Abstract
The regulation of the Plasmodium cell cycle is not understood. Although the Plasmodium falciparum genome is completely sequenced, about 60% of the predicted proteins share little or no sequence similarity with other eukaryotes. This feature impairs the identification of important proteins participating in the regulation of the cell cycle. There are several open questions that concern cell cycle progression in malaria parasites, including the mechanism by which multiple nuclear divisions is controlled and how the cell cycle is managed in all phases of their complex life cycle. Cell cycle synchrony of the parasite population within the host, as well as the circadian rhythm of proliferation, are striking features of some Plasmodium species, the molecular basis of which remains to be elucidated. In this review we discuss the role of indole-related molecules as signals that modulate the cell cycle in Plasmodium and other eukaryotes, and we also consider the possible role of kinases in the signal transduction and in the responses it triggers.
Keywords: Plasmodium, Malaria, Serpentine receptor, Calcium, Kinases
1. Introduction
The molecular mechanisms responsible for eukaryotic cell cycle control are likely to have been highly conserved during evolution. There is indeed evidence that fundamental principles are conserved between yeast and mammals [2]. However, yeast and metazoans are both members of the same phylum (Opisthokont) [3] and much more detailed analysis in different eukaryotic groups is needed before a full picture can be established. In addition to its importance in fundamental biology, research into cell cycle control carries a strong potential for application in drug discovery. Many elements of the cell cycle control machinery are important targets for the development of new drugs against cancer and other pathologies. The many unusual features of eukaryotic pathogens (including Plasmodium spp.) suggest that cell cycle control could be selectively targeted to create a new range of anti-parasitic drugs.
Plasmodium has a complex life cycle alternating between two hosts (1) mosquitoes, in which sexual reproduction occurs, and (2) a vertebrate, where Plasmodium invades and multiplies asexually in erythrocytes and hepatocytes. The parasite undergoes major metabolic and morphological changes as it exploits its two hosts, with periods of intensive cell division as it multiplies during sporozoite formation (sporogony) in the mosquito gut wall, and in the liver and red blood cells (schizogony) of the vertebrate host.
The intraerythrocytic phase is the cause of malaria pathogenesis. This phase consists of cycles of invasion, multiplication and reinfection. It begins with the invasion of erythrocytes by merozoites and continues into growth (ring and trophozoite stages), formation of multiple new merozoites (schizont stage) and finally release into the bloodstream of merozoites that in turn will infect new erythrocytes.
In Plasmodium falciparum, the most virulent of the four Plasmodium species that infect humans, and in the rodent malaria parasite P. chabaudi, the erythrocytic cycle events usually occur synchronously during in vivo infection but synchrony is lost in in vitro cultures, presumably because some defining factor present in the host is absent from the culture medium. There are artificial ways of restoring synchrony of P. falciparum in vitro, such as temperature elevation [4] or the addition of sorbitol [5] which, however is not related to normal control since it entails the killing of all but ring stages. Of greater biological significance is the reported modulation of synchrony of P. falciparum by host tryptophan-derived molecules [6].
The cell cycle comprises a range of highly ordered events that lead to mitosis and the formation of new cells. Regulation of these conserved processes is critical to normal cellular growth, differentiation and replication. According to Hammarton et al.[7] merozoites and rings are in G1, and the S phase begins when the parasite is at trophozoite stage, while in schizont stage, merozoites are produced by successive rounds of poorly understood mitosis [1]. To regulate these processes cells employ several mechanisms including phosphorylation, transcription control and degradation of regulatory proteins by the proteasome complex.
2. Receptors: the upstream part of signaling pathways
In many instances the sensing of environmental cues and ensuing signal transduction are crucial to initiate the cell cycle and/or cell differentiation [8]. These occur when a stimulus binds to a receptor and promotes downstream responses such as transcription regulation, alterations in metabolism, cell proliferation and apoptosis, all mediate by second messengers and effectors. Cells may sense a range of stimuli such as hormones, light, growth factors, cytokines and other molecules, and each cell type may express its own repertoire of receptors detecting specific signals. The more spread family of receptor is GPCR (G-protein coupled receptors) which are involves in a range of physiological processes.
Many organisms use external molecular signals to drive cell differentiation. Examples include several species of amoebae, which modulate encystation by sensing hormones such as catecholamines by means of transmembrane receptors [9], and fungi that secrete molecules inducing cell differentiation [10]. Indole is an important signaling molecule not only in eukaryotes, but also in bacteria, where it has been implicated in quorum sensing processes [11] and in the cell cycle control of Escherichia coli [12].
Within cells, second messengers like calcium and cAMP are able to promote a range of intracellular responses. An increase in cytosolic calcium concentration is caused by the release of calcium from intracellular pools such as the endoplasmic reticulum [13], mitochondria [14] and acidocalcisomes [15,16] and also by influx through the plasma membrane. In Plasmodium there are several reports from different labs implicating calcium signaling at several stages of the life cycle, including erythrocytic schizogony, gametogenesis, ookinete motility, [17,18]. Moreover increase of intracellular second messengers concentration as cAMP and calcium are involved in a range of signaling events in Plasmodium signaling such as in tryptophan-derived response [19,20] and in sporozoite apical regulated exocytosis [21]. cAMP also inhibits maturation of merozoites in RBCs [22] and in is able to promote sexual differentiation [23–25].
Since Martin et al. [26] reported that gametocytes of P. falciparum produce InsP3 during exflagellation it is possible that calcium increase via PLC could be caused by InsP3. Furthermore Passos and Garcia [27] promote in vitro calcium increase by adding InsP3 in P. falciparum culture.
Another second messenger, cGMP and subsequently PKG activation together with calcium release are crucial for xanthurenic acid-induced gametogenesis into mosquito [28].
