Abstract
The myocyte enhancer factor 2 (MEF2) transcription factors play important roles in neuronal, cardiac, and skeletal muscle tissues. MEF2 serves as a nuclear sensor, integrating signals from several signaling cascades through protein-protein interactions with kinases, chromatin remodeling factors, and other transcriptional regulators. Here, we report a novel interaction between the catalytic subunit of protein phosphatase 1α (PP1α) and MEF2. Interaction occurs within the nucleus, and binding of PP1α to MEF2 potently represses MEF2-dependent transcription. The interaction utilizes uncharacterized domains in both PP1α and MEF2, and PP1α phosphatase activity is not obligatory for MEF2 repression. Moreover, a MEF2-PP1α regulatory complex leads to nuclear retention and recruitment of histone deacetylase 4 to MEF2 transcription complexes. PP1α-mediated repression of MEF2 overrides the positive influence of calcineurin signaling, suggesting PP1α exerts a dominant level of control over MEF2 function. Indeed, PP1α-mediated repression of MEF2 function interferes with the prosurvival effect of MEF2 in primary hippocampal neurons. The PP1α-MEF2 interaction constitutes a potent locus of control for MEF2-dependent gene expression, having potentially important implications for neuronal cell survival, cardiac remodeling in disease, and terminal differentiation of vascular, cardiac, and skeletal muscle.
The myocyte enhancer factor 2 (MEF2) transcription factors play important roles in T-cell selection, neuronal survival, and terminal differentiation of cardiac and skeletal muscle (3, 47). The MEF2 family proteins are encoded by four genes, MEF2A to -D, which demonstrate tissue-specific and temporally dependent developmental expression patterns. Expression and activity of MEF2 factors in both cardiac and skeletal muscle lineages are vital for activation and maintenance of genes representing the structural components of sarcomeric muscle. Gene-targeted ablation of MEF2A or MEF2C results in aberrant heart formation and premature death (32, 46), whereas loss of the single MEF2 gene in Drosophila melanogaster (D-mef2) results in complete loss of all muscle tissues (31). In neurons, MEF2 transcriptional activity plays a critical role for prevention of apoptosis due to neurocytotoxicity signals (1, 5, 20, 37).
The amino-terminal (NT) region of MEF2 transcription factors is composed of a highly conserved MADS (MCM1, agamous, deficiens, and serum response factor) domain, responsible for DNA binding to the consensus DNA binding element (T/C)TA(A/T)4TA(G/A), and the MEF2 domain, which is required for homo- and heterodimerization of MEF2 factors. The carboxyl terminus (CT) of all MEF2 factors is subject to alternative splicing, represents the target for several signal transduction pathways, and is required for transcriptional activation properties (3).
MEF2 transcriptional activity is stimulated by the mitogen-activated protein kinase (MAPK) p38 signaling module (25, 50), Ca2+/calmodulin kinases (CaMKs) (52), extracellular signal-regulated kinase 5/BMK1 (59), p300 (27), and the calcium-activated serine/threonine phosphatase calcineurin (PP2B) (39). By contrast, MEF2-dependent transcription is repressed by cdk5 (20), protein kinase A (30), sumoylation (21), and direct interaction of the MADS/MEF2 domain with Cabin1/Cain (60) or histone deacetylases (HDACs) (22, 29, 35, 41). Repression of MEF2 transcriptional activity through HDAC interaction is alleviated by the direct action of CaMKs on HDACs, altering their subcellular distribution (24, 40). To date, no information exists with regard to activity of phosphatases that reverse the effects of signaling pathways on MEF2 activity.
Protein phosphatase families are classified on the basis of phospho-amino acid specificity, structure, and interaction with regulatory subunits (6, 11). The protein phosphatase 1 (PP1) family of phosphatases is composed of three ubiquitously expressed isoforms (α, β, and γ) that regulate a multitude of cellular functions (11, 14). Subcellular distributions and substrate specificities of PP1 phosphatases are achieved through interaction with regulatory subunits (R-subunits), which typically contain the highly conserved RVXF ([K/R]-X0-1-[V/I/L]-X-[F/W]) binding motif (12). PP1α phosphatase activity is regulated by interaction with inhibitory R-subunits or by direct phosphorylation of a C-terminal threonine residue (T320) by cyclin/cyclin-dependent kinase (CDK) complexes (2).
Here, we present a novel interaction between the catalytic subunit of protein phosphatase 1α (PP1α) and MEF2 transcription factors. Coexpression of PP1α represses MEF2-dependent transcriptional activation and the positive effects of calcineurin signaling and blocks the prosurvival function of MEF2 in primary hippocampal neurons. A role for PP1α in regulating the activity of tissue-restricted transcription factors constitutes a unique property of PP1α activity, and this is the first demonstration of MEF2-mediated transcriptional repression due to a direct interaction with a phosphatase.
MATERIALS AND METHODS
Yeast two-hybrid screen.
The yeast two-hybrid screen utilized amino acids 19 to 167 of rat MEF2D; details have been described elsewhere (59).
Plasmids and antibodies.
The plasmids used were as follows: pMT2 MEF2A and Gal4-MEF2A (amino acids 92 to 507 of human MEF2A fused to the Gal4 DNA binding domain [DBD]) (15), Gal4-C/EBPβ(1-4) (a gift from Andre Bedard), hemagglutinin (HA)-tagged PP1α, PP1β, and PP1γ, and full-length PP2B (calcineurin) were generated by reverse transcription-PCR and cloned in frame with a single copy of the HA tag within a pcDNA3 backbone (Invitrogen). T320 mutants of PP1α were generated by insertion of double-stranded oligonucleotides containing the necessary mutations. Truncated calcineurin was obtained by PCR from full-length protein and insertion of a stop codon after amino acid 398, yielding a constitutively active phosphatase. Activated CaMKIV was generated by PCR from full-length CaMKIV with Glu 318 being replaced with a stop codon and cloned into pcDNA3 lacking an epitope tag (tCaMKIV). MEF-luciferase (a gift from Ron Prywes) has a single MEF2 DNA binding element, GAL4-luciferase (five tandem copies of the Gal4-UAS) and CMV-β-Gal. FLAG-tagged HDAC4 and HDAC5 have been described elsewhere (23). HDAC4-GFP was a kind gift from X.-J. Yang (McGill University). The DsRed vector was generated by cloning the expression cassette of pDsRed2-N1 (Clontech) into a pBluescript (Stratagene) backbone. The green fluorescent protein (GFP) expression vector was generated by cloning the GFP coding region from pEGFP-N1 (Clontech) into pcDNA3. Expression vectors for I-1 and I-2 were generated by reverse transcription-PCR cloning with mutagenesis by oligonucleotide insertion of mutant sequences. All plasmids constructed for these studies were sequenced (York University Core Facility).
Antibodies used included the following: anti-HA, produced using the 12CA5 hybridoma cell line (supernatant diluted 1:10 for immunoblotting); anti-MEF2A and anti-MEF2C rabbit polyclonal antibodies, produced with the assistance of the York University Animal Care Facility; anti-MEF2D (1:1,000; BD Biosciences); anti-GFP (1:1,000; Santa Cruz Biotechnology); anti-β-tubulin III (Sigma).
Cell culture and transfections.
Cos1, Cos7, C2C12, HeLa, and 10T1/2 cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (HyClone), sodium pyruvate, l-glutamine, and penicillin-streptomycin. Cells were maintained in a humidified, 37°C incubator with a 5% CO2 atmosphere.
For transfections, cells were seeded 1 or 2 days prior to transfection and transfected according to the calcium phosphate method as previously described (53).
Embryonic hippocampal neuronal cell culture and transfection.
Primary hippocampal neuronal cells were generated from embryonic day 18 (E18) embryonic brains isolated from timed-pregnant Sprague-Dawley rats in accordance with the Institutional Animal Care and Use Committee of York University, Toronto, Canada. Cells were dissociated by trypsin digestion and mechanical tituration and seeded on poly-d-lysine-coated six-well tissue culture dishes at densities of 1.5 × 106 cells/well for fluorescence-activated cell sorting (FACS) analysis or at a density of 6 × 105 cells/well for luciferase reporter assays. Cells were maintained in neurobasal medium supplemented with B27 (2 ml/100 ml) and l-glutamine (1 ml/100 ml; Invitrogen) in a 37°C humidified incubator with a 5% CO2 atmosphere. Primary neurons were cultured for 7 days prior to calcium phosphate-mediated transient transfection with the indicated expression plasmids. Analyses were carried out 36 h posttransfection. This transfection method typically yields at least 50% transfected cells as determined by inclusion of a constitutively expressed GFP expression vector. Cells were refed with 2 ml/well fresh growth medium 24 h prior to transfection with 200 μl of transfection reagent. For each 1 ml of transfection mixture, 5 μg of reporter, β-galactosidase (β-Gal), and 7.5 μg of effectors was included. Cells were exposed to transfection reagent for 8 h, washed twice with Dulbecco's modified Eagle's medium, and refed with 2 ml/well of fresh culture medium. Cells were processed for FACS or luciferase assays 36 h after transfection.
FACS analysis.
