Abstract
We identify Kette, a key regulator of actin polymerization, as a substrate for Drosophila protein tyrosine phosphatase PTP61F, as well as for dAbl tyrosine kinase. We further show that dAbl is a direct substrate for PTP61F. Therefore, Kette phosphotyrosine levels are regulated both directly and indirectly by PTP61F. Kette and PTP61F genetically interact in the regulation of F-actin organization in pupal eye discs, suggesting that tyrosine phosphorylation is essential for the proper regulation of Kette-mediated actin dynamics. This hypothesis was confirmed by demonstrating the loss of Kette-mediated F-actin organization and lamella formation in S2 cells in a Kette Y482F mutant in which the dAbl phosphorylation site was eliminated. Our results establish for the first time that PTP61F and dAbl ensure proper actin organization through the coordinated and reversible tyrosine phosphorylation of Kette.
The actin cytoskeleton is regulated as a function of development, cell motility, intracellular transport, and the cell cycle by the polymerization of G-actin to F-actin (34). Correct regulation of actin cytoskeletal dynamics is essential to numerous differentiating and cellular processes in the nervous system (9) and musculature (42), among others. Actin polymerization is regulated by a number of proteins, among which is human NCK-associated protein 1 (NAP-1 [3, 4, 45]). It and its Drosophila orthologue, Kette (Hem in FlyBase), are critical components in both SCAR/WAVE and WASP complexes, which play essential roles in transducing Rac1 signals to initiate Arp2/3-dependent actin polymerization (6, 25, 40, 48). Murine NAP-1 interacts with NCK, an SH2-SH3 adaptor protein (4), and is essential for proper neuronal differentiation in the cortex (53). Neuronal differentiation and neural tube defects are observed in NAP-1 mutant mice, apparently due to reduced localization of WAVE1 to the cell membrane (53).
In Drosophila, loss of kette activity specifically results in the accumulation of cytosolic F-actin (6). Kette protein associates with F-actin in the cytosol, but also at focal contact sites, where it apparently antagonizes SCAR/WAVE function and activates WASP-dependent actin polymerization (6). Despite its role in repressing SCAR/WAVE function, Kette serves to protect the complex from proteosome-mediated degradation and is critical to its intracellular localization (25). At the level of the organism, kette alleles affect axonal growth and pathfinding due to aberrant actin cytoskeleton formation, for example, altering crossing of the embryonic ventral midline by VUM neuron axons, as well as generating aberrant axonal projections in both motor and sensory neurons (21). Like mammalian NAP-1, Drosophila kette also interacts with the fly NCK orthologue, dreadlocks (dock) (21). Other evidence for the conserved interaction of Kette with signaling cascades is provided by the observation that kette mutant phenotypes are partially rescuable by overexpression of the small G protein Rac1 (21). The interaction of kette with dock suggests the possibility of tyrosine phosphorylation in the regulation of Kette activity, but no evidence supporting this hypothesis has been reported.
Signaling by tyrosine phosphorylation in various metazoans controls numerous processes involved in cellular differentiation and proliferation. Many of the components regulating tyrosine phosphorylation have been identified and characterized using genetic, biochemical, molecular, and genomic sequence analyses (31). However, in contrast to the very well-characterized regulation of cellular processes by kinase-mediated tyrosine phosphorylation (15, 52), their regulation by dephosphorylation by protein tyrosine phosphatases (PTPs) has generally lagged behind. Although the functions of several receptor PTPs have been clearly defined as playing essential roles in axon guidance in both Drosophila (12, 23, 41, 47, 50) and mammals (44, 49), our understanding of nontransmembrane PTPs (NT-PTPs) is more limited. Only three of the eight putative Drosophila NT-PTPs have been characterized genetically. Corkscrew (Csw) acts as a downstream effector of various receptor protein tyrosine kinases (PTKs) and is essential for R7 photoreceptor development (35). PTP-enhancer of Ras1 has been characterized as an essential regulator antagonizing signaling mediated by Ras1, possibly through tyrosine dephosphorylation of mitogen-activated protein kinase (24, 36). More recently, it has been shown that PTP-meg participates in the establishment and maintenance of axon projections in the Drosophila brain (51). Other than these, the functions of Drosophila NT-PTPs remain largely unknown.
PTP61F was originally identified as an NT-PTP that contains one phosphatase domain in the N-terminal region and five proline-rich motifs in the C-terminal tail (29). It is the Drosophila orthologue of mammalian PTP1B and T-cell PTP (TC-PTP) (1), which have been implicated in the regulation of signaling by both insulin (39) and JAK/STAT (33). Two PTP61F isoforms due to alternative splicing possess unique sequences at the C terminus, which determine either internal membrane-association (PTP61Fm) or nuclear localization (PTP61Fn) (29). To date, limited data suggest that PTP61F may participate in the downregulation of JAK/STAT signaling (2, 32), although the underlying mechanism remains unexplored. While PTP61F may recognize the adaptor proteins DOCK (10) and Abi (20) as potential substrates, the signaling pathways involving these interactions have not been clearly defined. In this study, we demonstrate for the first time that the regulation of Kette, and hence the localization and polymerization of the actin cytoskeleton, is achieved by reversible tyrosine phosphorylation under the control of both PTP61F and the PTK dAbl.
MATERIALS AND METHODS
Plasmid constructs and purification of recombinant dPTP61F.
cDNA sequences corresponding to amino acids 1 to 339 of dPTP61F, or the trapping mutant D203A, were constructed with a hemagglutinin (HA) tag or a Myc tag at the N terminus and cloned into a pET-28a (for protein expression in Escherichia coli) or a pAc5.1A (for protein expression in S2 cells) vector. Protein purification from E. coli extracts was described previously (8). cDNA sequences corresponding to the full-length Kette (cytosolic) or KetteMyr (6) were constructed with a Flag tag at the N terminus and C terminus, respectively, and cloned into the pAc5.1A vector. The KetteMyr-Y180F, KetteMyr-Y482F, KetteMyr-Y180F+Y482F, and Kette (cytosolic)-Y482F mutant constructs were generated by site-directed mutagenesis following a standard procedure. The pAc5.1-HA-dAbl construct was a gift provided by J.-L. Juang (National Health Research Institutes, Taiwan). All cDNAs were confirmed by sequencing.
Genetics.
All crosses were performed at 25°C unless otherwise indicated. The following strains were obtained from various sources: upstream activation sequence (UAS)-ketteMyr/TM6B (6), UAS-dAbl/CyO (14), and UAS-dAblK417N/CyO (14). To generate the PTP61F-RNA interference (RNAi) construct, the PTP61F DNA (coding sequence +901 to +1415) was cloned as an inverted repeat into the pWIZ vector (28). To generate UAS-PTP61Fm, full-length cDNA encoding PTP61Fm was subcloned into the pUAST P-element vector and subsequently injected into Drosophila embryos. To generate progeny expressing the target gene in a tissue-specific pattern, we used the GAL4-UAS system (7).
In vitro substrate-trapping and MS-based analysis.
The detailed procedure for in vitro substrate-trapping and mass spectrometry (MS)-based analysis has been described recently (8), with some modifications applied in this study. Briefly, the HA-tagged wild-type (WT) or D203A (DA) mutant form of C-terminally truncated PTP61F recombinant protein was incubated with an aliquot of total lysate prepared from S2 cells that were stimulated with 100 μM pervanadate for 30 min. After incubation at 4°C for 30 min, an aliquot of immobilized anti-HA antibody-agarose beads was added for an additional 3 h of incubation. After extensive washes, the beads were either boiled in sodium dodecyl sulfate (SDS) sample buffer (for immunoblotting) or incubated with 500 mM NaCl at room temperature for 10 min (for substrate identification). The eluted proteins were analyzed by immunoblotting them with antiphosphotyrosine (anti-pTyr) antibody or were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and visualized by silver staining or Sypro Ruby staining for in-gel trypsin digestion (27), followed by liquid chromatography-tandem-MS analysis (27).
