ mutant and the complementary control also decreased, but to a lesser extent than wild-type Newman, suggesting that other autolysis regulators might be affected by SarZ-independent oxidation regulation pathways.
Contribution of sarZ to virulence
Previously, we reported that MgrA is a major virulence determinant in S. aureus and mgrA mutation leads to a dramatic drop of virulence (4-6 logs) (Chen et al., 2006). It had also been suggested that mutation of sarZ decreases the virulence of S. aureus but the attenuation (~10 fold) is much less significant than the mgrA mutant strain (Kaito et al., 2006). Since MgrA and SarZ regulations in S. aureus are largely independent of each other, despite sharing a similar oxidation sensing mechanism, we wondered whether deletions of sarZ and mgrA may be additive for virulence. A sarZ/mgrA double mutant strain was constructed and its effect on S. aureus virulence was compared with sarZ or mgrA single gene mutant in the murine abscess model (Fig. 6). Bacteria (Newman, sarZ, mgrA, mgrA/sarZ; 106 CFU, colony-forming units) were injected intravenously into mice (ten mice per strain). Five days post infection, animals were killed, and kidneys and livers were removed to measure bacterial loads in organs. Colony forming units were counted on agar plates after plating organ pulps. As expected, the mgrA mutant strain exhibited a dramatic reduction of virulence as bacterial loads were reduced by 3-5 and 4 logs in livers and kidneys, respectively. The sarZ mutant displayed a statistically insignificant decrease in virulence as compared to wild-type (a repeat of this experiment did show a statistically significant decrease in virulence in agreement with the previous report; Fig. S3) (Kaito et al., 2006). However, a double sarZ/mgrA mutant exhibited an epistatic phenotype over the mgrA mutation. Bacterial loads in kidneys and livers were further decreased as compared to infection with the mgrA mutants (Fig. 6). This result indicates that mgrA and sarZ regulate virulence pathways independently and perhaps can be targeted synergistically since a similar mechanism is used to induce the activity of both regulations.
Fig. 6.

Effect of the sarZ mutation on the virulence of S. aureus as tested using the murine abscess model of infection. S. aureus strain Newman (wild-type), sarZ, mgrA, and sarZ/mgrA mutant strains were used to infect ten mice each via retro-orbital injection. After 5 days, mice were killed and organs (kidneys and livers) were removed. Homogenized tissues were incubated on agar medium for S. aureus colony formation and enumeration. Each circle stands for one animal experiment. The horizontal bars indicate the mean. The CFU number was converted to log[CFU] and the arithmetic mean was obtained. The limit of detection was 100 CFU ml-1 in this case, as 10 μl of homogenates (1 ml total) were used for colony enumeration.
Conclusion
Broad attention has been brought to the study of thiol-based redox sensing and regulatory processes (Hausladen et al., 1996; Choi et al., 2001; Pomposiello and Demple, 2001; Kim et al., 2002; Sevier and Kaiser, 2002; Finkel 2003; Wood et al., 2003; Chander and Demple, 2004; Green and Paget, 2004; Poole et al., 2004; Liu et al., 2005; Morris et al., 2005; Jones, 2006; Lee and Helmann, 2006; Sevier and Kaiser, 2006; D’Autréaux and Toledano, 2007; Ilbert et al., 2007; Lee et al., 2007). We have recently shown that oxidation of a single Cys in MgrA has a dramatic effect on the antibiotic resistance regulation and perhaps also virulence regulation in S. aureus (Chen et al., 2006). Here we show that a parallel oxidation sensing and regulation pathway mediated through an MgrA homologue, SarZ, exists in S. aureus. We show that SarZ uses a Cys-based oxidation sensing and regulatory mechanism resembling MgrA. The two proteins share similar oxidative sensing properties to peroxides. Transcription profiling of the sarZ regulon indicated that ~87 genes, encoding proteins that perform a variety of functions in S. aureus, are controlled by this global regulator.
