Abstract
The serotonergic (5-HT) system in the human medulla oblongata is well-recognized to play an important role in the regulation of respiratory and autonomic function. In this study, using both immunocytochemistry (n=5) and tissue section autoradiography with the radioligand 125I-1-(2,5-dimethoxy-4-iodo-phenyl)2-aminopropane (n=7), we examine the normative development and distribution of the 5-HT2A receptor in the human medulla during the last part of gestation and first postnatal year when dramatic changes are known to occur in respiratory and autonomic control, in part mediated by the 5-HT2A receptor. High 5-HT2A receptor binding was observed in the dorsal motor nucleus of the vagus (preganglionic parasympathetic output) and hypoglossal nucleus (airway patency); intermediate binding was present in the nucleus of the solitary tract (visceral sensory input), gigantocellularis, intermediate reticular zone, and paragigantocellularis lateralis. Negligible binding was present in the raphé obscurus and arcuate nucleus. The pattern of 5-HT2A immunoreactivity paralleled that of binding density. By 15 gestational weeks, the relative distribution of the 5-HT2A receptor was similar to that in infancy. In all nuclei sampled, 5-HT2A receptor binding increased with age, with significant increases in the hypoglossal nucleus (p=0.027), principal inferior olive (p=0.044), and medial accessory olive (0.038). Thus, 5-HT2A receptors are concentrated in regions involved in autonomic and respiratory control in the human infant medulla, and their developmental profile changes over the first year of life in the hypoglossal nucleus critical to airway patency and the inferior olivary complex essential to cerebellar function.
Keywords: airway patency, autoradiography, hypoglossal nucleus, long-term facilitation, preBötzinger complex, sudden infant death syndrome
INTRODUCTION
Serotonergic (5-HT) neurons in the medulla oblongata comprise a critical system involved in the modulation of autonomic and respiratory effector neurons in a state-dependent manner (Azmitia, 1999; Kinney et al., 2007; Kinney et al., 2001; Lovick, 1997; Mason, 2001; Morrison, 2001). These 5-HT neurons are organized in midline (raphé), lateral (extra-raphé), and ventral surface regions such that they virtually encircle and project to the adjacent tegmental nuclei in the medulla that mediate respiration, upper airway patency, heart rate, blood pressure, thermoregulation, and arousal (Kinney et al., 2007). The specific effects of 5-HT upon the autonomic and respiratory effector nuclei are mediated via different 5-HT receptor subtypes, of which the most information relevant to homeostasis is available for the 5-HT1A, 5-HT2A, and 5-HT3 receptors (Cayetanot et al., 2002; Darnall et al., 2005; Helke et al., 1997; Helke et al., 1993; King and McCall, 1991; Knapp et al., 1998; Kubo et al., 1995; Lalley, 1994; Lalley et al., 1994; Li et al., 1999; Ling et al., 2001; Lovick, 1989; Nosjean and Guyenet, 1991; Onimaru et al., 1998; Paterson et al., 2006a; Pena and Ramirez, 2002; Penatti et al., 2006; Rose et al., 1995; Schwarzacher et al., 2002; Talley et al., 1997; Teng et al., 2003; Tryba et al., 2006; Wilken et al., 1997). There is increasing interest in the medullary 5-HT system in human pediatric neuropathology because of the evidence for 5-HT dysfunction in major developmental brainstem disorders, e.g., sudden infant death syndrome (SIDS) (Kinney et al., 2001; Kinney et al., 2005; Kinney et al., 2003; Machaalani et al., 2008; Ozawa and Okado, 2002; Panigrahy et al., 2000; Paterson et al., 2006b) and Rett syndrome (Paterson et al., 2005). Yet, little is known about the normative development of the different 5-HT receptor subtypes in the human brainstem—information that is essential towards defining 5-HT receptor pathology in pediatric disorders.
Previously we described the developmental distribution of the 5-HT1A receptor in the human infant medulla (Paterson et al., 2004). In the following study, we examine the normative development and distribution of the 5-HT2A receptor in the human medulla during the same developmental period. We chose to focus upon the 5-HT2A receptor because extensive experimental analysis indicates a critical role for the 5-HT2A receptor in respiration governed by the preBötzinger complex (Pena and Ramirez, 2002; Ramirez et al., 2004), gasping (Tryba et al., 2006), central cardiovascular regulation including the baroreceptor reflex (Comet et al., 2007; Dergacheva et al., 2007; Raul, 2003; Shen et al., 2007; Villalon and Centurion, 2007), the development of the medullary and spinal cord respiratory network (Belzile et al., 2002; Bou-Flores and Hilaire, 2000; Cayetanot et al., 2002; Kinkead et al., 2002), upper airway control (Cornea-Hebert et al., 1999; Fonseca et al., 2001; Ogasa et al., 2004; Zhan et al., 2002), and 5-HT-mediated adaption to intermittent hypoxia (Bach and Mitchell, 1996; Baker-Herman et al., 2004; Mitchell et al., 2001). In this study, we used tissue section autoradiography with 125I-1-(2,5-dimethoxy-4-iodo-phenyl)2-aminopropane (125I DOI) to determine quantitatively changes in the density of 5-HT2A receptors in the human medulla during infancy and a combination of single-and double-label immunocytochemistry to determine the cellular localization of 5-HT2A receptors in the medulla.
