Abstract
Our perception of the vitamin D system continues to evolve. Recent studies have re-evaluated the parameters for adequate vitamin D status in humans, revealing a high prevalence of insufficiency in many populations throughout the world. Other reports have highlighted the potential consequences of vitamin D insufficiency beyond established effects on bone homeostasis. Most notably, there is now strong evidence of a role for vitamin D in modulating innate and adaptive immunities, with insufficiency being linked to infectious disease and other immune disorders. To date, signaling pathways for these new responses to vitamin D have been based on established endocrine models for active 1,25-dihydroxyvitamin D, despite present evidence for more localized, intracrine modes of action. In the following review, we provide a fresh perspective on vitamin D signaling in non-classical target cells such as macrophages by highlighting novel factors associated with the transport and action of this pluripotent secosteroid.
Introduction
At the end of 2007, Time magazine listed the ‘Benefits of Vitamin D’ as one of its top ten ‘medical breakthroughs’ of the year. The reason for this they stated has been the recent remarkable increase in studies documenting new actions for vitamin D and the potential significance this may have for human health (http://www.time.com/time/specials/2007/top10/). A simple investigation using PubMed shows that since March 2003, there have been ~9500 entries for the keyword ‘vitamin D’, whereas ‘thyroid hormone’ generated almost 6800 entries over the same period of time. This contrasts with the preceding 5 years (1998–2003) where ‘vitamin D’ and ‘thyroid hormone’ were more or less equal at ~6300 entries each.
Two key concepts have fuelled the vitamin D renaissance. The first of these has been the complete change in our understanding of what constitutes normal vitamin D status. For many years, vitamin D deficiency in humans was defined by the serum concentration of 25-hydroxyvitamin D (25OHD, the main circulating form of vitamin D) associated with rickets (<8 ng/ml or 20 nM). However, in 1997 Chapuy et al. (1997) took a different approach by assessing the relationship between serum 25OHD and serum parathyroid hormone. They described an inverse correlation between these two factors at concentrations of 25OHD up to 78 nM.
This indicated that optimal vitamin D status was much higher than originally thought and prompted the introduction of a new term, ‘vitamin D insufficiency’, to describe individuals with serum 25OHD concentrations that are higher than ‘deficient’ (i.e. adequate to prevent rickets) but lower than optimal (Holick 2008). Although there is still a continuing discussion concerning the precise levels of 25OHD, which define these different categories of vitamin D status, there is broad agreement that vitamin D insufficiency is prevalent in populations across the globe (Holick 2007). This is particularly true for individuals with dark skin pigmentation or those living in Northern latitudes, where the capacity for u.v. light-induced epidermal synthesis of vitamin D is impaired.
The second seminal development in vitamin D research arose from the studies by Robert Modlin et al. aimed at defining the key genes involved in mediating innate immune responses to Mycobacterium tuberculosis (Liu et al. 2006). The DNA array analysis of macrophages activated with an M tuberculosis lipoprotein highlighted the induction of mRNA for both the vitamin D receptor (VDR) and 25-hydroxyvitamin D-1α-hydroxylase (CYP27b1), the enzyme that catalyzes the synthesis of active 1,25-dihydroxy-vitamin D (1,25(OH)2D) from 25OHD (Liu et al. 2006). As a consequence, the resulting macrophages demonstrated strong intracrine responses to 25OHD, most notably the induction of the antibacterial peptide cathelicidin (Liu et al. 2006). Subsequent studies have confirmed that this is a pivotal step in the innate immune response to infection by pathogens such as M tuberculosis (Liu et al. 2007b, Martineau et al. 2007). Crucially, ex vivo experiments showed that the induction of macrophage cathelicidin is significantly compromised when using serumfrom25OHD-insufficient donors (Liu et al. 2006), indicating that similar responses in vivo may also be dependent on vitamin D status.
The induction of macrophage function by locally synthesized 1,25(OH)2D has highlighted a link between vitamin D insufficiency and tuberculosis, as well as a potential role for vitamin D supplementation in preventing and/or treating this disease (Liu et al. 2007a). However, in view of the pluripotent nature of 1,25(OH)2D as an inducer of non-classical responses (Holick 2006, 2007, Johnson et al. 2006, Norman 2006, Spina et al. 2006, Adams & Hewison 2008) and the widespread expression of CYP27b1 in cells other than macrophages (Townsend et al. 2005, Hewison et al. 2007), it is likely that vitamin D insufficiency will have broad-ranging biological effects that extend beyond innate immunity. Thus, present work within the vitamin D field has expanded the analysis of the effects of vitamin D status on disease epidemiology and clinical outcomes (Holick 2007), while other studies have investigated novel targets for locally synthesized 1,25(OH)2D (Adams & Hewison 2008) or potential therapeutic uses for vitamin D metabolites (Trump et al. 2006).
Significantly, this new perspective on vitamin D physiology has also prompted a re-evaluation of the cellular and molecular mechanisms associated with this secosteroid. In particular, the broader acceptance of localized tissue-specific metabolism as a key component of non-classical vitamin D physiology suggests that established models for vitamin D signal transduction based on endocrine mechanisms are incomplete. For example, although there has been a remarkable increase in our understanding of how liganded VDR interacts with accessory proteins to facilitate chromatin remodeling and the recruitment of transcriptional machinery (Kato et al. 2004, Fujiki et al. 2005, Jurutka et al. 2007, Yamaoka et al. 2007), much less is known concerning the pathways that lead up to these nuclear events. In the following review, we have addressed this by providing a new perspective on vitamin D signal transduction, which goes beyond the already well-documented actions of CYP27b1 and VDR. The proposed model (see Fig. 1) has been designed specifically to illustrate novel factors that may be integrated to facilitate key intracrine effects of vitamin D, in particular the induction of antibacterial innate immune responses. Reflecting its title, the review also aims to illustrate the importance of comparative and evolutionary biology as a tool for better understanding the way in which vitamin D works. Specifically, the review focuses on how the analysis of the changes in vitamin D function, which have accompanied alterations in environmental exposure to u.v. light, has helped to identify new biological targets and modes of action for vitamin D.