Cell culture, transient transfection, immunoprecipitation, and immunoblotting.
Drosophila S2 cells were maintained in 1× Schneider medium (Gibco) supplemented with 10% fetal bovine serum at 24°C. For transient transfection with plasmid, Lipofectamine 2000 (Invitrogen) was used as a vehicle, following the manufacturer's directions. For RNAi-mediated knockdown experiments, double-stranded RNA (dsRNA) was added to serum-free culture medium immediately after synthesis, according to the established protocol (11). After 2 h of treatment with dsRNA, the cells were incubated in complete medium for 24 or 48 h and then lysed for immunoprecipitation or immunoblotting analysis. For in vivo substrate-trapping assays, S2 cells were subjected to transient transfection with the WT (PTP61FΔC-WT) or the substrate-trapping mutant (Asp203→Ala203; dPTP61ΔC-DA) (DA) form of HA-tagged PTP61Fm. The cells were harvested in the substrate-trapping buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM iodoacetic acid, and proteases inhibitors) and incubated at 4°C for 30 min in darkness. Immunoprecipitation was performed using anti-HA tag antibody. Proteins associated with the trapping mutant form of PTP61F were visualized by immunoblotting.
Immunofluorescence staining.
S2 cells were plated and transfected as described above. After 48 h of incubation, the cells were suspended and replated on concanavalin A (ConA) (0.5 mg/ml; C2010; Sigma)-coated glass coverslips for 7 min and then fixed, permeabilized, and stained with either anti-Flag antibody (rabbit polyclonal; F7425; Sigma) or anti-HA antibody HA-7 (mouse monoclonal; H9658; Sigma), followed by Cy2-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody or Cy5-conjugated goat anti-mouse IgG antibody (Jackson). For F-actin staining, cells were prepared as described above and stained with tetramethyl rhodamine isocyanate-conjugated phalloidin (Jackson). The samples were visualized using a Zeiss LSM 510 confocal microscope. Pupal imaginal discs isolated from pupae at the 40-h stage were fixed, permeabilized, and stained with anti-ELAV antibody (Developmental Studies Hybridoma Bank [DSHB]), followed by Cy3-conjugated goat anti-mouse IgG antibody (Jackson). For F-actin staining, discs were reacted with tetramethyl rhodamine isocyanate-conjugated phalloidin (Sigma). The samples were visualized using a Zeiss LSM 510 confocal microscope.
Immunolabeling of Drosophila embryos.
To examine the central nervous system, stage 16 to 17 embryos were dechorionated, fixed, rehydrated, and permeabilized. The embryos were then stained with anti-central nervous system axon antibody (BP102; DSHB) as previously described (19). Cy3-conjugated goat anti-mouse IgG (Jackson) was used as a secondary antibody. The samples were visualized using an Olympus BX51 microscope.
SEM.
The external morphology of adult compound eyes was visualized by scanning electron microscopy (SEM). Whole adult flies were subjected to gradient dehydration and critical-point drying using a Hitachi HCP-2 critical-point dryer. After being coated (with a Hitachi H-1010 ion sputter), samples were viewed and photographed using an FEI Quanta 200 scanning electron microscope at 20 kV.
RESULTS
Kette is a substrate of PTP61F.
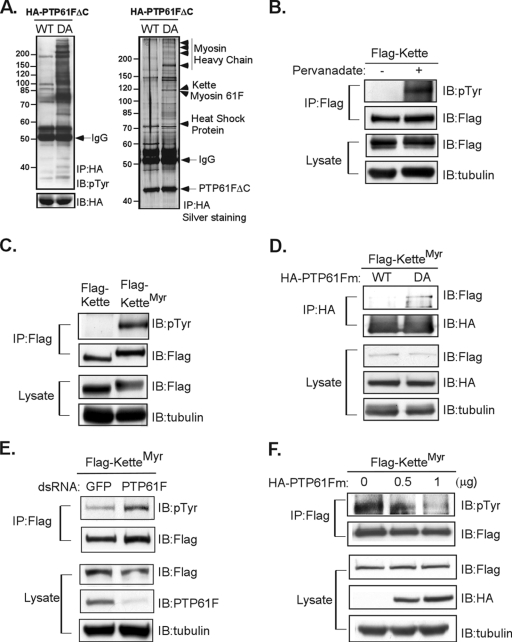
We identified Kette as a protein interacting with PTP61F using a substrate-trapping approach (13). Substrate trapping has been extensively used for revealing the physiological roles of PTPs across species (13). In substrate trapping, a mutant form of the PTP is generated and captures its cognate substrates for later identification. The C-terminally truncated variants (amino acids 1 to 339) of the WT form and the substrate-trapping DA mutant form of PTP61F, in which four proline-rich domains in the C-terminal region were removed to prevent protein association, were expressed in E. coli and then purified by affinity chromatography. Both the WT and the DA mutant forms of PTP61FΔC were incubated with total lysates prepared from S2 cells, which had been treated with pervanadate to activate pTyr signaling prior to lysis. After immunoprecipitation of PTP61FΔC, the associated proteins were eluted by a high-salt buffer (500 mM NaCl) from the immunocomplexes and then subjected to either immunoblotting with anti-pTyr antibody or SDS-PAGE for silver staining and for Sypro Ruby staining. The results from immunoblotting showed PTP61FΔC-DA to be associated with significantly more pTyr proteins than PTP61FΔC-WT (Fig. 1A). Consistently, under the same experimental conditions, multiple proteins were eluted from the DA mutant, whereas only a limited number of nonsubstrate proteins were dissociated from the WT form of PTP61F, as revealed by silver staining of SDS gels (Fig. 1A). Subsequent MS-based analysis identified several proteins associated specifically with the DA mutant (Fig. 1A). One in particular was the 120-kDa protein Kette. To further test whether Kette is tyrosine phosphorylated, the ectopically expressed full-length form of the protein was immunoprecipitated from S2 cells treated with pervanadate. The immunoblots were then probed with anti-pTyr antibody. As shown in Fig. 1B, a robust increase in pTyr levels was detected following treatment, indicating that the suppression of cellular PTP activity by pervanadate led to increased Kette tyrosine phosphorylation.
FIG. 1.
PTP61F recognizes Kette as a substrate. (A) S2 cells were treated with 100 mM pervanadate for 30 min. An aliquot of total lysate was incubated with either the WT or the D203A trapping mutant (DA) form of HA-tagged, affinity-purified PTP61FΔC protein. After immunoprecipitation (IP), PTP61FΔC and its associated proteins were subjected to SDS-PAGE for immunoblotting (IB) (left) or silver staining (right) and Sypro Ruby staining (data not shown). The proteins associated with the DA mutant, but not the WT, form of PTP61FΔC were excised from the gel stained with Sypro Ruby, digested with trypsin, and subsequently analyzed by MS. The identities of these proteins, as indicated by arrows, were revealed by tandem-MS sequencing (data not shown). (B) Flag-tagged cytosolic Kette-transfected S2 cells were either left untreated or stimulated with pervanadate. Immunoprecipitated Kette or an aliquot of total lysates was subjected to immunoblotting. (C) Flag-tagged cytosolic Kette or KetteMyr was ectopically expressed in S2 cells. Immunoprecipitated Kette or an aliquot of total lysates was subjected to immunoblotting. (D) Flag-tagged KetteMyr was ectopically coexpressed with the HA-tagged WT form or the DA mutant form of full-length PTP61Fm in S2 cells. After immunoprecipitation, PTP61Fm-associated proteins were analyzed by immunoblotting. An aliquot of total lysates was subjected to immunoblotting. (E) Endogenous PTP61F was ablated by treating S2 cells with specific dsRNA. As a control, S2 cells were treated with dsRNA for green fluorescent protein (GFP). The dsRNA-treated S2 cells were then transfected with Flag-tagged KetteMyr. Immunoprecipitated KetteMyr, or an aliquot of total lysates, was analyzed by immunoblotting. (F) Flag-tagged KetteMyr was ectopically coexpressed with various doses of the HA-tagged WT form of PTP61Fm in S2 cells. Immunoprecipitated KetteMyr and an aliquot of total lysates were analyzed by immunoblotting.
