Abstract
Monocyte chemoattractant protein 1 (MCP-1; CCL2) is a recently identified prominent regulator of prostate cancer growth and metastasis. The purpose of this study was to investigate the mechanistic role of CCL2 in prostate cancer growth in bone. The present study found that CCL2 was upregulated in osteoblasts (3-fold by PC3 and 2-fold by VCaP conditioned media) and endothelial cell (2-fold by PC3 and VCaP conditioned media). PTHrP treatment of osteoblastic cells upregulated CCL2 and was blocked by a PTHrP antagonist suggesting prostate cancer-derived PTHrP plays an important role in elevation of osteoblast derived CCL2. CCL2 indirectly increased blood vessel formation in endothelial cells through VEGF-A, which was up-regulated 2-fold with administration of CCL2 in prostate cancer cells. In vivo, anti-CCL2 treatment suppressed tumor growth in bone. The decreased tumor burden was associated with decreased bone resorption (serum TRAP5b levels were decreased by 50~60% in anti-CCL2 treated animals from VCaP or PC-3 cell osseous lesions) and micro-vessel density was decreased by 70% in anti-CCL2 treated animals with bone lesions from VCaP cells. These data suggest that a destructive cascade is driven by tumor cell-derived PTHrP mediated induction of CCL2, which facilitates tumor growth via enhanced osteoclastic and endothelial cell activity in bone marrow. Taken together, CCL2 mediates the interaction between tumor-derived factors and host-derived chemokines acting in cooperation to promote skeletal metastasis.
Keywords: CCL2, prostate cancer, parathyroid hormone related protein
Introduction
Monocyte chemoattractant protein 1 (MCP-1/CCL2) is a member of the CC chemokine family and was recently described as facilitating osteoclastogenesis (1, 2). Osteoclastogenesis and bone resorption are independent steps leading to the development of skeletal metastases and are mutually essential for prostate cancer establishment in the bone microenvironment. CCL2 can also directly support prostate carcinoma cell growth in vitro (3, 4). We further demonstrated that targeted inhibition of CCL2 effectively suppressed prostate cancer growth in vivo and suggested that CCL2 may enhance tumor growth through macrophage infiltration and angiogenesis (5). Previous findings suggest that CCL2 can directly mediate angiogenesis in endothelial cells which express CCR2, the receptor for CCL2 (6). Human brain endothelial cells, human umbilical cord vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs) were reported to express CCR2 and are able to respond to CCL2 by forming more vessel spouts in vitro (6, 7). Conversely, one study demonstrated that human umbilical cord vein and dermal microvascular endothelial cells do not express CC-chemokine receptors (8) and thus are unlikely to be regulated by CCL2. The presence of CCR2 and its angiogenic response to CCL2 may differ in endothelial cells depending on tissue sites. In addition, it is not clear if CCL2 can directly activate endothelial cells in the bone marrow, which would be more relevant to angiogenesis during bone metastasis. Interestingly, human bone marrow endothelial (HBME) cells were found to secrete high levels of CCL2 compared to human aortic endothelial cells (HAECs) and HDMECs (4), indicating an active role of CCL2 in the bone microenvironment. With recent studies highlighting a role for CCL2 in supporting the development of prostate cancer skeletal metastasis, a better understanding of how CCL2 facilitates tumor growth in bone is important to consider it as a novel therapeutic target for the treatment of bone-specific disease. There appears to be two distinct roles for CCL2 in the development and promotion of prostate cancer: a direct effect on prostate cancer epithelial cells, and an indirect effect on cells (i.e. osteoclasts and endothelial cells) at the metastatic site to support tumor growth. Here, we hypothesized that elevated CCL2 in the bone microenvironment contributes to prostate tumor growth by initiating osteoclastogenesis and angiogenesis to create a favorable niche for prostate cancer cells.
Materials and Methods
Materials
Recombinant human parathyroid hormone related protein, PTHrP (1–34) and (7–34) were obtained from Bachem (Torrance, CA). CNTO 888, a human antibody that neutralizes human CCL2, C1142, an anti-mouse CCL2 antibody, and C1322, a control antibody for C1142, were provided by Centocor, Inc. (Radnor, PA). CNTO 888 and C1142 do not cross-react with or neutralize mouse CCL2 or human CCL2, respectively (4). Anti-human IgG from R&D System (Minneapolis, MN) was used as control antibody for CNTO 888. Human rCCL2 was obtained from Peprotech (Rocky Hill, NJ).
Cell Culture
All cell lines are from ATCC (Manassas, VA) until otherwise noted. PC-3 and LnCaP were maintained in RPMI 1640 + 10% fetal bovine serum (FBS) (Invitrogen). VCaP and HBME cells were obtained from the Rapid Autopsy program at the University of Michigan (9). HAECs, HBMEs and VCaP cells were maintained in DMEM containing 10% FBS plus 1% antibiotics (Invitrogen). HDMVEC were maintained in EGM2 (Clonetics, Walkersville, MD) containing 10% FBS and 1 % antibiotics.
Human osteoblastic Saos2 cells (ATCC HTB-85) were maintained in αMEM (Invitrogen) containing 1 % antibiotics and 10% FBS. Saos2 cells were plated at 50,000 cells/cm2 and cultured to 80~90% confluence. Cells were subsequently treated with vehicle or PTHrP (10 nM) for 4h after serum starvation. The PTHrP antagonist, PTHrP (7–34) (2 µM) was added 30 min ahead of PTHrP treatment as indicated. Primary mouse calvarial cells were isolated as previously described (10). Cells were plated at 80,000 cells/well in 24-well-plate in αMEM with 10% FBS containing 1 % antibiotics. Primary cultures at 90% confluence were used without passage, and were treated with PTHrP or vehicle for 4h.
Protein detection
Bone marrow aspirates, sera and conditioned media from cell culture were used for protein detection. Bone marrow aspirates were prepared by flushing tibiae or femurs with 1 ml of saline. The number of total cells were counted and used to normalize protein levels. Bone marrow aspirates, sera separated from blood and conditioned media were aliquoted and kept at −20°C until assays were performed.