Cell survival analyses were performed using the annexin V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) apoptosis detection kit (Sigma) according to the manufacturer's instructions. Transiently transfected cells were washed with phosphate-buffered saline (PBS) and trypsinized for 3 to 4 min. Cells were collected by centrifugation and washed twice with ice-cold 1× PBS. On ice, cell pellets were resuspended in 100 μl of 1× annexin binding buffer per condition, followed by addition of staining with annexin V-FITC and PI for 15 min in the dark at 4°C. Cells were collected by centrifugation, resuspended in 500 μl of 1× annexin binding buffer, and analyzed immediately by flow cytometry. Ten thousand cells from each event were scanned using a FACSCalibur flow cytometer (Becton Dickinson) using the standard configuration and parameters. Data from quadrants demarcating unstained cells, cells PI positive, cells annexin V-FITC positive, and cells positive for both PI and annexin V-FITC were collected and analyzed using CellQuest computer software (Becton Dickinson). Necrotic cells are represented as PI positive, whereas apoptotic cells were positive for annexin V-FITC fluorescence only.
Protein extractions and reporter assays.
Protein extractions were performed as described previously (53). Cells were harvested using an NP-40 lysis buffer (0.5% NP-40, 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 10 mM sodium pyrophosphate, 1 mM EDTA [pH 8.0], 0.1 M sodium fluoride) containing 10 μg/ml leupeptin and aprotinin, 5 μg/ml pepstatin A, 0.2 mM phenylmethylsulfonyl fluoride, and 0.5 mM sodium orthovanadate. Protein concentrations were determined using the Bradford method (Bio-Rad) with bovine serum albumin (BSA) as a standard. Extracts used for immunoblotting were diluted in sample buffer containing β-mercaptoethanol and boiled.
Transcriptional assays using luciferase reporter plasmids were done as previously described (28). Luciferase values were normalized to β-galactosidase activity. Corrected luciferase values for control, reporter-alone transfections were arbitrarily set to 1.0, and average activation values were calculated. Bars represent the means and error bars represent standard errors of the means. For each luciferase figure presented, one representative experiment is shown consisting of a triplicate set. In all cases, experiments were done multiple times and were carried out in several cell lines, showing near-identical results as the data presented.
Hippocampal neurons were transiently transfected with 1 μg of 3X-MEF2-luciferase reporter plasmid, 1 μg pCMV-β-galactosidase (control for transfection efficiency), and 1.5 μg of MEF2D and/or HA-PP1α expression plasmid. The total amount of DNA for each experiment was kept constant by using empty vectors. Cells were harvested 36 h posttransfection and both β-galactosidase and luciferase activities were measured. Luciferase data were collected using a Berthold 9501 luminometer. All experiments using luciferase reporter plasmids were done in triplicate with data presented as means ± standard errors of the means.
Immunoblotting, coimmunoprecipitations, and GST pulldown experiments.
Immunoblotting, coimmunoprecipitations, and glutathione S-transferase (GST) pulldown experiments were done as previously described (53). For immunoblotting, 20 μg of total protein extracts was used for analysis, and for coimmunoprecipitation 200 to 500 μg of total cell extract was diluted to 0.8 μg/ml with NP-40 lysis buffer.
GST fusion proteins were obtained by standard protocols. Purified GST protein concentrations were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining using BSA to estimate concentrations. For pulldown experiments, 500 μg of cell extract was diluted to 650 μl with NP-40 lysis buffer and 5 μg of GST fusion protein was added. Glutathione-agarose beads (25 μl of a 50% slurry [Amersham]) were added and mixtures were incubated overnight at 4°C on a Nutator (BD Diagnostics). Beads were washed, diluted with sample buffer containing β-mercaptoethanol, boiled, and separated using 10% sodium dodecyl sulfate-polyacrylamide gels.
Immunofluorescence.
Cells transfected for immunofluorescence analysis were fixed 24 h after transfection for 10 min at room temperature with 4% paraformaldehyde in PBS, washed with PBS, and permeablilized with 0.3% Triton X-100 in PBS for 5 min. Cells were blocked with 10% goat serum in PBS for 30 min at 37°C and incubated overnight at 4°C on a rocker table with primary antibodies diluted in 0.1% BSA dissolved in PBS (affinity-purified MEF2A at 1:500 and anti-HA hybridoma supernatant at 1:1). Cells were washed with PBS and incubated at room temperature with secondary FITC-conjugated secondary antibodies (produced in goat; Sigma) diluted in 0.1% BSA.
Electrophoretic mobility shift assay (EMSAs).
DNA binding assays and extract preparation were carried out as previously described (28, 38, 51).
RESULTS
PP1α interacts directly with MEF2 transcription factors.
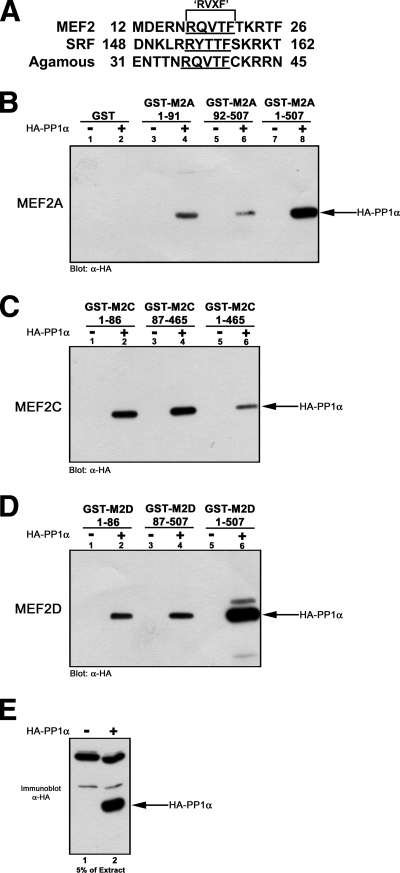
To examine MEF2 protein-protein interactions, we undertook a yeast two-hybrid genetic screen using amino acids 19 to 167 of rat MEF2D fused to the Gal4-DBD as bait (59). Our screen identified known MEF2-interacting factors, such as extracellular signal-regulated kinase 5 (59), HDAC4 (41), Cain/Cabin1 (60), and a novel interaction with the catalytic subunit of PP1α (see Fig. S1 in the supplemental material). This serine/threonine phosphatase regulates numerous cellular processes by interaction with a multitude of R-subunits via a conserved RVXF sequence motif (11). Initially, in silico analysis of MADS domain-containing proteins revealed the presence of a conserved PP1α binding site (RVXF motif) within the MADS DNA binding domains of MEF2, SRF, and Agamous (Fig. 1A).
FIG. 1.
PP1α binds to both the N- and C-terminal regions of MEF2A, -C, and -D. (A) Alignment of MEF2, SRF, and Agamous (amino acid regions marked by numbers) showing the RVXF motif (underlined) PP1α binding site within the MADS DNA binding domains. (B) Cell extracts from control (−) and HA-PP1α (+) transiently transfected Cos1 cells were assayed for the binding potential of GST fusion proteins containing different regions of MEF2. PP1α does not interact with GST (lanes 1 and 2). PP1α binding occurs with N-terminal (amino acids 1 to 91; lanes 3 and 4), C-terminal (amino acids 92 to 507; lanes 5 and 6), and full-length (amino acids 1 to 507; lanes 7 and 8) human MEF2A. (C and D) PP1α interacts with amino (lane 2), carboxyl (lane 4), and full-length (lane 6) fusions of canine MEF2C (C) and rat MEF2D (D). (E) Immunoblot of extracts indicating HA-PP1α expression levels. Shown is 5% of the extract used for pulldowns with an identical exposure.
Next, we analyzed the interaction of HA-tagged PP1α with full-length (FL), NT, and CT GST fusions of MEF2A, -C, and -D (Fig. 1B to D). HA-PP1α did not interact with GST (Fig. 1B, lanes 1 and 2) but was detected when NT (Fig. 1B, lanes 3 and 4), CT (Fig. 1B, lanes 5 and 6), or FL (Fig. 1B, lanes 7 and 8) GST fusions of MEF2A were assayed. Similarly, HA-PP1α was detected using FL, NT, and CT fusions of MEF2C (Fig. 1C) and MEF2D (Fig. 1D). An immunoblot using 5% of extract and an identical exposure as that for GST pulldown blot assays is shown in Fig. 1E. Control experiments using GST-MyoD or GST-ATF2 did not permit detection of HA-tagged PP1α (not shown). The data suggest a bipartite interaction of PP1α with both the N- and C-terminal regions of MEF2.
PP1α represses MEF2 transactivation properties.
To address the significance of PP1α binding to MEF2, we assessed the subcellular localization of both proteins to determine whether the interaction occurs within mammalian cells and to predict the functional consequence of PP1α interaction for MEF2 activity (see Fig. S2 in the supplemental material). We transfected combinations of MEF2A and HA-PP1α and determined their subcellular distributions by immunofluorescence, with transfected cells being marked by inclusion of a constitutively expressed DsRed2 expression plasmid. We found that HA-tagged PP1α in DsRed-positive cells showed both nuclear and cytoplasmic localization and coexpression of MEF2A and HA-PP1α did not alter the cellular distribution of either molecule (see Fig. S2A and B in the supplemental material). Indeed, coimmunofluorescence showed both MEF2A and HA-PP1α within the nucleus of cotransfected cells (see Fig. S2C in the supplemental material).