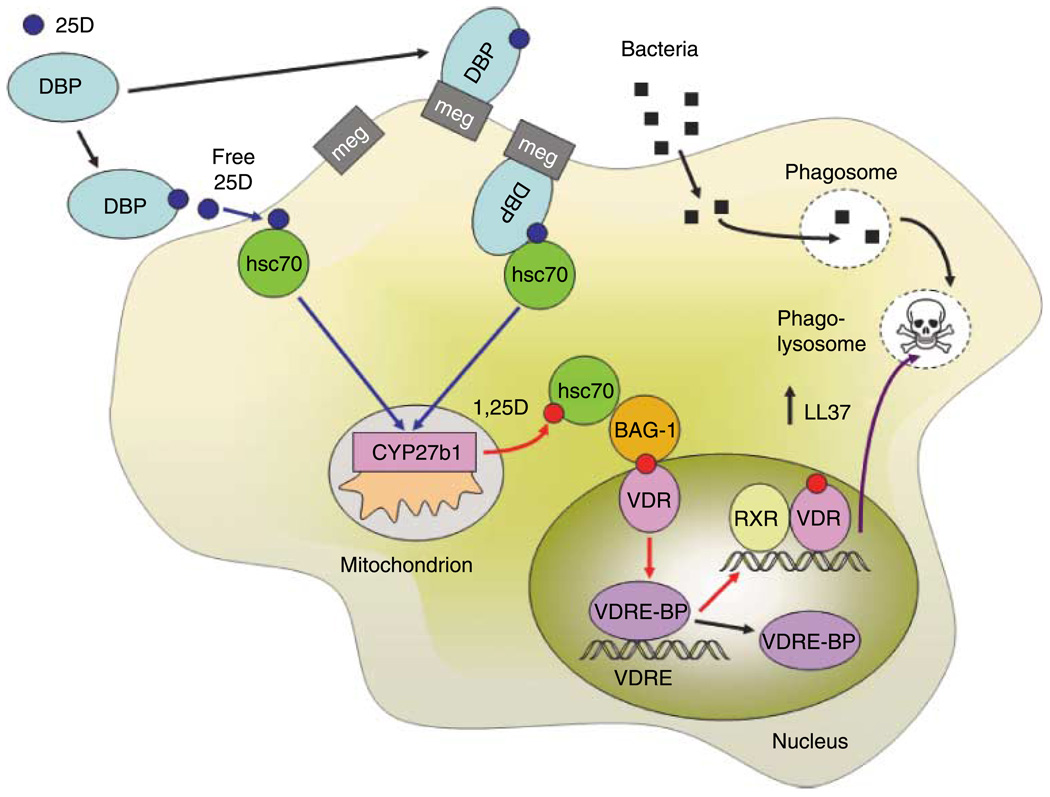
Figure 1.
Vitamin D signaling and the regulation of macrophage innate immunity. Proposed pathways for the extracellular entry, intracellular movement, mitochondrial metabolism, and nuclear response to vitamin D during macrophage innate immune responses. Serum 25-hydroxyvitamin D (25D) bound to vitamin D-binding protein (DBP) is internalized by macrophages either through passive diffusion of ‘free’ 25D or via megalin (meg)-mediated uptake. Intracellular 25D is then translocated to mitochondrial 25-hydroxyvitamin D-1α-hydroxylase (CYP27b1) in association with constitutive heat-shock protein 70 (hsc70). The resulting 1,25-dihydroxyvitamin D (1,25D) produced by CYP27b1 is then translocated to the nucleus in conjunction with hsc70 and Bcl-2-associated athanogene (BAG-1). Within the nucleus, 1,25D binds to the vitamin D receptor (VDR), which is then able to form a heterodimer with the retinoid X receptor (RXR). Interaction between the VDR-RXR dimer and vitamin D response elements (VDRE) in the promoters of target genes such as the antimicrobial protein cathelicidin (LL37) is controlled by the VDRE-binding protein (VDRE-BP). Intracrine 1,25D-mediated transcriptional regulation of LL37 in this fashion increases availability of the antimicrobial protein for killing of bacteria in phagosomes fused with lysosomes (phago-lysosomes).
