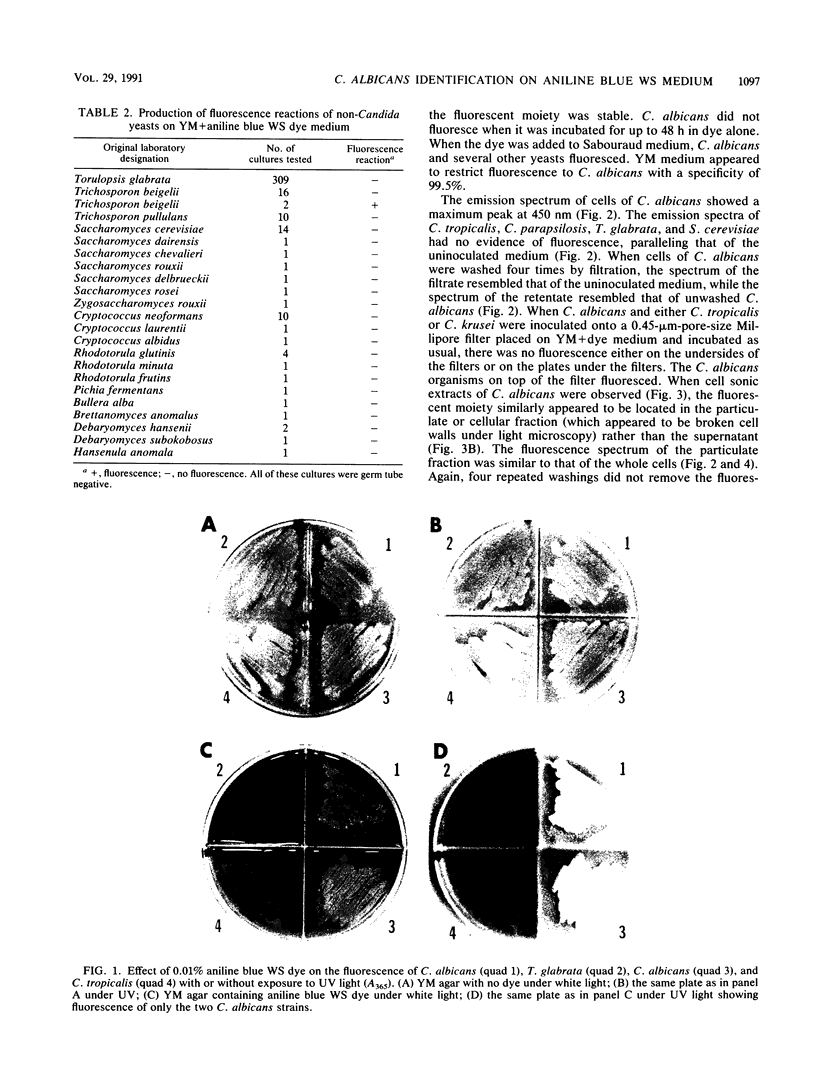
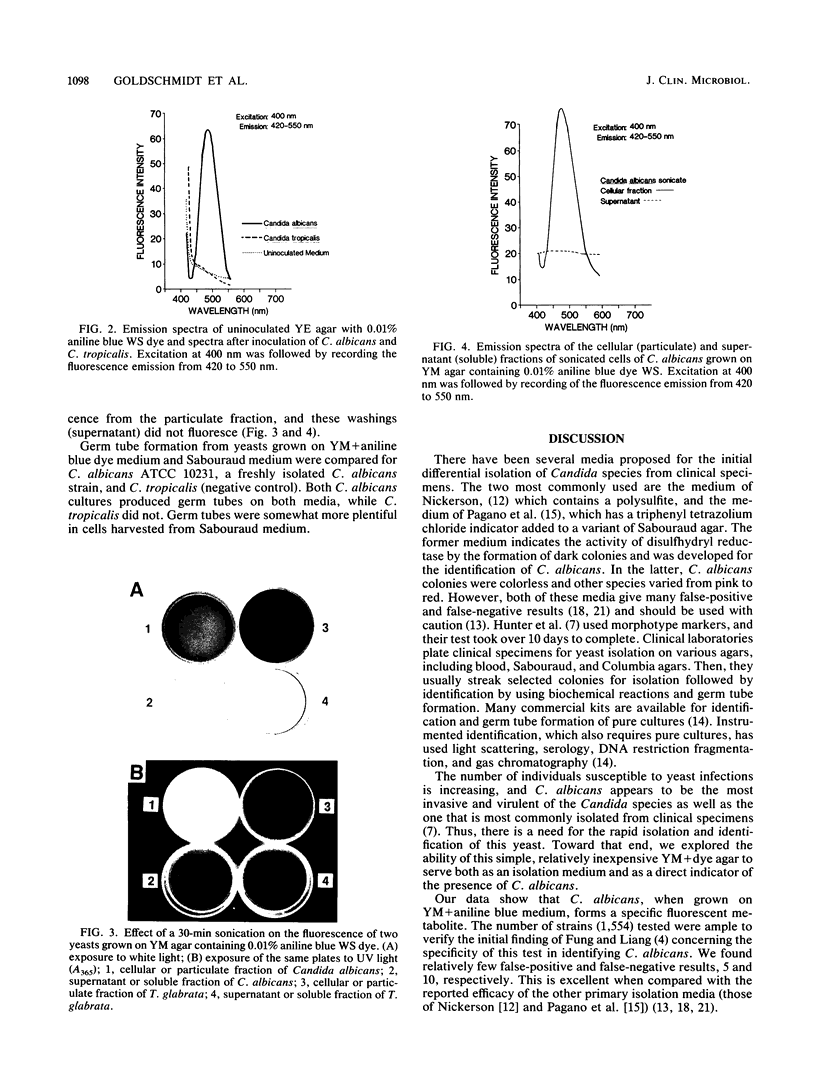
Abstract
Organic dyes have long been used in diagnostic microbiology to differentiate species by color reactions. We studied the ability of a new noninhibitory medium, YM agar containing 0.01% aniline blue WS dye, Colour Index 42780 (YMAB), to identify Candida albicans among 1,554 yeast specimens obtained from seven clinical laboratories. Appropriate American Type Culture Collection and other characterized strains served as controls. A total of 487 of the clinical strains were identified as C. albicans. The remainder were other Candida species and non-Candida yeasts. Clinical isolates and controls were grown on Sabouraud agar for 18 h at 30 degrees C and then transferred to YMAB. Plates were incubated for 12 to 18 h at 30 degrees C, and colonies were observed for yellow-green fluorescence under long-wave UV light (A365). All control strains of C. albicans and Candida stellatoidea fluoresced, as did 480 of the 490 isolates designated as C. albicans (which included 3 strains of C. stellatoidea). Cells of C. albicans grown on YMAB produced germ tubes in serum. Only five of the other 1,062 non-C. albicans yeasts fluoresced. The sensitivity and specificity were 98.0 and 99.5%, respectively, with a predictive value of 99.1%. A fluorescent metabolite was found in cell wall particulate fractions of C. albicans sonic extracts grown on YMAB but not in non-C. albicans yeasts. This metabolite showed the same spectral curve as those of metabolites from whole cells in a recording spectrofluorometer when it was excited at 400 nm and scanned from 420 to 550 nm. Thus, growth on YMAB generates the production of a fluorescent moiety that can be used to specifically identify C. albicans within 12 to 18 h.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fattorossi A., Nisini R., Pizzolo J. G., D'Amelio R. New, simple flow cytometry technique to discriminate between internalized and membrane-bound particles in phagocytosis. Cytometry. 1989 May;10(3):320–325. doi: 10.1002/cyto.990100311. [DOI] [PubMed] [Google Scholar]
- Folb P. I., Trounce J. R. Immunological aspects of candida infection complicating steroid and immunosuppressive drug therapy. Lancet. 1970 Nov 28;2(7683):1112–1114. doi: 10.1016/s0140-6736(70)92299-3. [DOI] [PubMed] [Google Scholar]
- Hunter P. R., Fraser C. A., Mackenzie D. W. Morphotype markers of virulence in human candidal infections. J Med Microbiol. 1989 Feb;28(2):85–91. doi: 10.1099/00222615-28-2-85. [DOI] [PubMed] [Google Scholar]
