Abstract
Peptides derived from conserved heptad repeat (HR) regions of paramyxovirus fusion (F) proteins inhibit viral fusion by interfering with the formation of the fusogenic six-helix bundle structure. Peptide efficacy is affected by the strength of the peptide association with the target virus's complementary HR region. Here, we show that a second basis for peptide efficacy lies in the kinetics of F activation by the homotypic attachment protein: efficient F activation by the attachment protein shortens the period during which antiviral molecules targeting intermediate states of F may act, thereby modulating the effectiveness of inhibitory peptides. These results highlight new issues to be considered in developing strategies for fusion inhibitors.
Paramyxoviruses: important childhood pathogens.
Paramyxoviruses cause important human illnesses, ranging from lower respiratory tract diseases in infants caused by human parainfluenza virus type 1 (HPIV1), HPIV2, and HPIV3 (3, 33) to highly lethal central nervous system diseases caused by the emerging paramyxoviruses Hendra virus (HeV) and Nipah virus (NiV), contributing significantly to global disease and mortality. No clinical therapies or vaccines for these paramyxovirus diseases exist, and furthermore, vaccines would be unlikely to protect the youngest infants. Antiviral agents, therefore, would be beneficial, particularly for the youngest age groups.
Paramyxovirus entry into target cells: the initial step in infection.
All paramyxoviruses possess two envelope glycoproteins directly involved in viral entry and pathogenesis: a fusion (F) protein and a receptor-binding protein (hemagglutinin-neuraminidase [HN], H, or G). In the cases of HPIV3, HeV, and NiV, the receptor-binding protein, HN (HPIV3) or G (HeV and NiV), binds to cellular surface receptors, positions the viral envelope in proximity to the plasma membrane, and activates the viral F protein at a neutral pH, initiating a series of conformational changes in F. This attachment protein-receptor interaction is required for the F protein to mediate the fusion of the viral envelope with the host cell membrane (17, 24, 25). The membrane-anchored subunit of the F protein contains two hydrophobic domains: the fusion peptide, which inserts into the cellular target membrane during fusion, and the transmembrane (TM)-spanning domain. Each of these domains is adjacent to one of two conserved heptad repeat (HR) regions: the fusion peptide is adjacent to the N-terminal HR (HRN), and the TM domain is adjacent to the C-terminal HR (HRC). Once F has been activated—in the case of HPIV, by means of the receptor-bound HN (25, 28)—the fusion peptide inserts into the target membrane, first generating a transient intermediate that is anchored to both viral and cell membranes and then refolding and assembling into a fusogenic six-helix bundle (6HB) structure as the HRN and HRC associate into a tight complex. This refolding of F into its final stable form relocates the fusion peptides and TM anchors to the same end of the coiled coil, brings the viral and cell membranes together, and is the driving force for membrane fusion (8). The key to these events is the initial activation step, wherein HN triggers F to initiate the fusion process. We have shown previously that a balance between the three functions of the HN molecule, binding, receptor cleaving, and F triggering, ultimately determines the outcome of infection (5, 6, 13, 15, 19, 22, 24). The efficiency of F triggering by HN critically influences the capacity for fusion mediated by F and, thus, the extent of viral entry (25). We propose that differences in the efficiency of F activation have an impact on the efficacy of potential antiviral molecules that target intermediate states of the fusion protein.
Peptide inhibitors of F activity.