Noticeably, SarZ controls genes involved in metabolic switching and peroxide response, which are mostly absent in the mgrA regulon. Previous study has indicated that such changes (for instance the turning on of pflA, pflB and ohr) should take place when S. aureus is challenged with H2O2 (Chang et al., 2006a); the bacterium is expected to change its cell wall thickness or morphology to protect itself and turn on anaerobic respiration upon challenges with oxidative stress. The observed dramatic upregulation of pflA and pflB and the change of cell wall properties (autolysis and resistance towards cell-wall targeting antibiotics) in the sarZ mutant strain are consistent with its role in oxidation sensing and response. These functions are actually regulated by SarZ instead of MgrA (Fig. 7). Therefore, the discovery of the SarZ-mediated regulation fills a missing gap for oxidation sensing and response in S. aureus. This study also suggested that thiol-based redox sensing and regulation may be more prevalent and play much broader roles in human pathogens as compared to many non-pathogenic bacteria (Collet and Bardwell, 2002; Imlay, 2003; Paget and Buttner, 2003; Poole et al., 2004; Poole, 2005; Imlay, 2008). Host immune systems respond to bacterial infections by generating reactive oxygen and nitrogen species. These oxidative stresses are sensed in parallel signaling pathways through SarZ and MgrA in S. aureus (Fig. 7). The high similarity of SarZ and MgrA and the synergistic effect of sarZ and mgrA on virulence certainly indicate that both pathways can be targeted for developing new therapeutic agents to treat S. aureus infection.
Fig. 7.
MgrA and SarZ play complementary roles in oxidation sensing and regulation in S. aureus. X stands for a covalent modification of the Cys resides.
Experimental procedures
Bacterial strains, plasmids and culture conditions
The human clinical isolate, S. aureus strain Newman, and its derivatives were used. Bacteria were grown with rotary shaking (250 rpm) in TSB (Tryptic soy broth, Difco) supplemented with 5 μg ml-1 nalidixic acid for Newman and 10 μg ml-1 erythromycin for mutant strains. E. coli strains DH5α and BL21 star (DE3) (Invitorogen) were used for DNA manipulation and protein expression, respectively. Vector pET28a (Novagen) was used for cloning in E. coli. E. coli-S. aureus shuttle vectors pYJ335 (Ji et al., 1999) and pHY300PLK (Takara) were used for complementary studies.
Construction of the mgrA-sarZ double mutant
In-frame deletions in the sarZ gene were generated by allelic replacement without antibiotic marker selection using pKOR1 (Bae and Schneewind 2006). DNA fragments containing 1 k b upstream and downstream sequences of sarZ were PCR-amplified from chromosomal DNA of Newman using the following primers: attB2-sarZ-up-F 5’-GGGGACCACTTTGTACAAGAAAGCTGGGTATGAAGCAGGATCAGCAAATGGTA-3’ and sarZ-up-HindIII 5’-ATAAGCTTAAGGTGATGGGCAGAAGTATGCATCGCTAT-3’ for the upstream fragment, and attB1-sarZ-CF 5’-GGGGACAAGTTTGTACAAAAAAGCAGGCTCACTAATGCAATAACAACTTGCAT-3’ and sarZ-CR-HindIII 5’-ATAAGCTTCCAATCACTCCTTGTTAAAATAAACAATAT-3’ for the downstream fragment. PCR products were digested with HindIII, mixed together and ligated by T4 DNA ligase (NEB). The ligation product was used for recombination with pKOR1 and recombination products were introduced to DH5α. The resulting plasmid pKORΔsarZ was transferred by electroporation first to S. aureus RN4220, and subsequently to Newman mgrA, generating strain Newman mgrA/pKORΔsarZ.
To integrate the plasmid, Newman mgrA/pKORΔsarZ was inoculated into 25 ml TSB with 10 μg ml-1 chloramphenicol (TSBCm10) and grown at 43 °C overnight. Culture aliquots were streaked on TSA (Tryptic soy agar, Difco) with 10 μg ml-1 chloramphenicol (TSACm10) and incubated at 43 °C overnight. From the resulting plate, one colony was picked, inoculated into 5 ml TSB and incubated at 30 °C overnight to facilitate plasmid excision. Cultures were then diluted 104-fold with sterile water and 0.1 ml aliquots were spread on TSA containing 0.1 μg ml-1 anhydrotetracycline (ATc, Clontech) (TSAATc) and incubated at 30 °C for 2 days.
To identify the desired deletion mutants on TSAATc, 8 large colonies were inoculated into TSB and incubated at 37 °C overnight. Chromosomal DNA was purified from the cultures and the sarZ gene was PCR-amplified using the primers attB2-sarZ-up-F and attB1-sarZ-CF. Four out of 8 picks carried a deletion. The allelic replacement mutants were isolated at frequencies of 50%.