Recent studies have suggested that the activity of Kette in facilitating actin polymerization is correlated with its plasma membrane localization (6). We therefore examined whether the membrane-localized, potentially active form of Kette was also tyrosine phosphorylated. A fusion form of Kette (KetteMyr) containing the first 88 amino acid residues of Drosophila Src kinase in the N-terminal region, and therefore capable of being myristoylated and hence membrane localized (6), was ectopically expressed in S2 cells. As demonstrated in Fig. 1C, membrane-tethered KetteMyr was tyrosine phosphorylated. To further test whether PTP61F recognized Kette as a substrate in vivo, the full-length WT or DA mutant form of PTP61Fm (the membrane-associated variant) was coexpressed with KetteMyr in S2 cells for substrate trapping. As expected, a substantial quantity of KetteMyr was associated with the DA mutant, but not with the WT form of PTP61Fm (Fig. 1D). Moreover, the tyrosine phosphorylation level of KetteMyr was robustly increased in S2 cells treated with dsRNA knocking down PTP61F (Fig. 1E). Additional evidence further demonstrated that the greater the amount of PTP61Fm ectopically expressed in S2 cells, the greater the loss of tyrosine phosphorylation in KetteMyr (Fig. 1F). Taken together, our findings suggest that PTP61F plays a critical role in regulating Kette activity through direct tyrosine dephosphorylation.
The organization of F-actin in eye imaginal discs is regulated by Kette and PTP61F.
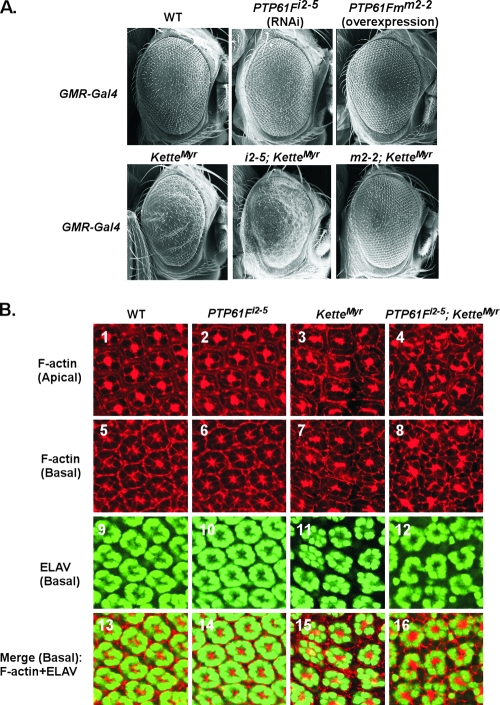
We next investigated whether the interaction between PTP61F and Kette was of biological relevance. To examine this possibility in vivo, we established multiple lines of PTP61F RNAi transgenic flies, which carry an inverted repeat driven by the UAS regulatory sequence. After extensive screening, the PTP61F-RNAi-i2-5 line (referred to as the i2-5 line hereafter) (see Fig S1 in the supplemental material) was selected for further analysis. Additional transgenic flies were also used in genetic studies. As shown in Fig. 2A, ectopic expression of KetteMyr led to a rough-eye phenotype. The severities of eye phenotypes were used as biological readouts to examine further genetic interactions between Kette and PTP61F. We observed that the eye roughness generated by the ectopic expression of KetteMyr was significantly increased when endogenous PTP61F was ablated, as demonstrated by the obvious loss of hexagonal ommatidia (Fig. 2A). Moreover, the rough-eye phenotype revealed in KetteMyr transgenic flies could be rescued completely through the ectopic expression of PTP61Fm (line m2-2, shown in Fig. 2A), demonstrating a clear antagonistic interaction between these gene products.
FIG. 2.
The actin cytoskeleton in eye imaginal discs is regulated by Kette and PTP61F. All transgenic constructs were driven by GMR-GAL4 and maintained at 25°C. (A) The phenotype of adult compound eyes was examined by SEM. Ectopic expression of KetteMyr resulted in a rough-eye phenotype. The roughness was enhanced upon RNAi-mediated knockdown of endogenous PTP61F in combination with KetteMyr (i2-5; KetteMyr). In contrast, forced expression of PTP61Fm rescued the KetteMyr-induced eye defect (m2-2; KetteMyr). (B) All images were prepared from the 40-h pupal eye imaginal discs stained with rhodamine-phalloidin (top row and second row from top) and anti-ELAV antibody (third row from top). F-actin organization is shown at both apical (top row) and basal (second row from top) levels, whereas the neuronal pattern of photoreceptor cells is shown at the basal level (third row from top). Merged images of F-actin and photoreceptor cells at the basal level are shown in the bottom row. The staining of F-actin (images 1 and 5) and photoreceptor cells (images 9 and 13) in the WT pupal eye discs reveals the organized pattern of ommatidia. This pattern became disorganized in response to ectopic expression of KetteMyr (images 3, 7, 11, and 15). In GMR-Gal4-driven i2-5; KetteMyr flies, severe defects in the F-actin organization (images 4, 8, and 16) and disturbed localization of photoreceptor cells (images 12 and 16) were observed.
Kette is regarded as a key regulator in the proper control of actin polymerization in the WASP- and SCAR/WAVE-mediated signaling pathways (5, 6, 25). Therefore, we hypothesized that abnormal Kette activity might affect F-actin organization in photoreceptor cells, resulting in developmental defects, such as the eye roughness we observed in adult flies (Fig. 2A). To test this hypothesis, we examined the F-actin organization in 40-h pupal eye imaginal discs isolated from WT or various transgenic flies. As shown in Fig. 2B, F-actin in WT pupal eye discs was found to have a star-like pattern at the center of each ommatidium, consistent with previously reported observations (30). In contrast, in ommatidia isolated from eye imaginal discs of KetteMyr-overexpressing flies, the pattern of F-actin became irregular. The condensed and star-like distribution shown in WT ommatidia (Fig. 2B, 1 and 5) was converted to a more diffused and irregular staining pattern (Fig. 2B, 3 and 7). RNAi-mediated knockdown of PTP61F, however, did not lead to significant abnormal phenotypes in ommatidia at this developmental stage (Fig. 2B, 2 and 6). Importantly, the defects in F-actin organization were increased significantly when overexpressed KetteMyr was combined with suppressed endogenous PTP61F (Fig. 2B, 4 and 8). F-actin was no longer confined to the center of each ommatidium but was distributed chaotically among adjacent ommatidia (Fig. 2B, 4 and 8), concomitant with the appearance of disorganized photoreceptor cells (Fig. 2B, 12 and 16). These results demonstrate that Kette and its interactions with PTP61F regulate F-actin organization.