Vitamin D-binding protein (DBP): passive carrier or active facilitator
In common with other steroid hormones, circulating vitamin D metabolites are bound to serum carrier proteins in the serum. Although this facet of vitamin D is well documented, relatively little is known about its impact on the tissue availability of vitamin D metabolites. Serum albumin and serum DBP are the principle carriers of vitamin D. The affinity of albumin for 25OHD (Ka=6×10−5 M) and 1,25(OH)2D (Ka=5.4×10−4 M) is substantially lower than that of DBP (7×10−8 M and 4×10−7 M respectively) (Bikle et al. 1985, 1986). The vast majority of vitamin D ligands are bound to DBP but, because of its relative abundance (650 µM compared with 5 µM for DBP), some vitamin D metabolites are also carried by albumin. Additionally, the vast majority of vitamin D carrier proteins are empty because of their comparatively high levels in relation to typical circulating levels of vitamin D metabolites (in humans 25OHDZ50 nM and 1,25(OH)2DZ0.1 nM). Despite the fact that almost all the available ligands are likely to be bound to DBP, it has generally been assumed that the relatively small free fraction of vitamin D metabolites is the fraction that enters target cells (White & Cooke 2000, Speeckaert et al. 2006). However, in some circumstances, binding to DBP may be a more active feature of vitamin D physiology. Nykjaer et al. (1999) showed that in the proximal tubules of the kidney megalin, a cell surface receptor binds and internalizes DBP via an endocytic pathway. Mice with the ablation of the megalin gene are unable to recover DBP in this fashion and thus lose vitamin D via the urine, with resulting rachitic bone disease (Nykjaer et al. 1999). Subsequent studies have further refined this mechanism to include cubilin (Nykjaer et al. 2001) and disabled-2 (Nagai et al. 2005) as facilitators of megalin action in the kidney.
Unlike the endocrine synthesis of 1,25(OH)2D in the kidney, autocrine responses to vitamin D appear to be highly dependent on the availability of substrate 25OHD. Thus, a key question concerns whether target cell delivery of 25OHD occurs as a consequence of receptor-mediated membrane transfer as is the case in the kidney or whether it simply involves passive transfer of the free metabolite. Two recent studies have supported both models. In the first of these, megalin-mediated uptake of DBP was described in T47D breast cancer cells (Rowling et al. 2006). Further studies confirmed that this mechanism was able to facilitate the uptake of 25OHD by these cells and subsequent intracrine activation to 1,25(OH)2D (Rowling et al. 2006). Although the general applicability of this mechanism for cell-specific acquisition of vitamin D metabolites has yet to be fully defined, it is interesting to note that the expression of megalin and cubilin has also been linked to intracrine synthesis of 1,25(OH)2D by bone-forming osteoblastic cells (van Driel et al. 2006, Atkins et al. 2007).
Normal vitamin D responses in DBP knockout (KO) mice
The second important re-evaluation of DBP function stems from the analysis of the in vivo effects of 1,25(OH)2D following DBP gene ablation in mice. DBP KO mice are generally healthy and able to reproduce like their wild-type counterparts despite demonstrating lower circulating levels of 25OHD and 1,25(OH)2D (Safadi et al. 1999). However, when placed on a vitamin D-deficient diet, DBP KO mice succumb to bone mineralization abnormalities much more rapidly than wild-type animals, endorsing a role for DBP in maintaining skeletal health through its effects on renal vitamin D homeostasis (White & Cooke 2000). What is less clear is whether DBP is also required for other, non-classical effects of vitamin D. This has been addressed, in part, through recent studies that have characterized the effects of DBP on responses to 1,25(OH)2D in vivo and in vitro. Zella et al. (2008) showed that although DBP KO mice had reduced the circulating levels of vitamin D metabolites, they exhibited intestinal tissue concentrations of 1,25(OH)2D, which were in fact higher than serum observed in wild-type mice. Moreover, when used as a cell culture supplement, serum from DBP KO mice supported more sensitive in vitro responses to 1,25(OH)2D than serum from wild-type mice (Zella et al. 2008). Collectively, these observations have endorsed the role of DBP in maintaining the endocrine levels of 1,25(OH)2D, but conversely suggest that it may be detrimental to the accumulation and action of 1,25(OH)2D in some peripheral tissues. As yet, it is unclear whether this facet of DBP will manifest itself with CYP27b1-active target cells such as macrophages or whether similar effects will be observed with 25OHD as a ligand rather than 1,25(OH)2D.
Effects of DBP gene variants on human physiology and evolution
From the recent studies outlined above, it is clear that DBP plays a pivotal role in how target cells ‘see’ vitamin D, either by actively facilitating the acquisition of 25OHD/1,25(OH)2D or by defining the amount of ‘free vitamin D’ that is available for passive cellular uptake. Thus, there has also been renewed interest in well-characterized genetic variations of DBP. There are three major polymorphisms in the gene for DBP, which is also known as group-specific component (GC). GC-1S contains a D416E amino acid change from the GC-1F form of DBP, whileGC-2 contains a T420Kamino acid change from GC-1F (Constans et al. 1985). These differences in amino acid primary sequence are associated with changes in DBP affinity for vitamin D ligands. GC-2 has the lowest affinity for 25OHD while GC-1S has twofold greater affinity and GC-1F has a fourfold increase in affinity for 25OHD relative to GC-2 (Arnaud & Constans 1993). At present, it is not clear whether there is any connection between these affinity differences and reported correlations between DBP polymorphisms and thyroid disorders, diabetes, obesity, and other diseases (White & Cooke 2000, Speeckaert et al. 2008).