- Ito-Kuwa S., Aoki S., Watanabe T., Ehara T., Osafune T. Fluorescence microscopic studies on mitochondria and mitochondrial nucleoids in a wild-type strain and respiratory mutants of Candida albicans. J Med Vet Mycol. 1988;26(4):207–217. doi: 10.1080/02681218880000301. [DOI] [PubMed] [Google Scholar]
- Krogh P., Holmstrup P., Vedtofte P., Pindborg J. J. Yeast organisms associated with human oral leukoplakia. Acta Derm Venereol Suppl (Stockh) 1986;121:51–55. [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuda S., Tegoshi T. Yeast glucan in the cyst wall of Pneumocystis carinii. J Protozool. 1989 Jan-Feb;36(1):21S–22S. doi: 10.1111/j.1550-7408.1989.tb02674.x. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. Reduction of inorganic substances by yeasts. I. Extracellular reduction of sulfite by species of Candida. J Infect Dis. 1953 Jul-Aug;93(1):43–56. doi: 10.1093/infdis/93.1.43. [DOI] [PubMed] [Google Scholar]
- PAGANO J., LEVIN J. D., TREJO W. Diagnostic medium for differentiation of species of Candida. Antibiot Annu. 1957;5:137–143. [PubMed] [Google Scholar]
- Perry J. L., Miller G. R., Carr D. L. Rapid, colorimetric identification of Candida albicans. J Clin Microbiol. 1990 Mar;28(3):614–615. doi: 10.1128/jcm.28.3.614-615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt M. L., Belville W. D., Stones C., Oberhofer T. R. Rapid bacteriuria screening in a urological setting: clinical use. J Urol. 1986 Nov;136(5):1044–1046. doi: 10.1016/s0022-5347(17)45202-5. [DOI] [PubMed] [Google Scholar]
- Schnell J. D. Investigations into the pathoaetiology and diagnosis of vaginal mycoses. Chemotherapy. 1982;28 (Suppl 1):14–21. doi: 10.1159/000238147. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Monga D. P., Prasad S. A combination of Gomori-Grocott methenamine silver nitrate and hematoxylene and eosin staining technique for the demonstration of Candida albicans in tissue. Quad Sclavo Diagn. 1988 Jan-Dec;24(1-4):129–132. [PubMed] [Google Scholar]
- Sinski J. T., Kelley L. M., Reed G. L. Pagano-Levin Candida test medium: evaluation using vaginal samples. J Clin Microbiol. 1975 Feb;1(2):206–211. doi: 10.1128/jcm.1.2.206-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitka C. M., Jackson S. G. Rapid fluorogenic assay for differentiation of the Candida parapsilosis group from other Candida spp. J Clin Microbiol. 1989 Jan;27(1):203–206. doi: 10.1128/jcm.27.1.203-206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]