RNA isolation
In triplicate, cultures of S. aureus strains grown overnight in BHI (Brain Heart Infusion, Difco)with appropriate antibiotics were diluted 1:100 into fresh BHI without antibiotics and grown at 37 °C with constant aeration for 2 hr and 5 hr respectively before being harvested. The method of harvesting samples for RNA isolation is to re-suspend bacterial culture with an equal volume of ice-cold ethanol-acetone (1:1) solution and store the mixtures at -80°C for 20 min or until ready to prepare RNA. The 2 hr and 5 hr time spots were chosen as it is well known that a variety of genes’ expression level in S. aureus are growth-phase dependent and dramatic changes had been observed between early-exponential and post-exponential growth phase conditions. The 2 hr samples represented the early-exponential growth phase with an OD600=0.6 and a doubling time about 20 min. The 5 hr samples represented the post-exponential growth phase with an OD600=2.0 and a doubling time about 4 hrs. OD data were obtained by using an Agilent 8453 UV-Vis Spectrophotometer with cuvettes of 1cm path length. For samples with OD600>1.0, appropriate dilutions were applied before OD being read.
The harvested mixture samples for RNA isolation mentioned above were centrifuged at 10,000 × g at 4°C for 10 min and cell pellets were air dried and resuspended on ice in 0.5 ml TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6). The cell suspensions were transferred to lysing matrix B tubes (MP Biomedicals) and processed in an FP120 FastPrep cell disruptor (MP Biomedicals) for 20 s at setting 5.0. After chilling on ice for 5 min, the tubes were repeated to process for 20 s at setting 4.5. The tubes were centrifuged at 17,000 × g in a tabletop centrifuge for 15 min at 4°C, and the upper phases were transferred to a 1.5-ml microtube. The RNA samples were further purified and treated with DNase I using the RNeasy mini kit (QIAGEN), according to the manufacturer’s instructions. The RNA was spectrophotometrically quantified.
Microarray profiling
RNA was converted to cDNA, and microarray analysis was performed according to the manufacturer’s instructions for antisense prokaryotic arrays (Affymetrix). To ensure reproducibility, three RNA samples from each strain and sampling time were prepared at each growth phase. Each RNA sample was independently hybridized to a GeneChip. One mutant 2 hr RNA sample was not included within microarray analysis, due to machinery malfunction; two replicates were used to analyze that particular strain/sampling time all others corresponded to three independent RNA sample measurements. For comparisons of each strain and sampling time, GeneChip signal intensity values were normalized to the total microarray signal, averaged, and compared using GeneSpring GX 7.3.1 software (Agilent Technologies), as previously described (Beenken et al., 2004; Bischoff et al., 2004; Weinrick et al., 2004; Luong et al., 2006). All microarray results have been deposited in the Gene Expression Omnibus (GEO) provisional number GSE13138. Genes with at least a two-fold difference (t test; P = 0.05) in RNA titer between the wild-type strain and the sarZ insertion mutant were considered differentially expressed in a sarZ-dependent manner.
Real-time RT-PCR
To confirm the microarray data, we selected genes from different functional categories to assay their relative expression levels by real-time RT (reverse transcription)-PCR. Briefly, one-step quantitative RT-PCR was performed by incubating DNase I-treated RNA with SuperScript III platinum SYBR Green One-Step qRT-PCR master mix (Invitrogen) using the ABI Prism 7300 detection system (Applied Biosystems). The cDNA was subjected to real-time PCR using the primer pairs listed in Table 2. Cycling conditions were 50 °C for 5 min and 95 °C for 5 min followed by 40 cycles of 95 °C for 15 sec and 60 °C for 35 sec and a dissociation step at 40 °C for 1 min, 95 °C for 15 sec, 60 °C for 30 sec, and 95 °C for 15 sec.