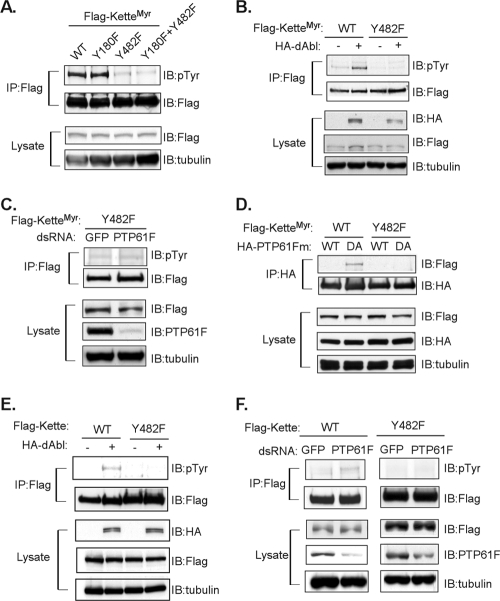
FIG. 5.
Kette Tyr482 is the primary regulatory site for dAbl and PTP61F. (A) The WT and various mutant forms of Flag-tagged KetteMyr were ectopically expressed in S2 cells. Immunoprecipitated (IP) KetteMyr or an aliquot of total lysates was analyzed by immunoblotting (IB). (B) The WT or Y482F mutant form of Flag-tagged KetteMyr was coexpressed with dAbl in S2 cells. Immunoprecipitated KetteMyr or an aliquot of total lysates was analyzed by immuoblotting. (C) S2 cells were treated with dsRNA for PTP61F or green fluorescent protein (GFP). The dsRNA-treated cells were then transfected with the Y482F mutant form of Flag-tagged KetteMyr. Immunoprecipitated KetteMyr or an aliquot of total lysates was analyzed by immunoblotting. (D) Both WT and Y482F mutant forms of Flag-tagged KetteMyr were coexpressed with either the WT or DA mutant form of HA-tagged PTP61Fm in S2 cells. PTP61Fm was immunoprecipitated with anti-HA antibody. The immunocomplex or an aliquot of total lysates was analyzed by immunoblotting. (E) All experimental procedures were as described for panel B with the exception of ectopic expression of Flag-tagged cytosolic Kette instead of KetteMyr. (F) All experimental procedures were as described for panel C with the exception of ectopic expression of Flag-tagged cytosolic Kette instead of KetteMyr.
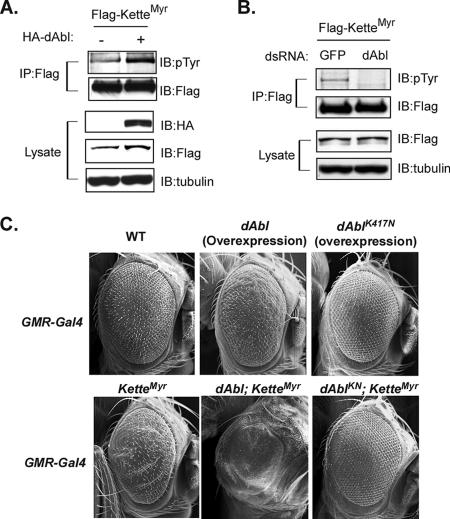
FIG. 3.
dAbl regulates Kette. (A) Flag-tagged KetteMyr was ectopically expressed in the absence or presence of HA-tagged dAbl in S2 cells. Immunoprecipitated (IP) KetteMyr or an aliquot of total lysates was subjected to immunoblotting (IB). (B) S2 cells were treated with dsRNA for dAbl or green fluorescent protein (GFP). dsRNA-treated S2 cells were transfected with Flag-tagged KetteMyr. Immunoprecipitated KetteMyr or an aliquot of total lysates was subjected to immunoblotting. (C) Ectopic expression of dAbl resulted in eye roughness. Coexpression of dAbl and KetteMyr (dAbl; KetteMyr) significantly enhanced the defect. On the other hand, expression of the catalytically inactive mutant form of dAbl (dAblK417N) rescued the rough-eye phenotype induced by KetteMyr (dAblKN; KetteMyr).
FIG. 7.
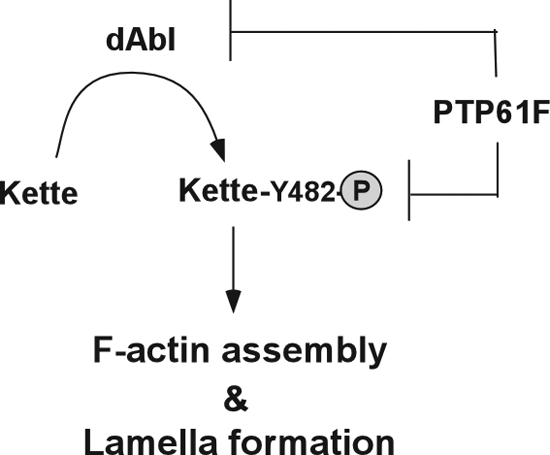
Proposed model for the organization of F-actin via concerted regulation of Kette by PTP61F and dAbl. Y482 phosphorylation (P) of Kette by dAbl kinase promotes F-actin assembly and lamella formation. This signaling event is negatively regulated by PTP61F, which dephosphorylates Y482 of Kette. Alternatively, PTP61F may downregulate the kinase activity of dAbl, resulting in lower phosphorylation of Kette.
FIG. 6.
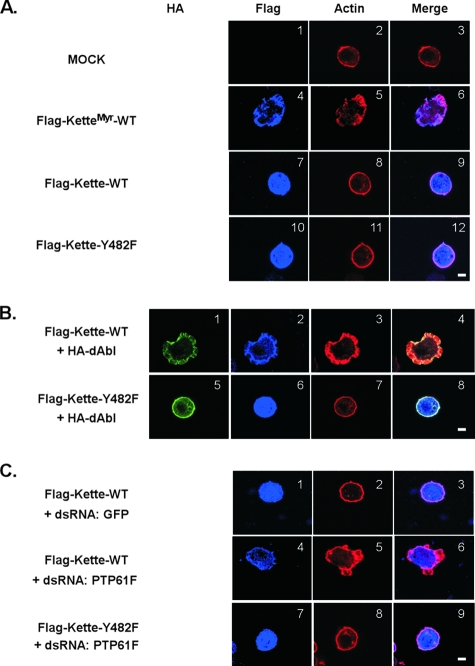
Subcellular localization of Kette, actin organization, and lamella formation in S2 cells on ConA. Transfected S2 cells were replated on ConA-coated coverslips for 7 min and then subjected to immunofluorescent staining. The images were obtained by confocal microscopy. (A) The cortical F-actin pattern was observed in mock transfectants attached on ConA (images 1 to 3). Ectopically expressed KetteMyr that was localized to the plasma membrane (image 4) led to reorganization of F-actin and formation of lamella (image 5). Upon cell spreading, KetteMyr and F-actin were colocalized near the plasma membrane (image 6). Ectopically expressed cytosolic Kette was distributed evenly in cells (image 7) without effects on the organization of F-actin or the formation of lamella (images 8 and 9). Similar results were observed when cytosolic Kette-Y482F was expressed in S2 cells (images 10 to 12). (B) In response to ectopic expression of dAbl (image 1), cytosolic Kette was localized to the plasma membrane (image 2), concomitant with F-actin reorganization and lamella formation (images 3 and 4). Upon cell spreading, dAbl, Kette, and F-actin were colocalized near the plasma membrane (image 4). In contrast, the cytosolic Kette-Y482F mutant was distributed evenly in cells when dAbl was coexpressed (images 5 and 6). The cortical F-actin was observed in transfectants expressing Y482F-Kette, independently of the levels of dAbl in cells (images 11 and 12). (C) dsRNA for green fluorescent protein (GFP) (images 1 to 3) or PTP61F (images 5 to 9) was used to treat S2 transfectants expressing cytosolic Kette or Kette-Y482F. In response to knockdown of PTP61F, cytosolic Kette was localized to the plasma membrane (image 4), concomitant with reorganization of F-actin and formation of lamella (images 5 and 6). When PTP61F was ablated, cytosolic Kette-Y482F was distributed evenly in cells (image 7) and F-actin remained in its cortical pattern (images 8 and 9). Under these conditions, S2 transfectants were unable to form lamella (images 7 and 9). Bars, 5 μM. The images are representative of multiple independent experiments with similar results. Under the experimental conditions applied, we routinely observed ∼90%, 50 to 60%, or 70 to 80% Flag-positive cells with the phenotype of lamella formation in response to KetteMyr overexpression, Kette overexpression combined with PTP61F knockdown, or Kette and dAbl cooverexpression, respectively.