Significantly, however, DBP polymorphisms show strong ethnic variations. Darker pigmented African, African-American, and Asian populations are far more likely to carry the GC-1F form of DBP while whites more frequently exhibit the GC-1S form. Conversely, the lower affinity GC-2 form is far more likely to be found in whites and rarely found in blacks (Constans et al. 1985). Darker skin complexions provide better protection against the damaging effects of exposure to u.v. light but conversely are less efficient in synthesizing vitamin D. This appears to have been a major factor in the migration of Homo sapiens from sunlight enriched, vitamin D-efficient Africa into less sunny Europe. Initially, the dark-skinned migrants may have compensated for the lack of sunlight-derived vitamin D by utilizing dietary sources such as fish oils. However, following the transition to agrarian societies ~10 000 years ago, vitamin D deficiency appears to have become a significant health issue: rachitic skeletal disease is known to result in the constriction of the pelvis with concomitant threat to normal childbirth. The potential role of vitamin D as a factor in recent human evolution has been highlighted by population analyses of the skin pigmentation gene SLC24A5, showing that decreased skin pigmentation in Europeans is likely to have taken place as recently as 6000–12 000 years ago (Sabeti et al. 2007). It has been postulated that such changes in skin pigmentation conferred a significant biological advantage by improving the efficiency of vitamin D synthesis in lighter pigmented skin under conditions of impaired exposure to u.v. light. In view of these observations, it is possible that DBP (as the major carrier of vitamin D metabolites) was also subject to evolutionary ‘pressure’, although as yet the significance of such changes remains unclear.
Alternative functions for DBP
In assessing the role of DBP in human physiology, it is also important to recognize that the DBP gene arose from a gene duplication ~580 million years ago, with subsequent gene duplications giving rise to heme-binding albuminoid proteins such as serum albumin and α-fetoprotein (Gibbs et al. 1998). Thus, the precise nature of the original function of DBP remains unclear and it is important to recognize that the modern day DBP has several putative properties beyond its capacity to bind vitamin D metabolites (Gomme & Bertolini 2004). In addition to its ligand-binding domain, the DBP protein also contains a domain near its C-terminus that facilitates high-affinity binding to globular (G)-actin, and thereby prevents the formation of filamentous (F)-actin (White & Cooke 2000). Although actin has well-recognized cytoskeletal functions, it is also released following cellular injury with potential detrimental effects on the vasculature. Thus, it has been proposed that DBP may have an alternative function in tissue homeostasis by acting as an ‘actin scavenger’ (Lee & Galbraith 1992). In the context of the innate immunity actions of vitamin D (see Fig. 1), it should also be noted that a deglycosylated form of DBP has been reported to function as a macrophage-activating factor (Yamamoto & Naraparaju 1996). However, the significance of this with respect to human innate immune responses is unclear in view of the fact that the amino acid targeted for deglycosylation (threonine 420) is altered to a non-glycosylated lysine in the 20% of the human population who carry the GC-2 allele (White & Cooke 2000).
Primate responses to vitamin D metabolites: New World versus Old World
New World primates (NWPs) such as marmosets, tamarins, and squirrel monkeys exhibit significantly higher circulating levels of steroid hormones than their Old World primate (OWP) counterparts (including humans), but they are also profoundly resistant to these hormones (Chrousos et al. 1986). In some instances, notably for glucocorticoids, end-organ resistance is associated with altered binding affinity of glucocorticoid receptors (GR) for its ligand cortisol (Brandon et al. 1989). A potential mechanism for this has been proposed involving differential interaction between GR and co-chaperones such as the immunophilins FKBP52 (humans and OWPs) and FKBP51 (NWPs) (Fuller et al. 2004). NWPs are also profoundly resistant to vitamin D. As with glucocorticoids, this appears to be due to impaired signaling via the cognate intracellular receptor, in this case the VDR. Initial studies suggested that the blockade of VDR action in NWPs was due to the elevated levels of a soluble protein that could act as a competitor for binding of ,25(OH)2D (Gacad & Adams 1991, 1992). The purification of this intracellular DBP (IDBP) revealed similarity to the constitutively expressed human heat-shock protein 70 (hsc70) (Gacad et al. 1997), and also indicated that hsc70 was capable of binding both 25OHD and 1,25(OH)2D, as well as gonadal steroids such as estradiol (Gacad & Adams 1998).
NWPs and the intracellular trafficking of vitamin D
The assumption was made that hsc70-like proteins act as an intracellular ‘decoy’ for the relatively high levels of vitamin D metabolites present in NWPs, thereby attenuating over-exuberant signaling via the cognate VDR. However, paradoxically, functional analyses using various in vitro models showed that the overexpression of hsc70-like IDBP cloned from NWP cells enhanced 1,25(OH)2D-VDR-mediated gene transcription in human cells (Wu et al. 2000). The IDBP also enhanced the metabolism of 25OHD to 1,25(OH)2D, indicating that its ability to facilitate the actions of vitamin D is not only restricted to nuclear responses but also incorporates effects on the mitochondrial enzyme CYP27b1 (Wu et al. 2000, 2002). Based on these observations, it has been proposed that rather than acting as simple VDR decoys IDBP/hsc70 functions as specific intracellular steroid chaperones, with the ability to traffic vitamin D metabolites from the cell membrane to individual organelles (Adams et al. 2003, 2004) (see Fig. 1).
