Abstract
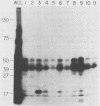
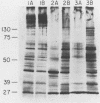
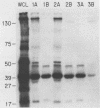
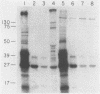
The presumed host defense against coagulase-negative staphylococci (ConS), recognized pathogens in hosts with compromised immunity or indwelling medical devices, is opsonophagocytosis. Targets for opsonization remain unclear. Using radiolabeling techniques, we identified the surface-exposed proteins of ConS and determined the innate humoral immune responses to them among healthy adults. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of surface proteins extrinsically labeled with 125I demonstrated 20 to 30 proteins with molecular weights of 15,000 to greater than 130,000. Five to ten of these proteins were immunogenic and recognized by normal human sera, including predominant 18-, 41-, 48-, and 51-kDa proteins. We also evaluated the humoral response of cancer patients with ConS bacteremia. Patients' sera obtained before bacteremic episodes demonstrated a pattern of reactivity similar to that of normal human sera. When patients' sera obtained after bacteremic episodes were used to determine whether an expanded immune response followed infection, only one of seven showed reactivity with more proteins than seen with the innate response. Western blot (immunoblot) analysis and whole-cell enzyme-linked immunosorbent assays were also evaluated. This study identifies (i) the surface-exposed proteins available for host interaction, (ii) the innate human antibody response to these proteins, and (iii) the immune response of cancer patients with ConS bacteremia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnie J. P., Lee W., Matthews R. C., Bayston R. Immunoblot fingerprinting of coagulase negative staphylococci. J Clin Pathol. 1988 Jan;41(1):103–107. doi: 10.1136/jcp.41.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnie J., Matthews R. C. Immunoblot analysis: a new method for fingerprinting hospital pathogens. J Immunol Methods. 1987 Jun 26;100(1-2):41–46. doi: 10.1016/0022-1759(87)90171-2. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. A., Easmon C. S. Opsonic requirements of Staphylococcus epidermidis. J Med Microbiol. 1986 Aug;22(1):1–7. doi: 10.1099/00222615-22-1-1. [DOI] [PubMed] [Google Scholar]
- Clink J., Pennington T. H. Staphylococcal whole-cell polypeptide analysis: evaluation as a taxonomic and typing tool. J Med Microbiol. 1987 Feb;23(1):41–44. doi: 10.1099/00222615-23-1-41. [DOI] [PubMed] [Google Scholar]
- Dickinson G. M., Bisno A. L. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob Agents Chemother. 1989 May;33(5):597–601. doi: 10.1128/aac.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E. Natural populations of the genus Staphylococcus. Annu Rev Microbiol. 1980;34:559–592. doi: 10.1146/annurev.mi.34.100180.003015. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Patrick C. C. Coagulase-negative staphylococci: pathogens with increasing clinical significance. J Pediatr. 1990 Apr;116(4):497–507. doi: 10.1016/s0022-3476(05)81593-8. [DOI] [PubMed] [Google Scholar]
- Patrick C. C., Kaplan S. L., Baker C. J., Parisi J. T., Mason E. O., Jr Persistent bacteremia due to coagulase-negative staphylococci in low birth weight neonates. Pediatrics. 1989 Dec;84(6):977–985. [PubMed] [Google Scholar]
- Patrick C. C., Plaunt M. R., Sweet S. M., Patrick G. S. Defining Staphylococcus epidermidis cell wall proteins. J Clin Microbiol. 1990 Dec;28(12):2757–2760. doi: 10.1128/jcm.28.12.2757-2760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson-Carter F. M., Pennington T. H. Characterization of coagulase-negative staphylococci by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analyses. J Clin Microbiol. 1989 Oct;27(10):2199–2203. doi: 10.1128/jcm.27.10.2199-2203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergeland H. I., Haaheim L. R., Natås O. B., Wesenberg F., Oeding P. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J Clin Microbiol. 1989 Jun;27(6):1286–1291. doi: 10.1128/jcm.27.6.1286-1291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bronswijk H., Verbrugh H. A., Heezius H. C., Renders N. H., Fleer A., van der Meulen J., Oe P. L., Verhoef J. Heterogeneity in opsonic requirements of Staphylococcus epidermidis: relative importance of surface hydrophobicity, capsules and slime. Immunology. 1989 May;67(1):81–86. [PMC free article] [PubMed] [Google Scholar]