FIG. 4.
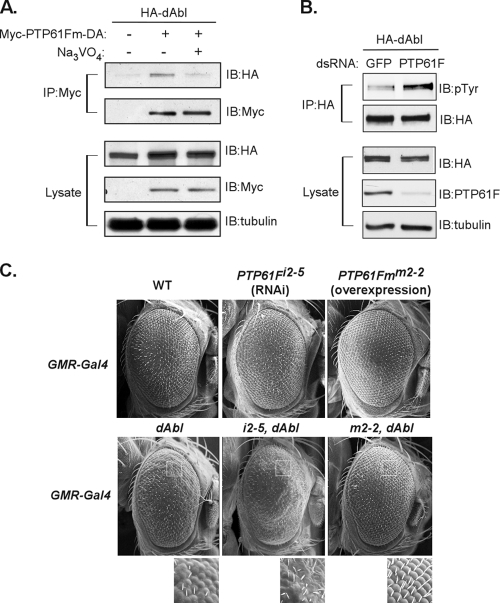
dAbl is a substrate of PTP61Fm. (A) HA-tagged dAbl was ectopically coexpressed with the Myc-tagged DA mutant form of PTP61Fm in S2 cells. Exogenous PTP61Fm was immunoprecipitated (IP) in the absence or presence of the substrate competitor sodium orthovanadate (Na3VO4; 10 mM). The immunocomplex or an aliquot of total lysates was analyzed by immunoblotting (IB). (B) S2 cells were treated with dsRNA for PTP61F or green fluorescent protein (GFP). dsRNA-treated S2 cells were then transfected with HA-tagged dAbl. Immunoprecipitated dAbl or an aliquot of total lysates was analyzed by immunoblotting. (C) The rough-eye phenotype caused by dAbl was enhanced in response to RNAi-mediated knockdown of PTP61F (i2-5, dAbl), whereas dAbl-induced eye roughness was rescued by cooverexpression of PTP61Fm (m2-2, dAbl). A magnified view of each genotype is shown to highlight the change in the organization of the ommatidial facets.
Kette is regulated by dAbl.
Our result suggests that a cellular PTK phosphorylates Kette (Fig. 1E). We investigated whether the cytosolic PTK dAbl, which is the orthologue of human c-Abl and has been proposed to regulate the SCAR/WAVE complex during actin polymerization (18, 22), is responsible for phosphorylating Kette. Accordingly, KetteMyr was ectopically expressed, alone or in combination with WT dAbl, in S2 cells and immunoprecipitated for analysis. As shown in Fig. 3A, the tyrosine phosphorylation levels of KetteMyr were increased in cells coexpressing dAbl. To further test the hypothesis that dAbl phosphorylates KetteMyr, its tyrosine phosphorylation levels were examined in S2 cells in which endogenous dAbl was ablated. Under these conditions, KetteMyr became unphosphorylated (Fig. 3B; see Fig. S2 in the supplemental material). Thus, our data demonstrate that dAbl functions in S2 cells as a predominant upstream PTK controlling the tyrosine phosphorylation levels of Kette.
We further investigated the regulatory role of dAbl in Kette signaling in vivo. As shown in Fig. 3C, ectopically expressed WT dAbl dramatically increased the eye roughness generated by KetteMyr. On the other hand, overexpression of the kinase-deficient mutant form of dAbl, dAblK417N, which did not cause an obvious phenotype when expressed on its own (Fig. 3C), suppressed the KetteMyr-induced rough-eye phenotype (Fig. 3C) and thus appeared to reduce signaling by Kette. This evidence suggests that Kette is indeed regulated by dAbl.
dAbl is a PTP61F substrate.
We also used the substrate-trapping technique to investigate whether the kinase dAbl was also regulated by PTP61F. As shown in Fig. 4A, dAbl was associated with the DA mutant form of membrane-associated PTP61Fm when both proteins were ectopically expressed in S2 cells. Consistent with this observation, incubation of the PTP61Fm immunocomplex with vanadate, which binds to the active pocket of PTPs, thus serving as a competitive inhibitor of PTPs in general, led to dissociation of dAbl from the trapping mutant form of the phosphatase (Fig. 4A). Furthermore, when endogenous PTP61F was ablated by RNAi, autophosphorylation levels of dAbl were increased significantly (Fig. 4B). dAbl and KetteMyr did not complex under the same experimental conditions (see Fig. S3 in the supplemental material), indicating that the association between dAbl and the DA mutant form of PTP61F was not mediated by Kette.
The role of PTP61F in regulating dAbl was further examined in vivo. As shown in Fig. 4C, the fused ommatidial phenotype resulting from overexpression of dAbl was strongly enhanced in response to ablation of endogenous PTP61F but reverted to the normal hexagonal formation when combined with overexpressed PTP61Fm, consistent with our observation that dAbl is a substrate of PTP61F (Fig. 4A and B). Taken together, our data not only demonstrate the opposing roles of PTP61F and dAbl in the regulation of Kette signaling in vivo (Fig. 1 to 3; see Fig. S4 in the supplemental material), but also reveal that dAbl-mediated signal transduction is directly negatively regulated by PTP61F (Fig. 4).
Identification of Kette Y482 as the primary phosphorylation site regulated by PTP61F and dAbl.