As already mentioned, synchrony of erythrocytic schizogony in some Plasmodium species occurs in vivo and is lost in vitro. In P. falciparum, treatment of trophozoites with melatonin activates a calcium/cAMP-dependent response that, at least in vitro, is able to synchronize their intraerythrocytic stages [29]. Other tryptophan-derivatives such as tryptamine, N-acetylserotonin, and serotonin also promote changes in the Plasmodium cell cycle [20,30]. N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), a product of melatonin degradation, is able to synchronize P. chabaudi and P. falciparum proliferation [31].
Furthermore, in P. chabaudi, erythrocyte rupture and reinvasion occur approximately between midnight and 3 a.m., which coincides with the circulating melatonin peak level. When P. chabaudi infects pinealectomized mice, which lack melatonin, synchrony is lost but melatonin administration is able to restore it; this effect of melatonin is inhibited by the addition of luzindole (a melatonin antagonist) [19]. Administration of a suboptimal concentration of the anti-malarial drug, chloroquine in addition to luzindole reduces significantly the mortality of mice infected with P. chabaudi [32].
Calcium release caused by melatonin treatment also activates several cysteine-proteases acting in erythrocyte rupture, hemoglobin and cytoskeletal proteins degradation [33]. Interestingly, it has been found in the laboratory of one of the authors that in contrast to P. chabaudi and P. falciparum, asynchronous P. berghei is insensitive to melatonin with respect to both [Ca2+] increase and cell cycle modulation [34].
In vertebrates, melatonin receptors belong to the family of G-protein coupled receptors (GPCRs), also called seven transmembrane (7TM) receptors [35]. This family is widespread in eukaryotes. To mention just two examples, Dictyostelium discoideum uses GPCR signaling for many cellular processes including development [36], and the parasitic helminth Schistosoma mansoni uses a GPCR to sense histamine and to trigger cell responses via calcium and cAMP [37].
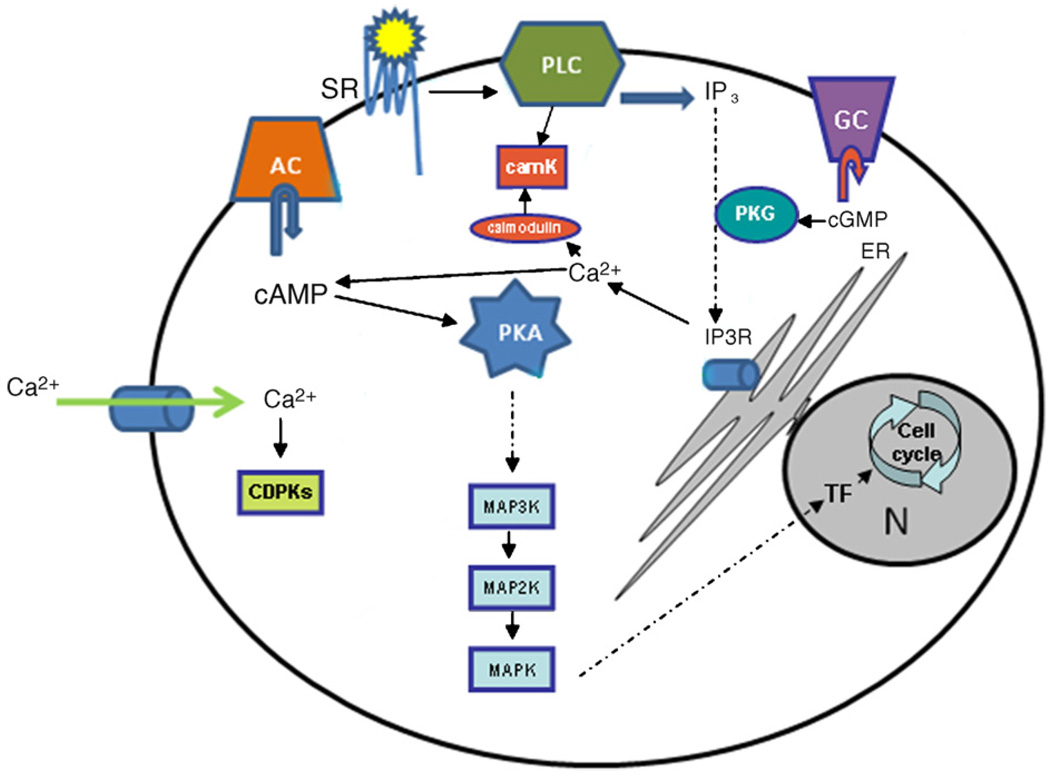
Madeira et al. [38] identified four putative serpentine receptors in P. falciparum and their functions are under analysis. Their predicted roles in sensing extracellular signals, possibly in the form of host hormones or other molecules, make these four putative receptors potentially very interesting, and elucidation of their detailed function may shed light on how the parasite modulates its life cycle in response to its environment Fig. 1 depicts schematic model for molecular signaling machineries in Plasmodium.
Fig. 1.
Schematic pathway of signaling in Plasmodium. AC adenylate cyclase, camK calcium/calmodulin kinase B, PDE phosphodiesterase, PLC phospholipase C, PKA protein kinase A, PKG protein kinase G PV parasitophorous vacuole, N nuclei, SR serpentine receptor. Tryptophan-derivatives are able to increase citoplasmic calcium through PLC. Calcium increase activates adenylate cyclase that convert AMP in cAMP once the concentration of such molecule is augmented in response to calcium increase by melatonin. cAMP is able to bind the regulatory subunit of PKA (cyclic AMP-dependent protein kinase) leading to an allosteric change in conformation which causes unleashing of the catalytic subunits becoming it activated and able to phosphorylate its targets. The molecular downstream effects of calcium and PKA in this pathway are proposed.
It has been proposed that hormones might influence malarial infection, for example in pregnant women infected with P. falciparum [39].
Although trimeric G-proteins are not found in Plasmodium genome, experiments with cholera and pertussis toxin brought evidences about its expression at the intraerythrocytic stage [25]. The inhibition of Gαs protein with peptides diminished P. berghei parasitemia [40]. On the other hand it is reported that Gαs present in erythrocytes is recruited to the malarial vacuole [41].