Peptides derived from the HRN and HRC regions of the F protein can interact with fusion intermediates of paramyxovirus F proteins (2, 11, 14, 26, 31, 34) and may provide a useful antiviral strategy. The HRC peptides of a number of paramyxoviruses, including Sendai, measles, Newcastle disease, and respiratory syncytial viruses, simian virus 5, HeV, and NiV, can inhibit the infectivity of a homologous virus (9, 11, 20, 21, 26, 32, 34-36). The ability of HR peptides to interfere with the fusion process mediated by the human immunodeficiency virus type 1 (HIV-1) fusion protein led to a clinically effective peptide inhibitor of HIV-1 infection (T-20, or enfuvirtide) (7, 10, 30, 31). The peptides bind to the complementary HR region, thereby preventing F from refolding into the stable 6HB structure required for fusion (2, 4, 28). Recently, we showed that peptides derived from the HRC region of the F protein of HPIV3 are effective inhibitors of HPIV3, HeV, and NiV fusion (21) and that, for HeV, the strength of association between the HRC peptides and the corresponding HRN region is a determinant of the antiviral efficacy of the peptides (20). However, peptides derived from the HPIV3 F protein HRC region inhibit HeV and NiV fusion more effectively than HPIV3 fusion, despite a stronger homotypic HRN-HRC interaction for HPIV3 (20, 21). HRC peptides inhibit fusion only at the stage when F has been activated to expose the fusion peptide but has not yet proceeded to fold into its final state. Therefore, we hypothesized that sensitivity to peptide inhibition may depend on the kinetics of fusion (as is the case for HIV [27, 29]). HN molecules that possess an enhanced F-triggering function may lessen the inhibitory effects of HRC peptides. Peptide efficacy would therefore be modulated by the kinetics of fusion activation, which depends on HN for HPIV and on G for HeV/NiV.
In order to investigate the relationship between the kinetics of F activation and sensitivity to peptide inhibitors, we used HPIV3 HN variants with specifically altered F-triggering phenotypes (19, 22, 23, 25), as well as live HeV and NiV (1). We sought to determine whether individual HN properties affect the sensitivity of HPIV3 to fusion inhibition by peptides and whether differences among the rates of triggering of the HPIV3, HeV, and NiV fusion processes account for the differences in inhibitor sensitivity among these viruses. Results from experiments varying the time of addition of the inhibitor during the course of F activation reveal that for HPIV3, the rate of F triggering determines the window for inhibitor sensitivity and that the slower-triggering HeV/NiV G allows a longer period during which an inhibitor may act. Moreover, by varying the inhibitor concentration and temperature and utilizing variant HN molecules with modified F activation properties, we showed that fusion kinetics is a major determinant for peptide effectiveness. Thus, the kinetics of F activation is likely to underlie the differences among the efficacies of inhibitory peptides for different paramyxoviruses and virus variants and needs to be considered in the development of strategies for fusion inhibitors.
Time points for effective intervention with an inhibitory peptide depend on the speed of F activation for HPIV3, HeV, and NiV.
We have proposed previously that the kinetic window for F activation is shorter for HPIV3 than for HeV/NiV (20). We now tested this idea, to identify whether differences in the time line of F activation between viruses affect peptide efficacy. HRC peptide inhibition ceases when triggering is complete (and F has folded). Thus, the exact time postinfection at which peptides no longer can inhibit fusion pinpoints the end of the F activation process and the window of time for peptide action. If specific attachment proteins have an inherent advantage in F activation, peptide inhibition will cease sooner after infection.