Secreted CCL2 proteins levels in Saos2, PC-3, endothelial cells or primary cell culture media were measured by ELISA assay system from BD Biosciences (San Jose, CA). Values were calculated from standard curves set up for each assay. Data were based on triplicate experiments performed independently. Serum TRAP5b activity was measured by ELISA (Immunodiagnostic Systems, Inc., Fountain Hills, AZ) following the manufacturer’s instructions. VEGF-A levels in the conditioned media of PC-3 cells were measured using an ELISA assay system from R&D Systems.
In vivo prostate cancer models
Five week old CB17 severe combined immunodeficient mice obtained from Charles River Laboratories (Portage, MI) were used for subcutaneous and intratibial tumor implantation. VCaP and PC-3 cells were cultured in T-75 flasks to 100% confluence, trypsinized and enumerated. Mice were sedated with 1.7% isofluorane mixed with air for subcutaneous and intratibial tumor implantation. Xenograft tumors were established by subcutaneous injection of 106 VCaP or PC-3 prostate cancer cells in 200 µl of growth factor reduced Matrigel (BD Biosciences) as previously described (11). Animals were sacrificed after 4 weeks. There were no significant body weight differences in the animals with or without s.c. tumors at the time of sacrifice. For intratibial tumor injection, a 27 gauge needle was used to bore a hole in the marrow cavity through the tibial plateau, into the left tibia as described previously (5). A 28 gauge Hamilton Syringe was used to inject 5 × 105 cells in a 10µL volume into the marrow cavity. Animals were sacrificed after 8 weeks. Tibias were removed, placed in 10% formaldehyde for 24 hours, and then transferred to 70% ethanol. All animal studies were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Treatment with anti-CCL2 (C1142)
Prior to intratibial injection, mice were pre-treated with 2 mg/kg anti-mouse CCL2 (C1142). Control mice were treated with either 2 mg/kg isotype control antibody (C1322) or PBS. Following intratibial injection with PC-3 or VCaP prostate cancer cells as described above, mice continued treatment twice weekly until the end of the experiment.
Histology and immunohistochemistry
Xenograft tumors were harvested and placed in fresh 10% formalin. Tibiae were decalcified in 10% EDTA prior to paraffin embedding. Specimens were sectioned (5 µm) and stained with either hematoxylin and eosin (H&E), trichrome (to highlight bone), tartate resistant acid phosphotase (TRAP) (to identify osteoclasts) (Acid Phosphatase, Leukocyte Kit, Sigma, St. Louis, MO), or immunohistochemistry performed for von Willebrand factor (vWF) (NeoMarkers, Fremont, CA). Standard indirect immunoperoxidase procedures were used for immunohistochemistry using the AEC Cell and Tissue staining system (R&D systems), with Mayer’s hematoxylin (Sigma) used for counterstaining.
For microvessel density analysis (MVD), after vWF staining, four random areas per tumor section were selected. Any single or cluster of positively stained endothelial cells that was clearly separated from adjacent microvessels was considered as one countable microvessel. The average MVD was determined for each specimen.
Endothelial sprout network formation assay
Growth factor-reduced Matrigel (BD Biosciences) was placed in 8-well chamber slides and polymerized at 37°C for 30 min. Eight thousand (8×103) endothelial cells were added to the top of the Matrigel in each well. After 24h incubation at 37°C, the slides were fixed, stained with Hema3 STAT PACK (Protocol, Kalamazoo, MI) and analyzed by enumerating the sprouts under a light microscope.
