Abstract
Enhanced delivery and expression of genes in specific neuronal systems is critical for the development of genetic models of neurodegenerative disease and potential gene therapy. Recent discovery of new recombinant adeno-associated viral (rAAV) capsid serotypes has resulted in improved transduction efficiency, but expression levels, spread of transgene, and potential toxicity can differ depending on brain region and among species. We compared the transduction efficiency of titer-matched rAAV 2/1, 2/5 and 2/8 to the commonly used rAAV2/2 in the rat nigrostriatal system via expression of the reporter transgene, enhanced green fluorescent protein (EGFP). Newer rAAV serotypes 2/1, 2/5 and 2/8 demonstrated marked increase in transduction and spread of EGFP expression in dopaminergic nigrostriatal neurons and projections to the striatum and globus pallidus compared to rAAV2/2 at 2 weeks post injection. The number of nigral cells transduced was greatest for rAAV2/1, but for serotypes 2/5 and 2/8 was still 2 to 3-fold higher than that for 2/2. Enhanced transduction did not cause an increase in glial cell response or toxicity. New rAAV serotypes thus promise improved gene delivery to nigrostriatal system with the potential for better models and therapeutics for Parkinson disease and other neurodegenerative disorders.
Keywords: adeno-associated virus, transduction, substantia nigra, striatum, gene therapy, Parkinson disease
Introduction
Parkinson disease (PD) is the most common neurodegenerative movement disorder and affects nearly 1% of the population over 60 years (Lee & Trojanowski 2006). Despite several treatment options, continued disease progression causes these to become less effective and fraught with complications. Experimental models of parkinsonism are critical for elucidating mechanisms of disease and developing new therapeutics. Several PD models exist and employ toxins (6-OHDA, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine [MPTP]) (Jenner et al. 1984, Kirik et al. 1998), pesticides (Hoglinger et al. 2006), or herbicides (Thiruchelvam et al. 2000). Chemical models are efficient and result in relatively specific dopaminergic toxicity and behavioral deficits that recapitulate features of PD (Ulusoy et al. 2008). The recent discovery of several genes associated with familial parkinsonism has renewed interest in molecular causes of PD and has led to the development of genetic models. These models are key to understanding the molecular and genetic causes of parkinsonism, as well as for discovery of potential gene therapies. Transgenic mouse models have been extremely useful, but are limited both in targeting of affected neural systems and control of transgene expression. In contrast, viral vector-mediated gene delivery has several advantages, including use in multiple species, more control over transgene expression (in particular, targeting the adult brain), expression in discrete brain regions, and increased transgene expression (Ulusoy et al. 2008). Increasing the efficiency of viral-vector mediated gene transfer and expression is a major goal for the development of genetic models of neurodegenerative disorders, such as Parkinson disease, and the delivery of potential gene therapeutics.
Both recombinant adeno-associated virus (rAAV) and lentivirus have been used to express transgenes within specific target systems, such as the nigrostriatal circuit in Parkinson disease models (Kirik et al. 2002, Klein et al. 2002, Lo Bianco et al. 2002). However, in the nigrostriatal system, viral-mediated expression has had varied results with differences in tropism, time to expression, and neural toxicity (Davidson et al. 2000, Klein et al. 2006, St Martin et al. 2007, Lo Bianco et al. 2002). Recombinant AAV serotype 2 has most commonly been used to transduce mammalian brain, but transduction volume is typically limited and high titers are required to achieve effective expression levels. Increasing evidence suggests that cross-packaging, or pseudotyping, rAAV2 with capsids from different AAV serotypes results in increased neural tropism and spread of the viral transgene (Burger et al. 2004, Paterna et al. 2004, Broekman et al. 2006). At least 10 new AAV serotypes have been cloned and used for packaging of rAAV2 (Rabinowitz et al. 2002, Gao et al. 2005). Of these, AAV1, 5, 7, and 8 have excellent neural tropism and increased expression and spread of the viral transgene, when compared to AAV2 (Broekman et al. 2006, Burger et al. 2004, Klein et al. 2006, Taymans et al. 2007). However, transduction efficiency varies between different brain regions, such as the hippocampus and substantia nigra, and among species. Another issue is potential toxicity and the immune response associated with higher levels of rAAV transduction and gene expression (Klein et al. 2006). Gliosis and microglial response to striatal injection of newer rAAV serotypes is reported, but few have characterized the response in the substantia nigra (Reimsnider et al. 2007).
In this study we compare the transduction efficiency of rAAV serotypes 2/1, 2/2, 2/5, and 2/8 expressing the enhanced green fluorescent protein (EGFP) in the nigrostriatal system of the adult rat brain. In Parkinson disease models, dopaminergic neurons within the substantia nigra are the primary target. We thus specifically examined tropism for tyrosine-hydroxylase immunoreactive neurons and expression levels within nigrostriatal terminals. We also compared the glial response among different rAAV serotypes injected into the adult rat substantia nigra. Our findings provide evidence that newer rAAV serotypes 2/1, 2/5 and 2/8 enhance gene delivery to the nigrostriatal system without increasing inflammatory, glial response.