The tyrosine phosphorylation of a specific residue(s) in Kette, which is regulated by PTP61F and dAbl, must be critically important to determine signaling outputs, such as the phenotypes we observed in eye development (Fig. 2 to 4). We therefore focused on identification of any phosphorylated tyrosine residues in Kette. Since Kette has not previously been shown to be a phosphoprotein, potential phosphorylation sites were identified based on conserved motif sequences. The YXXP motif has been proposed as a consensus phosphorylation site for mammalian Abl (17). There are two such YXXP motifs in Kette, located at tyrosine 180 and tyrosine 482, which might be phosphorylated by dAbl and dephosphorylated by PTP61F. To test this hypothesis, we examined the tyrosine phosphorylation levels of KetteMyr ectopically expressed in S2 cells in its WT and various mutant forms. As shown in Fig. 5A, there were no obvious differences in pTyr phosphorylation levels between the WT and the Y180F mutant form of KetteMyr, indicating that Y180 is not a major phosphorylation site. In contrast, KetteMyr-Y482F showed a dramatic decrease in tyrosine phosphorylation, similar to that found in the Y180F/Y482F double mutant (Fig. 5A). These observations suggest that Y482 plays a critical role in KetteMyr tyrosine phosphorylation. KetteMyr-Y482F was then coexpressed with dAbl in S2 cells. As anticipated, WT KetteMyr was robustly tyrosine phosphorylated in the presence of dAbl, while the Y482F mutant was not (Fig. 5B). Thus, Y482 is the primary dAbl phosphorylation site. We further examined whether the same tyrosine residue is also targeted by PTP61F. For this, KetteMyr-Y482F was ectopically expressed in S2 cells in which endogenous PTP61F was ablated by RNAi. In response to the depletion of PTP61F, KetteMyr-Y482F was not tyrosine phosphorylated (Fig. 5C), whereas its WT form showed upregulation of tyrosine phosphorylation (Fig. 1E). Additional substrate-trapping experiments illustrated the necessity of Y482 as the recognition site of PTP61F. As expected, the WT form of KetteMyr was associated with the trapping mutant form (DA) of PTP61F when coexpressed in S2 cells (Fig. 5D). However, KetteMyr-Y482F was absent from the immunocomplex of the PTP61F DA mutant (Fig. 5D), indicating that PTP61F could no longer recognize the mutant KetteMyr as a substrate. We also examined the role of Y482 in the cytosolic form of Kette involved in dAbl- and PTP61F-regulated tyrosine phosphorylation signaling. Consistent with the observation for KetteMyr, the Y482F mutant of cytosolic Kette was not tyrosine phosphorylated when coexpressed with dAbl in S2 cells (Fig. 5E). Moreover, the Y482F mutant form of cytosolic Kette remained unphosphorylated in S2 cells when endogenous PTP61F was ablated (Fig. 5F), in contrast to an increase of phosphorylation levels in the WT form of cytosolic Kette under the same conditions (Fig. 5F). Together, our data suggest that dAbl and PTP61F regulate Kette-mediated signal transduction primarily through the control of tyrosine 482 phosphorylation.
PTP61F and dAbl control Kette-mediated actin organization and lamella formation in spreading S2 cells through a Tyr482 phosphorylation-dependent mechanism.
To determine whether tyrosine phosphorylation of Y482 controls the function of Kette in regulating actin dynamics, we examined the process of lamella formation in S2 cells. When plated on glass coverslips coated with the lectin ConA, S2 cells attach to the ConA substrate within 10 min and to begin to form actin-based lamella structures ∼30 min after being plated (37, 38). We observed that S2 cells ectopically expressing KetteMyr spread on a ConA-coated coverslip 7 min after being plated, concomitant with actin assembly at the cell periphery (Fig. 6A). Mock-transfected control S2 cells showed only normal cortical actin structure within this short time, as anticipated (Fig. 6A). These results suggested that the early-spreading assay in S2 cells might provide some insight into Kette-mediated actin organization and lamella formation. We therefore examined actin cytoskeleton morphology at 7 min post-cell plating in all subsequent experiments. As shown in Fig. 6A, cytosolic WT or Y482F mutant Kette was distributed evenly in the cytoplasm without any detectable effects on the actin cytoskeleton when ectopically expressed in S2 cells, consistent with its low tyrosine phosphorylation levels (Fig. 5).
We next asked whether tyrosine-phosphorylated Kette could promote actin polymerization near the plasma membrane. To do this, cytosolic Kette was either ectopically expressed together with dAbl or in combination with knockdown of PTP61F. Under the condition of dAbl coexpression, tyrosine-phosphorylated WT Kette (Fig. 5E) was translocated and concentrated at the cell periphery (Fig. 6B), concomitant with a rearranged actin cytoskeleton and lamella formation (Fig. 6B). In contrast, coexpression of dAbl was unable to promote tyrosine phosphorylation (Fig. 5E) and plasma membrane localization (Fig. 6B) of Kette-Y482F and showed no effect on actin assembly and lamella formation in early-spreading S2 transfectants (Fig. 6B). Moreover, in response to RNAi-mediated ablation of endogenous PTP61F, WT Kette was tyrosine phosphorylated (Fig. 5F) and translocated to the proximity of the plasma membrane (Fig. 6C). The translocation of WT Kette was concurrent with actin assembly and lamella formation at the cell periphery (Fig. 6C). Importantly, ablation of PTP61F by RNAi failed to induce tyrosine phosphorylation of Kette-Y482F (Fig. 5F) and its localization to the cell periphery (Fig. 6C). Consistently, knockdown of PTP61F showed no effect on actin rearrangement (Fig. 6C) and lamella formation (Fig. 6C) in cells expressing Y482F mutant Kette. Together, these data demonstrate that tyrosine phosphorylation at Y482 determines Kette's subcellular localization and its functions in actin organization.
DISCUSSION
PTP61F was identified 15 years ago as an active tyrosine phosphatase with a typical signature motif of the PTP superfamily (29), but to date, our understanding of the function and regulation of PTP61F is surprisingly limited. In the present study, we identified two novel substrates (Kette and dAbl) of PTP61F. We further showed that tyrosine dephosphorylation activity of PTP61F in Kette and dAbl plays an important role in controlling developmental processes. Moreover, we demonstrated the molecular basis for the interactions among PTP61F, Kette, and dAbl, which coordinate to regulate actin dynamics. To the best of our knowledge, this is the first report delineating the role of a PTP in regulating the actin cytoskeleton in Drosophila.
The most significant finding of this study is that there is a reversible tyrosine phosphorylation network that controls Kette's regulation of actin organization (Fig. 7). Kette was initially identified as a key regulator of cytoskeletal dynamics during axon pathfinding (21). Despite the accumulated data revealing the critical role of Kette in promoting actin organization in proximity to the plasma membrane, the underlying mechanism for controlling this process remained unclear. Here, we have provided direct evidence that an increased tyrosine-phosphorylated level of Kette through either activation of dAbl or ablation of PTP61F is concomitant with plasma membrane association of Kette, F-actin assembly, and lamella formation at the cell periphery. Our data also demonstrate that the phosphorylation of Tyr482 is a critical step in Kette's translocation from the cytoplasm to the cell periphery, where Kette facilitates actin polymerization.
In addition to participating in the WASP-mediated signaling pathway, Kette also regulates SCAR/WAVE-dependent actin nucleation (25). Interestingly, a recent investigation has demonstrated that ConA-stimulated spreading and lamella formation of S2 cells, the same experimental conditions used in our current study, is a SCAR/WAVE-dependent, not a WASP-mediated, process (38). Based on those findings (5, 38) and ours (Fig. 6), we hypothesize a possible mechanism that may illustrate the functional role of Kette in regulating SCAR/WAVE signaling. In the resting state, SCAR/WAVE forms a complex with Kette, Abi, Sra-1, and HSP300 in the cytoplasm, where the activity of SCAR/WAVE is suppressed (16, 22). Studies have suggested that there may be two possible extracellularly stimulated signaling events that could activate SCAR/WAVE (16, 22). In the first model, SCAR/WAVE is dissociated from the complex if other components of the complex function as inhibitors. In this case, the free form of SCAR/WAVE may translocate to the cell periphery, where it facilitates Arp2/3-mediated actin polymerization. A second possibility is that the pentameric complex is recruited to the proximity of the plasma membrane, where SCAR/WAVE present in the complex may gain access to Arp2/3 for activating the formation of actin filaments. In the present study, we observed that the translocation of WT Kette to the plasma membrane occurred concomitantly with F-actin assembly and lamella formation in S2 cells once they had spread on a ConA-coated surface, whereas the mutant form of Kette without the capability for membrane translocation was unable to activate actin organization. Thus, we propose that tyrosine-phosphorylated Kette may be recruited, together with SCAR/WAVE, to the proximity of the plasma membrane in response to ConA stimulation. This step seems to be critically important for activating subsequent actin assembly and lamella formation. Taken collectively, our data suggest that the second model proposed above may be more likely to explain the regulation of the SCAR/WAVE complex.