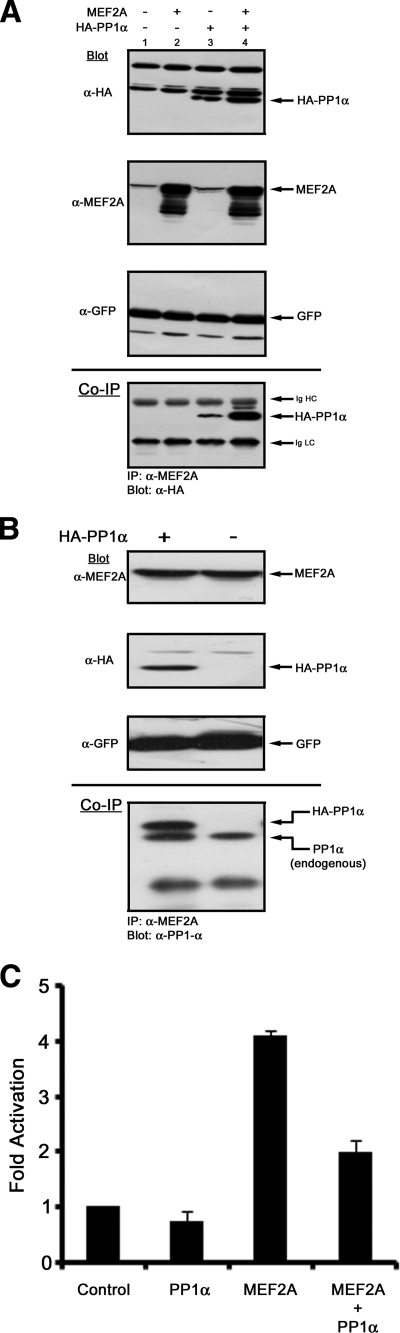
Next, we transiently transfected Cos1 cells and confirmed that a MEF2-PP1α interaction occurred within a cellular context by coimmunoprecipitation (Fig. 2A). Immunoblot tests showed that steady-state levels of HA-PP1α and MEF2A were unaffected due to coexpression (Fig. 2A, upper panels). Levels of GFP, a control of transfection efficiency, were unchanged, suggesting no cellular effects due to HA-PP1α or MEF2A overexpression (Fig. 2A, GFP blot). Coimmunoprecipitation of MEF2A from cell extracts demonstrated that HA-PP1α is readily detectable (Fig. 2A, bottom panel). Detection of HA-PP1α from extracts not overexpressing MEF2A is due to endogenous MEF2A expression (Fig. 2A, MEF2A blot lanes 1 and 3). Increased HA-PP1α detection when MEF2A is ectopically expressed supports a specific interaction between MEF2A and PP1α (Fig. 2A, co-IP panel, compare lanes 3 and 4). Coimmunoprecipitation of endogenous MEF2A from HeLa cells transfected with HA-PP1α and GFP or with GFP alone indicates that endogenous and exogenous PP1α are detectable, showing that this interaction occurs in the absence of overexpressed factors (Fig. 2B).
FIG. 2.
PP1α binding to MEF2 represses transcriptional activity. (A) Coimmunoprecipitation of HA-PP1α with MEF2A. Cos1 cells were transiently transfected with GFP and combinations of MEF2A and HA-PP1α expression plasmids (as indicated). Immunoblots (upper three panels) show expression levels of HA-PP1α, MEF2A, and GFP (a marker of transfection efficiency). The bottom panel (coimmunoprecipitation [co-IP]) shows that HA-PP1α is readily detected when MEF2A is immunoprecipitated from extracts (lanes 3 and 4). The immunoglobulin G heavy chain (Ig HC) and light chain (Ig LC) are indicated. (B) Coimmunoprecipitation of HA-PP1α and endogenous PP1α. HeLa cells were transiently transfected with HA-PP1α and GFP or with GFP alone. Immunoblots (upper three panels) show expression levels of endogenous MEF2A, HA-PP1α, and GFP (control for transfection efficiency). The bottom panel (immunoprecipitation [IP] with MEF2A; blot with PP1α) shows that both endogenous PP1α and HA-PP1α are readily detected when endogenous MEF2A is immunoprecipitated. The Ig light chain is indicated. (C) Cos7 cells were transiently transfected as indicated. MEF2A transcriptional activity was assessed using a 1X-MEF-luciferase reporter vector.
We next investigated the effects of PP1α on MEF2A transcriptional activity by using a MEF2-dependent luciferase reporter plasmid (Fig. 2C). MEF2A overexpression effectively activated MEF2-dependent transcription (Fig. 2C). While PP1α expression had little effect on basal activity of the reporter, MEF2-mediated transcriptional activity was reduced (Fig. 2C).
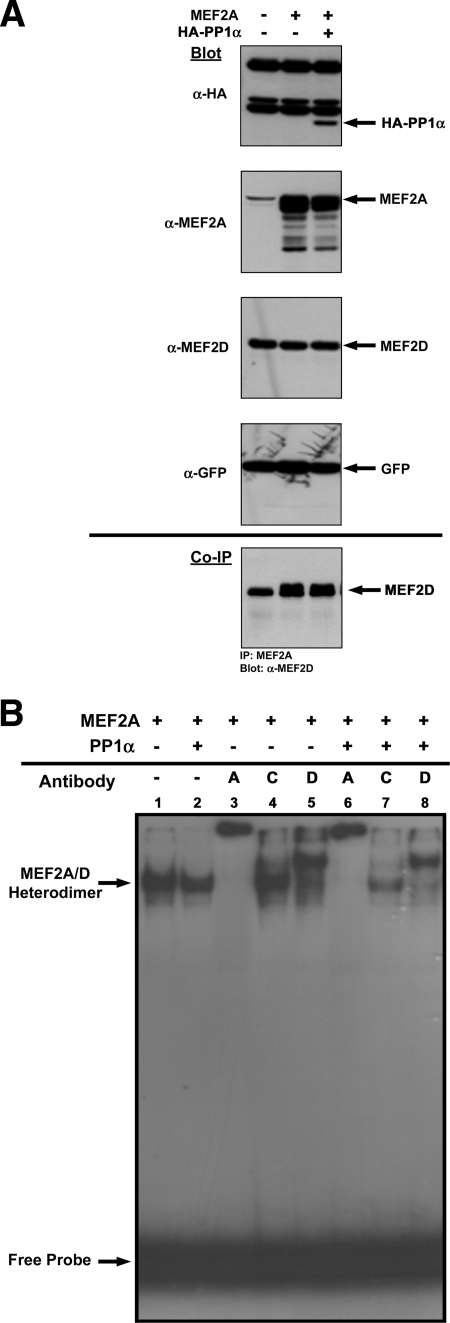
Implicit to MEF2-dependent transcriptional activity is homo- or heterodimerization and DNA binding to an A/T-rich MEF2 box. To investigate whether PP1α affected MEF2 transcription at these levels, we performed coimmunoprecipitation assays and EMSAs to elucidate the repressive nature of PP1α on MEF2 transactivation properties (Fig. 3). Immunoblot assays showed expression levels of transfected molecules were essentially equivalent, with GFP showing near-identical transfection efficiencies and no alterations in the levels of endogenous MEF2D (Fig. 3A, top panels). Coimmunoprecipitation of MEF2A and probing for MEF2D indicated PP1α does not interfere with the heterodimerization potential of MEF2A or MEF2D (Fig. 3A, bottom panel). Similarly, when we assessed DNA binding in an EMSA, both dimer composition and DNA binding of MEF2A/D heterodimers were essentially unaffected by PP1α coexpression (Fig. 3B, compare lanes 1 and 2). Supershift analyses using antibodies to MEF2A, -C, or -D supported the notion that all dimeric pairs in MEF2A-overexpressing Cos cells contain both MEF2A and MEF2D (Fig. 3B, lanes 3 and 5) and coexpression of PP1α does not alter the dimeric composition (Fig. 3B, compare lanes 3 and 5 with lanes 6 and 8). The inability of MEF2C antibody to supershift MEF2 binding complexes supports our finding that MEF2C expression is not detectable in Cos cells (data not shown).
FIG. 3.
PP1α does not interfere with MEF dimerization or DNA binding. (A) MEF2A heterodimerization with endogenous MEF2D is unaffected by HA-PP1α coexpression. Cos1 cells were transiently transfected with the indicated plasmids, and immunoblots showing levels of expression of transfected molecules are shown (HA-PP1α, MEF2A, and GFP). Levels of endogenous MEF2D remained unchanged, and GFP levels showed similar transfection efficiencies. Coimmunoprecipitation (co-IP; bottom panel) showed that endogenous MEF2A and MEF2D are found in a heterodimeric pair (left lane). Overexpression of MEF2A permits increased detection of MEF2D, and inclusion of HA-PP1α does not affect the heterodimeric composition of MEF2A or MEF2D obtained from transfected extracts (bottom panel, middle and right lanes). (B) PP1α does not affect MEF2 DNA binding. An EMSA and supershift analysis from Cos1-transfected cells showed that overexpression of MEF2A alone or with HA-PP1α results in similar levels of dimerization and DNA binding (lanes 1 and 2; MEF2A/-D DNA binding complexes are indicated with an arrow). All DNA binding appears to be mediated by MEF2A/-D heterodimers, as inclusion of antibodies against MEF2A (lanes 3 and 6) or MEF2D (lanes 5 and 8) led to supershifts of all detectable binding complexes. Coexpression of HA-PP1α does not alter the MEF2 heterodimeric composition, as identical results were observed when HA-PP1α was coexpressed (compare lanes 3 and 6 for MEF2A supershift and lanes 5 and 8 for MEF2D supershift). The lack of MEF2C expression in these cells was confirmed by the inability of MEF2C antibody to supershift any of the DNA binding complex. Free probe is indicated at the bottom of the panel.