Co-chaperones and the intracellular trafficking of vitamin D
Subsequent studies have shown that non-VDR intracellular binding of vitamin D metabolites is not restricted to hsc70. Heat-shock proteins also exhibit specific binding sites for ATP, which produces the necessary conformational changes required for the protein–protein interactions with hsc70 or hsp70. It was therefore interesting to note that under these conditions the association between hsc70 and vitamin D metabolites is also altered, indicating that ATP may alter binding kinetics sufficiently to facilitate the transfer of 25OHD or 1,25(OH)2D to another protein (Chun et al. 2005). Potential targets would include VDR, CYP27b1, or other vitamin D targets such as the feedback control enzyme 24-hydroxylase (CYP24). An alternative hypothesis is that hsc70 may transfer 25OHD or 1,25(OH)2D to more downstream proteins that are then able to confer more organelle-specific localization of the vitamin D metabolites. An immediate candidate for the interaction with hsc70 is the co-chaperone Bcl-2-associated athanogene (BAG-1) that interacts with hsc70 as part of the protein-folding activities of the latter (Takayama et al. 1997). Like hsc70, BAG-1 can also act as an intracellular binding site for vitamin D metabolites. However, BAG-1 showed higher affinity for 1,25(OH)2D than 25OHD and the overexpression of the co-chaperone increased VDR-mediated transactivation but not CYP27b1- or CYP24-mediated metabolism (Chun et al. 2007). Thus, it is possible to postulate a model whereby chaperones such as hsc70 provide a conduit for the cytosolic translocation of vitamin D metabolites following their cellular entry, while co-chaperones such as BAG-1 facilitate organelle-specific transfer (see Fig. 1). The broader applicability of such a mechanism for other steroid hormones is as yet unclear, although another member of the heat-shock protein family, hsp27, has been shown to bind estradiol with high affinity (Chen et al. 2008).
Collectively, these studies suggest that the movement of vitamin D metabolites from the cell membrane to specific intracellular destinations is not a random process but instead is strongly influenced by chaperone and co-chaperone proteins that share the ability to interact with each other as well as 25OHD and 1,25(OH)2D. Clearly, many questions remain to be answered. For example, although hsc70 appears to be a highly effective intracellular conduit for vitamin D metabolites, it is uncertain how hsc70 acquires these ligands either in free form or bound to internalized megalin. Likewise, a mitochondrion-specific co-chaperone associated with vitamin D metabolism or catabolism has yet to be identified. Finally, a key question posed at the onset of these studies concerns the paradox of why NWPs express such high levels of vitamin D-potentiating chaperone proteins despite the fact that these animals are profoundly resistant to steroid hormones in general. This latter question is addressed in greater detail in the following section.
The vitamin D response element, cathelicidin, and the evolution of innate immunity in primates
As outlined above and in Fig. 1, the antimicrobial peptide cathelicidin LL37 is a direct target for liganded VDR. The transcriptional regulation of LL37 by 1,25(OH)2D has been described in a variety of cell types including macrophages (Liu et al. 2006, 2007b), myeloid cells (Gombart et al. 2005), keratinocytes (Schauber et al. 2007), decidual cells (Evans et al. 2006), and lung epithelial cells (Yim et al. 2007). Although this appears to be a fundamental facet of innate immunity in humans, similar responses are not apparent in lower orders of mammals such as mice (Gombart et al. 2005). Gombart et al. (2005) have shown that the transcriptional regulation of LL37 by 1,25(OH)2D involves a classical direct repeat 3-type VDR element (VDRE) within the proximal promoter of the LL37 gene, 615 bp upstream of the transcriptional start site. Comparative analysis indicated that this piece of the LL37 promoter is composed of a short interspersed nuclear element (SINE) that is conserved only in higher primates such as humans and chimpanzees (Gombart et al. 2005). Thus, it would appear that the ability of vitamin D to influence this facet of innate immunity is a relatively recent event in the evolutionary pathway. In humans, SINEs make up as much as 13% of the genome. They consist of repeat DNA sequences (most commonly Alu repeats in humans) and function as retrotransposons in that they are genetic elements that can amplify themselves through RNA intermediaries (Wicker et al. 2007). Because of their ability to amplify DNA within the genome, SINEs and other transposable elements are thought to play a pivotal role in gene evolution. In this context, it is interesting to speculate that this particular LL37 promoter SINE may have conferred significant advantages given the relatively high circulating levels of 1,25(OH)2D in subhuman primates. The VDRE-containing SINE appears to be conserved in NWPs as well as OWPs but is not present in prosimians, the most ancestral extant group of primates (Gombart personal communication). Thus, the regulation of LL37 expression by vitamin D appears to have emerged ~55 million years ago.
VDRE-binding proteins (VDRE-BPs) and the regulation of VDR-induced gene transcription
As outlined above, decreased VDR function in NWPs involves diminished nuclear activity of the liganded receptor (Gacad & Adams 1991). This was initially attributed to the elevated levels of IDBPs in NWPs, which were thought to act as decoy-binding sites for 1,25(OH)2D (Gacad et al. 1997, Gacad & Adams 1998). However, as already described, subsequent studies showed that the opposite was true in that IDBPs such as hsc70 were potent enhancers of VDR-mediated signaling (Wu et al. 2000). An alternative mechanism for vitamin D resistance in NWPs was proposed based on the experiments showing nuclear extracts from NWP cells contained a soluble factor that was able to compete with the VDR-retinoid X receptor (RXR) complex for binding to VDRE during 1,25(OH)2D-VDR-directed gene regulation (Arbelle et al. 1996). This factor was subsequently referred to as the VDRE-BP. Similar observations have also been made for estrogen response element (ERE) responses in NWPs (Chen et al. 1997). In this case, the offending ERE-binding protein (ERE-BP) was shown to be homologous with a human heterogeneous nuclear ribo-nucleoprotein C (hnRNPC)-like protein (Chen et al. 1998) and competed with the estrogen receptor (ERα) for binding to EREs.