The identification of dAbl as a substrate of PTP61F is a surprising and interesting result. As illustrated in Fig. 7, PTP61F may dephosphorylate both dAbl and Kette upon termination of the signaling event, thus halting actin polymerization. Thus, a phosphatase is capable of controlling the extent and duration of signaling events via synergistic dephosphorylation of a kinase and its substrate. It is also important to note that, in addition to the direct regulation of Kette, PTP61F may participate in control of the actin cytoskeleton indirectly through inactivation of dAbl, which may phosphorylate SCAR/WAVE for regulation of cell spreading, lamellipodium formation, and cell migration, as evidenced in mammals (43, 46). If this is the case, to terminate such signaling cascades, PTP61F may simply dephosphorylate dAbl, thus downregulating the activity of SCAR/WAVE indirectly. More studies at the biochemical and genetic levels are required to clarify the underlying mechanism that controls SCAR/WAVE-dependent actin dynamics through the interaction between PTP61F and dAbl.
The role of PTP61F in regulating the actin cytoskeleton may not be confined to tyrosine dephosphorylation of Kette and dAbl. There is biochemical evidence suggesting that PTP61F-dependent dephosphorylation of Abi may play a role in actin organization in S2 cells (20). In addition to Kette, dAbl, and Abi, we have recently identified many other potential substrates of PTP61F through a large-scale MS-based analysis of PTP61F-associated proteins (8). Importantly, a number of components of the SCAR/WAVE complex, including Kette and Abi, as well as Sra-1 and SCAR/WAVE itself, are among the substrates of PTP61F (8). By combining these approaches with the use of cell-based and genetic analyses, it should be possible to characterize specific interactions between PTP61F and its substrates and to gain insight into their physiological roles in regulating the actin cytoskeleton.
The data provided by the present study open a new avenue for understanding of the roles of human PTP1B and TC-PTP in controlling important biological processes through the regulation of actin dynamics. Based on our results, it is tempting to hypothesize that PTP1B or TC-PTP may recognize NAP-1, the mammalian orthologue of Kette (3), as a potential substrate. We propose that tyrosine dephosphorylation of NAP-1 by PTP1B or TC-PTP might lead to a change in WAVE activity, further affecting Arp2/3-mediated actin polymerization. Alternatively, PTP1B or TC-PTP may participate in the control of actin cytoskeleton dynamics through an indirect route by tyrosine dephosphorylation and inactivation of c-Abl, an upstream kinase promoting WAVE activity. It has been shown that the oncogenic form of the Bcr-Abl chimeric protein is a substrate of PTP1B (26). Therefore, it is also possible that PTP1B may recognize c-Abl as a substrate. Through tyrosine dephosphorylation and inactivation of c-Abl, the activities of WAVE and other components of the WAVE complex, such as NAP-1, may be downregulated, thus affecting actin organization. Although more experiments are needed to support this hypothesis, using Drosophila as a model organism, our study has already shed some light on a pivotal role that PTPs may play in controlling the actin cytoskeleton through tyrosine phosphorylation-dependent signal transduction.
Supplementary Material
Acknowledgments
This work was supported by grants from Taiwan's National Science Council (95-2311-B-001-051-MY3 to T.-C.M. and 96-2311-B-001-033-MY3 to G.-C.C) and by funding from the Université Paris Sud 11 and the CNRS (to L.R.). T.-C.M. and G.-C.C. also received additional funding from Academia Sinica.
We thank C. Klämbt, B. Hassan, J.-L. Juang, the Vienna Drosophila RNAi Center, and the DSHB for reagents. Special thanks are due to the staff of Taiwan's NRPGM Core Facilities for Proteomic Research for MS-based analysis, C. C. Hung for confocal microscopy assistance, and W. N. Jane for EM assistance.
Footnotes
Published ahead of print on 27 April 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Andersen, J. N., R. L. Del Vecchio, N. Kannan, J. Gergel, A. F. Neuwald, and N. K. Tonks. 2005. Computational analysis of protein tyrosine phosphatases: practical guide to bioinformatics and data resources. Methods 3590-114. [DOI] [PubMed] [Google Scholar]
- 2.Baeg, G. H., R. Zhou, and N. Perrimon. 2005. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 191861-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner, S., D. Martin, R. Chiquet-Ehrismann, J. Sutton, A. Desai, I. Huang, K. Kato, and R. Hromas. 1995. The HEM proteins: a novel family of tissue-specific transmembrane proteins expressed from invertebrates through mammals with an essential function in oogenesis. J. Mol. Biol. 25141-49. [DOI] [PubMed] [Google Scholar]
- 4.Bladt, F., E. Aippersbach, S. Gelkop, G. A. Strasser, P. Nash, A. Tafuri, F. B. Gertler, and T. Pawson. 2003. The murine Nck SH2/SH3 adaptors are important for the development of mesoderm-derived embryonic structures and for regulating the cellular actin network. Mol. Cell. Biol. 234586-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan, S., O. Grewe, M. Strunk, A. Mertens, and C. Klambt. 2004. Sra-1 interacts with Kette and Wasp and is required for neuronal and bristle development in Drosophila. Development 1313981-3989. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, S., and C. Klambt. 2003. Kette regulates actin dynamics and genetically interacts with Wave and Wasp. Development 1304427-4437. [DOI] [PubMed] [Google Scholar]
- 7.Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118401-415. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. C., S. Y. Lin, S. Y. Liang, K. T. Pan, C. C. Chou, C. H. Chen, C. L. Liao, K. H. Khoo, and T. C. Meng. 2008. Tyrosine phosphoproteomics and identification of substrates of protein tyrosine phosphatase dPTP61F in Drosophila S2 cells by mass spectrometry-based substrate trapping strategy. J. Proteome Res. 71055-1066. [DOI] [PubMed] [Google Scholar]
- 9.Cingolani, L. A., and Y. Goda. 2008. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9344-356. [DOI] [PubMed] [Google Scholar]
- 10.Clemens, J. C., Z. Ursuliak, K. K. Clemens, J. V. Price, and J. E. Dixon. 1996. A Drosophila protein-tyrosine phosphatase associates with an adapter protein required for axonal guidance. J. Biol. Chem. 27117002-17005. [DOI] [PubMed] [Google Scholar]
- 11.Clemens, J. C., C. A. Worby, N. Simonson-Leff, M. Muda, T. Maehama, B. A. Hemmings, and J. E. Dixon. 2000. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 976499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai, C. J., N. X. Krueger, H. Saito, and K. Zinn. 1997. Competition and cooperation among receptor tyrosine phosphatases control motoneuron growth cone guidance in Drosophila. Development 1241941-1952. [DOI] [PubMed] [Google Scholar]
- 13.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 941680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogerty, F. J., J. L. Juang, J. Petersen, M. J. Clark, F. M. Hoffmann, and D. F. Mosher. 1999. Dominant effects of the bcr-abl oncogene on Drosophila morphogenesis. Oncogene 18219-232. [DOI] [PubMed] [Google Scholar]
- 15.Giannakou, M. E., and L. Partridge. 2007. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 32180-188. [DOI] [PubMed] [Google Scholar]
- 16.Goley, E. D., and M. D. Welch. 2006. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7713-726. [DOI] [PubMed] [Google Scholar]