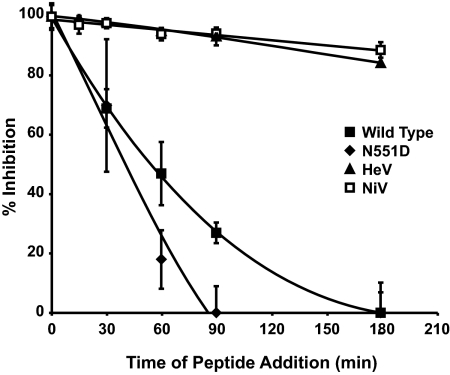
HPIV3 HRC peptides were synthesized and purified as described previously (20), and concentrations were determined by UV spectrophotometry with an Ultrospec 2000 instrument (Pharma Biotech). For this experiment, in addition to wild-type (wt) HPIV3 (16) and wt HeV and NiV (1), we used an HPIV3 variant bearing an HN with the N551D mutation (designated HN-N551D), known to be more efficient at triggering HPIV3 F (19, 22). The effects of peptides on plaque numbers were assessed by a plaque reduction test (12) for HPIV3 and by a viral detection assay (1) for HeV/NiV. For the experiment with results presented in Fig. 1, permissive cells were infected with wt HPIV3, HN-N551D-bearing HPIV3, or HeV/NiV at 37°C. At specific time points, 10 μM inhibitory F peptide (HPIV3 wt HRC [20]) was added. Since the HRC peptide acts by binding to the exposed HRN region and, thus, can act only once the triggering process has started and the F peptide has inserted into the target membrane (18, 28), peptides that are added after F triggering has occurred will not be inhibitory. As indicated in Fig. 1, the addition of the peptide and virus concurrently (at time zero) resulted in complete inhibition for all viruses observed. The inhibition of HeV and NiV by the HRC peptide was extended over a greater period of time—up to and beyond 180 min after infection—than that of HPIV3. For HPIV3 wt virions, HRC peptide added at 180 min postinfection failed to inhibit, while for the faster-fusing HPIV3 HN-N551D variant, the HRC peptide completely failed to inhibit as soon as 90 min after infection. This difference in the period of time during which peptide addition is inhibitory reflects the different speeds with which HPIV3 HN variants and HeV/NiV activate F. Faster activation, as that by HPIV3 HN-N551D, means that the time period during which F exists in an intermediate state and the peptide target (HRN region) is accessible, but before fusion progresses, is shorter. The results furthermore support the hypothesis that the time frame for HeV/NiV F activation is longer than that for HPIV3 F activation. These data explain the previously observed greater efficacy of HPIV3 peptide inhibitors for HeV/NiV infections than for HPIV3 infections (20, 21).
FIG. 1.
Inhibition of infection by HPIV3 HRC peptides added at different times after infection reveals differences in the F activation rate. CV1 cells were infected with wt or HN-N551D-bearing HPIV3 (N551D) at a multiplicity of infection of 6.7 × 10−4 or with HeV or NiV at a multiplicity of infection of 0.25. The HPIV3 HRC peptide was added at the time points noted for a final concentration of 10 μM. For HPIV, cells were overlaid with agarose 90 min later, and plaques were stained and counted at 18 h postinfection. For HeV/NiV, immunodetection of viral antigen was performed at 24 h postinfection (1). The percent inhibition of viral entry, normalized to 100% inhibition at time zero, is shown as a function of the time of HRC peptide addition. Data points are means (± standard deviations) for triplicate samples. These data are representative of results from three to five experiments.
Slowing the fusion kinetics by modulating temperature alters the time frame of peptide inhibition.
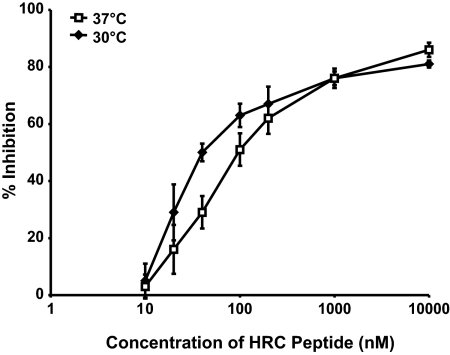
We have shown previously that HPIV3 HN/F-mediated fusion is modulated by temperature (22, 24) and that the temperature of triggering can be used as an indicator of the activation energy required. HNs more efficient at F triggering lower the activation energy required to activate F and promote fusion at lower temperatures (22, 24, 25). Using a series of HN glycoproteins with specific alterations that have an impact on F triggering, an approach different than that taken in the present study, we showed previously that the kinetics of HPIV3 HN/F-mediated fusion is slower at lower temperatures (22, 24). By coexpressing equal levels of these variant HN molecules with