Quantitative Polymerase Chain Reaction (QPCR) Assay
Total RNA was isolated from cultured cells using Tri reagent (Sigma) and 1 µg total RNA was reverse transcribed in a 20-µL reaction volume containing random hexamers with a reverse transcription assay system (Applied Biosystems, Foster City, CA). Quantitative RT-PCR was performed using the ABI PRISM 7700 with a ready-to-use mix of primers and FAM-labeled probe assay system (Applied Biosystems). Data were calculated using the standard curve method and expressed as a ratio to the GAPDH reference.
Statistical Analysis
Student's t test for independent analysis was applied to evaluate differences using the GraphPad Instat software program (GraphPad Software, Inc., San Diego, CA). The value p<0.05 was considered statistically significant. In vitro assays were repeated a minimum of 2 times with similar results. For in vivo assays, N indicates the number of independent samples from different mice.
Results
Prostate tumors elevate CCL2 expression in bone marrow, osteoblasts and endothelial cells
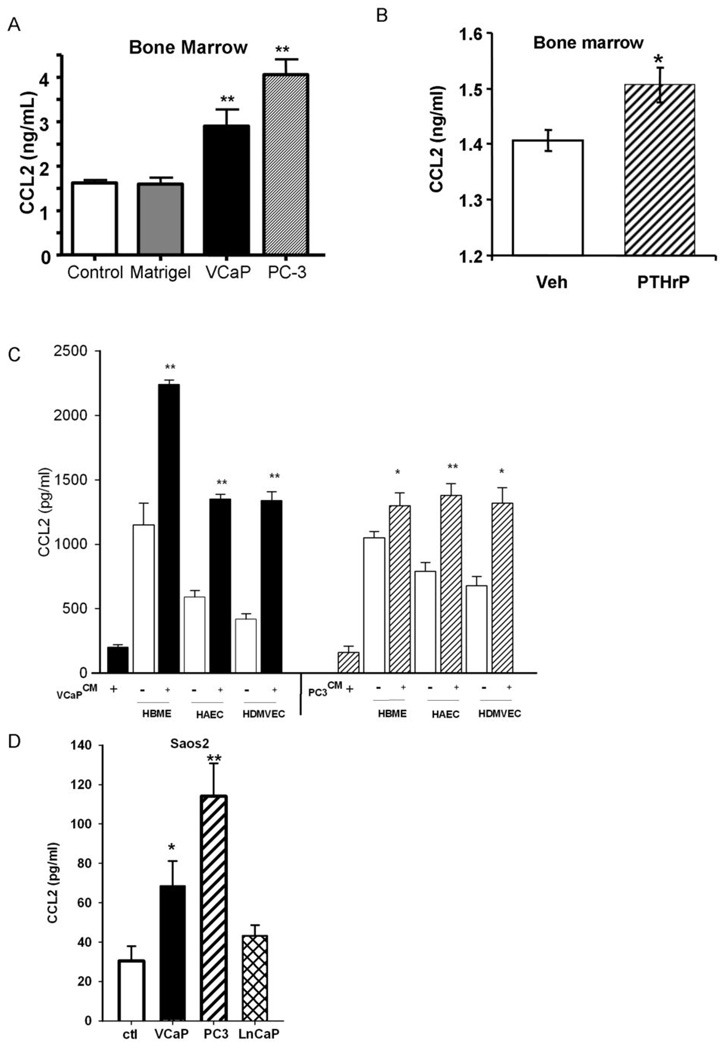
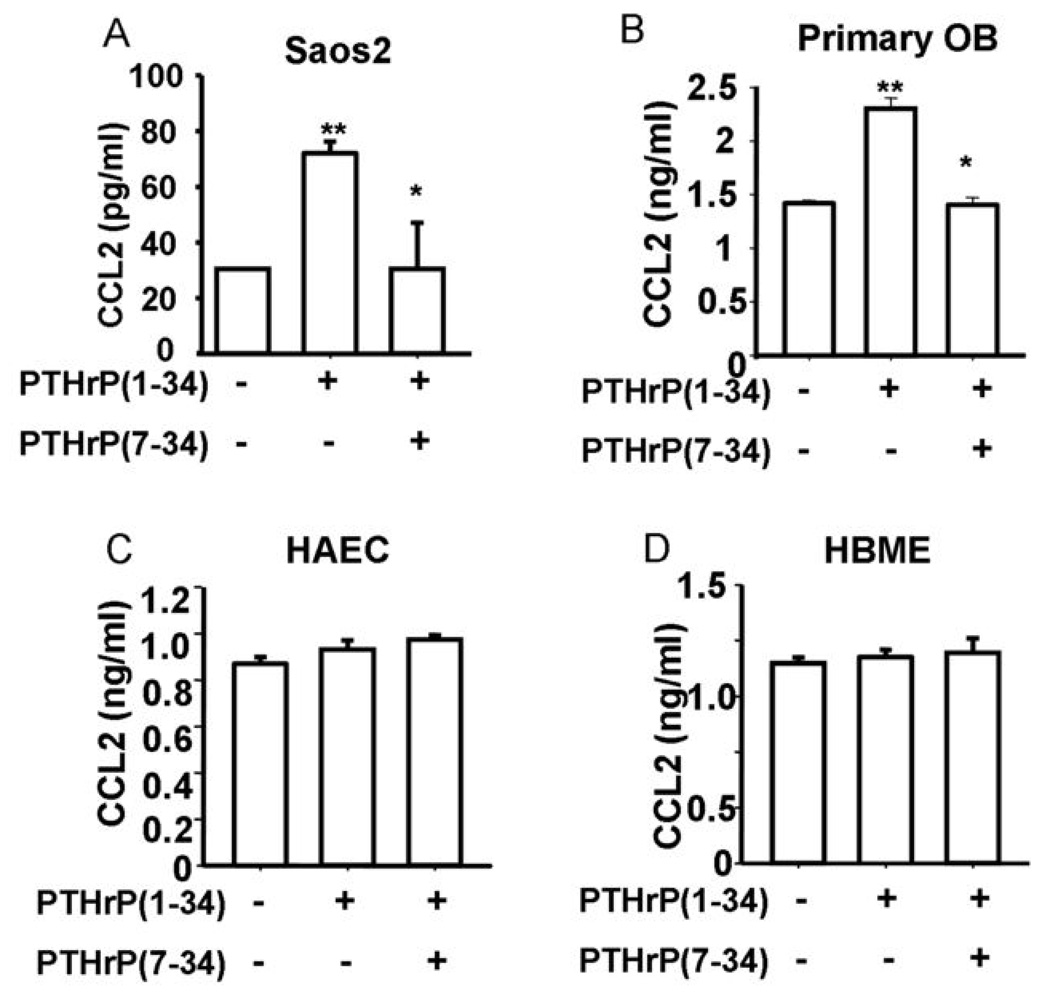
To determine the ability of a primary tumor to alter the bone microenvironment as a pre-metastatic event, PC-3 and VCaP cell xenografts were implanted subcutaneously and allowed to develop for 4 weeks. At 4 weeks, bone marrow aspirates were collected from the tibiae, and analyzed for CCL2 expression. The presence of PC-3 and VCaP cell xenografts significantly increased the levels of CCL2 approximately 2-fold in the bone marrow compared to control animals (Figure 1A). In separate experiments, subcutaneous injection of PTHrP also significantly upregulated CCL2 in the bone marrow (Figure 1B) and serum (Supplementary Figure 1), which is consistent with the hypothesis that PTHrP, secreted from primary tumors, mediates upregulation of CCL2 in the bone marrow. Previously, we demonstrated that CCL2 is expressed at high levels by bone marrow endothelial cells (4). Here, we measured the CCL2 levels in vitro when endothelial cells were cultured with prostate cancer cell conditioned media. The conditioned media from prostate cancer cell lines (PC-3, VCaP or LnCaP) increased the levels of CCL2 produced by endothelial cells (bone marrow endothelial cells – HBME, aortic endothelial cells – HAEC and dermal microvascular endothelial cells - HDMVEC) (Figure 1C and Supplementary Figure 2). A significant increase of CCL2 was also found in human osteoblastic (Saos2) cells cultured with conditioned media from prostate cancer cell lines PC-3 (3-fold) and VCaP (2-fold), both of which secrete high levels of PTHrP. Conditioned media from the low PTHrP-secreting prostate cancer cell line, LnCaP, failed to stimulate CCL2 secretion from Saos2 cells (Figure 1D). Further, stimulation of primary osteoblasts and Saos2 cells but not endothelial cells (HAEC and HBME) with PTHrP (10 nM) increased secretion of CCL2 by 2.5-fold (Figure 2). To confirm that the increase in CCL2 was due to PTHrP, PTHrP(7–34) was used as an antagonist to suppress PTHrP signaling. PTHrP(7–34) competes with PTHrP for binding to its receptor but does not activate cAMP and hence blocks signaling (12). PTHrP(7–34) suppressed the PTHrP-induced CCL2 increase in osteoblasts while it had no effect on endothelial cells. This suggests that tumor cell-derived PTHrP increases CCL2 production by osteoblasts.