Subsequent studies revealed that vitamin D resistance in NWPs was associated with the overexpression of a response element-binding hnRNP-like protein (Chen et al. 2000). Thus, it appears that NWPs have a complex intracellular system for dealing with vitamin D. On the one hand, the relatively high levels of circulating 25OHD and 1,25(OH)2D in these animals may require equally elevated levels of chaperone IDBPs to facilitate effective cellular handling of the vitamin D metabolites. On the other hand, in order to prevent over-elaboration of VDR-mediated responses in the face of high circulating levels of 1,25(OH)2D, it may also be beneficial to have increased levels of a VDRE-BP that will more adequately manage access to VDREs in target genes. With this in mind, it is interesting to note that the identification of IDBP and VDRE-BP proteins in NWPs occurred in response to an outbreak of rickets in a cohort of these animals within the Los Angeles Zoo (Adams et al. 2003). Circulating levels of 1,25(OH)2D in these animals were, by present human standards, still relatively high but were shown to be too low to effectively displace the abundant VDRE-BP levels that are characteristic of the NWPs.
VDRE-BP and vitamin D resistance in humans
Significantly, a human VDRE-BP was cloned and sequenced from a patient who presented with symptoms that were highly reminiscent of hereditary vitamin D resistant rickets (HVDRR), despite the fact that she did not have a mutation in the VDR gene as is the usual case for this disease (Hewison et al. 1993). Instead, the patient demonstrated overexpression of an hnRNP-like VDRE-BP that suppressed normal VDR-VDRE function (Chen et al. 2003). Purification and cloning showed that the VDRE-BP was almost identical to hnRNPC1/C2, which was abundantly expressed in cells from the vitamin D-resistant patient (Chen et al. 2006).
Although overexpression of hnRNPC1/C2 appears to be the underlying cause of vitamin D resistance in this particular patient with rickets, it is important to recognize that the hnRNPs are among the most common of all nuclear proteins. Consisting of more than 20 variants, the hnRNP family plays a key role in RNA processing by interacting with pre-mRNA complexes (Dreyfuss et al. 1996). Despite their classical interaction with chromatin-associated single-stranded RNA, several studies have shown that hnRNPs are pluripotent proteins. In particular, they may also function as transregulatory factors through interaction with double-stranded DNA (Gao et al. 2005), raising the question as to whether they may also be part of the normal VDR-mediated gene regulation machinery. Chromatin immunoprecipitation (ChIP) studies from normal 1,25(OH)2D-sensitive cells indicate that this is indeed the case, with the human hnRNPC1/C2 (VDRE-BP) occupying the VDRE prior to treatment with 1,25(OH)2D, and then being displaced from the promoter by the liganded VDR-RXR complex (Chen et al. 2006). This reciprocal relationship between VDR and VDRE-BP occurs in a time-dependent cyclical fashion following exposure to 1,25(OH)2D, suggesting that hnRNPC1/C2 may play a pivotal role in the spatio-temporal organization of the receptor–response element complex.
A similar relationship has also been recently observed for ERα which, when liganded, is able to displace the hnRNPC-like ERE-BP from its basal occupancy of the ERE, thereby initiating the cyclical occupancy of the response element required for normal estradiol-directed gene transactivation (Chen et al. 2008). For both ERα and the VDR, overexpression of their specific hormone response element-binding protein either naturally (as is the case for the VDRE-BP in the HVDRR patient) or by cDNA transfection resulted in a dysregulated ChIP pattern of response element occupancy and concomitant suppression of transcription (Chen et al. 2006, 2008).
Conclusions
The vitamin D synthetic and response mechanisms are phylogenetically ancient. Vitamin D is produced by both single-cell plants and animals (Holick 1992); DBP is the most primitive of all the albuminoid proteins (Fasano et al. 2007) and an ancestral VDR first appears in nematodes (Carosa et al. 1998). These observations provide an invaluable platform for better understanding the role of vitamin D in modern human physiology. For example, a functional VDR has been identified in lampreys, the most ancient of the extant vertebrates (Whitfield et al. 2003, Reschly et al. 2007). The fact that this animal has a cartilaginous skeleton has endorsed the idea that the original function of vitamin D may have been unrelated to its classical modern day role in the homeostasis of calcified skeletons. One possibility is that vitamin D is involved in regulating immune responses in lampreys. Another possibility, based on the evolutionary link between VDR and another ancient steroid hormone receptor, the pregnane X receptor, is that vitamin D may have originally acted as an inducer of cytochrome P450-mediated detoxification pathways (Whitfield et al. 2003). In the case of the latter, it is interesting to note other studies showing that toxic bile salt derivatives can also act as ligands for the VDR (Makishima et al. 2002). The take-home message from these and other studies has been that we can gain a better understanding of the role of vitamin D in modern day human physiology through improved knowledge of its history.