- 17.Grossmann, A. H., K. S. Kolibaba, S. G. Willis, A. S. Corbin, W. S. Langdon, M. W. Deininger, and B. J. Druker. 2004. Catalytic domains of tyrosine kinases determine the phosphorylation sites within c-Cbl. FEBS Lett. 577555-562. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez, S. E., M. Krishnaswami, A. L. Miller, and A. J. Koleske. 2004. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 1436-44. [DOI] [PubMed] [Google Scholar]
- 19.Hill, K. K., V. Bedian, J. L. Juang, and F. M. Hoffmann. 1995. Genetic interactions between the Drosophila Abelson (Abl) tyrosine kinase and failed axon connections (fax), a novel protein in axon bundles. Genetics 141595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, C. H., T. Y. Lin, R. L. Pan, and J. L. Juang. 2007. The involvement of Abl and PTP61F in the regulation of Abi protein localization and stability and lamella formation in Drosophila S2 cells. J. Biol. Chem. 28232442-32452. [DOI] [PubMed] [Google Scholar]
- 21.Hummel, T., K. Leifker, and C. Klambt. 2000. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 14863-873. [PMC free article] [PubMed] [Google Scholar]
- 22.Ibarra, N., A. Pollitt, and R. H. Insall. 2005. Regulation of actin assembly by SCAR/WAVE proteins. Biochem. Soc. Trans. 331243-1246. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, K. G., and D. Van Vactor. 2003. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 831-24. [DOI] [PubMed] [Google Scholar]
- 24.Karim, F. D., and G. M. Rubin. 1999. PTP-ER, a novel tyrosine phosphatase, functions downstream of Ras1 to downregulate MAP kinase during Drosophila eye development. Mol. Cell 3741-750. [DOI] [PubMed] [Google Scholar]
- 25.Kunda, P., G. Craig, V. Dominguez, and B. Baum. 2003. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr. Biol. 131867-1875. [DOI] [PubMed] [Google Scholar]
- 26.LaMontagne, K. R., Jr., A. J. Flint, B. R. Franza, Jr., A. M. Pandergast, and N. K. Tonks. 1998. Protein tyrosine phosphatase 1B antagonizes signalling by oncoprotein tyrosine kinase p210 bcr-abl in vivo. Mol. Cell. Biol. 182965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, C. L., H. H. Hsiao, C. W. Lin, S. P. Wu, S. Y. Huang, C. Y. Wu, A. H. Wang, and K. H. Khoo. 2003. Strategic shotgun proteomics approach for efficient construction of an expression map of targeted protein families in hepatoma cell lines. Proteomics 32472-2486. [DOI] [PubMed] [Google Scholar]
- 28.Lee, Y. S., and R. W. Carthew. 2003. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30322-329. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin, S., and J. E. Dixon. 1993. Alternative splicing gives rise to a nuclear protein tyrosine phosphatase in Drosophila. J. Biol. Chem. 2686839-6842. [PubMed] [Google Scholar]
- 30.Menzel, N., D. Schneeberger, and T. Raabe. 2007. The Drosophila p21 activated kinase Mbt regulates the actin cytoskeleton and adherens junctions to control photoreceptor cell morphogenesis. Mech. Dev. 12478-90. [DOI] [PubMed] [Google Scholar]
- 31.Morrison, D. K., M. S. Murakami, and V. Cleghon. 2000. Protein kinases and phosphatases in the Drosophila genome. J. Cell Biol. 150F57-F62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller, P., D. Kuttenkeuler, V. Gesellchen, M. P. Zeidler, and M. Boutros. 2005. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436871-875. [DOI] [PubMed] [Google Scholar]
- 33.Myers, M. P., J. N. Andersen, A. Cheng, M. L. Tremblay, C. M. Horvath, J. P. Parisien, A. Salmeen, D. Barford, and N. K. Tonks. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 27647771-47774. [DOI] [PubMed] [Google Scholar]
- 34.Paavilainen, V. O., E. Bertling, S. Falck, and P. Lappalainen. 2004. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 14386-394. [DOI] [PubMed] [Google Scholar]
- 35.Perkins, L. A., I. Larsen, and N. Perrimon. 1992. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell 70225-236. [DOI] [PubMed] [Google Scholar]
- 36.Rintelen, F., E. Hafen, and K. Nairz. 2003. The Drosophila dual-specificity ERK phosphatase DMKP3 cooperates with the ERK tyrosine phosphatase PTP-ER. Development 1303479-3490. [DOI] [PubMed] [Google Scholar]
- 37.Rogers, S. L., and G. C. Rogers. 2008. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat. Protoc. 3606-611. [DOI] [PubMed] [Google Scholar]
- 38.Rogers, S. L., U. Wiedemann, N. Stuurman, and R. D. Vale. 2003. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 1621079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmeen, A., J. N. Andersen, M. P. Myers, N. K. Tonks, and D. Barford. 2000. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell 61401-1412. [DOI] [PubMed] [Google Scholar]
- 40.Schenck, A., A. Qurashi, P. Carrera, B. Bardoni, C. Diebold, E. Schejter, J. L. Mandel, and A. Giangrande. 2004. WAVE/SCAR, a multifunctional complex coordinating different aspects of neuronal connectivity. Dev. Biol. 274260-270. [DOI] [PubMed] [Google Scholar]
- 41.Schindelholz, B., M. Knirr, R. Warrior, and K. Zinn. 2001. Regulation of CNS and motor axon guidance in Drosophila by the receptor tyrosine phosphatase DPTP52F. Development 1284371-4382. [DOI] [PubMed] [Google Scholar]
- 42.Simske, J. S., and J. Hardin. 2001. Getting into shape: epidermal morphogenesis in Caenorhabditis elegans embryos. Bioessays 2312-23. [DOI] [PubMed] [Google Scholar]
- 43.Sossey-Alaoui, K., X. Li, and J. K. Cowell. 2007. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J. Biol. Chem. 28226257-26265. [DOI] [PubMed] [Google Scholar]
- 44.Stepanek, L., A. W. Stoker, E. Stoeckli, and J. L. Bixby. 2005. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J. Neurosci. 253813-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stradal, T. E., K. Rottner, A. Disanza, S. Confalonieri, M. Innocenti, and G. Scita. 2004. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 14303-311. [DOI] [PubMed] [Google Scholar]
- 46.Stuart, J. R., F. H. Gonzalez, H. Kawai, and Z. M. Yuan. 2006. c-Abl interacts with the WAVE2 signaling complex to induce membrane ruffling and cell spreading. J. Biol. Chem. 28131290-31297. [DOI] [PubMed] [Google Scholar]
- 47.Sun, Q., B. Schindelholz, M. Knirr, A. Schmid, and K. Zinn. 2001. Complex genetic interactions among four receptor tyrosine phosphatases regulate axon guidance in Drosophila. Mol. Cell Neurosci. 17274-291. [DOI] [PubMed] [Google Scholar]
- 48.Takenawa, T., and S. Suetsugu. 2007. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 837-48. [DOI] [PubMed] [Google Scholar]
- 49.Uetani, N., M. J. Chagnon, T. E. Kennedy, Y. Iwakura, and M. L. Tremblay. 2006. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J. Neurosci. 265872-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Vactor, D., A. M. O'Reilly, and B. G. Neel. 1998. Genetic analysis of protein tyrosine phosphatases. Curr. Opin. Genet. Dev. 8112-126. [DOI] [PubMed] [Google Scholar]
- 51.Whited, J. L., M. B. Robichaux, J. C. Yang, and P. A. Garrity. 2007. Ptpmeg is required for the proper establishment and maintenance of axon projections in the central brain of Drosophila. Development 13443-53. [DOI] [PubMed] [Google Scholar]
- 52.Yamaoka, K., P. Saharinen, M. Pesu, V. E. Holt III, O. Silvennoinen, and J. J. O'Shea. 2004. The Janus kinases (Jaks). Genome Biol. 5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokota, Y., C. Ring, R. Cheung, L. Pevny, and E. S. Anton. 2007. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron 54429-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.