Materials & Methods
Virus production
Pseudotyping was used to produce rAAV vectors packaged with AAV 1, 2, 5, or 8 plasmid capsid proteins (Howard et al. 2007, Hildinger et al. 2001). Recombinant AAV vectors were constructed with a transgene cassette encoding the CMV promoter and enhanced green fluorescent protein, CMV-EGFP, flanked by human β-globin sequences containing a polyA site and inserted between AAV2 inverted terminal repeats (ITRs). AAV were generated via tripartite transfection of the cis-transgene, packaging (encoding rep and cap genes), and helper plasmids into HEK 293A cells. Viral particles were purified by heparin column chromatography for serotype 2, or iodixanol density gradient for serotypes 1, 5, and 8. Concentrated virus was isolated and titered by dot blot hybridization, yielding 6.2×1011 to 1.0×1013 gc/mL (virus kindly provided by the Harvard Gene Therapy Initiative, Harvard Medical School.
Stereotaxic viral injections
All protocols and procedures were approved by the MGH Subcommittee on Research Animal Care. The nigrostriatal system is essentially unilateral, thus each animal received bilateral stereotaxic injections of rAAV into the substantia nigra (SN) for direct comparison of different AAV serotypes (2/1, 2/2, 2/5, and 2/8). Three to four animals were injected for each condition. Sprague Dawley rats (300–350 g) were anesthetized with ketamine HCl/xylazine, placed in a Kopf stereotax, and after midline skin incision, bilateral small skull holes were drilled to expose the dura over the injection sites. Coordinates for SN injections from bregma were AP −5.2, ML ±2.0, and DV −7.4 from the dural surface and targeted the central SN pars compacta. Viral titers for each rAAV subtype were matched by common dilution in 0.1M phosphate buffered saline (pH 7.4) to 6.2×1011 gc/mL. Equal viral particles, 6.2×108 gc in 1 µL, of each rAAV serotype (2/1, 2/2, 2/5, or 2/8) were injected at 0.2 µL/min using a microinjection pump (Stoelting Co., Wood Dale, IL) with 10 µL Hamilton syringe with 33-gauge needle. After injection the syringe remained in situ for 5 min before withdrawal.
Tissue preparation and immunohistochemistry
After 2 weeks, rats were deeply anesthetized with ketamine HCl/xylazine and transcardially perfused with cold 0.1M phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in PBS. Brains were postfixed for 24 hours, then cryoprotected in 30% sucrose/PBS, and serially sectioned at 40 µm on a sliding microtome into 12 wells (forebrain and midbrain were separately blocked). Sections were collected and stored in cryoprotectant, 30% sucrose and 30% ethylene glycol in PBS, until processed and analyzed. Briefly, free-floating sections were rinsed with PBS, then treated 3–5 min. with 10% methanol and 3% H2O2 to inhibit endogenous peroxidases, permeablized with 0.3% Triton X-100 in PBS, and blocked in 5% normal goat serum. To assess rAAV transduction efficiency, coronal sections through the striatum and nigra were immunostained with primary antibodies to GFP (rabbit, 1:3000 dilution; Abcam, Cambridge, MA) and tyrosine hydroxylase (TH, mouse; 1:1000 dilution; Sigma-Aldrich, St. Louis, MO) overnight at 4 °C. After washing, immunostaining was visualized with fluorescent secondary (1:200 dilution; Alexa Fluor 488, Molecular Probes, Eugene, OR; or Cy3, Jackson ImmunoResearch, West Grove, PA). Previous studies have shown that GFP-immunostaining is more sensitive in detecting early AAV-mediated GFP expression (Reimsnider et al. 2007). Indeed, as expected immunodetection of GFP in midbrain cells and nigrostriatal projections was more sensitive than direct observation of EGFP-immunofluorescence (data not shown). To identify neuronal and glial cell reaction, some midbrain sections were immunostained with NeuN (1:1000 dilution; Millipore, Billerica, MA), glial acidic fibrillary protein (1: 750 dilution; GFAP, Sigma-Aldrich), or the microglial marker, Iba1 (1:1000 dilution; Wako, Richmond, VA) and again visualized with fluorescent secondary (Alexa Fluor 350, Molecular Probes; or Cy3). All immunostained sections were washed, mounted on Superfrost slides, and then coverslipped with GVA mount (Zymed Laboratories, Inc., San Francisco, CA).
Stereology and Microscopy
GFP, TH, and glial-cell immunostaining were observed using an Olympus BX51 microscope with epifluorescence attachment. Photomicrographs were taken with an Olympus DP70 digital camera and adjusted only for suitable contrast and brightness. For wide-field striatal images, photomontages were made from multiple 4x-objective images using Adobe Photoshop CS2. The same exposure settings and adjustments were used for comparison between all rAAVs.
Transduction efficiency of rAAV serotypes within the nigrostriatal system was assessed by counting GFP and TH-immunoreactive cells in the SN pars compacta, including the adjacent pars lateralis and caudal dense cell group ventral to the medial lemniscus and retrorubral area, using unbiased stereology according to the optical fractionator principle (West et al. 1991). At least 8 sections though the SN for each case were analyzed and counted using the Olympus CAST Stereology System. A coefficient of error less than 0.1 was allowed.