Figure 1. Tumor cells increased CCL2 expression in vivo and in vitro.
A) PC-3 and VCaP cell xenografts or Matrigel (control) were implanted subcutaneously and allowed to establish. At 4 weeks, bone marrow aspirates were collected from the tibias and analyzed for CCL2 expression by ELISA. Bars show mean±SEM (** p<0.01 versus control, n=5). B) Four-week-old C57/B6 mice were treated with recombinant PTHrP (1–34) at 50µg/kg/day or Vehicle twice a day for 7 days. One hour after the last injection, bone marrow aspirates were collected from the tibia and analyzed for CCL2 expression by ELISA. Bars show mean±SEM (* p<0.05 versus, n=8). C–D) Cells were maintained and passaged as described in Materials and Methods. Confluent Saos2, HAECs, HBMEs and HDMVEC cells were co-cultured with conditioned media from confluent VCaP and PC-3 cells overnight as indicated for CCL2 measurement. C) CCL2 levels in HAECs, HBMEs and HDMVEC supernatants cultured with or without VCaP or PC-3 conditioned media; D) Saos2 cultured with (VCaP, PC-3 and LnCaP) or without (Control, ctl) conditioned media, respectively. Data are shown as mean ± SEM (*p<0.05, n=3, ** p<0.01, n=3).
Figure 2. PTHrP increases CCL2 in osteoblasts but not endothelial cells.
Saos2 cells (A) were plated at 50,000 cells/cm2, endothelial cells (C and D) were plated at 30,000 cells/cm2, grown to 80~90% confluence and were subsequently treated with vehicle or PTHrP(1–34) at 10 nM for 4h after serum starvation. PTHrP antagonist, PTHrP(7–34) (2 µM) was added 30 min before PTHrP(1–34) treatment. Primary mouse osteoblasts (B) were treated with PTHrP or vehicle for 4h without serum starvation. CCL2 levels in the media were measured by ELISA. Data are shown as mean ± SEM. (** p<0.01 versus vehicle control and * p<0.05 versus PTHrP treated group, n=3)
Antibody to CCL2 reduces tumor burden and microvessel density
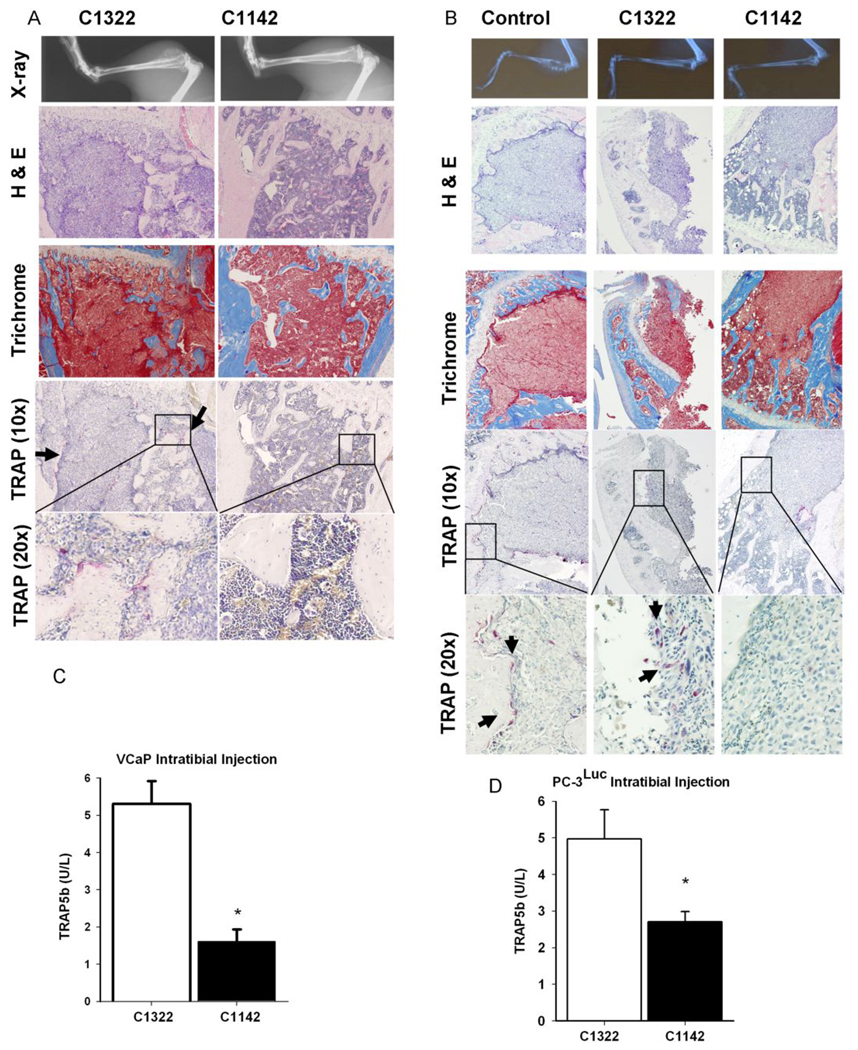
To determine the role of CCL2 in prostate cancer metastatic bone establishment and growth, systemic inhibition of CCL2 was accomplished using a neutralizing antibody that targets mouse CCL2 (C1142). Mice were inoculated with either PC-3 or VCaP prostate cancer cells. VCaP cells were originally isolated from a vertebral metastatic lesion obtained via the University of Michigan Rapid Autopsy Program (9). PC-3 cells are osteolytic, androgen receptor negative and androgen-independent while VCaP cells express a wild type androgen receptor and are androgen sensitive. Both PC-3 and VCaP cells are known to express the CCL2 receptor, CCR2 (4). Mice were injected with PC-3 or VCaP cells by intratibial injection and bone lesions were monitored weekly using radiographic imaging. PC-3 cells established visible osteolytic lesions by week 6 and VCaP cells established a mixed osteolytic/osteoblastic lesion by week 10.