Together, these data support a novel interaction between the catalytic subunit of PP1α and MEF2 that serves to repress MEF2-mediated transcriptional activity without affecting subcellular distribution, dimerization, or DNA binding properties of MEF2 factors.
The C terminus of MEF2 is required for PP1-dependent regulation.
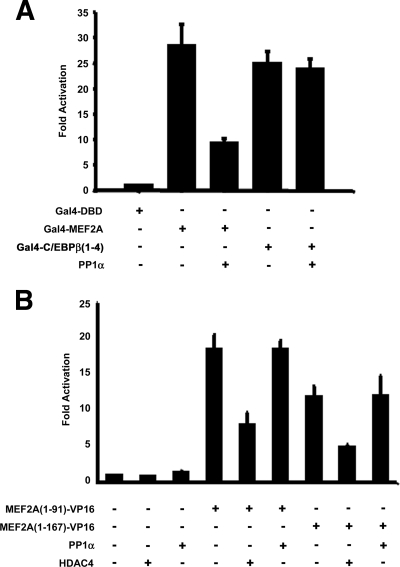
To characterize PP1α-mediated regulation of MEF2 transactivation properties, we examined the transcriptional activation properties of the CT transactivation domain (TAD) and NT DNA binding/dimerization domain of MEF2 (Fig. 4). A Gal4-DBD fusion of the MEF2A CT TAD (amino acids 92 to 507 of MEF2A) potently activated the Gal4-luciferase reporter gene (Fig. 4A). Coexpression of PP1α suppressed this activity (Fig. 4A), and coimmunoprecipitations indicated that HA-PP1α interacts with Gal4-MEF2A 92-507 (not shown). The specificity of repression was demonstrated by the inability of PP1α to affect a Gal4-DBD fusion of the unrelated transcription factor C/EBPβ(1-4) (Fig. 4A).
FIG. 4.
PP1 represses MEF2 through the C-terminal transactivation domain. (A) Cos7 cells were transiently transfected as indicated and the activity of Gal4-MEF2A (amino acids 92 to 507) and C/EBPβ(1-4) was assessed using a 5X-Gal4-luciferase reporter vector. (B) 10T1/2 mouse fibroblasts were transiently transfected with the indicated plasmids, and fusions of the N terminus of MEF2A containing the MADS/MEF2 domain (aa 1 to 91) or a more-C-terminal extension (aa 1 to 167) to the VP16 acidic transactivation domain were assayed for activation of the 1X-MEF-luciferase reporter vector.
Next, we replaced the CT TAD of MEF2A with the VP16 acidic TAD. We generated two fusions of MEF2 utilizing the first 91 amino acids and a longer fusion containing both RVXF and the putative C-terminal interaction domain (amino acids 1 to 167) and assessed transcriptional activity using the MEF2-luciferase reporter gene (Fig. 4B). An HDAC4 expression vector was included as a positive control for repression. Both fusions efficiently activated the MEF2-luciferase reporter vector (Fig. 4B). While HDAC4 exerted a negative effect on the MADS/MEF2-VP16 chimeras, overexpression of PP1α had no effect (Fig. 4B). These data show that while the MADS domain RVXF motif is not utilized for PP1α-mediated repression, the CT TAD (amino acids 168 to 507) of MEF2 appears to be required for PP1α regulation.
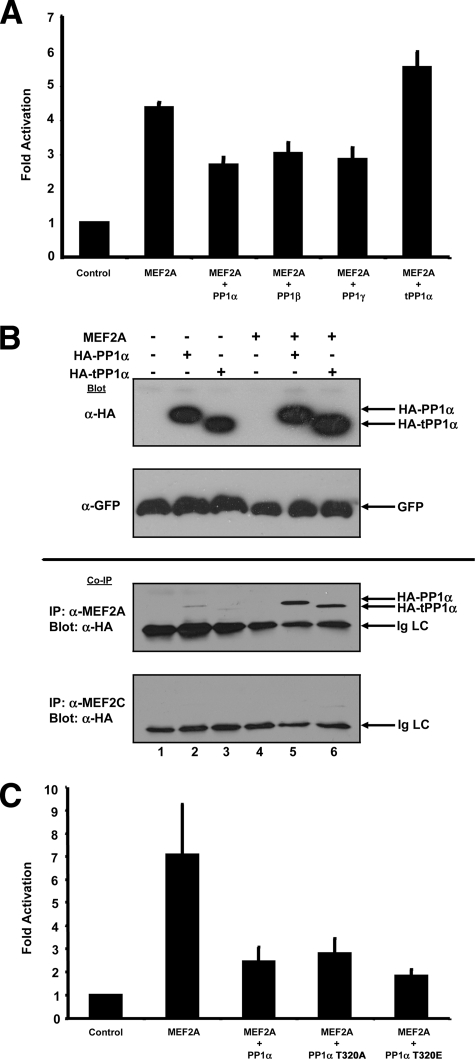
PP1 isoforms regulate MEF2 independently of phosphatase activity.
The three PP1 forms (α, β, and γ) show tissue-specific expression patterns and R-subunit binding specificity (11), and the necessary regions of PP1α involved in R-subunit interaction have been well characterized (19). Coexpression of PP1 isoforms and a truncated PP1α (truncated at tyrosine-306 [tPP1α]) demonstrated that all three PP1 isoforms repress MEF2 transactivation activity, whereas tPP1α exerted no effect (Fig. 5A). Interestingly, coimmunoprecipitation demonstrated that HA-tPP1α is capable of interacting with MEF2A (Fig. 5B). While tPP1α has increased phosphatase activity, essentially normal RVXF binding (19), and normal subcellular localization (not shown), the inability to negatively regulate MEF2 suggests the far C terminus of PP1α is required for regulation of MEF2-dependent transcriptional activity. Since the C terminus of PP1 contains an important regulatory threonine residue (T320), we examined PP1α mutants that are constitutively active (threonine 320 mutated to alanine [T320A]) (2) or a phospho-mimetic, inactive phosphatase (T320 mutated to glutamic acid [T320E]) (Fig. 5C). It can be seen that both T320 mutants repressed MEF2-dependent transcriptional activity (Fig. 5C) and coexpression of PP1α inhibitors did not rescue MEF2 activity (data not shown, but see Fig. 6A, below), supporting the notion that PP1α phosphatase activity is not required for regulation of MEF2. Together, these data suggest that phosphatase activity is dispensable for regulation and the far CT region of PP1α is necessary for regulating MEF2-dependent transcriptional activity but not interaction.
FIG. 5.
MEF2 regulation requires the C-terminal region of PP1α but not phosphatase activity. (A) C2C12 myoblasts were transfected as indicated and MEF2 transcriptional activity was determined by activation of the 1X-MEF2-luciferase reporter vector. Coexpression of all three isoforms of PP1 (α, β, and γ) represses MEF2A transcriptional activity. Coexpression of tPP1α is unable to regulate MEF2. (B) Coimmunoprecipitation of full-length and C-terminal-truncated HA-tagged PP1α (HA-tPP1α) with MEF2A. Cos7 cells were transiently transfected with the indicated expression plasmids. Immunoblots (upper two panels) show expression levels of HA-PP1α, HA-tPP1α, and GFP (included as a control for transfection efficiency) (upper panels). Coimmunoprecipitation (Co-IP; bottom two panels) showed detection of both full-length HA-PP1α and truncated HA-tPP1α when MEF2A was immunoprecipitated from extracts. A control IP using an antibody raised against MEF2C demonstrated essentially no detection of HA-tagged PP1α molecules (bottom Co-IP panel). (C) Cos7 cells were transfected as indicated and MEF2 transcriptional activity was assessed by activation of the 1X-MEF-luciferase reporter vector. Mutants of PP1α that cannot be inactivated by cell cycle-dependent cyclin/cdk phosphorylation (T320A) or PP1α mutant simulating constitutive inactivation (T320E) repress the MEF2 transcriptional activity seen with wild-type PP1α.
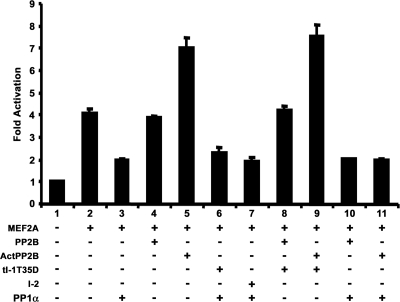
FIG. 6.