Nigrostriatal GFP
Semi-quantitative analysis of GFP density in nigrostriatal terminals for each rAAV serotype was performed via optical density measurement of immunofluorescence intensity scans using a Scan Array Express (Perkin Elmer, Boston, MA). All sections for analysis were handled, washed, and immunostained at the same time, using common reagents to minimize interanimal variability. Striatal sections approximately 1 mm rostral to the decussation of the anterior commissure were immunostained with antibody to GFP and fluorescent secondary, Alexa Fluor 488 (Molecular Probes), and scanned using 488-laser excitation. Using ImageJ (NIH), mean gray value of fluorescence intensity within the dorsolateral striatum was obtained for each section and normalized to background, corpus callosum.
Statistical analysis
All data were expressed as group mean ± SEM. Stereological estimates of GFP and TH-immunoreactive cells in the SN and mean striatal terminal density measurements were analyzed using one-way ANOVA with Bonferonni/Dunn post-hoc (StatView 5.01, SAS Institute Inc, Cary, NC) unless otherwise stated.
Results
Transduction pattern after injection of different rAAV serotypes into the substantia nigra (SN)
To compare the transduction efficiency of rAAV serotypes within the nigrostriatal system, we performed stereotaxic injections of titer-matched rAAV-CMV-EGFP vectors (6.2×1011 gc/mL), packaged and pseudotyped serotypes 2/1, 2/2, 2/5, and 2/8, into the rat dorsal SN, targeting the pars compacta (pc). At 2 weeks post injection, direct observation of EGFP fluorescence within cell bodies was limited to the SN, and few transduced fibers and nigrostriatal terminals could be seen. To enhance detection of viral EGFP expression, sections were immunostained for GFP. Staining revealed numerous transduced cells surrounding the nigral injection site in all cases, as well as extensive GFP in processes and terminals within the globus pallidus and striatum.
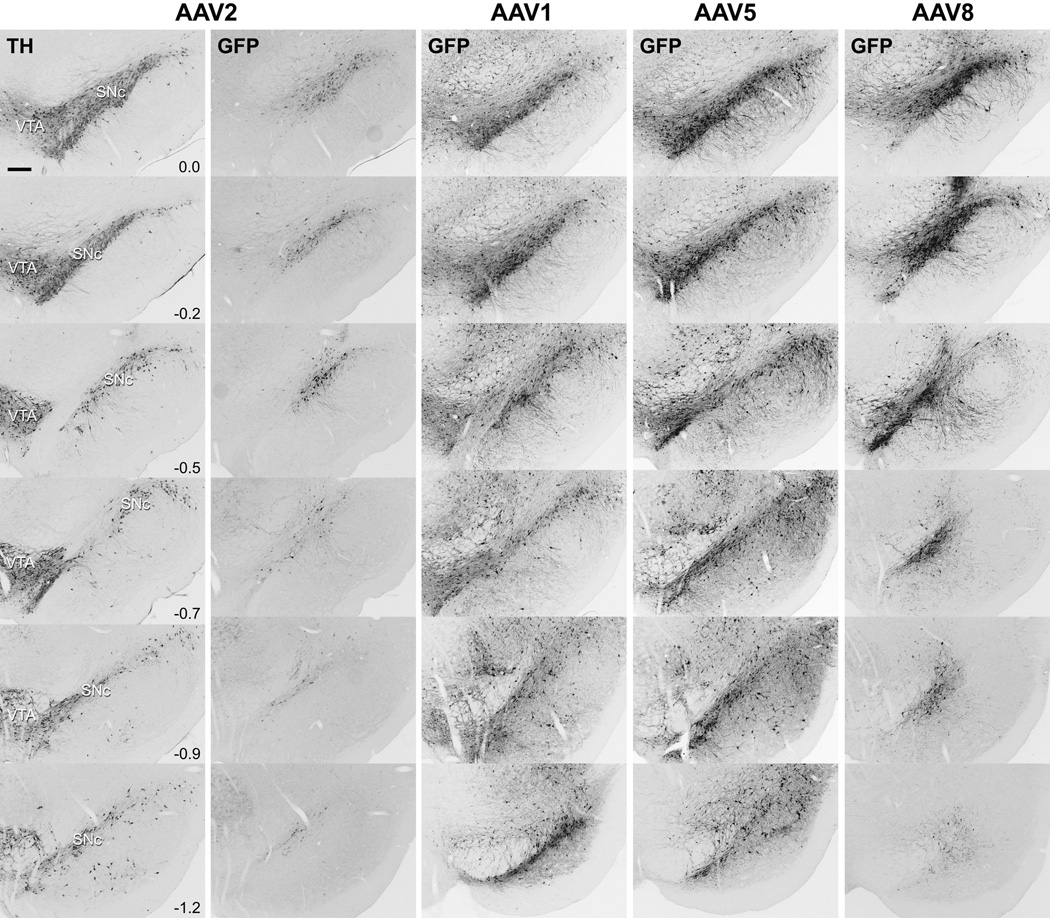
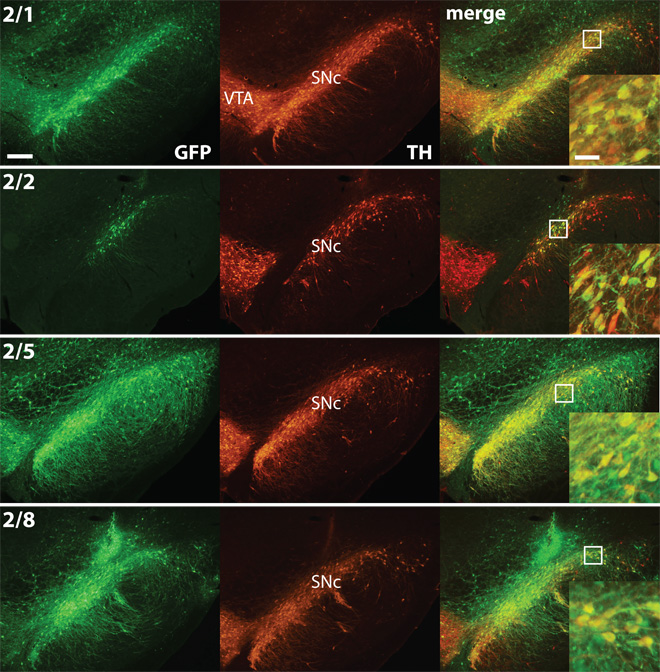
The distribution and extent of transduced cells (GFP-positive) within the SN for each pseudotyped rAAV is shown in Figure 1. Compared to rAAV2/2, injection of rAAV serotypes 2/1, 2/5 and 2/8 resulted in significantly increased volume of SN transduced. The extent of GFP-positive cells within the nigra for rAAV2/2 was limited largely to the injection site in SNpc. Few cells were transduced outside the pars compacta and GFP-positive projections did not extend significantly into the pars reticulata. Furthermore, overall EGFP expression with rAAV2/2 per cell was not as robust. In contrast, rAAV 2/1, 2/5, and 2/8 injection