Chemical characterization and quantification of thiol modification
The reduced form of purified His6-tagged SarZ (SarZ-SH) served as starting material and was generated by incubating with a large excess of DTT (100 mM) for 1 h, which was followed by running the sample through desalting columns (HiTrap desalting column, Amersham Biosciences) three times at 4 °C. Assays were performed in a buffer with 100 mM KH2PO4/K2HPO4, 200 mM NaCl and 1 mM EDTA at pH 7.0. The SarZ-SOH modification was confirmed by the NBD-Cl assay (Ellis and Poole, 1997; Chen et al., 2006): aliquots of 100 μl reduced SarZ (50 μM in 10 mM Tris at pH 7.4 with 100 mM NaCl) were treated with 4 equiv of CHP (200 μM) at room temperature for 10 min followed by washing with thiol-assay buffer three times to generate the oxidized SarZ. All the washing steps in the DTNB assay and the NBD-Cl assay followed the same procedure by adding 500 μl thiol-assay buffer to 100 μl sample solution and concentrating down to the original volume with Microcon YM-10 (Amicon) ultrafiltration device (small molecules in the sample will be diluted by six fold after each time of washing). Both the oxidized and reduced SarZ were incubated with excess amounts of NBD-Cl (1 mM) for 1 h at room temperature in dark before being washed with thiol-assay buffer three times. The spectra were then taken in an Agilent 8453 UV-vis spectrophotometer against a blank of the buffer solution. Free thiol quantification was conducted by DTNB assay (Riddles et al., 1983; Chen et al., 2006): the reduced SarZ (50 μM) was treated with varying equivalents of CHP or H2O2 at room temperature for 10 min followed by washing with the thiol-assay buffer three times to generate the oxidized sample. The reduced SarZ can be regenerated by treating aliquots of the oxidized SarZ samples with 20 mM DTT for 1 h followed by removing extra DTT using Micro Bio-Spin 6 chromatography columns. Finally, 100 μl of all the oxidized and reduced SarZ samples were incubated with 1 mM DTNB at room temperature for 15 min before the UV-vis spectra were taken. The absorption at 412 nm from each sample was recorded and the free thiol concentrations were calculated by using the previously published methods (Riddles et al., 1983).
Electrophoretic mobility shift assays
The electrophoretic mobility shift experiments were performed by using 8-32 pmol of SarZ in 20 μl of binding buffer (20 mM Hepes-KOH at pH 7.6, 50 mM KCl, 5 mM Magnesium Acetate, 1 mM EDTA, 0.5 mg ml-1 BSA, 10 μg ml-1 Salmon sperm DNA, 0.005% Triton X-100 and 20% glycerol). The probe DNA was labeled using a T4 polynucleotide kinase (Invitrogen) and [γ-32P] ATP (Perkin Elmer). DNA probe (1 pmol) was added to the mixture and incubated at room temperature for 30 min. CHP was added to the binding reaction and incubated for another 30 min at room temperature. When indicated, either 250 μM CoA or 1 mM DTT was added into the solution and incubation continued at room temperature for 30 min before the samples were used for the shift assay. The 8% native polyacrylamide gel was pre-run for 30 min before the binding samples were loaded, and the gel was then continuously run at 150 V and room temperature. The gels were dried and subjected to autoradiography using the storage phosphor screen (Image Screen-K, Kodak) and the Molecular Imager PharosFX Plus System (Bio-Rad). Oligonucleotides sequences used in the assays are: ohr1 5’-TTTTCGAATGGGTAAAGCATAAATGTATTTTAAATTAGGAGGTTATAAGT-3’, ohr2 5’-ACTTATAACCTCCTAATTTAAAATACATTTATGCTTTACCCATTCGAAAA-3’.
Peroxide and antibiotic sensitivity assays
S. aureus wild-type Newman and the sarZ mutant strains were grown at 37 °C overnight in TSB with appropriate antibiotics to ensure plasmid maintenance. Overnight, cultures were diluted 100-fold into the same medium and grown at 37 °C for 3 h to reach an OD600 ~0.6. After further incubation at 37 °C for ~2 h, the numbers of bacterial cells from all strains were normalized to approximately 5×108 CFU ml-1 with fresh TSB, followed by six 10-fold serial dilutions. Then 5 μl of each strain of bacterial samples were spotted onto the TSA plates containing CHP, H2O2, chloramphenicol (Cm), or vancomycin (Vm). All plates were incubated at 37 °C for 24 h before being read.