PP1α blocks calcineurin-dependent activation of MEF2. C2C12 myoblasts were transiently transfected with different combinations (as indicated) of MEF2A, HA-PP1α (PP1α), wild-type full-length calcineurin (PP2B), constitutively activated calcineurin (Act PP2B), or inhibitors of PP1α function inhibitor 2 (I-2) or a truncated activated form of inhibitor 1 (tI-1T35D). Transcriptional activity of MEF2A was assessed using a 1X-MEF2 luciferase reporter vector.
Hierarchy of PP1α-mediated MEF2 regulation.
While some of our analyses suggested PP1α phosphatase activity is dispensable for regulation of MEF2, we analyzed the potential that some aspects of MEF2A function are affected by phosphatase-active PP1α. We treated purified TAP-MEF2A (15) with PP1α in vitro and used mass spectrometry to determine that the CDK5-targeted serine-408 (S408) of MEF2A was a detectable site of dephosphorylation (data not shown). To examine whether this site was the basis for PP1α regulation of MEF2, S408A and S408D mutants were tested; however, we still observed full repression by PP1α coexpression (data not shown). Reports indicate S408 is a target for calcineurin-mediated activation of MEF2 (21). Since calcineurin activates PP1α through inhibitor-1 (I-1), we assessed whether calcineurin modulates MEF2 through PP1α (Fig. 6). Coexpression of PP1α repressed MEF2-dependent transcriptional activity (Fig. 6, lane 3), whereas activated calcineurin enhanced MEF2-dependent transcriptional activity (Fig. 6, lane 5). Inclusion of PP1α inhibitors did not rescue PP1α-mediated repression of MEF2 transcriptional activity (Fig. 6, lanes 6 and 7). Expression of a potent inhibitor of PP1α, truncated I-1 T35D (tI-1T35D), did not alter the effects of calcineurin, suggesting that calcineurin acts independently of PP1α (Fig. 6, lanes 8 and 9). Coexpression of PP1α with full-length or activated calcineurin blocked MEF2 transcriptional activity to levels observed with only PP1α (Fig. 6, compare lanes 10 and 11 with lane 3). This suggests PP1α-mediated repression of MEF2 is dominant to calcineurin-mediated activation.
These data indicate that PP1α regulates MEF2-dependent transcriptional activity by direct interaction, PP1α phosphatase activity is dispensable for this regulation, and PP1α-dependent repression of MEF2 overrides calcineurin-mediated activation of MEF2-dependent transcriptional activity.
PP1α can recruit HDAC4 to MEF2.
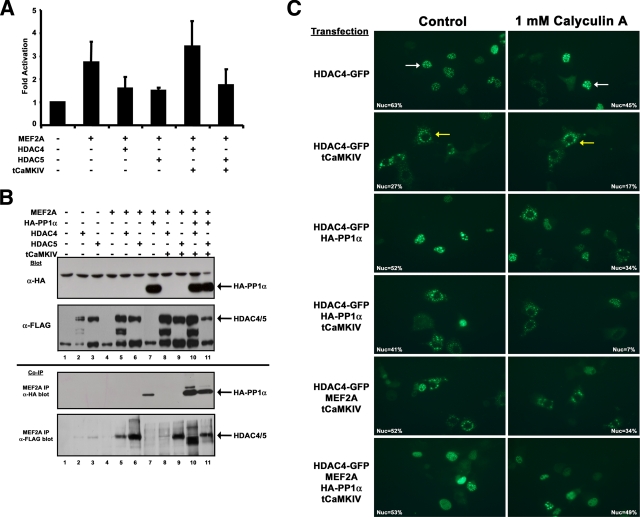
We next examined whether the MEF2-PP1α interaction permits recruitment of cofactors to MEF2 transcriptional complexes. PP1α interacts with HDAC1, -6, and -10, but not HDAC3, -4, or -5 (8), and nuclear retention of HDAC4 is sensitive to calyculin A, an inhibitor of PP1α (34). We examined the possibility that the MEF2-PP1α interaction might modulate the interaction with HDAC4 or -5, known repressors of MEF2 activity (Fig. 7). Initially, we confirmed HDAC regulation by assessing MEF2-dependent transcriptional activity when coexpressed with HDAC4 or -5 and a constitutively active CaMKIV (tCaMKIV) (Fig. 7A). Both HDACs efficiently repressed MEF2A transcriptional activity, whereas addition of tCaMKIV restored MEF2A transcriptional activity only in the case of HDAC4 (Fig. 7A). The reason for the difference between the rescue of MEF2 activity with HDAC4 but not HDAC5 is not clear; however, differences in nuclear export of HDAC4 and -5 have been seen in isolated skeletal muscle fibers under increased calcium signaling due to electrical stimulation (34).
FIG. 7.
PP1α-mediated recruitment of HDAC4 to the MEF2 transcriptional complex. (A) 10T1/2 mouse fibroblasts were transfected as indicated with MEF2A, HDAC4 or -5, and an activated form of CaMKIV (tCaMKIV). MEF2-mediated gene activation was assessed using a 1X-MEF2-luciferase reporter vector. (B) Cos7 cells were transiently transfected with the indicated plasmids, and immunoblot assays for HA-PP1α and FLAG-HDAC4 and -5 were performed (top two panels). Interaction of HA-PP1α (coimmunoprecipitation [co-IP]; anti-HA blot) or FLAG-tagged HDAC4 or -5 (co-IP; anti-FLAG blot) were detected after immunoprecipitation of MEF2A from cell extracts. (C) Cos7 cells were transiently transfected with combinations of GFP-HDAC4, tCaMKIV, HA-PP1α, and MEF2A expression plasmids (labels on left side). Left panels show control cells and right panels show cells treated for 4 h with 1 nM calyculin A (indicated at top). A cell with nuclear-localized HDAC4-GFP is marked by a white arrow (upper left panel) and cytoplasmic-localized HDAC4-GFP is indicated by a yellow arrow (upper right panel). The percentages of transfected cells with HDAC4-GFP solely localized within the nucleus are indicated within each photomicrograph. Photomicrographs were taken at 20× magnification using a Zeiss Axiovert microscope, and digital images were obtained using a Canon camera.
To examine whether PP1α can influence association of HDACs with a MEF2-containing transcriptional complex, we coexpressed these molecules and performed coimmunoprecipitation analyses (Fig. 7B). We observed that overexpressed HA-PP1α and FLAG-tagged HDACs are readily detected by immunoblot analysis (Fig. 7B, top panels). Interaction of HA-PP1α with MEF2A was confirmed (Fig. 7B, co-IP panel, anti-HA blot, lane 7) and when coexpressed with tCaMKIV or HDAC4/5 (Fig. 7B, co-IP anti-HA blot, lanes 10 and 11). We readily detected interactions of HDAC4 and -5 with endogenous MEF2A (Fig. 7B, lanes 2 and 3) and under conditions of MEF2A overexpression (Fig. 7B, lanes 5 and 6). By contrast, inclusion of tCaMKIV abrogated a MEF2-HDAC4 interaction but not a HDAC5-MEF2A interaction (Fig. 7B, co-IP anti-FLAG blot, lanes 8 and 9, respectively), congruent with the reporter assay data (Fig. 7A). Loss of the MEF2A-HDAC4 interaction due to CaMKIV activity was restored by coexpression of HA-PP1α, and the HDAC5/MEF2A complex remained unchanged (Fig. 7B, co-IP anti-FLAG blot, lanes 10 and 11, respectively). The faster migration of HDAC4 in the presence of HA-PP1α is likely due to dephosphorylation; however, we have not addressed this experimentally.
To further delineate the role of PP1α in regulating HDAC4, we analyzed a GFP-HDAC4 fusion protein under cotransfection conditions with tCaMKIV, HA-PP1α, and MEF2A in the absence and presence of the PP1 pharmacological inhibitor calyculin A (Fig. 7C). To quantitatively assess the role of both exogenously expressed proteins and the effects of calyculin A, we counted the number of cells that had solely nuclear localized HDAC4-GFP relative to the total number of transfected cells and presented this percentage on each photomicrograph (Fig. 7C). Cells expressing HDAC4-GFP show nuclear speckles and reduced fluorescence and increased cytoplasmic localization upon treatment with calyculin A (Fig. 7C, upper panels). Coexpression of tCaMKIV led to nuclear exclusion of HDAC4-GFP, which was increased in the presence of calyculin A treatment (Fig. 7C, HDAC4-GFP/tCaMKIV-transfected cells). Coexpression with HA-PP1α and treatment of cells with calyculin A showed similar results as for HDAC-GFP alone (Fig. 7C, HDA4-GFP/HA-PP1α). Coexpression of both tCaMKIV and HA-PP1α resulted in a range of nuclear and cytoplasmic localization, with calyculin A treatment causing reductions in both the intensity of fluorescence and HDAC4-GFP nuclear localization (Fig. 7C, HDAC4-GFP/tCaMKIV/HA-PP1α). Next, we overexpressed MEF2A in the absence and presence of HA-PP1α. While calyculin A treatment demonstrated a reduction of fluorescence intensity and reduced nuclear localization of HDAC4-GFP in the absence of HA-PP1α (Fig. 7C, HDAC4-GFP/tCaMKIV/MEF2A), inclusion of HA-PP1α showed retention of HDAC4-GFP in the nucleus in the presence of calyculin A (Fig. 7C, bottom panels). This supports the notion that PP1α phosphatase activity is dispensable for regulation of MEF2A and demonstrates that retention of HDAC4 within the nucleus does not require PP1α phosphatase in the presence of MEF2A. Nuclear retention of HDAC4 under conditions of tCaMKIV signaling is a unique trans-dominant property of the MEF2-PP1α interaction. Together, these data suggest that interaction of PP1α with MEF2A can reverse, or block, the effects of CaMKIV signaling on HDAC4 subcellular localization and interaction with a MEF2-containing transcriptional complex (Fig. 8B).