resulted in widespread, intense EGFP expression throughout the SN, including GFP-positive cells in the reticulata and adjacent ventral tegmental area (VTA). For rAAV 2/1 and 2/5 the entire rostral-caudal extent of SN contained GFP+ cells. Although the area transduced for rAAV2/8 was not quite as extensive as that for rAAV 2/1 and 2/5, the level of EGFP expression within the nigra appeared similar and included intense staining of cell bodies and projections.
Figure 1.
Comparison of EGFP expression and spread and expression after rAAV injection in the SN. Serial coronal sections through the SNc, immunostained with TH and GFP (grayscale photos inverted), show widespread, rostral-caudal infection of the SN for newer rAAV serotypes 2/1, 2/5, and 2/8 compared to that for rAAV2/2. Nigral sections for TH are at approximately the same level as GFP sections. Numbers represent distance (mm) between sections. (Bar = 250 µm)
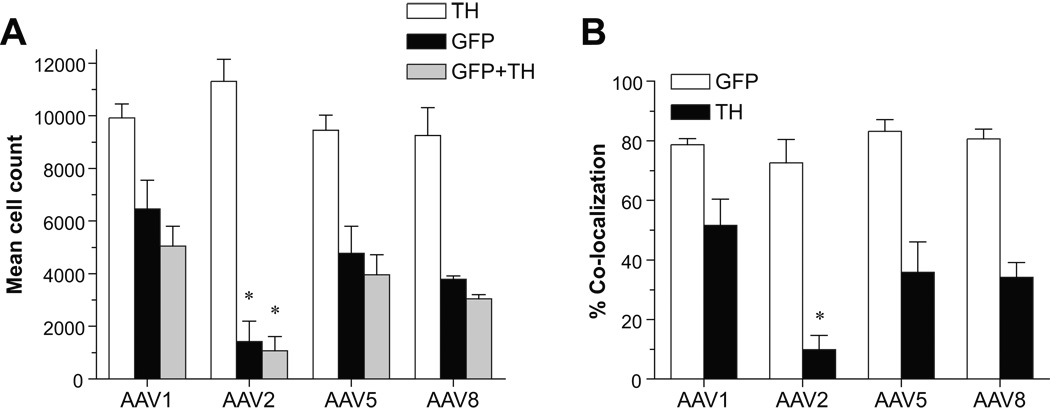
To accurately compare numbers of cells transduced in the SN by different rAAV serotypes, we performed unbiased stereological estimates of GFP-positive cells within the SNpc and adjacent pars lateralis, as identified by tyrosine hydroxylase (TH) immunostaining (Figure 2A). We compared the GFP cell counts among the different serotypes using ANOVA/Bonferroni multiple comparison test. Injection of rAAV2/1 resulted in the greatest number of GFP-positive cells (mean 6467 ± 1085.5 SEM) and was significantly increased (p < 0.05) compared to that of rAAV2/2 (1427 ± 767.8). Although mean GFP-positive cell counts within the SN for rAAV 2/5 (4773 ± 1030.3) and 2/8 (3787 ± 127.2) were lower than that for rAAV2/1, they were still approximately 3-fold higher than that of rAAV2/2, yet did not achieve significance.
Figure 2.
Comparison of viral transduction efficiency among rAAV serotypes. A. Graph shows stereological estimates of nigral TH-positive cells versus GFP and GFP+TH co-labeled cells within the SNpc. Mean values (n = 3–4) are reported and error bars represent SEM. Post-hoc analyses demonstrated significant differences between rAAV2/1 and rAAV2/2 for GFP (F[3,8] = 6.23 p = 0.017) and GFP+TH (F[3,9] = 7.85, p = 0.009) co-labeled cells in the SN (*p < 0.05). B. The graph illustrates the percent (mean ± SEM) of TH or GFP-positive cells within the SN that co-express TH and EGFP for each rAAV serotype. The proportion of GFP+ cells that also co-express TH (white bars) was comparable for all serotypes (~80%). In contrast, the proportion of TH-positive cells within the SN that express EGFP was markedly decreased for rAAV2/2 (*p < 0.05) compared to newer serotypes (F[3,8] = 5.74, p = 0.022).