Autolysis assay
Triton X-100 induced autolysis assays were performed as previously described (Manna et al., 2004). In brief, overnight cultures were diluted 1:200 to fresh TSB containing 1 M NaCl and grown at 37 °C with shaking till OD600 reached 1.0. Cells were pelleted by centrifugation, washed twice with ice-cold water and then resuspended in the same volume of 50 mM Tris-HCl (pH7.2) supplemented with and without 0.05% (v/v) Triton X-100. The cells were then incubated at 30 °C with shaking and the lysis activity was monitored by measuring OD600 versus time. To assay Triton X-100 induced autolysis under the oxidants challenged conditions, the freshly diluted bacteria TSB culture containing 1M NaCl were allowed to grow at 37 °C with shaking for 3 hrs (OD600=0.7). Then 400 μM H2O2 (final concentration)were added to the bacteria culture and the cells were incubated at 37 °C with shaking for another 30 min (OD600=1.0) before being harvested. The pelleted cells were manipulated and assayed in the same way as mentioned above. All assays were performed in triplicate and the standard deviation of three independent experiments was <10%.
Murine abscess model
Newman and mutant strains were grown at 37 °C overnight in TSB or TSB containing 10 μg ml-1 of erythromycin (TSBerm10). The cultures were diluted 100-fold with fresh TSB or TSBerm10 and incubated at 37 °C for about 2 h until OD600 reached 1.0. Bacteria were collected by centrifugation, washed and suspended in phosphate-buffered saline (PBS) to an OD600 of 0.4. Viable staphylococci were enumerated by colony formation on TSA plates to measure the infection dose (4 × 106 to 6 × 106 CFU). 0.1 ml of bacterial suspension was administered via retro-orbital injection into BALB/c nu/nu (nude) mice (6–8 weeks). Mice were killed by CO2 asphyxiation 5 days after the injection, and kidneys and liver were removed. The organs were homogenized in 1 ml of PBS, and 10 μl of dilutions of the homogenates were plated on TSA.
Acknowledgments
We want to thank Mr. Z. Li for providing PEG-alk. S. N. is supported in parts by the Mochida Memorial Foundation for Medical Pharmaceutical Research. This work was funded by NIAID, NIH through grant R01 AI074658 (C.H.). A.C. acknowledges support from the Medical Scientist National Research Service Award Grant T32GM07281 at the University of Chicago.
Supplementary Material
References
- Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, Olson PD, Projan SJ, Dunman PM. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol. 2006;188:6739–6756. doi: 10.1128/JB.00609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ, Blevins JS, Smeltzer MS. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–4684. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bachi B, Projan S. Microarray-based analysis of the Staphylococcus aureus σB regulon. J Bacteriol. 2004;186:4085–4099. doi: 10.1128/JB.186.13.4085-4099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner S, Monteil H, Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev. 2004;28:183–200. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Buis JM, Broderick JB. Pyruvate formate-lyase activating enzyme: elucidation of a novel mechanism for glycyl radical formation. Arch Biochem Biophys. 2005;433:288–296. doi: 10.1016/j.abb.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Chander M, Demple B. Functional analysis of SoxR residues affecting transduction of oxidative stress signals into gene expression. J Biol Chem. 2004;279:41603–41610. doi: 10.1074/jbc.M405512200. [DOI] [PubMed] [Google Scholar]
- Chang W, Small DA, Toghrol F, Bentley WE. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J Bacteriol. 2006a;188:1648–1659. doi: 10.1128/JB.188.4.1648-1659.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Toghrol F, Bentley WE. Toxicogenomic response of Staphylococcus aureus to peracetic acid. Environ Sci Tech. 2006b;40:5124–5131. doi: 10.1021/es060354b. [DOI] [PubMed] [Google Scholar]
- Chang MW, Toghrol F, Bentley WE. Toxicogenomic response to chlorination includes induction of major virulence genes in Staphylococcus aureus. Environ Sci Tech. 2007;41:7570–7575. doi: 10.1021/es070929k. [DOI] [PubMed] [Google Scholar]
- Chen PR, Bae T, Williams WA, Duguid EM, Rice PA, Schneewind O, He C. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat Chem Biol. 2006;2:591–595. doi: 10.1038/nchembio820. [DOI] [PubMed] [Google Scholar]
- Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40:1–9. doi: 10.1016/S0928-8244(03)00309-2. [DOI] [PubMed] [Google Scholar]
- Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol. 2008;40:355–361. doi: 10.1016/j.biocel.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Kim SJ, Mukhopadhyay P, Cho S, Woo JR, Storz G, Ryu SE. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- Chuchue T, Tanboon W, Prapagdee B, Dubbs JM, Vattanaviboon P, Mongkolsuk S. ohrR and ohr are the primary sensor/regulator and protective genes against organic hydroperoxide stress in Agrobacterium tumefaciens. J Bacteriol. 2006;188:842–851. doi: 10.1128/JB.188.3.842-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet JF, Bardwell JC. Oxidative protein folding in bacteria. Mol Microbiol. 2002;44:1–8. doi: 10.1046/j.1365-2958.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- D’Autréaux B, Toledano MB. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- delCardayré SB, Stock KP, Newton GL, Fahey RC, Davies JE. Coenzyme A disulfide reductase, the primary low molecular weight disulfide reductase from Staphylococcus aureus. Purification and characterization of the native enzyme. J Biol Chem. 1998;273:5744–5751. doi: 10.1074/jbc.273.10.5744. [DOI] [PubMed] [Google Scholar]
- Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HR, Poole LB. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997;36:15013–15018. doi: 10.1021/bi972191x. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Fuangthong M, Atichartpongkul S, Mongkolsuk S, Helmann JD. OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J Bacteriol. 2001;183:4134–4141. doi: 10.1128/JB.183.14.4134-4141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuangthong M, Helmann JD. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc Natl Acad Sci USA. 2002;99:6690–6695. doi: 10.1073/pnas.102483199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Pane-Farre J, Kohler C, Hecker M, Engelmann S. Anaerobic gene expression in Staphylococcus aureus. J Bacteriol. 2007;189:4275–4289. doi: 10.1128/JB.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino P, Engelmann S, Bischoff M. σB activity depends on RsbU in Staphylococcus aureus. J Bacteriol. 2001;183:1843–1852. doi: 10.1128/JB.183.6.1843-1852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Paget MS. Bacterial redox sensors. Nat Rev Microbiol. 2004;2:954–966. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- Groicher KH, Firek BA, Fujimoto DF, Bayles KW. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J Bacteriol. 2000;182:1794–1801. doi: 10.1128/jb.182.7.1794-1801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. Nitrosative stress: activation of the transcription factor OxyR. Cell. 1996;86:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Helmann JD, Wu MF, Gaballa A, Kobel PA, Morshedi MM, Fawcett P, Paddon C. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J Bacteriol. 2003;185:243–253. doi: 10.1128/JB.185.1.243-253.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Fuangthong M, Helmann JD, Brennan RG. Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family. Mol Cell. 2005;20:131–141. doi: 10.1016/j.molcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilbert M, Horst J, Ahrens S, Winter J, Graf PCF, Lilie H, Jakob U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nature Structural & Molecular Biology. 2007;14:556–563. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Ann Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular Defenses Against Superoxide and Hydrogen Peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingavale SS, Van Wamel W, Cheung AL. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol Microbiol. 2003;48:1451–1466. doi: 10.1046/j.1365-2958.2003.03503.x. [DOI] [PubMed] [Google Scholar]
- Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect Immun. 2005;73:1423–1431. doi: 10.1128/IAI.73.3.1423-1431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Marra A, Rosenberg M, Woodnutt G. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J Bacteriol. 1999;181:6585–6590. doi: 10.1128/jb.181.21.6585-6590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signaling. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Kaatz GW, Thyagarajan RV, Seo SM. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob Agents Chemother. 2005;49:161–169. doi: 10.1128/AAC.49.1.161-169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaito C, Morishita D, Matsumoto Y, Kurokawa K, Sekimizu K. Novel DNA binding protein SarZ contributes to virulence in Staphylococcus aureus. Mol Microbiol. 2006;62:1601–1617. doi: 10.1111/j.1365-2958.2006.05480.x. [DOI] [PubMed] [Google Scholar]
- Kim SO, Merchant K, Nudelman R, Beyer WF, Keng T, DeAngelo J, Hausladen A, Stamler JS. OxyR: A molecular code for redox-related signaling. Cell. 2002;109:383–396. doi: 10.1016/s0092-8674(02)00723-7. [DOI] [PubMed] [Google Scholar]
- Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, Ahn WS, Yu MH, Storz G, Ryu SE. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11:1179–1185. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- Lee JW, Soonsanga S, Helmann JD. A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc Natl Acad Sci USA. 2007;104:8743–8748. doi: 10.1073/pnas.0702081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtio L, Goldman A. The pyruvate formate lyase family: sequences, structures and activation. Protein Eng Des Sel. 2004;17:545–552. doi: 10.1093/protein/gzh059. [DOI] [PubMed] [Google Scholar]