F on the cell surface, we demonstrated that the altered kinetics of fusion triggering is not attributable to a variation in HN densities but is an inherent property of the molecules themselves (22). Combined with the finding, shown in Fig. 1 as described above, that the inhibitory potential of the HRC peptide is greater for wt HPIV3 than for the faster-fusing variant with HN-N551D, these data led us to hypothesize that by decreasing the temperature and thereby decreasing the kinetics of fusion, we could augment the inhibitory potential of the HRC peptide against wt HPIV3 infection. In the experiment with results illustrated in Fig. 2, permissive cells were infected with wt HPIV3 virions at 30 or 37°C in the presence of different concentrations of inhibitory peptides. The 50% inhibitory concentrations (IC50s) calculated for each temperature were 36 nM at 30°C and 79 nM at 37°C. A paired t test (performed using GraphPad Prism) revealed that the mean values for each point at each temperature represented in Fig. 2 are significantly different, with a P value of 0.012. The enhanced peptide inhibition seen at 30°C compared to that at 37°C indicates that decreasing the kinetics of fusion increases the effectiveness of inhibitory peptides. These data imply that slowing the kinetics of fusion lengthens the window of time during which the inhibitory peptides act, thereby resulting in greater inhibition, and support the notion that peptide activity varies depending on the kinetics of F triggering. Next, we tested a series of variants with different kinetics of fusion for their sensitivities to peptide inhibition.
FIG. 2.
Temperature modulates HRC peptide inhibitory efficacy. CV1 cells were infected with wt HPIV3 at a multiplicity of infection of 6.7 × 10−4 in the presence of increasing concentrations of HPIV3 HRC peptide. After either a 90-min incubation at 37°C or a 150-min incubation at 30°C, cells were overlaid with agarose, and plaques were stained and counted at 18 h postinfection. The percent inhibition of viral entry (compared to results for control cells infected in the absence of inhibitors) is shown as a function of the (log-scale) concentration of HPIV3 HRC peptide. Data points are means (± standard deviations) for triplicate samples. These data are representative of results from three to five experiments.
Kinetics of HN′s F triggering is a major determinant for inhibitory peptide effectiveness.
Previous work on HIV has identified the kinetics of fusion as a determinant for inhibition by HRC peptides (29). However, in those instances, the fusion kinetics was modulated by changes in the fusion protein that also altered the strength of the interaction between the inhibitory peptide and its target. In order to examine the impact of the fusion kinetics on peptide inhibition without changing the peptide target, we utilized HPIV3 variants carrying mutations in the HN protein but not the F protein. Figure 3a shows that the triggering-defective virus bearing HN-P111S/D216N, an HN variant with a stalk mutation (P111S/D216N) that renders it triggering impaired (25), is inhibited at lower concentrations of HRC peptide than the wt virus. The triggering-enhanced virus, a virus bearing the HN-N551D variant with enhanced F activation (22), requires a higher peptide concentration for effective inhibition. The results for these variants also added support to the temperature data shown for wt HN in Fig. 2; for each variant, the IC50 for peptide inhibition at each of four temperatures (25, 30, 35, and 40°C) was determined and the IC50 was found to vary inversely with temperature. For all HNs, the IC50 for peptide inhibition was higher at 35°C than at 30°C, and the IC50 values for all variants were highest at 40°C. Furthermore, as expected, the IC50 values for peptide inhibition at all temperatures were lowest for the slow-F-triggering HN-P111S/D216N variant and highest for the fast-F-triggering HN-N551D variant (data not shown).
FIG. 3.