The knowledge of the downstream mechanisms involved in signal transduction pathways in Plasmodium is fundamental to understand parasite biology. Protein kinases (PKs) are an important family of proteins that are expected to regulate diverse cellular activities. In the sections below we present a brief overview of current knowledge on Plasmodium kinases.
3. Protein kinases: signal transducers
The P. falciparum genome sequence contains 85 or 99 protein kinases (PK)-related sequences, depending on the study [42,43]. The number is rather low in comparison to the size of the kinome in Saccharomyces cerevisiae: while the yeast genome contains a similar number of total genes to that of P. falciparum, it possesses significantly more PKs. This low number might be attributed to the intracellular lifestyle of the parasite, which is likely to receive less environmental cues than a free-living unicellular eukaryote. However, complex life cycle stages of malaria parasites in mosquito and the vertebrate host entail intricate regulatory mechanisms where protein kinases are expected to be key molecules. Therefore, it is possible that malaria PKs may possess particularly complex cellular functions. Given that many Plasmodium PKs have atypical features compared to their eukaryotic homologues they are predicted to be promising targets for antimalarial development [42,44].
3.1. Calcium modulated protein kinases
Calcium-mediated intracellular signaling is increasingly being found to be important for an assortment of cellular function in apicomplexans, and calcium signaling has been implicated in the response of Plasmodium to melatonin [29,45]. A major class of downstream effector of Ca2+-mediated signal transduction in Plasmodium is the CDPK family. These proteins have a conserved NH2-terminal ser/thr protein kinase domain that is fused to a COOH-terminal calmodulin-like domain containing four EF-hand calcium-binding sites; proteins sharing a similar domain organization are found in plants and Alveolates, but not in metazoans. The majority of PfCDPK genes exhibit significant expression in the sexual stages [42]. Elegant reverse genetics work in P. berghei by Billker et al. showed that CDPK4 is a key enzyme in male gamete formation, regulating entry into S phase during gametocyte activation [17]. CDPK4 also exhibits a potential role in sporogonic development because there is a significant reduction of mosquito infectivity of ookinetes derived from ΔCDPK4 macrogametes [17]. The Plasmodium CDPK3 is exclusively expressed in the ookinetes [46]. Although cdpk3 disrupted P. berghei lines exhibited normal exflagellation and development into ookinetes, their transmission efficiency is severely affected as evidenced by reduction of the number of oocyst in the midgut [46,47]. The reason for this drop in oocyst number was shown to be due to a defect in gliding motility [46,47]. CDPK6 is another CDPK that is dispensable for the asexual cycle; P. berghei parasites lacking CDPK6 are competent for sporozoite formation, but the sporozoites are significantly less infective for hepatocytes than wild-type parasites [48]. In contrast, PfCDPK1 is essential for erythrocytic schizogony [49] and is localized to parasite or parasitophorous vacuolar membrane [50], consistent with a proposed role in motility. The targeting of PfCDPK1 to these membranes was found to be dependent on the N-terminal dual acylation and basic residue motifs. Interestingly, Raf kinase inhibitor protein (RKIP) ortholog, which is also a protein kinase C (PKC) substrate in mammals, modulates PfCDPK1 activity in vitro [51]. Whether or not PfCDPK1 functions like PKC in Plasmodium remains to be established, but these results highlights atypical properties of Plasmodium kinases that cannot be discerned simply based on homology because many of these proteins are expected to possess parasite-specific functions. It is interesting to note that a receptor for activated protein kinase C (PfRack) has been identified in Plasmodium [52].
The importance of Ca2+ in the intracellular signaling of malaria parasites is exemplified by the existence of a novel Ca2+/calmodulin-regulated protein kinase B (PfPKB). Unlike the mammalian PKB, the PfPKB does not contain the N-terminal pleckstrin homology domain that interacts with phosphoinositides to regulate its activity. Instead this novel N-terminal region of PfPKB exhibits Ca2+-dependent interaction with calmodulin for its activation [53]. Involvement of phospholipase C (PLC) in PfPKB activation was suggested as U73122, a specific inhibitor of phospholipase C, inhibited kinase activity [53]. Recently, it has been shown that treatment with a peptide inhibitor that competes with calmodulin binding to PKB and A443654, a small-molecule inhibitor of PKB, inhibits invasion of merozoites [54]. PfPKB was also shown to phosphorylate PfGAP45, a glideosome-associated protein, suggesting its role in invasion [54].
It has been also shown that the cGMP-dependent protein kinase (PKG) in P. falciparum may function upstream of events that mobilize Ca2+ and is likely to be a key regulator of gametogenesis [28]. While the anticoccidial PKG inhibitor compound 1 inhibits gametocyte rounding up and subsequent exflagellation, gametocytes in which inhibitor-insensitive PKG has been incorporated in the genome, through allelic replacement, rounds up normally [28].
It is evident that Ca2+ is an important second messenger that regulates various cellular processes in Plasmodium (Fig. 1). To elucidate the cellular response to Ca2+ signals in malaria parasites it will be important to understand the mechanism by which the Ca2+ level is regulated and the role of different Ca2+-regulated proteins.
3.2. Cyclic nucleotide-dependent pathway
Cyclic nucleotide monophosphates, cAMP and cGMP are important second messengers in eykaryotic cell synthesized by adenylyl cyclases (PfAC) and guanylyl cyclases (PfGC), respectively. Malaria genome encodes two distinct PfACs [55]. PfACα contains six potential transmembrane domains at the N-terminus that have structural features of voltage-gated K+ channel and a C-terminal adenylyl cyclase domain. The unique features of PfACα suggest that the changes in ion conductance may be coupled to cAMP synthesis. The PfACβ is related to a family of soluble ACs found in photosynthetic bacteria and humans [56]. It has been shown that cAMP may have a role in sexual differentiation of the parasite [24,57]. In eukaryotes, one of the major roles of cAMP synthesized by ACs is to activate cAMP-dependent protein kinase (PKA) by binding to the inhibitory regulatory subunit PKAr. P. falciparum PKA catalytic (PKAc) [58] and the regulatory subunits have been characterized [59]. Using patch-clamp technique in infected erythrocytes, it has been shown that either addition of PfPKAr or overexpression of PfPKAr in trans leads to down-regulation of host cell anion conductance. Furthermore, PfPKAr overexpressing line exhibits reduced growth, which could be corrected by increasing the intracellular cAMP level [59].