FIG. 8.
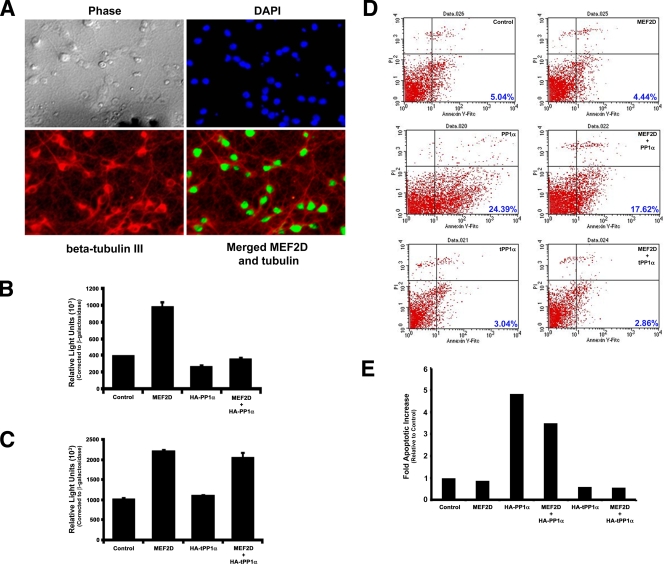
PP1α blocks the prosurvival effect of MEF2 in primary hippocampal neurons. (A) Primary hippocampal neurons express MEF2D. Primary hippocampal neurons were processed for coimmunofluorescence detection using β-tubulin III (a marker of neurons) and MEF2D. Phase-contrast and 4′,6-diamidino-2-phenylindole (DAPI) panels show all cells present, and β-tubulin III demarcates neuronal cells. The merged panel shows coexpression of MEF2D within the nuclei of β-tubulin III-positive cells. (B) Primary hippocampal neurons were transiently transfected with combinations of MEF2D and HA-PP1α plasmids (as indicated), and MEF2-mediated transcriptional activity was assessed using a 3X-MEF-luciferase reporter plasmid, normalized to β-galactosidase activity. (C) Primary hippocampal neurons were transiently transfected with combinations of MEF2D and HA-tPP1α plasmids (as indicated), and MEF2D transcriptional activity was assessed using a 3X-MEF-luciferase reporter plasmid, normalized to β-galactosidase activity. (D) PP1α blocks prosurvival activity of MEF2D. Primary hippocampal neurons were transiently transfected as indicated in the upper right corner of each panel (black lettering), and apoptosis was determined using annexin V-FITC or propidium iodide staining and FACS analysis (the percent apoptosis is shown in blue numbers in the bottom right corner of each panel). (Upper panels) Control and MEF2D-transfected cells; (middle panels) HA-PP1α and MEF2D/HA-PP1α; (bottom panels) HA-tPP1α and MEF2D/HA-tPP1α. Overexpression of HA-PP1α in the absence and presence of MEF2D increases the percentage of apoptotic, annexin V-positive cells. (E) Graphical representation of the FACS data shown in panel D, showing the change in numbers of apoptotic cells relative to control transfected cells. Note that the data are from one representative experiment. Replicate experiments showed similar results.
PP1α promotes neuronal apoptosis by overriding MEF2's prosurvival function.
MEF2-dependent transcriptional activity plays a key prosurvival role in preventing neuronal apoptosis induced by neurocytotoxic stimuli (1, 5, 20, 37). To establish the importance of PP1α regulation of MEF2 function, we examined the role of PP1α in regulating neuronal apoptosis in primary hippocampal neurons (Fig. 8). These primary cultures contained a high proportion of neuronal cells, as determined by β-tubulin III expression, and these cells expressed readily detectable levels of MEF2D (Fig. 8A). Moreover, transient transfection of these cells using a modified calcium phosphate methodology routinely results in expression of exogenously transfected plasmids in approximately 50% of cells (see Fig. S3 in the supplemental material). Similar to transient transfections in other cell lines, coexpression of HA-PP1α led to a repression of MEF2D-dependent transcriptional activation of a MEF2-dependent reporter plasmid that contained three copies of a consensus MEF2 enhancer element (3X-MEF-luciferase) (Fig. 8B). As seen earlier, coexpression of truncated PP1α does not negatively regulate MEF2D-mediated transcriptional activity, supporting a novel regulatory role for the far C terminus of PP1α for regulating MEF2 activity (Fig. 8C). This activity of PP1α is specific for MEF2, as similar experiments using a luciferase reporter plasmid lacking MEF2 enhancer element show it is not regulated by HA-PP1α overexpression (data not shown).
Next, we examined whether PP1α overexpression affects the survival of primary hippocampal neurons by using annexin-V/propidium iodide staining and FACS analysis (Fig. 8D). Control transfected cells or cells transfected with only MEF2D showed similar percentages of apoptotic cells (Fig. 8D, upper panels). By contrast, cells transfected with HA-PP1α showed an increase in the number of apoptotic cells (Fig. 8D, middle left panel), and coexpression of MEF2D with PP1α did not rescue the proapoptotic effect of PP1α (Fig. 8D, middle right panel). By contrast, expression of truncated PP1α alone did not affect neuronal survival in the absence (Fig. 8D, bottom left panel) or presence of coexpressed MEF2D (Fig. 8D, bottom panels). Indeed, PP1α promotes apoptotic cell death nearly fivefold above control levels. Inclusion of MEF2D has only a small effect on reversing PP1α-mediated cell death, consistent with PP1α's trans-dominant repression of MEF2 activity seen in other assays (Fig. 8E). By contrast, there is essentially no change, or a modest decrease, when cells express a truncated PP1α (Fig. 8E) which does not regulate MEF2 (Fig. 5A). These data are congruent with the reporter assay data (Fig. 8B and C), which showed that the truncated PP1α cannot repress MEF2, supporting the notion that MEF2-dependent transcriptional activity is important for neuronal cell survival. Together, these data demonstrate that PP1α represses the transcriptional activity of MEF2 in primary hippocampal neurons and suggest PP1α can negatively impact primary hippocampal neuron survival by preventing MEF2D from eliciting its prosurvival or antiapoptotic effects.
DISCUSSION
As an integrator of several signaling pathways, MEF2 transcriptional activity is regulated through protein-protein interactions and direct targeting by signal transduction pathways. In this report, we document a novel interaction between MEF2 and the catalytic subunit of protein phosphatase 1α that exerts a strong repressive effect on MEF2 transcriptional activity (Fig. 9). The interaction requires uncharacterized regions in both proteins, and PP1α phosphatase activity is dispensable for regulation, indicating direct binding is sufficient for regulation. Interestingly, while both full-length PP1α and truncated PP1α are capable of direct interaction with MEF2, only the full-length form represses MEF2-dependent transcriptional activity. This suggests the far C-terminal domain of PP1α is uniquely required for regulation of MEF2. This, in addition to an uncharacterized region of MEF2, demonstrates unique regulatory functions for PP1α and MEF2.
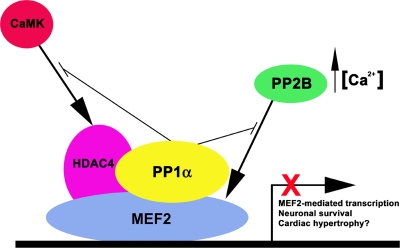
FIG. 9.
Models of PP1α-dependent regulation of MEF2 transcriptional activity. The schematic shows a MEF2-PP1α interaction that blocks MEF2 activity and abrogates both PP2B- and CaMK-mediated activation by HDAC4 phosphorylation. Direct interaction of PP1α with MEF2 blocks MEF2-dependent transcription and MEF2-mediated neuronal survival.
Our analyses regarding potential targets of dephosphorylation revealed serine 408 of MEF2A as a target of PP1α-mediated dephosphorylation. This site has been implicated for activation of MEF2 transcriptional activity via a signaling network including calcineurin, p300, and sumoylation (17, 21). Our investigations into the relationship between calcineurin and PP1α revealed that activation of MEF2-mediated gene expression is independent of PP1α. However, the positive influence of calcineurin was blocked by coexpression of PP1α. We further established that the phosphorylation status of serine 408 did not impact PP1α-mediated repression of MEF2 (data not shown).