(a) The kinetics of HN′s F triggering influences peptide efficacy, as revealed by HPIV3 virions bearing variant HNs. CV1 cells were infected with HPIV3 variants (the wt and the HN-P111S/D216N [P111S/D216N] and HN-N551D [N551D] mutants) at a multiplicity of infection of 6.7 × 10−4 in the presence of different concentrations of peptides and incubated at 37°C. After 90 min, cells were overlaid with agarose, and plaques were stained and counted 18 to 36 h postinfection. The percent inhibition of viral entry (compared to results for control cells infected in the absence of inhibitors) is shown as a function of the (log-scale) concentration of HPIV3 HRC peptide. Data points are means (± standard deviations) for triplicate samples. These data are representative of results from three to five experiments. (b) Preincubation with HPIV3 HRC peptide does not affect viral infection, revealing that the peptide-binding sites of F are not exposed prior to HN′s receptor engagement. HPIV3 virions (the wt and the HN-P111S/D216N and HN-N551D mutants) were incubated with or without 5 μM HPIV3 HRC peptide for 45 min at 37°C. The viral mixture was then diluted 10-fold before being used to infect CV1 cells at a multiplicity of infection of 6.7 × 10−4. “Preincubation” and “infection” refer to HRC peptide concentrations during the preincubation and infection steps. After 90 min, cells were overlaid with agarose, and plaques were stained and counted 18 to 36 h postinfection. The data are normalized to the observed infectivity of virus not exposed to HRC peptide and represent the means (± standard deviations) for triplicate samples.
These data support the idea that peptide efficacy correlates inversely with the kinetics of F triggering. The results of this experiment, revealing that the kinetics of F activation influences the sensitivity of viral infection to peptide inhibition, offer a possible explanation for the finding that despite the strong interaction between HPIV3 HRC and HRN peptides, the HPIV3 peptide inhibits HPIV3 infection less effectively than it does HeV infection.
An alternate interpretation of the experimental results shown in Fig. 3a, however, may be that the P111S/D216N mutation in HN may alter the conformation of the F protein, prematurely generating the transient intermediate state and thereby exposing the peptide-binding site prior to receptor binding. In order to assess this possibility, we preincubated HPIV3 virions with inhibitory peptides before permitting receptor engagement and, therefore, before HN could activate F. We found that preincubating the virions with peptide, even at a high HRC peptide concentration (5 μM), before diluting for infection did not significantly enhance or affect the level of peptide inhibition for any of the HN variant virions (Fig. 3b). These data indicate that the peptide-binding site is not exposed prior to receptor engagement for any of the HPIV3 HN mutants. In addition, since we have shown that the three HN molecules—the wt and the P111S/D216N and N551D mutants—have equivalent receptor avidities under these experimental conditions (22, 25), the kinetics of viral attachment to the cell does not impact fusion inhibition by peptides. Thus, the variation in HRC peptide inhibitory activities for the virions with different HNs is due to differences in the rate of F triggering by HN.
Implications for antiviral development.
The paramyxovirus family encompasses prevalent and particularly infectious viruses, including the measles virus, HPIV, respiratory syncytial virus, and HeV and NiV. This viral family is defined by the presence of a fusion protein (F) that mediates viral and host cell membrane fusion at a neutral pH. Thus, methods of perturbing viral entry and fusion may potentially be widely applicable to members within the paramyxovirus family and to other viruses with similar entry pathways.
Inhibition by HRC peptides requires the interaction of these peptides with a specific intermediate stage of F during the fusion activation process. Only during the putative extended state, after triggering but before refolding into the 6HB, is the HRC peptide target accessible. Depending on the virus, the time spent by the fusion protein in this intermediate stage can vary (8), and thus, peptide inhibitors have different windows of time in which they may access the target. Using the example of HPIV3, we have now shown that for paramyxoviruses, the receptor-binding protein regulates the length of time spent by the fusion protein in the peptide-accessible state. As shown schematically in Fig. 4, a receptor-binding protein with a more efficient F-triggering function decreases the activation energy of F triggering, illustrated by the relative height of the first peak in each panel, and thus the amount of time F exists in its intermediate, extended state before refolding into a collapsed state. In a setting in which the F proteins are identical and the peptide inhibitor affinity is unchanged, an alteration in the F activation properties of HN alters the kinetics of F's progression through its conformational changes, thus altering inhibitor efficacy. Once the extended intermediate stage has passed and fusion proceeds, peptide inhibitors are ineffective. This means that in order to develop effective antiviral inhibitors, it is not sufficient to examine the strength of inhibitor-F interaction; the parameter of the kinetics of F triggering—which determines the access of peptide to its target—must also be considered. This parameter for paramyxoviruses depends on the efficiency of a second protein, the receptor-binding protein: HN for HPIV and G for HeV/NiV in the examples used in this study. It will be of interest to determine whether variants with faster F-triggering kinetics may emerge under the selective pressure of peptide inhibitors. The design of effective inhibitors may require either targeting an earlier stage of F activation or increasing the concentration of the inhibitor at the location of receptor binding to enhance the inhibitor's access to and association with the intermediate-stage fusion protein.