Two guanylyl cyclases, PfGCα and PfGCβ with catalytic activity have been identified in P. falciparum [60]. Interestingly, the PfGCs appear to be bifunctional as they also contain P-ATPase domain at the N-terminal extension [56,60]. The PfGCα gene is expressed in both asexual and sexual stages [61] and cannot be deleted [62] suggesting its essentiality. Although previous pharmacological studies suggested the role of cGMP in exflagellation, recently it has been shown that the disruption of PfGCβ has no effect on gametogenesis [62]. However, disruption of phosphodiesterase PfPDE) δgene affects gametogenesis. This suggests the importance of PfPDE in maintaining the level of cGMP during sexual development of the parasite.
3.3. MAP kinase pathway
The mitogen-activated protein (MAP) kinases play a central role in coordinating activity of multiple intracellular mediators. P. falciparum genome encodes two homologues of MAP kinases [42], pfmap-1 and pfmap-2, and both loci have been disrupted to understand their function. While pfmap-1 knock-out lines do not have any phenotype in erythrocytic schizogony and sporogony, pfmap-2 is essential for asexual growthand the loci can only be disrupted when an episomal copy of pfmap-2 is present suggesting its essentiality [63]. Interestingly, the P. berghei homologue of PfMAP-2 was shown to be nonessential in asexual stages and gametocytes but is important for male gamete formation [64,65]. It is noteworthy that there are no unambiguous orthologues of MAP kinase kinase (MAPKK) or MEK in P. falciparum. PfPK7 appears to be a novel chimeric protein whose C-terminal region has identity with MEKs, whereas the N-terminal lobe shows homology to fungal protein kinase A [66]. Furthermore, PfPK7 does not contain the activation site in its T-loop and is insensitive to MEK and PKA inhibitors [67]. Recent determination of PfPK7 structure at 3.7Å resolution showed, however, that its structure is similar to TAO2 kinase, a MAP3KKK [68]. Although PfPK7 was not essential for the asexual growth, malaria parasites in which pfpk7 locus was disrupted grows slowly with a reduced number of merozoites per segmenters compared to the wild type [66]. PfPK7 deficient parasite lines also have severe defects in oocysts production [66].
Recently, it has been suggested that PfNek3, one of the P. falciparium homologue of NIMA-like kinases that are involved in cell cycle regulation, particularly G2/M transition, in eukaryotes [69] activates PfMAP2 in vitro through phosphorylation, a feature that had previously been described for Pfnek-1 [70–72]. These results underscore unique function of Plasmodium kinases that will be difficult to perceive by homology analysis. NIMA-related kinases (NEKs) usually regulate cell cycle progression in eukaryotes [73]. Plasmodium genome encodes four homologues of these proteins that are expressed mainly in gametocytes [74]. Although PfNek4 is expressed in gametocytes, its disruption in the P. berghei has no influence in gamete formation or fertilization of gametes but differentiation of zygotes to ookinetes is interrupted [75]. PbNek4 was shown to be essential for the replication of diploid zygote genome before meiosis ensues.
3.4. CDK-like kinases and other putative cell cycle kinases
Although the developmental stages of malaria parasite are unique and complex, it is expected that proteins belonging to the CDK-related subfamily will be key regulators in the Plasmodium similar to eukaryotic cell cycle. Among the Pf protein kinases clustering within the CMGC group (to which CDKs and MAP kinases belong), PfPK5 clusters with CDK1/2 and Pfcrk-1 with CDK10/11. PfPK5 was the first CDK-like kinase characterized in P. falciparum with 60% identity to human CDK1 [76]. PfPK5 is expressed throughout erythrocytic schizogony, and immunoprecipitation experiments using synchronized parasite extracts showed that PfPK5 activity peaks at the schizont stage around 36 h post-invasion [77,78]. PfPK5 also colocalizes with the nuclear stain [78]. The structure of PfPK5 has been determined to a resolution of 1.9Å [79]. PfPK5 has structurally homology to human CDK2, the only other monomeric CDK structure solved.
PfPK6 is novel protein showing identity to both CDKs and MAP kinases by differential display RT-PCR of mRNA samples undergoing transition from ring to schizonts [80]. Molecular modeling data suggests that PfPK6 is more closely related to the CDKs [81,82] rather than MAP kinases. The PSTAIRE motif is replaced by a SKCILRE sequence in PfPK6, but the sites of regulatory phosphorylation are conserved. PfPK6 appears to be a novel cyclin-independent kinase. Another CDK-related kinase identified was Pfmrk, a homologue of the Mo15/CDK7 CDK-activating kinase [83]. Recombinant Pfmrk displays very little histone kinase activity as a monomer, but can be activated by the presence of human cyclin H and Pfcyclin-1 [84,85]. Recently, it was shown that Pfmrk is activated by PfMAT1 homologue in presence of cyclin, similar to what is observed with CDK7/cyclinH-MAT complex in other eukaryotes [86]. Pfcrk-1, Pfcrk-3, and Pfcrk-5 are other CDK-like kinases [81]. Pfcrk-1 is not expected to be a functional homolog of eukaryotic CDK1/2; instead it belongs to the p58GTA gene family that is a negative regulator of cell growth [87]. Pfcrk-1 exhibits peak expression in gametocytes, but the P. berghei orthologue was shown to be essential for completion of the asexual cycle [88]. Four P. falciparum cyclin homologues, Pfcyc1–4, have been identified [84,89]. Pfcyc1 has maximum homology to the cyclin H family, an activator ofCDK7. As expected, Pfcyc1 activated Pfmrk (a putative CDK7 homologue) [85] but, surprisingly, Pfcyc-1 also activated PfPK5 [84]. Members of the cyclin H family are specific activators of CDK7 and not CDK1 or CDK5 (to which PfPK5 has the highest homology). PfPK5 has also been shown to be activated by mammalian cyclin A, p25 and RINGO [74,84,89]; such promiscuity for various cyclin-related proteins has not been reported for mammalian or yeast CDKs. Both p25 and RINGO are non-cyclin CDK activators from vertebrates. Three additional cyclins, Pfcyc2, Pfcyc3, and Pfcyc4 have been identified recently [90]. Pull-downand co-immunoprecipitation experiments showed that these cyclins associate with histone H1 kinase activity in parasite extracts. Furthermore, Pfcyc3 activates PfPK5 in vitro.