To compare the transduction efficiency of each rAAV serotype in dopaminergic nigrostriatal neurons, we performed estimates of TH-positive cells that co-express EGFP within the SN (Figure 2A). All newer rAAV serotypes, particularly rAAV2/1, resulted in an increased number of co-labeled cells (GFP+TH) compared to rAAV2/2 (F[3,8] = 7.851, p = 0.009). In all cases a high proportion of GFP-positive cells, 72.5–83.9%, was also TH-immunoreactive (Figure 2B), indicating similar tropism for dopaminergic nigral neurons for each rAAV serotype. However, within the SN the proportion TH cells transduced—that were also GFP-positive—after rAAV 2/1, 2/5, and 2/8 injections was clearly greater than that for rAAV2/2 (F[3,8] = 5.74, p = 0.022). rAAV2/1 transduced the largest proportion of nigral TH cells (51.6% ± 8.8), which was significantly greater that that transduced by rAAV2/2 (9.8% ± 4.8) [p < 0.05]. The proportion of TH cells transduced by rAAV 2/5 (43.1% ± 10.1) and 2/8 (34.1% ± 5.1) was approximately 3.5–4 times that of rAAV2/2.
Transduction outside of the SN: comparison of GFP transport in nigral projections
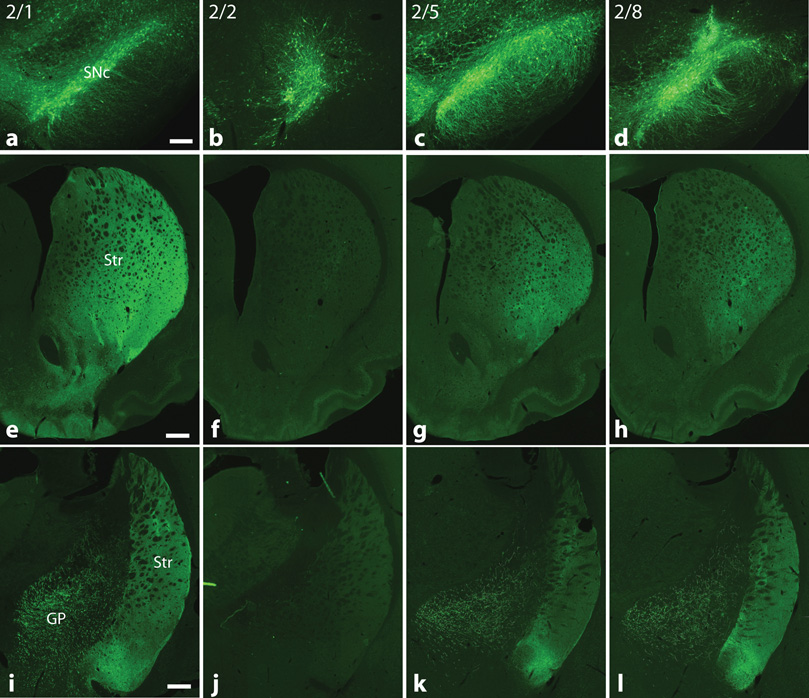
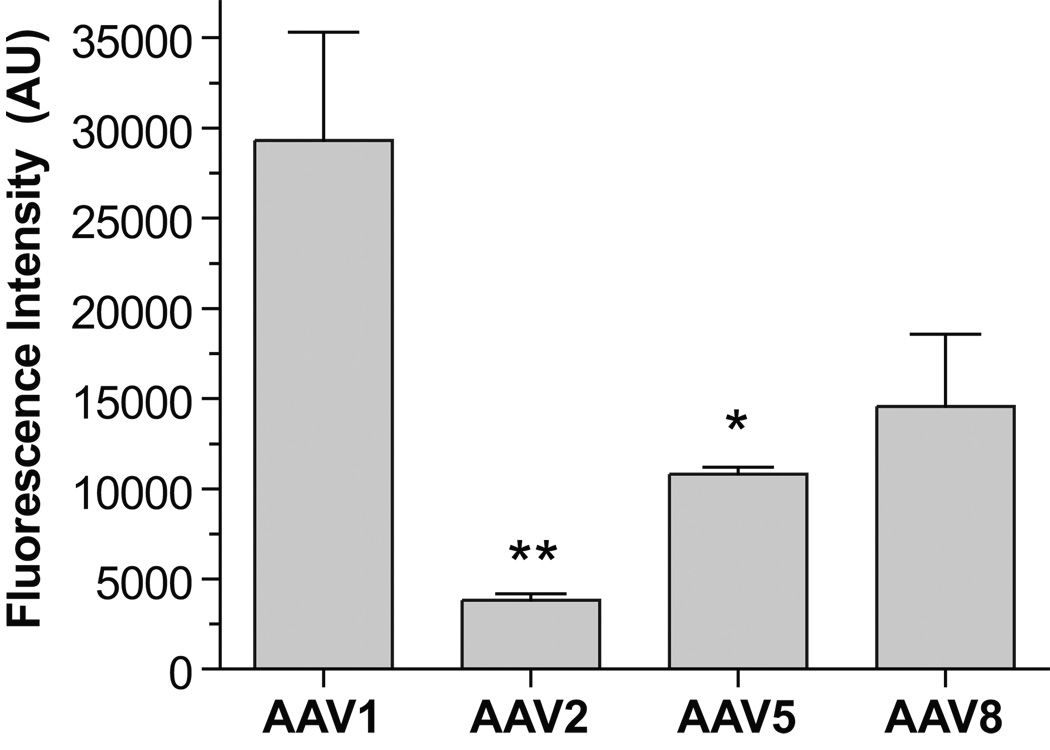
At 2 weeks post injection, only newer AAV serotypes 2/1, 2/5, and 2/8 resulted in widespread anterograde GFP-labeling of projection fibers to both the striatum and pallidum (Figure 3). GFP-positive terminal density at the level of the striatum, as measured by fluorescence intensity, was significantly higher for rAAV2/1 than rAAV serotypes 2/2 (p < 0.01) and 2/5 (p < 0.05) when analyzed by ANOVA (F[3,8] = 8.81, p = 0.007). However, there was no difference between rAAV2/1 and 2/8 (Figure 4). Both rAAV serotypes 2/5 and 2/8 resulted in a similar distribution of fiber labeling compared to rAAV2/1, but GFP-terminal densities were