Acknowledgments
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nature Clinical Practice. Endocrinology and Metabolism. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Chen H, Chun RF, Nguyen L, Wu S, Ren SY, Barsony J, Gacad MA. Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. Journal of Cellular Biochemistry. 2003;88:308–314. doi: 10.1002/jcb.10333. [DOI] [PubMed] [Google Scholar]
- Adams JS, Chen H, Chun R, Gacad MA, Encinas C, Ren S, Nguyen L, Wu S, Hewison M, Barsony J. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. Journal of Steroid Biochemistry and Molecular Biology. 2004;89–90:461–465. doi: 10.1016/j.jsbmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Arbelle JE, Chen H, Gaead MA, Allegretto EA, Pike JW, Adams JS. Inhibition of vitamin D receptor-retinoid X receptor-vitamin D response element complex formation by nuclear extracts of vitamin D-resistant New World primate cells. Endocrinology. 1996;137:786–789. doi: 10.1210/endo.137.2.8593831. [DOI] [PubMed] [Google Scholar]
- Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Human Genetics. 1993;92:183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O’Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. Journal of Clinical Endocrinology and Metabolism. 1985;61:969–975. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. Journal of Clinical Endocrinology and Metabolism. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- Brandon DD, Markwick AJ, Chrousos GP, Loriaux DL. Glucocorticoid resistance in humans and nonhuman primates. Cancer Research. 1989;49:2203s–2213s. [PubMed] [Google Scholar]
- Carosa E, Fanelli A, Ulisse S, Di Lauro R, Rall JE, Jannini EA. Ciona intestinalis nuclear receptor 1: a member of steroid/thyroid hormone receptor family. PNAS. 1998;95:11152–11157. doi: 10.1073/pnas.95.19.11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporosis International. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- Chen H, Arbelle JE, Gacad MA, Allegretto EA, Adams JS. Vitamin D and gonadal steroid-resistant New World primate cells express an intracellular protein which competes with the estrogen receptor for binding to the estrogen response element. Journal of Clinical Investigation. 1997;99:669–675. doi: 10.1172/JCI119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hu B, Gacad MA, Adams JS. Cloning and expression of a novel dominant-negative-acting estrogen response element-binding protein in the heterogeneous nuclear ribonucleoprotein family. Journal of Biological Chemistry. 1998;273:31352–31357. doi: 10.1074/jbc.273.47.31352. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu B, Allegretto EA, Adams JS. The vitamin D response element-binding protein. A novel dominant-negative regulator of vitamin D-directed transactivation. Journal of Biological Chemistry. 2000;275:35557–35564. doi: 10.1074/jbc.M007117200. [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Hu B, Adams JS. Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: a cause of vitamin D resistance. PNAS. 2003;100:6109–6114. doi: 10.1073/pnas.1031395100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hewison M, Adams JS. Functional characterization of heterogeneous nuclear ribonuclear protein C1/C2 in vitamin D resistance: a novel response element-binding protein. Journal of Biological Chemistry. 2006;281:39114–39120. doi: 10.1074/jbc.M608006200. [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Adams JS. Control of estradiol-directed gene transactivation by an intracellular estrogen-binding protein and an estrogen response element-binding protein. Molecular Endocrinology. 2008;22:559–569. doi: 10.1210/me.2007-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Loriaux DL, Tomita M, Brandon DD, Renquist D, Albertson B, Lipsett MB. The new world primates as animal models of glucocorticoid resistance. Advances in Experimental Medicine and Biology. 1986;196:129–144. doi: 10.1007/978-1-4684-5101-6_9. [DOI] [PubMed] [Google Scholar]
- Chun R, Gacad MA, Hewison M, Adams JS. Adenosine 5′-triphosphate-dependent vitamin D sterol binding to heat shock protein-70 chaperones. Endocrinology. 2005;146:5540–5544. doi: 10.1210/en.2005-0579. [DOI] [PubMed] [Google Scholar]
- Chun RF, Gacad M, Nguyen L, Hewison M, Adams JS. Co-chaperone potentiation of vitamin D receptor-mediated transactivation: a role for Bcl2-associated athanogene-1 as an intracellular-binding protein for 1,25-dihydroxyvitamin D3. Journal of Molecular Endocrinology. 2007;39:81–89. doi: 10.1677/JME-07-0042. [DOI] [PubMed] [Google Scholar]
- Constans J, Hazout S, Garruto RM, Gajdusek DC, Spees EK. Population distribution of the human vitamin D binding protein: anthropological considerations. American Journal of Physical Anthropology. 1985;68:107–122. doi: 10.1002/ajpa.1330680110. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Hentze M, Lamond AI. From transcript to protein. Cell. 1996;85:963–972. doi: 10.1016/s0092-8674(00)81298-2. [DOI] [PubMed] [Google Scholar]
- van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1α-hydroxylase expression and activity in human bone cells. FASEB Journal. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biology of Reproduction. 2006;75:816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- Fasano M, Fanali G, Leboffe L, Ascenzi P. Heme binding to albuminoid proteins is the result of recent evolution. IUBMB Life. 2007;59:436–440. doi: 10.1080/15216540701474523. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO Journal. 2005;24:3881–3894. doi: 10.1038/sj.emboj.7600853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fuller PJ, Smith BJ, Rogerson FM. Cortisol resistance in the New World revisited. Trends in Endocrinology and Metabolism. 2004;15:296–299. doi: 10.1016/j.tem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Gacad MA, Adams JS. Endogenous blockade of 1,25-dihydroxyvitamin D-receptor binding in New World primate cells. Journal of Clinical Investigation. 1991;87:996–1001. doi: 10.1172/JCI115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacad MA, Adams JS. Specificity of steroid binding in New World primate B95-8 cells with a vitamin D-resistant phenotype. Endocrinology. 1992;131:2581–2587. doi: 10.1210/endo.131.6.1446602. [DOI] [PubMed] [Google Scholar]
- Gacad MA, Adams JS. Proteins in the heat shock-70 family specifically bind 25-hydroxyvitamin D3 and 17beta-estradiol. Journal of Clinical Endocrinology and Metabolism. 1998;83:1264–1267. doi: 10.1210/jcem.83.4.4725. [DOI] [PubMed] [Google Scholar]