FIG. 4.
Model for HN modulation of fusion inhibitor peptide efficacy. The schematic diagram illustrates the free energy changes (energy of activation [EA]) during F-mediated fusion (the heights of the kinetics barriers are arbitrary and should be viewed relative to one another). Receptor engagement by a triggering-enhanced HN (HN-N551D) that is more efficient than wt HN at activating F leads to lowering of the first energy barrier compared to that for wt HN. A slower-triggering HN (HN-P111S/D216N) is less effective than wt HN at overcoming the energy barrier to F activation, resulting in a longer period of action for an inhibitory peptide. (Adapted with permission of the publisher of reference 8.)
Acknowledgments
This work was supported by Public Health Service grants AI076335 and AI31971 from the National Institutes of Health (NIAID) to A.M., NIH (NIAID) Northeast Center of Excellence for Bio-Defense and Emerging Infections Disease Research U54AI057158 Developmental and Innovation Research Grants to A.M. (principal investigator for Center of Excellence grant, W. I. Lipkin), and a March of Dimes research grant to A.M.
We are grateful to Ashton Kutcher and Jonathan Ledecky for their support, to Dan and Nancy Paduano for support of innovative research projects, and to the Friedman Family Foundation for renovation of our laboratories at Weill Cornell Medical College. We thank Zuhair Salah for assistance.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Aljofan, M., M. Porotto, A. Moscona, and B. A. Mungall. 2008. Development and validation of a chemiluminescent immunodetection assay amenable to high throughput screening of antiviral drugs for Nipah and Hendra virus. J. Virol. Methods 14912-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3309-319. [DOI] [PubMed] [Google Scholar]
- 3.Collins, P., R. Chanock, and K. McIntosh. 1996. Parainfluenza viruses, p. 1205-1241. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 4.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4309-319. [DOI] [PubMed] [Google Scholar]
- 5.Corey, E. A., A. M. Mirza, E. Levandowsky, and R. M. Iorio. 2003. Fusion deficiency induced by mutations at the dimer interface in the Newcastle disease virus hemagglutinin-neuraminidase is due to a temperature-dependent defect in receptor binding. J. Virol. 776913-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng, R., Z. Wang, P. J. Mahon, M. Marinello, A. Mirza, and R. M. Iorio. 1999. Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology 25343-54. [DOI] [PubMed] [Google Scholar]
- 7.Eckert, D. M., and P. S. Kim. 2001. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc. Natl. Acad. Sci. USA 9811187-11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison, S. C. 2008. Viral membrane fusion. Nat. Struct. Mol. Biol. 15690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi, S. B., R. E. Dutch, and R. A. Lamb. 1998. A core trimer of the paramyxovirus fusion protein: parallels to influenza virus hemagglutinin and HIV-1 gp41. Virology 24820-34. [DOI] [PubMed] [Google Scholar]
- 10.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 41302-1307. [DOI] [PubMed] [Google Scholar]
- 11.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. Petteway, Jr. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 932186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin Perlman, S., M. Jordan, R. Brossmer, O. Greengard, and A. Moscona. 1999. The use of a quantitative fusion assay to evaluate HN-receptor interaction for human parainfluenza virus type 3. Virology 26557-65. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., V. R. Melanson, A. M. Mirza, and R. M. Iorio. 2005. Decreased dependence on receptor recognition for the fusion promotion activity of L289A-mutated Newcastle disease virus fusion protein correlates with a monoclonal antibody-detected conformational change. J. Virol. 791180-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 21075-1082. [DOI] [PubMed] [Google Scholar]