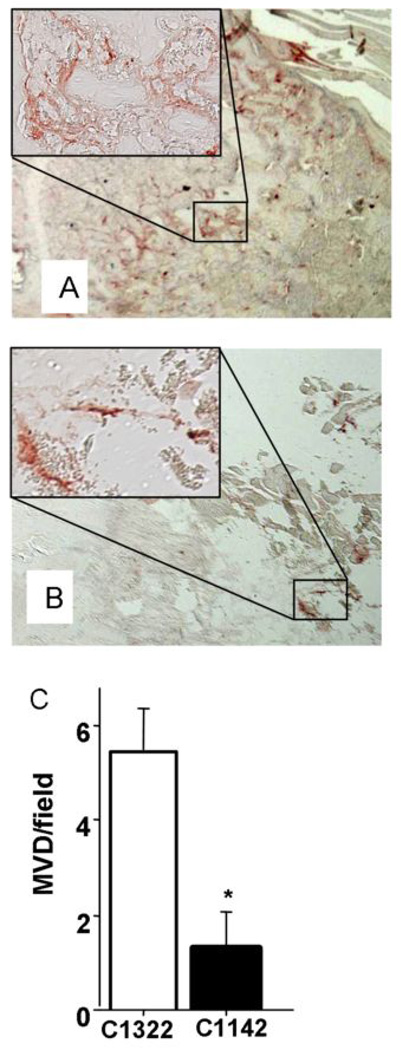
Tibias were harvested and examined via immunohistochemical analysis (Figure 3). Overall tumor burden was significantly decreased in tibias of mice receiving C1142 (anti-mouse CCL2) antibody compared to control antibody (C1322). Bone destruction was decreased, as observed by trichrome staining for mineralized bone matrix, in animals receiving C1142 (VCaP: Figure 3A). Additionally, inhibition of CCL2 attenuated TRAP-positive osteoclast staining suggesting an inhibition of osteoclast activity (VCaP: Figure 3A; PC-3: 3B). Interestingly, mice inoculated with VCaP cells failed to develop obvious osteolytic lesions when treated with the anti-CCL2 antibody. Analysis of serum markers of osteoclast activity revealed a significant decrease in TRAP5b serum levels in animals receiving anti-mouse CCL2 antibody compared to controls (Figure 3C and D). Immunostaining for vWF in tibial sections of mice that had received intratibial injections of VCaP tumor cells revealed decreased microvessel density (MVD) in animals receiving anti-mouse CCL2 antibody compared to controls (Figure 4).
Figure 3. CCL2 antibody treatment decreased tumor burden and bone resorption.
Representive images of tibial sections from mice with intratibial injection of VCaP (A) or PC-3 tumor cells (B) with H&E staining, Masson’s Trichrome staining and TRAP staining as indicated. (C–D) serum TRAP5b levels in mice treated with anti-mouse CCL2 antibody (C1142) or control antibody (C1322) * p<0.001 versus C1322, values are mean±SEM; n=4.
Figure 4. Blocking CCL2 decreased microvessel density (MVD) in intratibia VCaP bone lesions.
Representive images of vWF staining in tibia of mouse treated with control antibody (C1322) (A) or anti-mouse CCL2 antibody (C1142) (B) after intratibial inoculation of VCaP cells. (C) Total number of blood vessels was counted per section of tibia with bone lesions. * p<0.01, values are mean±SEM; n=4.
Pro-angiogenic impact of CCL2
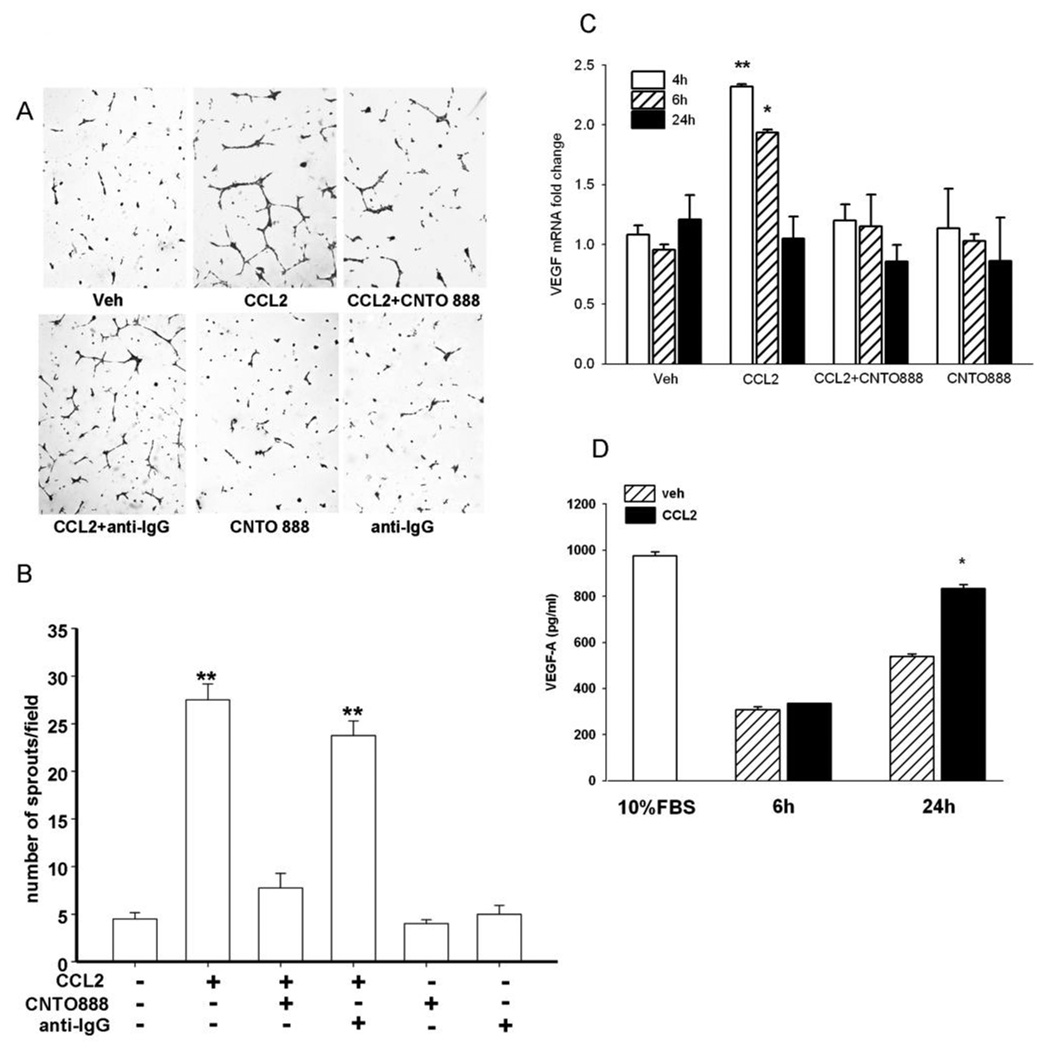
It has been reported that some human endothelial cells express the receptor for CCL2 (CCR2) and respond to CCL2 (6), but it is not clear whether HBMEs respond to CCL2. This is particularly relevant since bone marrow endothelial cells are the most abundant endothelial cell type in skeletal lesions. Our study demonstrated that CCL2 stimulated tumor cells to produce a pro-angiogenic factor(s) instead of directly stimulating bone marrow endothelial cells (Figure 5). Conditioned media from CCL2-treated PC-3 cells significantly increased sprout formation in HBMEs while pre-treating PC-3 cells