3.5. Novel FIKK kinases
Of all PfPKs identified, the presence of a novel family of 20 PKs is particularly noteworthy [42,90]. This family of kinases is termed FIKK based on a conserved amino acid sequence motif present [42]. All family members contain a non-conserved N-terminal domain and a conserved kinase domain in the C-terminus. The FIKK kinases contain all residues that are important for catalytic activity except the Glycine triad in subdomain I. The N-terminal domain is not conserved among paralogs and this region contains a stretch of hydrophobic residues corresponding to a predicted trans-membrane or signal sequence [90].A recently described host-targeting (HT) signal motif RxSRILAExxx [91] is present in six FIKK paralogs, whereas the Plasmodium export element (Pexel) RxLx(D, E, Q) [92] can be detected in all FIKK paralogs downstream of the signal sequence. Because HT/PEXEL motifs have been shown to mediate export of proteins beyond the parasitophorous vacuole into the erythrocyte cytoplasm [91,92], it is expected that FIKK kinases are trafficked to erythrocytes. Indeed, GFP-fusion protein of one of the FIKK kinases, FIKK12, was shown to be exported to the erythrocytes and associates with Maurer’s clefts [93]. Although protein kinase activity of FIKK12 was detected in immunoprecipitates [93], recombinant FIKK 11 and FIKK 10.1 did not show any protein kinase activity using a peptide phosphorylation motif array [Turk and Chakrabarti, unpublished]. FIKK kinases may have a role in parasite-induced signaling events, given that members of this family are exported into the erythrocytes, associate with Maurer’s clefts, and one of the paralogs, R45, is trafficked to the host cell membrane [94].
4. Concluding remarks
The knowledge of signaling transduction pathways in Plasmodium is fundamental to aid the design of new strategies against malaria. The finding that Plasmodium possesses serpentine receptors [38] opens new possibilities to dissect the upstream mechanisms through which Plasmodium senses the environment. On the other hand, the use of second messengers by parasites such as cAMP and calcium has long been suggested in the literature and finding their target could bring invaluable information regarding Plasmodium cell biology. Together with downstream mechanisms for signaling in Plasmodium they will provide a more complete picture of how Plasmodium signaling handling machinery is put in action.
Precise delineation of Plasmodium protein kinase functions as key regulators of cellular events will be a major challenge of the post-genome project era. It is apparent from earlier discussions that it will be difficult to ascertain physiological roles of Pf kinases simply based on homology because many of these proteins are expected to possess parasite-specific function as a means of regulating complex life cycle events. Therefore, characterization of physiological function of Plasmodium kinases will not be a mere repetition of what is already known in model organisms but will provide novel parasite specific information and fill a major gap in our understanding of the malaria parasite life cycle. Studies on the malarial protein kinases, their regulators and substrates will also provide new avenues of drug design targeting intraerythrocytic stages. Targeting protein kinase substrates rather than typical ATP-binding pocket will allow us to inhibit specific physiological events. Although targeting protein–protein interactions can be challenging because of complexity and diversity of binding surfaces, there have been recent progresses towards developing such therapeutic intervention approaches [95]. One such method is known as ‘fragment assembly’ that probes large chemical space as seen with interacting surfaces between proteins [96–98]. Alternatively, interfering peptidomimetics can also be developed. A long-term goal of this project is to use similar approaches can be utilized to identify molecular entity targeting malaria parasite kinases.
Acknowledgements
We thank Fundação de Amparo à pesquisa de São Paulo (Fapesp) and MS-CNPq for funding C.R.S.G. F.C.K. received fellowship from FAPESP. The work in D.C. laboratory is supported in part by a National Institutes of Health Grant AI73795. We thank Dr. Christian Doerig for critical reading the manuscript.