Functionally, a MEF2-HDAC interaction regulates skeletal myogenesis (36), cardiac hypertrophy (61), and MEF2-dependent transcriptional activity in neurons (1). Several kinases have been shown to target HDACs and activate MEF2-dependent transcription due to 14-3-3-mediated nuclear export of phosphorylated HDACs (24, 40). PP1α directly interacts with class I HDAC1, -6, and -10 but not with HDAC2, -3, -4, or -5 (8). However, calyculin A blocks nuclear retention of HDAC4, implicating a functional role for PP1α (34) (Fig. 5C). Our analyses demonstrated that PP1α can block CaMKIV-mediated activation of MEF2 transcription and appears to maintain a repressive HDAC4-MEF2 transcriptional complex. This suggests one level of regulation where a MEF2-PP1α complex permits recruitment of molecules not normally recognized as PP1α substrates. Interestingly, our data demonstrated that in the absence of sufficient levels of MEF2, nuclear retention of HDAC4 is dependent upon PP1α phosphatase activity. However, when both MEF2A and PP1α are present in sufficient quantities, the complexes formed lead to HDAC4 nuclear retention in a phosphatase-independent manner (Fig. 7C). It will be interesting to determine whether PP1α can recruit other HDACs, such as HDAC1, to MEF2-dependent genes. Indeed, other reports have shown an HDAC3-MEF2 interaction, indicating a role for class I HDACs in regulating MEF2 transcriptional activity (22). Moreover, HDAC1 bridges an interaction between CREB and PP1α, leading to downregulation of CREB-mediated transcription via PP1-mediated dephosphorylation of serine 133 (9), establishing an indirect role of PP1α for regulating transcription factor activity. Our data demonstrate a unique and more direct role for PP1α-mediated repression of transcription factor activity.
Regulating survival of neuronal cells represents an important physiological role for MEF2 transcription factors (1, 37). This prosurvival role for MEF2 is dependent upon increased transcriptional activity (1, 37) that can be abrogated by direct targeting of MEF2-dependent gene activation (5, 20, 55, 56). Our analyses of primary hippocampal neurons indicates PP1α overexpression increases apoptosis in these cells. Consistent with PP1α's trans-dominant repression of MEF2 activity, exogenous MEF2D supplementation is unable to overcome this effect, suggesting that direct interaction of PP1α with MEF2 and repression of MEF2-mediated transcriptional activity interfere with the prosurvival function of MEF2 in neurons. Indeed, coexpression of truncated PP1α, which interacts with MEF2 but does not repress transcriptional activity, has no effect on hippocampal neuronal cell survival, which corresponds to the inability of tPP1α to repress MEF2-mediated transcriptional activity in these cells. Recent reports indicate that timing of PP1 activation and the type of neurotoxic stimulus are key determinants of a positive or negative role for PP1 in neuronal cell survival (18, 26). This lends support to our finding that PP1α-mediated repression of MEF2 may be a key determinant in regulation of neuronal survival. It will be of great interest to further understand the importance of our data in light of the fact that both PP1α and MEF2 have been implicated in neural structural plasticity (17, 45, 54). Our data may provide a nuclear link through which PP1 can act to alter changes in neuronal cell gene expression and structural plasticity.
MEF2 factors cooperate with tissue-restricted transcriptional regulators, such as MyoD (42), GATA (43), HAND (44), myocardin/MASTR (16), and the nuclear factor of activated T cells (4, 33). Here, we present a mechanism by which direct binding of PP1α phosphatase represses transcriptional activity via MEF2. It will be of great interest to examine the effects of this type of regulation within the context of cooperative activity. The transition from proliferating myoblast to terminally differentiated muscle fibers requires the formation of a transcriptional complex composed of MyoD, MEF2, and pRb (49). Inclusion of PP1α within this complex would provide for an elegant molecular switch for dephosphorylation and recruitment of pRb. In this model, activation of PP1α phosphatase activity would occur due to reduction of cyclin/cdk activity, recruitment of pRb, cell cycle arrest, and initiation of myogenic gene expression. Within a cardiovascular context, the progression of heart disease involves a rise in PP1 activity (48). While transgenic and gene-targeted mouse models demonstrate that restoring intracellular calcium signaling through PP1 inhibition blocks disease progression (7, 10), there are indications that effects of increased PP1 encompass more than just altered signaling (13). Our data may represent a key molecular clue as to how PP1 influences heart disease at the level of altered gene expression. Moreover, blocking MEF2 activity, as we observed for hippocampal neurons, may play a vital role in cardiac remodeling, cardiomyocyte cell survival (57, 58), and T-cell apoptosis (60).
In summary, we have presented a novel interaction between PP1α and MEF2 transcriptional regulators that abrogates MEF2-dependent transcriptional activity. PP1α-mediated regulation of MEF2-mediated gene activation is dominant to calcineurin and serves to retain HDAC4 in the nuclear compartment. Importantly, PP1α promotes apoptotic cell death in primary hippocampal neurons, consistent with its dominant repressive role on MEF2 activity. The PP1α-MEF2 interaction represents a potent locus of control for MEF2-dependent gene expression, with important implications for cell division, apoptosis, cardiac disease, and terminal differentiation.
Supplementary Material
Acknowledgments
We truly miss our dear friend, colleague, and coauthor, Joseph Chan. We thank Joseph for his years of dedication to the progress of the McDermott lab and its many past and present members.
We thank Michael Scheid and Tetsuaki Miyake for critical reading of the manuscript. We thank Tamara Korolnek and Nathaniel Nowaki for technical assistance. We thank Samuel Benchimol and members of his laboratory for assistance and use of equipment for FACS analysis.
This work was supported by research grants held by J.C.M. from the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research. Salary support for R.L.S.P. was in part provided by a joint Postdoctoral Research Fellowship Award from the Canadian Institutes of Health Research and the Muscular Dystrophy Association of Canada. Salary support for H.I. was provided for by a scholarship award from the Heart and Stroke Foundation of Canada.
Footnotes
Published ahead of print on 13 April 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Belfield, J. L., C. Whittaker, M. Z. Cader, and S. Chawla. 2006. Differential effects of Ca2+ and cAMP on transcription mediated by MEF2D and cAMP-response element-binding protein in hippocampal neurons. J. Biol. Chem. 28127724-27732. [DOI] [PubMed] [Google Scholar]
- 2.Berndt, N., M. Dohadwala, and C. W. Y. Liu. 1997. Constitutively active protein phosphatase 1α causes Rb-dependent G1 arrest in human cancer cells. Curr. Biol. 7375-386. [DOI] [PubMed] [Google Scholar]
- 3.Black, B. L., and E. N. Olson. 1998. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14167-196. [DOI] [PubMed] [Google Scholar]
- 4.Blaiser, F., N. Ho, R. Prywes, and T. A. Chatila. 2000. Ca2+-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem. 275197-209. [DOI] [PubMed] [Google Scholar]
- 5.Bolger, T. A., X. Zhao, T. J. Cohen, C.-C. Tsai, and T.-S. Yao. 2007. The neurodegenerative disease protein ataxin-1 antagonizes the neuronal survival function of myocyte enhancer factor-2. J. Biol. Chem. 28229186-29192. [DOI] [PubMed] [Google Scholar]
- 6.Bollen, M., and M. Beullens. 2002. Signaling by protein phosphatases in the nucleus. Trends Cell. Biol. 12138-145. [DOI] [PubMed] [Google Scholar]
- 7.Braz, J. C., K. Gregory, A. Pathak, W. Zhao, B. Sahin, R. Klevitsky, T. F. Kimball, J. N. Lorenz, A. C. Nairn, S. B. Liggett, I. Bodi, S. Wang, A. Schwartz, E. G. Lakatta, A. A. DePaoli-Roach, J. Robbins, T. E. Hewett, J. A. Bibb, M. V. Westfall, E. G. Kranias, and J. D. Molkentin. 2004. PKC-α regulates cardiac contractility and propensity toward heart failure. Nat. Med. 10248-254. [DOI] [PubMed] [Google Scholar]
- 8.Brush, M. H., A. Guardiola, J. H. Connor, T.-P. Yao, and S. Shenolikar. 2004. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J. Biol. Chem. 2797685-7691. [DOI] [PubMed] [Google Scholar]
- 9.Canettieri, G., I. Morantte, E. Guzmán, H. Asahara, S. Herzig, S. D. Anderson, J. R. Yates III, and M. Montminy. 2003. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat. Struct. Biol. 10175-181. [DOI] [PubMed] [Google Scholar]
- 10.Carr, A. N., A. G. Schmidt, Y. Suzuki, F. del Monte, Y. Sato, C. Lanner, K. Breeden, S.-H. Jing, P. B. Allen, P. Greengard, A. Yatani, B. D. Hoit, I. L. Grupp, R. J. Hajjar, A. A. DePaoli-Roach, and E. G. Kranias. 2002. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell. Biol. 224124-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceulemans, H., and M. Bollen. 2004. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 841-39. [DOI] [PubMed] [Google Scholar]
- 12.Ceulemans, H., W. Stalmans, and M. Bollen. 2002. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays 24371-381. [DOI] [PubMed] [Google Scholar]
- 13.Champion, H. C. 2005. Targeting protein phosphatase 1 in heart failure. Circ. Res. 96708-710. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, P. T. 2002. Protein phosphatase 1: targeted in many directions. J. Cell Sci. 115241-256. [DOI] [PubMed] [Google Scholar]