with anti-CCL2 antibody abolished this effect (Figure 5A and B). CCL2 itself failed to stimulate capillary tube formation of HBMEs when added directly (Supplementary Figure 3). CNTO 888 alone, the antibody to human CCL2, had no effect on capillary tube formation. CCL2 increased VEGF-A mRNA levels from PC-3 cells after 4h and 6h (Figure 5C). The stimulation of VEGF-A mRNA in PC-3 cells was specifically mediated by CCL2 since it could be blocked with pre-treatment of the neutralizing antibody to the tumor cells. The increase of VEGF-A protein in CCL2-stimulated PC-3 cells was confirmed by ELISA (Figure 5D) resulting in a 60% increase of VEGF-A after serum starved PC-3 cells were incubated with CCL2 for 24h.
Figure 5. CCL2 has pro-angiogenic effects on endothelial cells through elevated tumor-derived VEGF-A.
(A) Representative images of in vitro vascular sprouting assay of HBMEs in the presence of conditioned media from serum starved PC-3 cells treated with vehicle, CCL2 (50 ng/ml), anti-human CCL2 antibody (CNTO 888) (30 µg/ml) before CCL2, control antibody (anti-IgG) (30 µg/ml) before CCL2, CCL2 antibody and control antibody alone; (B) Conditioned media from CCL2-treated PC-3 cells increased sprout formation in HBMEs while pre-treating PC-3 cells with CNTO 888 abolished the effect; (C) Quantitative-RT-PCR for VEGF-A mRNA expression in serum starved PC-3 cells after 4h (open bars), 6h (hatched bars) and 24h (solid bars) incubation with or without CCL2 and its antibody. Data were standardized to GAPDH. (D) VEGF-A expression in 24h-serum-starved PC-3 cells after 6h and 24h incubation with vehicle (hatched bars) or CCL2 (solid bars). The open bar indicates the level of VEGF-A of PC-3 cells cultured with 10% FBS media.* p<0.01; ** p<0.001 vs. vehicle (Veh); values are mean±SEM; n=3.
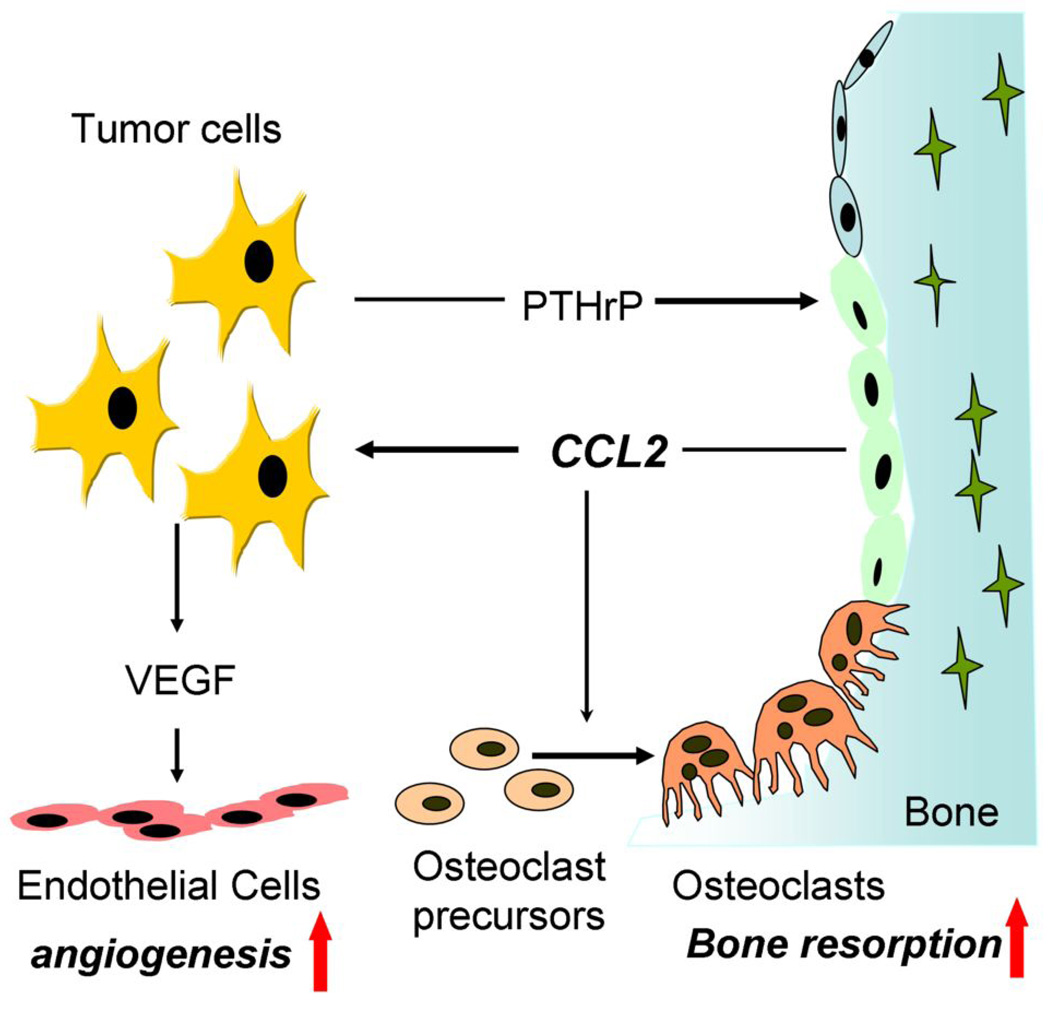
Taken together, our model proposes an explanation of how CCL2 facilitates prostate cancer growth in bone (Figure 6). PTHrP released from prostate cancer stimulates osteoblastic CCL2 expression (Figure 1 and Figure 2) which causes increased osteoclastic bone resorption (refs. 1, 2 and Figure 3) and in turn, facilitates prostate cancer tumor localization in bone (4, 5). Meanwhile, CCL2 binds to its receptor on prostate cancer cells and stimulates tumor cells to release the pro-angiogenic factor VEGF-A (Figure 5). VEGF-A enhances the formation of blood vessels to support further tumor growth (Figure 4 and Figure 5). Thus, CCL2 plays a key role in the destructive cascade which favors tumor growth via activation of tumor cells, bone resorption and angiogenesis.