not significantly higher than that of rAAV2/2. However, for rAAV2/2 few GFP-positive fibers could be traced beyond the level of the midbrain. By contrast, cell labeling by retrograde transport of GFP was rarely seen outside the nigra and adjacent mesencephalic nuclei (some cells were likely transduced by leakage of virus along the injection track) for all rAAV serotypes.
Figure 3.
Comparison of rAAV-EGFP expression pattern in nigral projections to the striatum and globus pallidus. Comparable sections through the rostral and caudal striatum and globus pallidus, immunostained for GFP, show the extent of anterograde GFP labeling. SN injections with newer rAAV serotypes 2/1, 2/5, and 2/8 result in marked GFP labeling of nigrostriatal and pallidal projections, whereas few GFP-positive fibers are seen outside the SN for rAAV2/2. (a–d, Bar = 250 µm; e–l, Bar = 500 µm)
Figure 4.
Comparison of GFP-positive nigrostriatal terminal density in the dorsal striatum as measured by relative fluorescence intensity. Mean values and SEM are plotted for each rAAV serotype. Striatal fluorescence intensity for rAAV2/1 was significantly higher than that of rAAV 2/2 and 2/5, but not 2/8, *p < 0.05, ** p < 0.01 (F[3,8] = 8.81, p = 0.007).
Recombinant AAV serotype tropism and phenotype of transduced cells within the substantia nigra (SN)
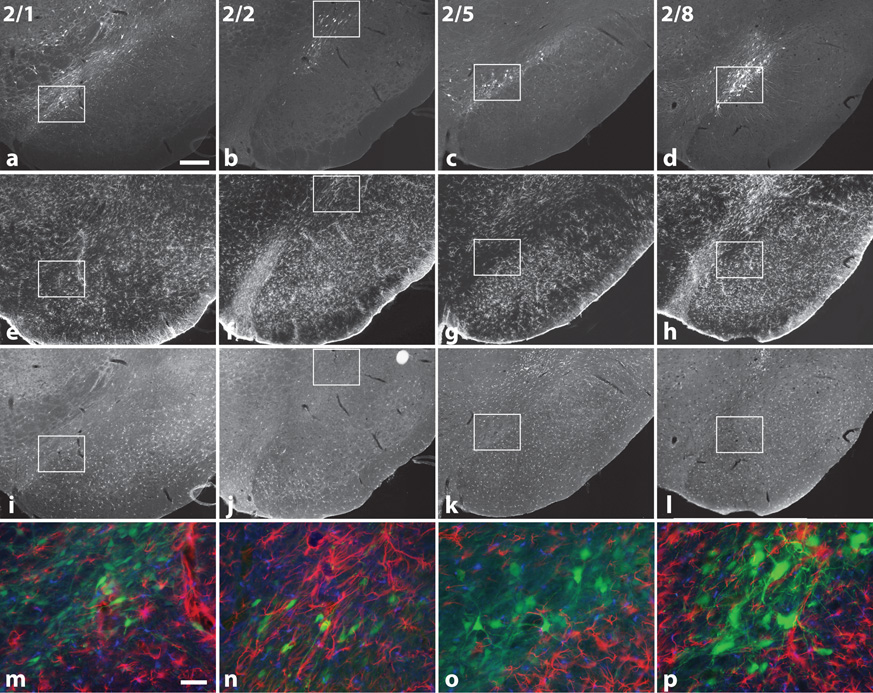
For all serotypes, the majority of GFP-positive cells in the SN were large, fusiform, had processes, and also expressed TH (72.5–83.9%), consistent with transduction of midbrain dopaminergic neurons (Figure 5). These cells also stained for the neuronal marker, NeuN (not shown). Co-staining for glial cell markers, GFAP (glial fibrillary acidic protein) and Iba1 (a microglial marker), did not co-localize with GFP-positive cells (see Figure 6).
Figure 5.