- 15.Cox, D. M., M. Du, M. Marback, C. C. Yang, J. Chan, K. W. M. Siu, and J. C. McDermott. 2003. Phosphorylation motifs regulating the stability and function of myocyte enhancer factor 2A. J. Biol. Chem. 27815297-15303. [DOI] [PubMed] [Google Scholar]
- 16.Deng, K., D. Z. Ewton, S. E. Mercer, and E. Friedman. 2005. Mirk/dyrk1B decreases the nuclear accumulation of class II histone deacetylases during skeletal muscle differentiation. J. Biol. Chem. 2804894-4905. [DOI] [PubMed] [Google Scholar]
- 17.Flavell, S. W., C. W. Cowan, T.-K. Kim, P. L. Greer, Y. Lin, S. Paradis, E. C. Griffith, L. S. Hu, C. Chen, and M. E. Greenberg. 2006. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 3111008-1012. [DOI] [PubMed] [Google Scholar]
- 18.Gee, C. E., and I. M. Mansuy. 2005. Protein phosphatases and their potential implications in neuroprotective processes. Cell. Mol. Life Sci. 621120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons, J. A., D. C. Weiser, and S. Shenolikar. 2005. Importance of a surface hydrophobic pocket on protein phosphatase-1 catalytic subunit in recognizing cellular regulators. J. Biol. Chem. 28015903-15911. [DOI] [PubMed] [Google Scholar]
- 20.Gong, X., X. Tang, M. Wiedmann, X. Wang, J. Peng, D. Zheng, L. A. C. Blair, J. Marshall, and Z. Mao. 2003. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 3833-46. [DOI] [PubMed] [Google Scholar]
- 21.Grégoire, S., A. M. Tremblay, L. Xiao, Q. Yang, K. Ma, J. Nie, Z. Mao, J. Wu, V. Giguère, and X.-J. Yang. 2006. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J. Biol. Chem. 2814423-4433. [DOI] [PubMed] [Google Scholar]
- 22.Grégoire, S., L. Xiao, J. Nie, X. Zhang, M. Xu, J. Li, J. Wong, E. Seto, and X.-J. Yang. 2007. Histone deacetylase interacts with and deacetylates myocyte enhancer factor 2. Mol. Cell. Biol. 271280-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grozinger, C. M., C. A. Hassig, and S. L. Schreiber. 1999. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 964868-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grozinger, C. M., and S. L. Schreiber. 2000. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. USA 977835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han, J., Y. Jiang, V. V. Kravchenko, and R. J. Ulevitch. 1997. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386299. [DOI] [PubMed] [Google Scholar]
- 26.Hédou, G. F., K. Koshibu, M. Farinelli, E. Kilic, C. E. Gee, U. Kilic, K. Baumgärtel, D. M. Hermann, and I. M. Mansuy. 2008. Protein phosphatase 1-dependent bidirectional synaptic plasticity controls ischemic recovery in the adult brain. J. Neurosci. 28154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J.-D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 167054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kollias, H. D., R. L. S. Perry, Y. Miyake, A. Aziz, and J. C. McDermott. 2006. Smad7 promotes and enhances skeletal muscle differentiation. Mol. Cell. Biol. 266248-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemercier, C., A. Verdel, B. Galloo, S. Curtet, M.-P. Brocard, and S. Khochbin. 2000. mHDA1/HDAC5 histone deacetylase interacts with and represses MEF2A transcriptional activity. J. Biol. Chem. 27515594-15599. [DOI] [PubMed] [Google Scholar]
- 30.Li, L., R. Heller-Harrison, M. P. Czech, and E. N. Olson. 1992. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol. Cell. Biol. 124478-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilly, B., B. Zhao, G. Ranganayakulu, B. M. Paterson, R. A. Schulz, and E. N. Olson. 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267688-693. [DOI] [PubMed] [Google Scholar]
- 32.Lin, Q., J. Schwarz, C. Bucana, and E. N. Olson. 1997. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 2761404-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, S., P. Liu, A. Borras, T. Chatila, and S. H. Speck. 2007. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated by a novel pathway involving a MEF2 family member. EMBO J. 16143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, Y., W. R. Randall, and M. F. Schneider. 2005. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 168887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu, J., T. A. McKinsey, R. L. Nicol, and E. N. Olson. 2000. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc. Natl. Acad. Sci. USA 974070-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, J., T. A. McKinsey, C.-L. Zhang, and E. N. Olson. 2000. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell 6233-244. [DOI] [PubMed] [Google Scholar]
- 37.Mao, Z., A. Bonni, F. Xia, M. Nadal-Vicens, and M. E. Greenberg. 1999. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286785-790. [DOI] [PubMed] [Google Scholar]
- 38.McDermott, J. C., M. C. Cardoso, Y.-T. Yu, V. Andres, D. Leifer, D. Krainc, S. A. Lipton, and B. Nadal-Ginard. 1993. hMEF2C gene encodes skeletal muscle- and brain-specific transcription factors. Mol. Cell. Biol. 132564-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinsey, T. A., C. L. Zhang, and E. N. Olson. 2001. Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet. Dev. 11497-504. [DOI] [PubMed] [Google Scholar]
- 40.McKinsey, T. A., C.-L. Zhang, J. Lu, and E. N. Olson. 2000. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408106-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miska, E. A., C. Karlsson, E. Langley, M. J. Nielsen, J. Pines, and T. Kouzarides. 1999. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 185099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molkentin, J. D., B. L. Black, J. F. Martin, and E. N. Olson. 1995. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 831125-1136. [DOI] [PubMed] [Google Scholar]
- 43.Morin, S., R. Charron, L. Robataille, and M. Nerner. 2000. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 192046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morin, S., G. Pozzulo, J. Cross, and M. Nerner. 2005. MEF2-dependent recruitment of the HAND1 transcription factor results in synergistic activation of target promoters. J. Biol. Chem. 28032272-32278. [DOI] [PubMed] [Google Scholar]
- 45.Munton, R. P., S. Vizi, and I. M. Mansuy. 2004. The role of protein phosphatase-1 in the modulation of synaptic and structural plasticity. FEBS Lett. 567121-128. [DOI] [PubMed] [Google Scholar]
- 46.Naya, F. J., B. L. Black, H. Wu, R. Bassel-Duby, J. A. Richardson, J. A. Hill, and E. N. Olson. 2002. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat. Med. 81303-1309. [DOI] [PubMed] [Google Scholar]
- 47.Naya, F. J., and E. N. Olson. 1999. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell. Biol. 11683-688. [DOI] [PubMed] [Google Scholar]
- 48.Neumann, J., T. Eschenhagen, L. R. Jones, B. Linck, W. Schmitz, H. Scholz, and N. Zimmermann. 1997. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 29265-272. [DOI] [PubMed] [Google Scholar]
- 49.Novitch, B. G., D. B. Spicer, P. S. Kim, W. L. Cheung, and A. B. Lassar. 1999. pRB is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol. 9449-459. [DOI] [PubMed] [Google Scholar]
- 50.Ornatsky, O. I., D. M. Cox, P. Tangirala, J. J. Andreucci, Z. A. Quinn, J. L. Wrana, R. Prywes, Y.-T. Yu, and J. C. McDermott. 1999. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 272646-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ornatsky, O. I., and J. C. McDermott. 1996. MEF2 protein expression, DNA binding specificity and complex composition, and transcriptional activity in muscle and non-muscle cells. J. Biol. Chem. 27124927-24933. [DOI] [PubMed] [Google Scholar]
- 52.Passier, R., H. Zeng, N. Frey, F. J. Naya, R. L. Nicol, T. A. McKinsey, P. Overbeek, J. A. Richardson, S. R. Grant, and E. N. Olson. 2000. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Investig. 1051395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry, R. L. S., M. H. Parker, and M. A. Rudnicki. 2001. Activated MEK1 binds the nuclear MyoD transcriptional complex to repress transactivation. Mol. Cell 8291-301. [DOI] [PubMed] [Google Scholar]
- 54.Shalizi, A., B. Gaudiliere, Z. Yuan, J. Stegmüller, T. Shirogane, Q. Ge, Y. Tan, B. Schulman, J. W. Harper, and A. Bonni. 2006. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 3111012-1017. [DOI] [PubMed] [Google Scholar]
- 55.Smith, P. D., M. P. Mount, R. Shree, S. Callaghan, R. S. Slack, H. Anisman, I. Vincent, X. Wang, Z. Mao, and D. S. Park. 2006. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 26440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, X., X. Wang, X. Gong, M. Tong, D. Park, Z. Xia, and Z. Mao. 2005. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 254823-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Oort, R. J., E. van Rooij, M. Bourajjaj, J. Schimmel, M. A. Jansen, R. van der Nagel, P. A. Doevendans, M. D. Schneider, C. J. A. van Echteld, and L. J. De Windt. 2006. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation 114298-308. [DOI] [PubMed] [Google Scholar]
- 58.Xu, J., N. L. Gong, I. Bodi, B. J. Aronow, P. H. Backx, and J. D. Molkentin. 2006. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J. Biol. Chem. 2819152-9162. [DOI] [PubMed] [Google Scholar]
- 59.Yang, C.-C., O. I. Ornatsky, J. C. McDermott, T. F. Cruz, and C. A. Prody. 1998. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 264771-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn, H.-D., L. Sun, R. Prywes, and J. O. Liu. 1999. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science 286790-793. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, C. L., T. A. McKinsey, S. Chang, C. L. Antos, J. A. Hill, and E. N. Olson. 2002. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.