Figure 6. The destructive cascade of prostate cancer derived PTHrP and its downstream action on CCL2 bone resorption and angiogenesis in skeletal lesions.
Prostate tumor cells release PTHrP, which stimulates CCL2 expression from osteoblasts. CCL2 causes increased osteoclastic bone resorption to facilitate prostate cancer tumor growth in bone. Meanwhile, CCL2 binds to its receptor on prostate tumor cells and stimulates the pro-angiogenic factor VEGF-A release from tumor cells. VEGF-A can further support tumor growth with enhanced neo-blood vessel formation. Thus, CCL2 plays a key role in the destructive cascade which favors tumor growth via activation of tumor cells, bone resorption and angiogenesis.
Discussion
Prostate cancer characteristically forms osteoblastic lesions when it metastasizes to bone. It is becoming clear that osteoclast activation is an essential component of prostate cancer skeletal metastases, although the mechanism, timing and relationship to the osteoblastic component remains unclear. Growth factors and calcium released from the bone matrix during resorption subsequently support tumor growth in bone. Here we present data that demonstrate the importance of the chemokine, CCL2, in developing prostate cancer metastatic bone lesions using a subcutaneous and an intratibial model of prostate cancer. Subcutaneous tumor implantation induced elevation of CCL2 in the bone marrow compartment prior to tumor metastasis in vivo. CCL2 is released from osteoblasts and bone marrow endothelial cells endogenously, and is further induced by tumor cell-derived factors. In osteoblasts, CCL2 was stimulated in response to PTHrP. Metastatic prostate carcinoma in bone secretes high levels of PTHrP that are important in the establishment of prostate cancer in the bone microenvironment (13–16). Tumor-derived PTHrP is known to upregulate receptor activator of NF kappaB ligand (RANKL), an essential factor in osteoclastogenesis. Furthermore, CCL2 has been shown to directly stimulate the proliferation of prostate cancer cells and favors osteoclastogenesis by facilitating the fusion of osteoclast precursor cells (1–4). It has been shown that PTH regulates CCL2 expression in bone through osteoblasts (2). PTHrP which acts on the same receptor (PTHR1) as parathyroid hormone was found here to regulate osteoblastic expression of CCL2. The stimulation of CCL2 by PTHrP in osteoblasts and the high level of CCL2 secreted by bone marrow endothelial cells could activate osteoclasts and enhance bone resorption to favor tumor cell localization.
It was reported that CCL2 released from endothelial cells and osteoblasts mediates prostate cancer induced bone resorption (17). An in vitro system used in that study showed that PC-3 prostate cancer cell conditioned medium induced bone resorption in human bone marrow mononuclear cells (HBMC), and bone resorption was inhibited with CCL2 neutralizing antibodies. The present study applied in vivo experiments which targeted CCL2 with a neutralizing antibody and elucidated mechanisms for the CCL2 impact on tumorigenesis. Significantly decreased bone resorption and tumor burden were found with CCL2 neutralizing antibody treatments. PC-3 cells are known to form highly lytic lesions compared to VCaP cells which form a mixture of osteolytic and osteoblastic lesions. It is interesting that the anti-CCL2 neutralizing antibody partially reduced PC-3 tumor growth and TRAP-positive osteoclast activity and almost completely inhibited osteoclast activation and establishment of VCaP tumors. This may suggest that osteolysis is an important early event in the establishment of prostate cancer in the bone compartment. The purely lytic PC-3 cells may retain the ability to establish bone lesions with anti-CCL2 challenge while anti-CCL2 completely inhibited the ability of VCaP cells to establish bone tumors. The decreased tumor growth was accompanied by a decrease in TRAP5b serum levels supporting the inhibition of osteoclast activity by inhibiting CCL2.
In addition, systemic inhibition of CCL2 with a neutralizing antibody was found to inhibit the growth and establishment of prostate cancer cells in the bone microenvironment in association with suppression of angiogenesis. Whether CCL2 directly stimulates angiogenesis in endothelial cells is controversial (6, 18–21). Different tissue-derived endothelial cells may respond distinctively to CCL2. It has been reported that human umbilical endothelial cells (HUVEC) and HDMVEC do not expression receptors for CCL2 nor do they respond to CCL2 (8). However, another study reported that HUVECs express receptors for CCL2 and respond to CCL2 (6). We tested several endothelial cells including HBMEs, HAECs and HDMVECs and did not find chemokine receptor 2 (CCR2), the primary receptor for CCL2 (data not shown), nor did they respond to CCL2. So it is unlikely that CCL2 exerts direct endotheliotropic effects in these cells. Furthermore, we did not see any effect of CCL2 alone on angiogenesis in HBMEs. However, the absence of evidence to support direct effects of CCL2 on endothelial cells does not diminish the important role of CCL2 in angiogenesis during tumor growth. CCL2 was found to stimulate the expression of VEGF-A in tumor cells in vitro and conditioned media from tumor cells treated with CCL2 potently induced angiogenesis of HBMEs. The stimulation of VEGF-A expression by CCL2 has been reported in several cell types including endothelial cells (22) and vascular smooth muscle cells (23). Animals with intratibial tumor cell inoculation and treated with neutralizing antibody for CCL2 had decreased blood vessel density in bone lesions. Consistent with these findings, the correlation of CCL2 expression with microvessel density and tumor invasion has been demonstrated in breast carcinomas (24), head and neck squamous cell carcinoma (25) and gastric carcinomas (26).