Newer rAAV serotypes 1, 5, and 8 show markedly increased transduction of TH cells within the SN compared to rAAV2/2. Photos are of coronal sections through the SN immunostained for GFP (Alexa Fluor 488) and TH (Cy3) and merged in Photoshop (bar = 250 µm). High power inset shows expression of EGFP in TH+ neurons (bar = 50 µm).
Figure 6.
Recombinant AAV serotypes cause minimal inflammatory reaction. Photos show immunostaining for GFP (a, b, c, d), astrocytes (GFAP-immunoreactivity, [e, f, g, h]), the microglial marker, Iba1 (i, j, k, l), and high power merged images (m, n, o, p). In merged image GFP is pseudocolored green, GFAP is red, and Iba1 is blue. GFP is not co-localized with either marker. (Bars = 250 µm and 50 µm for blowups)
Inflammatory response to rAAV injection of the SN
Glial reaction for each rAAV serotype was examined by GFAP-immunostaining for astrocytosis and Iba1 antibody for microglial activation (Figure 6). While there was gliosis along the injection tract, injury was minimal and gliosis at the nigral injection site minor. GFAP staining in the SN was similar for each rAAV serotype. Iba1-immunostaining revealed microglial activation in all cases within the SN, but qualitatively appeared similar to that seen throughout the midbrain, particularly at the periphery of each section. We observed no differences in microglial staining between rAAV serotypes.
Discussion
The properties of recombinant AAV make them highly suited for in vivo gene delivery, including development of genetic disease models and potential therapeutics (Rabinowitz & Samulski 1998, Burger et al. 2005, McCown 2005). For neurodegenerative disorders, such as Parkinson disease, viral studies have focused on efficient transduction of nigrostriatal neurons (Tenenbaum et al. 2004, Taymans et al. 2007, Paterna et al. 2004, Burger et al. 2004). To that end, newer rAAV serotypes are being explored for their potential to increase neural tropism, transgene expression, and spread over the standard rAAV2 (Gao et al. 2005, Wu et al. 2006). In this study, we compare the transduction efficiency of titer-matched rAAV serotypes 2/1, 2/2, 2/5, and 2/8 expressing EGFP in the rat nigrostriatal system at 2 weeks post injection. We demonstrate that newer serotypes 2/1, 2/5, and 2/8 transduce a much larger volume of the SN compared to rAAV2/2, including cells in the pars reticulata. Furthermore, stereological estimates of GFP and TH-positive cells in the SN suggest that newer rAAV serotypes transduce a greater number of dopaminergic nigrostriatal neurons. The number of TH cells transduced was significantly increased for rAAV2/1, and counts for serotypes 2/5 and 2/8 were 3-fold higher than that for 2/2, although not significant owing to the limited sample size. Newer rAAV serotypes also resulted in extensive anterograde transport of EGFP in nigral projections to both the striatum and globus pallidus. In contrast, EGFP expression after rAAV2/2 injection was highly restricted and did not extend much beyond the SN.
Our findings extend those of previous studies in both mice and rats and highlight differences among species and viral constructs. Taymans et al. (2007) examined rAAV expression of EGFP in adult mice and similarly found superior transduction efficiency for newer stereotypes over rAAV 2/2 in both the striatum and SN. They reported the relative efficiency of rAAV 2/7 and 2/5 as equal to, or better than that of rAAV2/8. In the rat rAAV2/1 and 2/5 also enhance nigral transduction compared to rAAV2/2 (Burger et al. 2004, Paterna et al. 2004). AAV serotype 8 likewise efficiently transduces neuronal tissue and has the highest level of transgene expression compared to rAAVs 1, 2, and 5 in hippocampal extracts (Klein et al. 2006). However, injection of relatively high-titer rAAV2/8-EGFP (1.0×1010 gc) into the rat SN results in neurotoxicity. These findings appear related to enhanced transgene expression by rAAV2/8, as no toxicity is seen for EGFP packaged in either rAAV2/5 or 2/1 and delivered at similar high-titers. At lower titers, though, it is unclear if there is an advantage of rAAV2/8 transduction in rat nigra over that of rAAV 2/1 or 2/5. In our study, we saw no apparent toxicity with rAAV2/8-EGFP, but the titer used was also relatively low (6.2×108 gc, to match titers), supporting a possible dose effect for toxicity (Howard et al. 2007).
Use of relatively low rAAV titer also reduced the likelihood of saturating expression levels, but resulted in proportionately lower numbers of transduced TH neurons in the SN compared to that reported in prior studies. In particular, rAAV2/2 transduced only about 10% of nigral TH neurons, but transduction efficiencies of up to 95% have been reported for AAV2, albeit using higher titers, a different viral construct and transgene (Kirik & Bjorklund 2003). We used the cytomegalovirus (CMV) promoter to drive EGFP expression, but increasingly AAV constructs are made with the hybrid CMV/chicken B-actin promoter (Niwa et al. 1991) combined with the 3’ woodchuck hepatitis post-transcriptional response element, which enhances transgene expression and stability (Donello et al. 1998). Long-term expression is primarily affected, and in the first 2 weeks these differences are unlikely to contribute to significant changes in transgene expression among rAAV serotypes. Furthermore, all of our rAAVs were based on the same vector construct.