- Liang XD, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji YD. Global regulation of gene expression by ArIRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J Bacteriol. 2005;187:5486–5492. doi: 10.1128/JB.187.15.5486-5492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark DG, Paolella P, Wood NP. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J Biol Chem. 1969;244:3605–3612. [PubMed] [Google Scholar]
- Liu HJ, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circulation Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Medical progress - Staphylococcus aureus infections. New Eng J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Luong TT, Newell SW, Lee CY. mgr, a novel global regulator in Staphylococcus aureus. J Bacteriol. 2003;185:3703–3710. doi: 10.1128/JB.185.13.3703-3710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J Bacteriol. 2006;188:1899–1910. doi: 10.1128/JB.188.5.1899-1910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998;180:2636–2643. doi: 10.1128/jb.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, Schoolnik G, Thompson CJ. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2005;102:12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry KJ, Fuangthong M, Panmanee W, Mongkolsuk S, Brennan RG. Structural mechanism of organic hydroperoxide induction of the transcription regulator OhrR. Mol Cell. 2007;28:652–664. doi: 10.1016/j.molcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- Oh SY, Shin JH, Roe JH. Dual role of OhrR as a repressor and an activator in response to organic hydroperoxides in Streptomyces coelicolor. J Bacteriol. 2007;189:6284–6292. doi: 10.1128/JB.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget MS, Buttner MJ. Thiol-based regulatory switches. Annu Rev Genet. 2003;37:91–121. doi: 10.1146/annurev.genet.37.110801.142538. [DOI] [PubMed] [Google Scholar]
- Pomposiello PJ, Demple B. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotech. 2001;19:109–114. doi: 10.1016/s0167-7799(00)01542-0. [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- Poole LB. Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch Biochem Biophys. 2005;433:240–254. doi: 10.1016/j.abb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rice KC, Nelson JB, Patton TG, Yang SJ, Bayles KW. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J Bacteriol. 2005;187:813–821. doi: 10.1128/JB.187.3.813-821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol. 2006;61:927–939. doi: 10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- Riddles PW, Blakeley RL, Zerner B. Reassessment of Ellman Reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Riordan JT, Muthaiyan A, Van Voorhies W, Price CT, Graham JE, Wilkinson BJ, Gustafson JE. Response of Staphylococcus aureus to salicylate challenge. J Bacteriol. 2007;189:220–227. doi: 10.1128/JB.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saïd-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, Arvidson S, Foster TJ, Projan SJ, Kreiswirth BN. Global regulation of Staphylococcus aureus genes by Rot. J Bacteriol. 2003;185:610–619. doi: 10.1128/JB.185.2.610-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G, Bock A. Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J Bacteriol. 1988;170:5330–5336. doi: 10.1128/jb.170.11.5330-5336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- Sevier CS, Kaiser CA. Conservation and diversity of the cellular disulfide bond formation pathways. Antioxid Redox Signal. 2006;8:797–811. doi: 10.1089/ars.2006.8.797. [DOI] [PubMed] [Google Scholar]
- Sukchawalit R, Loprasert S, Atichartpongkul S, Mongkolsuk S. Complex regulation of the organic hydroperoxide resistance gene (ohr) from Xanthomonas involves OhrR, a novel organic peroxide-inducible negative regulator, and posttranscriptional modifications. J Bacteriol. 2001;183:4405–4412. doi: 10.1128/JB.183.15.4405-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Bolduc QC, Zhang XM, Hooper DC. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J Bacteriol. 2003;185:3127–3138. doi: 10.1128/JB.185.10.3127-3138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol. 2005;187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker U, Andersen KK, Antelmann H, Devine KM, Hecker M. One of two OsmC homologs in Bacillus subtilis is part of the σB-dependent general stress regulon. J Bacteriol. 1998;180:4212–4218. doi: 10.1128/jb.180.16.4212-4218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrick B, Dunman PM, McAleese F, Murphy E, Projan SJ, Fang Y, Novick RP. Effect of mild acid on gene expression in Staphylococcus aureus. J Bacteriol. 2004;186:8407–8423. doi: 10.1128/JB.186.24.8407-8423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.