References
- 1.Naughton JA, Bell A. Studies on cell-cycle synchronization in the asexual erythrocytic stages of Plasmodium falciparum. Parasitology. 2007;134(Pt 3):331–337. doi: 10.1017/S0031182006001466. [DOI] [PubMed] [Google Scholar]
- 2.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldauf SL. The deep roots of eukaryotes. Science. 2003;300(5626):1703–1706. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med. 1989;169(1):357–361. doi: 10.1084/jem.169.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418–420. [PubMed] [Google Scholar]
- 6.Hotta CT, Markus RP, Garcia CR. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz J Med Biol Res. 2003;36(11):1583–1587. doi: 10.1590/s0100-879x2003001100016. [DOI] [PubMed] [Google Scholar]
- 7.Hammarton TC, Mottram JC, Doerig C. The cell cycle of parasitic protozoa: potential for chemotherapeutic exploitation. Prog Cell Cycle Res. 2003;5:91–101. [PubMed] [Google Scholar]
- 8.Garcia CR, de Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L. Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. Int Rev Cell Mol Biol. 2008;266:85–156. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- 9.Coppi A, Merali S, Eichinger D. The enteric parasite Entamoeba uses an autocrine catecholamine systemduring differentiation into the infectious cyst stage. J Biol Chem. 2002;277(10):8083–8090. doi: 10.1074/jbc.M111895200. [DOI] [PubMed] [Google Scholar]
- 10.Kugler S, Schurtz Sebghati T, Groppe Eissenberg L, Goldman WE. Phenotypic variation and intracellular parasitism by histoplasma Capsulatum. Proc Natl Acad Sci USA. 2000;97(16):8794–8798. doi: 10.1073/pnas.97.16.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Jayaraman A, Wood TK. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 2007;7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chant EL, Summers DK. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol. 2007;63(1):35–43. doi: 10.1111/j.1365-2958.2006.05481.x. [DOI] [PubMed] [Google Scholar]
- 13.Varotti FP, Beraldo FH, Gazarini ML, Garcia CR. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium. 2003;33(2):137–144. doi: 10.1016/s0143-4160(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 14.Gazarini ML, Garcia CR. The malaria parasite mitochondrion senses cytosolic Ca2+ fluctuations. Biochem Biophys Res Commun. 2004;321(1):138–144. doi: 10.1016/j.bbrc.2004.06.141. [DOI] [PubMed] [Google Scholar]
- 15.Garcia CR, Ann SE, Tavares ES, Dluzewski AR, Mason WT, Paiva FB. Acidic calcium pools in intraerythrocytic malaria parasites. Eur J Cell Biol. 1998;76(2):133–138. doi: 10.1016/S0171-9335(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 16.Marchesini N, Luo S, Rodrigues CO, Moreno SN, Docampo R. Acidocalcisomes and a vacuolar H+-pyrophosphatase in malaria parasites. Biochem J. 2000;347(Pt 1):243–253. [PMC free article] [PubMed] [Google Scholar]
- 17.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117(4):503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 18.Caldas ML, Wasserman M. Cytochemical localisation of calcium ATPase activity during the erythrocytic cell cycle of Plasmodium falciparum. Int J Parasitol. 2001;31(8):776–782. doi: 10.1016/s0020-7519(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 19.Hotta CT, Gazarini ML, Beraldo FH, et al. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol. 2000;2:466–546. doi: 10.1038/35017112. [DOI] [PubMed] [Google Scholar]
- 20.Beraldo FH, Garcia C. Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J Pineal Res. 2005;39:224–230. doi: 10.1111/j.1600-079X.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 21.Ono T, Cabrita-Santos L, Leitao R, et al. Adenylyl cyclase a and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog. 2008;4(2):e1000008. doi: 10.1371/journal.ppat.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inselburg J. Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. J Parasitol. 1983;69:584–591. [PubMed] [Google Scholar]
- 23.Kaushal DC, Carter R, Miller LH, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286:490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 24.Trager W, Gill GS. Plasmodium falciparum gametocyte formation in vitro: its stimulation by phorbol diesters and by 8-bromo cyclic adenosine monophosphate. J Protozool. 1989;36:451–454. doi: 10.1111/j.1550-7408.1989.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 25.Dyer M, Day K. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol Biochem Parasitol. 2000;110:437–448. doi: 10.1016/s0166-6851(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 26.Martin SK, Jett M, Schneider I. Correlation of phosphoinositide hydrolysis with exflagellation in the malaria microgametocyte. J Parasitol. 1994;80(3):371–378. [PubMed] [Google Scholar]
- 27.Passos A, Garcia C. Inositol 1,4,5-trisphosphate induced Ca2+ release from chloroquine-sensitive and -insensitive intracellular stores in the intraerythrocytic stage of the malaria parasite P. chabaudi. Biochem Biophys Res Commun. 1998;245:155–160. doi: 10.1006/bbrc.1998.8338. [DOI] [PubMed] [Google Scholar]
- 28.McRobert L, Taylor CJ, Deng W, et al. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008 June 6;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazarini ML, Thomas AP, Pozzan T, Garcia CR. Calcium signaling in a lowcalcium environment: how the intracellular malaria parasite solves the problem. J Cell Biol. 2003;161(1):103–110. doi: 10.1083/jcb.200212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beraldo FH, Mikoshiba K, Garcia CR. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J Pineal Res. 2007;43(4):360–364. doi: 10.1111/j.1600-079X.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 31.Budu A, Peres R, Bueno VB, Catalani LH, Garcia CR. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J Pineal Res. 2007;42(3):261–266. doi: 10.1111/j.1600-079X.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 32.Bagnaresi P, Markus RP, Hotta CT, Pozzan T, Garcia CRS. Desynchronizing plasmodium cell cycle increases chloroquine protection at suboptimal doses. Open Parasitol J. 2008;2:55–58. [Google Scholar]