In contrast to previous studies, rAAV2/1 yielded the widest spread and transduced the greatest number of dopaminergic nigral neurons compared to rAAV 2/5 and 2/8. Despite differences in expression, there was no difference in affinity for dopaminergic nigrostriatal neurons among the serotypes tested, including rAAV2/2 (nearly 80% cells transduced in SN were TH+). One possible explanation for our findings may be the early time-point (2 weeks) at which we observed EGFP expression. Previous studies have established that rAAV2/2 is optimally expressed at about 4 weeks post-infection (Klein et al. 2006). However, more recent data indicate a gradual increase in GFP expression and transduced neuronal number in the first 2 weeks for rAAV 2/5 and 2/8 infection, whereas for serotypes 2/1 and 2/2 GFP expression rapidly increases and reaches a steady-state by 7 days post-infection (Reimsnider et al. 2007). These findings suggest that early time points may not reflect stable levels of rAAV 2/5 and 2/8 transgene expression. However, detection of viral EGFP expression by GFP-immunostaining stabilizes by 2 weeks for rAAV 2/5 and 2/8 and is sensitive enough to detect transgene expression even as early as 4 days post infection (Reimsnider et al. 2007). Similarly, GFP-immunostaining enhances detection of EGFP by rAAV2/2 and reveals cellular expression at 2 weeks comparable to that seen by native GFP fluorescence at later time points. Thus, use of GFP-immunostaining in this study for detection of viral EGFP expression at 2 weeks accurately reflects the differences in transduction efficiency between the rAAV serotypes used. However, the early time point and lower relative levels of EGFP expression could explain the lack of retrogradely labeled GFP cells.
In addition to transduction efficiency, the differential immune response to rAAV serotypes is equally important in determining successful viral gene delivery and sustained expression. Although circulating antibodies to rAAV serotypes 1, 5, and 8 are rare (Erles et al. 1999), antibodies to rAAV2/2 have been shown to block rAAV2/2 transduction of the striatum (Burger et al. 2004). Moreover, in the rat intrastriatal injection of all rAAV serotypes by 1 week results in significant astrocytosis and microgliosis, which resolve over months (Reimsnider et al. 2007). A dose dependent response is hypothesized as lower titer striatal rAAV injections result in little to no inflammatory response. Indeed, to match rAAV titers we also injected relatively low viral copies into the SN and did not see marked astrocytosis or microgliosis at 2 weeks. Gliosis was prominent only adjacent to the injection tract, suggesting low inflammatory response despite widespread nigral transduction and high EGFP expression, especially for newer rAAV serotypes 1, 5, and 8. Later time points and higher rAAV titers were not assessed, but the pattern and time course of inflammatory reaction within the SN, in particular that associated with neurotoxicity (i.e. rAAV2/8), is clearly important for development of transgenic models and delivery of therapeutic genes and yet to be fully characterized.
In summary, we demonstrate an advantage to use of newer rAAV serotypes 2/1, 2/5, and 2/8 over 2/2 for delivery and expression of genes in the rat nigrostriatal system. Newer rAAV serotypes, in particular rAAV2/1, show increased tropism, transgene expression, and spread of the transgene product in the SN and projections to the striatum and globus pallidus. By contrast, rAAV2/2 had the most restricted expression that can be useful for targeting a small area. On the other hand, increased transduction efficiency may lead to toxicity given the vulnerability of nigral dopamine neurons and careful dose titration, in particular for rAAV2/8 is recommended (Klein et al. 2006). In all, new rAAV serotypes promise enhanced gene delivery to the nigrostriatal system and are critical to the development of better viral-vector based models of PD and other neurodegenerative disorders PD, as well as delivery of potential gene therapies.
Acknowledgements
This work was supported by NIH NS038372A-08. N.R.M. is supported by the American Parkinson Disease Association (APDA).
References
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther. 2005;16:781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J.Virol. 1998;72:5085. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J Med Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Oertel WH, Hirsch EC. The rotenone model of parkinsonism–the five years inspection. J Neural Transm Suppl. 2006:269–272. doi: 10.1007/978-3-211-45295-0_41. [DOI] [PubMed] [Google Scholar]
- Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2007 doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Rupniak NM, Rose S, Kelly E, Kilpatrick G, Lees A, Marsden CD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the common marmoset. Neurosci Lett. 1984;50:85–90. doi: 10.1016/0304-3940(84)90467-1. [DOI] [PubMed] [Google Scholar]
- Kirik D, Bjorklund A. Modeling CNS neurodegeneration by overexpression of disease-causing proteins using viral vectors. Trends Neurosci. 2003;26:386–392. doi: 10.1016/S0166-2236(03)00164-4. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum Gene Ther. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci U S A. 2002;99:10813. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2005;5:333–338. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Feldon J, Bueler H. Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol. 2004;78:6808–6817. doi: 10.1128/JVI.78.13.6808-6817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Samulski J. Adeno-associated virus expression systems for gene transfer. Curr Opin Biotechnol. 1998;9:470–475. doi: 10.1016/s0958-1669(98)80031-1. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- St Martin JL, Klucken J, Outeiro TF, et al. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J.Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6 Suppl 1:S212–222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res. 2000:873–225. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Bjorklund T, Hermening S, Kirik D. In vivo gene delivery for development of mammalian models for Parkinson's disease. Exp Neurol. 2008;209:89–100. doi: 10.1016/j.expneurol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]