- Gacad MA, Chen H, Arbelle JE, LeBon T, Adams JS. Functional characterization and purification of an intracellular vitamin D-binding protein in vitamin D-resistant new world primate cells. Amino acid sequence homology with proteins in the hsp-70 family. Journal of Biological Chemistry. 1997;272:8433–8440. doi: 10.1074/jbc.272.13.8433. [DOI] [PubMed] [Google Scholar]
- Gao C, Guo H, Mi Z, Wai PY, Kuo PC. Transcriptional regulatory functions of heterogeneous nuclear ribonucleoprotein-U and -A/B in endotoxin-mediated macrophage expression of osteopontin. Journal of Immunology. 2005;175:523–530. doi: 10.4049/jimmunol.175.1.523. [DOI] [PubMed] [Google Scholar]
- Gibbs PE, Witke WF, Dugaiczyk A. The molecular clock runs at different rates among closely related members of a gene family. Journal of Molecular Evolution. 1998;46:552–561. doi: 10.1007/pl00006336. [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB Journal. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends in Biotechnology. 2004;22:340–345. doi: 10.1016/j.tibtech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Hewison M, Rut AR, Kristjansson K, Walker RE, Dillon MJ, Hughes MR, O’Riordan JL. Tissue resistance to 1,25-dihydroxyvitamin D without a mutation of the vitamin D receptor gene. Clinical Endocrinology. 1993;39:663–670. doi: 10.1111/j.1365-2265.1993.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. Journal of Steroid Biochemistry and Molecular Biology. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Holick MF. Evolutionary biology and pathology of vitamin D. Journal of Nutritional Science and Vitaminology. 1992:79–83. doi: 10.3177/jnsv.38.special_79. Spec No. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: its role in cancer prevention and treatment. Progress in Biophysics and Molecular Biology. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of Epidemiology. 2008 doi: 10.1016/j.annepidem.2007.12.001. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Muindi JR, Hershberger PA, Trump DL. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Research. 2006;26:2543–2549. [PubMed] [Google Scholar]
- Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, Hsieh JC, Kaczmarska M, Haussler CA, Haussler MR. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. Journal of Bone and Mineral Research. 2007;22:V2–V10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- Kato S, Fujiki R, Kitagawa H. Vitamin D receptor (VDR) promoter targeting through a novel chromatin remodeling complex. Journal of Steroid Biochemistry and Molecular Biology. 2004;89–90:173–178. doi: 10.1016/j.jsbmb.2004.03.100. [DOI] [PubMed] [Google Scholar]
- Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. New England Journal of Medicine. 1992;326:1335–1341. doi: 10.1056/NEJM199205143262006. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends in Molecular Medicine. 2007a;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. Journal of Immunology. 2007b;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. Journal of Immunology. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- Nagai J, Christensen EI, Morris SM, Willnow TE, Cooper JA, Nielsen R. Mutually dependent localization of megalin and Dab2 in the renal proximal tubule. American Journal of Physiology-Renal Physiology. 2005;289:F569–F576. doi: 10.1152/ajprenal.00292.2004. [DOI] [PubMed] [Google Scholar]
- Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) PNAS. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschly EJ, Bainy AC, Mattos JJ, Hagey LR, Bahary N, Mada SR, Ou J, Venkataramanan R, Krasowski MD. Functional evolution of the vitamin D and pregnane X receptors. BMC Evolutionary Biology. 2007;7:222. doi: 10.1186/1471-2148-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxy-cholecalciferol actions in human mammary cells. Journal of Nutrition. 2006;136:2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, Xie X, Byrne EH, McCarroll SA, Gaudet R, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. Journal of Clinical Investigation. 1999;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. Journal of Clinical Investigation. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clinica Chimica Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Speeckaert MM, Wehlou C, Vandewalle S, Taes YE, Robberecht E, Delanghe JR. Vitamin D binding protein, a new nutritional marker in cystic fibrosis patients. Clinical Chemistry and Laboratory Medicine. 2008;46:365–370. doi: 10.1515/CCLM.2008.084. [DOI] [PubMed] [Google Scholar]
- Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Research. 2006;26:2515–2524. [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO Journal. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Research. 2006;26:2551–2556. [PubMed] [Google Scholar]
- White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends in Endocrinology and Metabolism. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- Whitfield GK, Dang HT, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144:2704–2716. doi: 10.1210/en.2002-221101. [DOI] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, et al. A unified classification system for eukaryotic transposable elements. Nature Reviews. Cancer. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Wu S, Ren S, Chen H, Chun RF, Gacad MA, Adams JS. Intracellular vitamin D binding proteins: novel facilitators of vitamin D-directed transactivation. Molecular Endocrinology. 2000;14:1387–1397. doi: 10.1210/mend.14.9.0523. [DOI] [PubMed] [Google Scholar]
- Wu S, Chun R, Gacad MA, Ren S, Chen H, Adams JS. Regulation of 1,25-dihydroxyvitamin D synthesis by intracellular vitamin D binding protein-1. Endocrinology. 2002;143:4135. doi: 10.1210/en.2002-220568. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Naraparaju VR. Role of vitamin D3-binding protein in activation of mouse macrophages. Journal of Immunology. 1996;157:1744–1749. [PubMed] [Google Scholar]
- Yamaoka K, Shindo M, Iwasaki K, Yamaoka I, Yamamoto Y, Kitagawa H, Kato S. Multiple co-activator complexes support ligand-induced transactivation function of VDR. Archives of Biochemistry and Biophysics. 2007;460:166–171. doi: 10.1016/j.abb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Yim S, Dhawan P, Ragunath C, Christakos S. Diamond G Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxy-vitamin D(3) Journal of Cystic Fibrosis. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008 doi: 10.1210/en.2008-0042. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]