- 33.Farias SL, Gazarini ML, Melo RL, et al. Cysteine-protease activity elicited by Ca2+ stimulus in Plasmodium. Mol Biochem Parasitol. 2005;141(1):71–79. doi: 10.1016/j.molbiopara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Bagnaresi P, Alves E, da Silva HB, Epiphanio S, Mota MM, Garcia CRS. Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P. yoelii asynchronous infections are not affected by melatonin. Int J Gen Med. 2009 doi: 10.2147/ijgm.s3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubocovich ML. Melatonin receptors: are there multiple subtypes? Trends Pharmacol Sci. 1995;16(2):50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- 36.Prabhu Y, Mondal S, Eichinger L, Noegel AA. A GPCR involved in post aggregation events in Dictyostelium discoideum. Dev Biol. 2007;312(1):29–43. doi: 10.1016/j.ydbio.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 37.Hamdan FF, Abramovitz M, Mousa A, Xie J, Durocher Y, Ribeiro P. A novel Schistosoma mansoni G protein-coupled receptor is responsive to histamine. Mol Biochem Parasitol. 2002;119(1):75–86. doi: 10.1016/s0166-6851(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 38.Madeira L, Galante PA, Budu A, Azevedo MF, Malnic B, Garcia CR. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS ONE. 2008;3(3):e1889. doi: 10.1371/journal.pone.0001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes MC, Scherf A. Plasmodium falciparum during pregnancy: a puzzling parasite tissue adhesion tropism. Parasitology. 2007;134(Pt 13):1863–1869. doi: 10.1017/S0031182007000133. [DOI] [PubMed] [Google Scholar]
- 40.Harrison T, Samuel BU, Akompong T, et al. Erythrocyte G protein-coupled receptor signaling in malarial infection. Science. 2003;301:1734. doi: 10.1126/science.1089324. [DOI] [PubMed] [Google Scholar]
- 41.Lauer S, VanWye J, Harrison T, et al. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 2000;19(14):3556–3564. doi: 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genom. 2004;5(1):79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anamika, Srinivasan N, Krupa A. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins. 2005;58(1):180–189. doi: 10.1002/prot.20278. [DOI] [PubMed] [Google Scholar]
- 44.Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta. 2004;1697(1–2):155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 46.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59(4):1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 47.Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol. 2006;60(6):1355–1363. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppi A, Tewari R, Bishop JR, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microb. 2007;2(5):316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato N, Sakata T, Breton G, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4(6):347–356. doi: 10.1038/nchembio.87. [DOI] [PubMed] [Google Scholar]
- 50.Moskes C, Burghaus PA, Wernli B, Sauder U, Durrenberger M, Kappes B. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Mol Microbiol. 2004;54(3):676–691. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- 51.Kugelstadt D, Winter D, Pluckhahn K, Lehmann WD, Kappes B. Raf kinase inhibitor protein affects activity of Plasmodium falciparum calcium-dependent protein kinase 1. Mol Biochem Parasitol. 2007;151(1):111–117. doi: 10.1016/j.molbiopara.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Madeira L, DeMarco R, Gazarini ML, Verjovski-Almeida S, Garcia CR. Human malaria parasites display a receptor for activated C kinase ortholog. Biochem Biophys Res Commun. 2003;306(4):995–1001. doi: 10.1016/s0006-291x(03)01074-x. [DOI] [PubMed] [Google Scholar]
- 53.Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum: II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. J Biol Chem. 2006;281(37):27126–27133. doi: 10.1074/jbc.M601914200. [DOI] [PubMed] [Google Scholar]
- 54.Vaid A, Thomas DC, Sharma P. Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. J Biol Chem. 2008;283(9):5589–5597. doi: 10.1074/jbc.M708465200. [DOI] [PubMed] [Google Scholar]
- 55.Muhia DK, Swales CA, Eckstein-Ludwig U, et al. Multiple splice variants encode a novel adenylyl cyclase of possible plastid origin expressed in the sexual stage of the malaria parasite Plasmodium falciparum. J Biol Chem. 2003;278(24):22014–22022. doi: 10.1074/jbc.M301639200. [DOI] [PubMed] [Google Scholar]
- 56.Baker DA, Kelly JM. Purine nucleotide cyclases in the malaria parasite. Trends Parasitol. 2004;20(5):227–232. doi: 10.1016/j.pt.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Kaushal DC, Carter R, Miller LH, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286(5772):490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 58.Syin C, Parzy D, Traincard F, et al. The H89 cAMP-dependent protein kinase inhibitor blocks Plasmodium falciparum development in infected erythrocytes. Eur J Biochem. 2001;268(18):4842–4849. doi: 10.1046/j.1432-1327.2001.02403.x. [DOI] [PubMed] [Google Scholar]
- 59.Merckx A, Nivez MP, Bouyer G, et al. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathog. 2008;4(2):e19. doi: 10.1371/journal.ppat.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carucci DJ, Witney AA, Muhia DK, et al. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J Biol Chem. 2000;275(29):22147–22156. doi: 10.1074/jbc.M001021200. [DOI] [PubMed] [Google Scholar]
- 61.Young JA, Fivelman QL, Blair PL, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143(1):67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Taylor CJ, McRobert L, Baker DA. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol Microbiol. 2008;69(1):110–118. doi: 10.1111/j.1365-2958.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorin-Semblat D, Quashie N, Halbert J, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol Microbiol. 2007;65(5):1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 64.Rangarajan R, Bei AK, Jethwaney D, et al. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Rep. 2005;6(5):464–469. doi: 10.1038/sj.embor.7400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005;58(5):1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 66.Dorin-Semblat D, Sicard A, Doerig C, Ranford-Cartwright L. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum. Eukaryot Cell. 2008;7(2):279–285. doi: 10.1128/EC.00245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorin D, Semblat JP, Poullet P, et al. PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2005;55(1):184–196. doi: 10.1111/j.1365-2958.2004.04393.x. [DOI] [PubMed] [Google Scholar]