Besides stimulating VEGF-A release from tumor cells to facilitate angiogenesis, CCL2 may act as an indirect inflammation-associated inducer of angiogenesis through activation of macrophage infiltration (5, 26–30). In addition, CCL2 could stimulate angiogenesis through enhanced osteoclastogenesis which results in release of bone matrix bound angiogenic factors, such as PDGF, FGF-1, FGF-2, and TGF-β (31). Both mechanisms could contribute to the neo-blood vessel formation during the development of bone lesions from prostate carcinomas. Taken together with the fact that CCL2 can also contribute to tumor progression through direct activation of cell adhesion and motility in prostate cancer cells (32), targeting CCL2 provides a promising therapeutic strategy for prostate cancer skeletal metastasis.
Supplementary Material
Acknowledgements
We thank Amy Koh for critical reading of the manuscript and Chris Strayhorn (University of Michigan, School of Dentistry Histology Core Facility) for technical assistance in processing histological sections.
This work was supported by the National Cancer Institute award P01-CA093900.
Abbreviations
- MCP-1/CCL2
monocyte chemoattractant protein 1
- HUVECs
human umbilical cord vein endothelial cells
- HAECs
human aortic endothelial cells
- HBME
human bone marrow endothelial
- PTHrP
parathyroid hormone related protein
- MVD
microvessel density
- CCR2
C-C chemokine receptor-2
- VEGF
vascular endothelial growth factor
References
- 1.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-κB ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J Biol Chem. 2007;282:33098–33106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 4.Loberg RD, Day LL, Harwood J, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 7.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247:38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 9.Korenchuk S, Lehr JE, L MC, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163–168. [PubMed] [Google Scholar]
- 10.Chen HL, Demiralp B, Schneider A, et al. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- 11.Loberg RD, St John LN, Day LL, Neeley CK, Pienta KJ. Development of the VCaP androgen-independent model of prostate cancer. Urol Oncol. 2006;24:161–168. doi: 10.1016/j.urolonc.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKee RL, Goldman ME, Caulfield MP, et al. The 7–34-fragment of human hypercalcemia factor is a partial agonist/antagonist for parathyroid hormone-stimulated cAMP production. Endocrinology. 1988;122:3008–3010. doi: 10.1210/endo-122-6-3008. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty KM, Blomme EA, Koh AJ, et al. Parathyroid hormone-related protein as a growth regulator of prostate carcinoma. Cancer Res. 1999;59:6015–6022. [PubMed] [Google Scholar]
- 14.Keller ET, Zhang J, Cooper CR, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333–349. doi: 10.1023/a:1015599831232. [DOI] [PubMed] [Google Scholar]
- 15.Blomme EA, Dougherty KM, Pienta KJ, Capen CC, Rosol TJ, McCauley LK. Skeletal metastasis of prostate adenocarcinoma in rats: morphometric analysis and role of parathyroid hormone-related protein. Prostate. 1999;39:187–197. doi: 10.1002/(sici)1097-0045(19990515)39:3<187::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Liao J, McCauley LK. Skeletal metastasis: Established and emerging roles of parathyroid hormone related protein (PTHrP) Cancer Metastasis Rev. 2006;25:559–571. doi: 10.1007/s10555-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Cai Z, Xiao G, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 18.Stamatovic SM, Keep RF, Mostarica-Stojkovic M, Andjelkovic AV. CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. J Immunol. 2006;177:2651–2661. doi: 10.4049/jimmunol.177.4.2651. [DOI] [PubMed] [Google Scholar]
- 19.Dzenko KA, Song L, Ge S, Kuziel WA, Pachter JS. CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2. Microvasc Res. 2005;70:53–64. doi: 10.1016/j.mvr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 21.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:220–224. doi: 10.1002/ijc.10705. [DOI] [PubMed] [Google Scholar]
- 22.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 23.Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1978–H1984. doi: 10.1152/ajpheart.00414.2003. [DOI] [PubMed] [Google Scholar]
- 24.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- 25.Strauss L, Volland D, Guerrero A, Reichert T. Antiangiogenic and anti-immunosuppressive therapeutic strategies in human head and neck squamous cell carcinoma (HNSCC) Mund Kiefer Gesichtschir. 2005;9:273–281. doi: 10.1007/s10006-005-0635-3. [DOI] [PubMed] [Google Scholar]
- 26.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–778. [PubMed] [Google Scholar]
- 27.Zheng M, Sun G, Cai S, Mueller R, Mrowietz U. Significant reduction of T-cell chemotaxis to MCP-1 in patients with primary and metastatic melanoma. Chin Med J (Engl) 1999;112:493–496. [PubMed] [Google Scholar]
- 28.Wong MP, Cheung KN, Yuen ST, et al. Monocyte chemoattractant protein-1 (MCP-1) expression in primary lymphoepithelioma-like carcinomas (LELCs) of the lung. J Pathol. 1998;186:372–377. doi: 10.1002/(SICI)1096-9896(199812)186:4<372::AID-PATH204>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Sica A, Saccani A, Bottazzi B, et al. Defective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinoma. J Immunol. 2000;164:733–738. doi: 10.4049/jimmunol.164.2.733. [DOI] [PubMed] [Google Scholar]
- 30.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–527. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 31.Cackowski FC, Roodman GD. Perspective on the osteoclast: an angiogenic cell? Ann N Y Acad Sci. 2007;1117:12–25. doi: 10.1196/annals.1402.073. [DOI] [PubMed] [Google Scholar]
- 32.Loberg RD, Tantivejkul K, Craig M, Neeley CK, Pienta KJ. PAR1-mediated RhoA activation facilitates CCL2-induced chemotaxis in PC-3 cells. J Cell Biochem. 2007;101:1292–1300. doi: 10.1002/jcb.21252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.