- 15.Melanson, V. R., and R. M. Iorio. 2004. Amino acid substitutions in the F-specific domain in the stalk of the Newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J. Virol. 7813053-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscona, A., and R. W. Peluso. 1992. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J. Virol. 666280-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moscona, A., and R. W. Peluso. 1991. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J. Virol. 652773-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netter, R. C., S. M. Amberg, J. W. Balliet, M. J. Biscone, A. Vermeulen, L. J. Earp, J. M. White, and P. Bates. 2004. Heptad repeat 2-based peptides inhibit avian sarcoma and leukosis virus subgroup A infection and identify a fusion intermediate. J. Virol. 7813430-13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palermo, L. M., M. Porotto, O. Greengard, and A. Moscona. 2007. Fusion promotion by a paramyxovirus hemagglutinin-neuraminidase protein: pH modulation of receptor avidity of binding sites I and II. J. Virol. 819152-9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porotto, M., P. Carta, Y. Deng, G. Kellogg, M. Whitt, M. Lu, B. Mungall, and A. Moscona. 2007. Molecular determinants of antiviral potency of paramyxovirus entry inhibitors. J. Virol. 8110567-10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porotto, M., L. Doctor, P. Carta, M. Fornabaio, O. Greengard, G. Kellogg, and A. Moscona. 2006. Inhibition of Hendra virus membrane fusion. J. Virol. 809837-9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porotto, M., M. Fornabaio, G. Kellogg, and A. Moscona. 2007. A second receptor binding site on the human parainfluenza 3 hemagglutinin-neuraminidase contributes to activation of the fusion mechanism. J. Virol. 813216-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porotto, M., O. Greengard, N. Poltoratskaia, M.-A. Horga, and A. Moscona. 2001. Human parainfluenza virus type 3 HN-receptor interaction: the effect of 4-GU-DANA on a neuraminidase-deficient variant. J. Virol. 767481-7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porotto, M., M. Murrell, O. Greengard, L. Doctor, and A. Moscona. 2005. Influence of the human parainfluenza virus 3 attachment protein's neuraminidase activity on its capacity to activate the fusion protein. J. Virol. 792383-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porotto, M., M. Murrell, O. Greengard, and A. Moscona. 2003. Triggering of human parainfluenza virus 3 fusion protein (F) by the hemagglutinin-neuraminidase (HN): an HN mutation diminishing the rate of F activation and fusion. J. Virol. 773647-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapaport, D., M. Ovadia, and Y. Shai. 1995. A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 145524-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 9916249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2001. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 204024-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steger, H. K., and M. J. Root. 2006. Kinetic dependence to HIV-1 entry inhibition. J. Biol. Chem. 28125813-25821. [DOI] [PubMed] [Google Scholar]
- 30.Wild, C., T. Oas, C. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 8910537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 919770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wild, T. F., and R. Buckland. 1997. Inhibition of measles virus infection and fusion with peptides corresponding to the leucine zipper region of the fusion protein. J. Gen. Virol. 78107-111. [DOI] [PubMed] [Google Scholar]
- 33.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao, Q., and R. W. Compans. 1996. Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 223103-112. [DOI] [PubMed] [Google Scholar]
- 35.Young, J. K., R. P. Hicks, G. E. Wright, and T. G. Morrison. 1997. Analysis of a peptide inhibitor of paramyxovirus (NDV) fusion using biological assays, NMR, and molecular modeling. Virology 238291-304. [DOI] [PubMed] [Google Scholar]
- 36.Young, J. K., D. Li, M. C. Abramowitz, and T. G. Morrison. 1999. Interaction of peptides with sequences from the Newcastle disease virus fusion protein heptad repeat regions. J. Virol. 735945-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]