- 68.Merckx A, Echalier A, Langford K, et al. Structures of P. falciparum protein kinase 7 identify an activation motif and leads for inhibitor design. Structure. 2008;16(2):228–238. doi: 10.1016/j.str.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Osmani SA, Pu RT, Morris NR. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- 70.Lye YM, Chan M, Sim TS. Pfnek3: an atypical activator of a MAP kinase in Plasmodium falciparum. FEBS Lett. 2006;580(26):6083–6092. doi: 10.1016/j.febslet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Low H, Lye YM, Sim TS. Pfnek3 functions as an atypical MAPKK in Plasmodium falciparum. Biochem Biophys Res Commun. 2007;361(2):439–444. doi: 10.1016/j.bbrc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 72.Dorin D, Le Roch K, Sallicandro P, et al. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum biochemical properties and possible involvement in MAPK regulation. Eur J Biochem. 2001;268(9):2600–2608. doi: 10.1046/j.1432-1327.2001.02151.x. [DOI] [PubMed] [Google Scholar]
- 73.O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13(5):221–228. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 74.Le Roch KG, Zhou Y, Blair PL, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 75.Reininger L, Billker O, Tewari R, et al. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem. 2005;280(36):31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- 76.Ross-Macdonald PB, Graeser R, Kappes B, Franklin R, Williamson DH. Isolation and expression of a gene specifying a cdc2-like protein kinase from the human malaria parasite Plasmodium falciparum. Eur J Biochem. 1994;220(3):693–701. doi: 10.1111/j.1432-1033.1994.tb18670.x. [DOI] [PubMed] [Google Scholar]
- 77.Graeser R, Wernli B, Franklin RM, Kappes B. Plasmodium falciparum protein kinase 5 and the malarial nuclear division cycles. Mol Biochem Parasitol. 1996;82(1):37–49. doi: 10.1016/0166-6851(96)02716-8. [DOI] [PubMed] [Google Scholar]
- 78.Graeser R, Franklin RM, Kappes B. Mechanisms of activation of the cdc2-related kinase PfPK5 from Plasmodium falciparum. Mol Biochem Parasitol. 1996;79(1):125–127. doi: 10.1016/0166-6851(96)02643-6. [DOI] [PubMed] [Google Scholar]
- 79.Holton S, Merckx A, Burgess D, Doerig C, Noble M, Endicott J. Structures of P. falciparum PfPK5 test the CDK regulation paradigm and suggestmechanisms of small molecule inhibition. Structure. 2003;11(11):1329–1337. doi: 10.1016/j.str.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 80.Bracchi-Ricard V, Barik S, Delvecchio C, Doerig C, Chakrabarti R, Chakrabarti D. PfPK6, a novel cyclin-dependent kinase/mitogen-activated protein kinase-related protein kinase from Plasmodium falciparum. Biochem J. 2000;347(Pt 1):255–263. [PMC free article] [PubMed] [Google Scholar]
- 81.Doerig C, Endicott J, Chakrabarti D. Cyclin-dependent kinase homologues of Plasmodium falciparum. Int J Parasitol. 2002;32(13):1575–1585. doi: 10.1016/s0020-7519(02)00186-8. [DOI] [PubMed] [Google Scholar]
- 82.Manhani KK, Arcuri HA, da Silveira NJ, Uchoa HB, de Azevedo WF, Jr, Canduri F. Molecular models of protein kinase 6 from Plasmodium falciparum. J Mol Model. 2005;12(1):42–48. doi: 10.1007/s00894-005-0002-1. [DOI] [PubMed] [Google Scholar]
- 83.Li JL, Robson KJ, Chen JL, Targett GA, Baker DA. Pfmrk, a MO15-related protein kinase from Plasmodium falciparum. Gene cloning, sequence, stagespecific expression and chromosome localization. Eur J Biochem. 1996;241(3):805–813. doi: 10.1111/j.1432-1033.1996.00805.x. [DOI] [PubMed] [Google Scholar]
- 84.Le Roch K, Sestier C, Dorin D, et al. Activation of a Plasmodium falciparum cdc2-related kinase by heterologous p25 and cyclin H. Functional characterization of a P. falciparum cyclin homologue. J Biol Chem. 2000;275(12):8952–8958. doi: 10.1074/jbc.275.12.8952. [DOI] [PubMed] [Google Scholar]
- 85.Waters NC, Woodard CL, Prigge ST. Cyclin H activation and drug susceptibility of the Pfmrk cyclin dependent protein kinase from Plasmodium falciparum. Mol Biochem Parasitol. 2000;107(1):45–55. doi: 10.1016/s0166-6851(99)00229-7. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y, Jirage D, Caridha D, et al. Identification of an effector protein and gain-of-function mutants that activate Pfmrk, a malarial cyclin-dependent protein kinase. Mol Biochem Parasitol. 2006;149(1):48–57. doi: 10.1016/j.molbiopara.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Doerig C, Horrocks P, Coyle J, et al. Pfcrk-1, a developmentally regulated cdc2-related protein kinase of Plasmodium falciparum. Mol Biochem Parasitol. 1995;70(1–2):167–174. doi: 10.1016/0166-6851(95)00033-w. [DOI] [PubMed] [Google Scholar]
- 88.Rangarajan R, Bei A, Henry N, et al. Pbcrk-1, the Plasmodium berghei orthologue of P. falciparum cdc-2 related kinase-1 (Pfcrk-1), is essential for completion of the intraerythrocytic asexual cycle. Exp Parasitol. 2006;112(3):202–207. doi: 10.1016/j.exppara.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Merckx A, Le Roch K, Nivez MP, et al. Identification and initial characterization of three novel cyclin-related proteins of the human malaria parasite Plasmodium falciparum. J Biol Chem. 2003;278(41):39839–39850. doi: 10.1074/jbc.M301625200. [DOI] [PubMed] [Google Scholar]
- 90.Schneider AG, Mercereau-Puijalon O. A new Apicomplexa-specific protein kinase family: multiple members in Plasmodium falciparum, all with an export signature. BMC Genom. 2005;6(1):30. doi: 10.1186/1471-2164-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hiller NL, Bhattacharjee S, van Ooij C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306(5703):1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 92.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306(5703):1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 93.Nunes MC, Goldring JP, Doerig C, Scherf A. A novel protein kinase family in Plasmodium falciparum is differentially transcribed and secreted to various cellular compartments of the host cell. Mol Microbiol. 2007;63(2):391–403. doi: 10.1111/j.1365-2958.2006.05521.x. [DOI] [PubMed] [Google Scholar]
- 94.Bonnefoy S, Guillotte M, Langsley G, Mercereau-Puijalon O. Plasmodium falciparum: characterization of gene R45 encoding a trophozoite antigen containing a central block of six amino acid repeats. Exp Parasitol. 1992;74(4):441–451. doi: 10.1016/0014-4894(92)90206-p. [DOI] [PubMed] [Google Scholar]
- 95.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3(4):301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 96.Boehm HJ, Boehringer M, Bur D, et al. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J Med Chem. 2000;43(14):2664–2674. doi: 10.1021/jm000017s. [DOI] [PubMed] [Google Scholar]
- 97.Shuker SB, Hajduk PJ, Meadows RP, Fesik SW. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274(5292):1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 98.Erlanson DA, Braisted AC, Raphael DR, et al. Site-directed ligand discovery. Proc Natl Acad Sci USA. 2000;97(17):9367–9372. doi: 10.1073/pnas.97.17.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]