MATERIALS AND METHODS
Clinical Cases
Infant brainstem specimens were accrued from the autopsy services of the Department of Pathology, Children’s Hospital Boston, MA, and the office of the Chief Medical Examiner, San Diego, CA. This study was approved by the Committee on Clinical Investigation at Children’s Hospital Boston. For tissue receptor autoradiography, we examined the medullae of 7 infants. The cases ranged in age from 39 postconceptional weeks (term birth) to 82 postconceptional weeks (approximately 10 postnatal months), with a median age of 5 months. Fetal cases were not available. These infant cases had a median postmortem interval of 15 hours with a range of 8–20 hours. The causes of death were: congenital heart disease (n=3), acute pneumonia (n=1), intestinal obstruction (n=1), acute asphyxia (n=1), and Potter’s syndrome (n=1). The brainstems did not demonstrate pathologic changes by standard criteria. For immunocytochemical analysis, we examined formalin-fixed, paraffin-embedded sections of the medulla of 5 fetuses and infants ranging in age from 15 gestational weeks through 10 postnatal months, with a median age of 38 weeks (term birth). The median postmortem interval was 14 hours. The causes of death in this autopsy population were: extreme prematurity (n=2), congenital neuroblastoma (n=1), congenital heart disease (n=1), and pulmonary veno-occlusive disease (n=1). Again, in no case was there brainstem pathology by standard histopathologic criteria.
5-HT2A Receptor Autoradiography
Tissue preparation
Our procedures for tissue preparation have been described in detail previously (Panigrahy et al., 2000; Paterson et al., 2004; Paterson et al., 2006b). Briefly, unfixed brainstems were stored frozen at −80°C, and subsequently sectioned at 20 μm on a Leitz motorized cryostat and thaw-mounted onto Superfrost Plus glass microscope slides (Thermo Fisher Sceintfic, NH).
125I-DOI Binding to 5-HT2A Receptors
The autoradiography procedure for determination of 125I-DOI binding to 5-HT2A receptors was performed on 20 μm sections of frozen medulla according to a previously described protocol (Lopez-Gimenez et al., 2002). Sections were preincubated in 50mM Tris-HCl (pH 7.4), 0.1% ascorbic acid, and 4mM CaCl2 for 30 minutes at room temperature followed by incubation in the same buffer containing 86.3pM 125I DOI (PerkinElmer Inc, Wellesley, Mass) for 60 minutes at room temperature to determine total 5-HT2A receptor binding density. Nonspecific binding was determined by addition of 10μM ritanserin to the incubation solution. Sections were then washed 2 × 10 minutes in ice cold buffer and dried in warm air before being placed in cassettes and exposed to 125I-sensitive film (Kodak BMR) for 42 hours along with a set of 125I standards (Amersham) for conversion of optical density of silver grains to fmol/mg tissue.
Quantitative Analysis of brainstem autoradiograms
Film autoradiograms were generated according to standard laboratory procedure for development of light-sensitive film. For each specimen receptor binding density (expressed as the specific activity of tissue-bound ligand in fmol/mg protein) was analyzed in a total of eleven medullary nuclei, including the raphé obscurus (Rob), nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus (DMX), hypoglossal nucleus (HG), intermediate reticular zone (IRZ), gigantocellularis nucleus (GC), paragigantocellularis lateralis nucleus (PGCL), dorsal accessory olive (DAO), principle inferior olive (PIO), medial accessory olive (MAO) and arcuate nucleus (Arc) at a defined level of the brainstem according to the atlas of Olszewski and Baxter (1954) with the exception of the raphé nuclei, which were classified according to Tork and Hornung (1990). Total receptor binding was determined in 2 sections and non-specific receptor binding in 1 section for each nucleus analyzed. Specific receptor binding density was determined by subtracting nonspecific binding from total binding. Quantitative densitometry of autoradiograms was performed using an MCID 5+ imaging system (Imaging Research, Ontario, Canada). Linear regression analysis was used to determine the effect of age on 125I-DOI binding across infancy. To examine a possible influence on postmortem interval (PMI) on binding density, linear regression analysis of binding (fmol/mg) versus PMI was also performed.
Photomicrograph production
Images of 5-HT2A receptor binding in the human infant medulla were generated as TIFF files from the autoradiography film by the MCID 5+ imaging system (Imaging Research). All images were then imported into PhotoShop 6.0 (Adobe Systems, San Jose, CA) where they were scaled relative to each other and appropriate labels were added to form composite images.
Receptor Immunocytochemistry
Single-label immunocytochemistry for 5-HT2Areceptors
Immunocytochemistry for 5-HT2A receptors was performed using a goat polyclonal antibody SR2A A-15 (sc-15073, Santa Cruz Biotechnology Inc., Santa Cruz, CA) raised against amino acids 10–60 [protein accession number P28223] near the n-terminus of the human 5-HT2A receptor that has previously been reported to label 5-HT2A receptors in human tissue (Vikman and Edvinsson, 2006). Four micron thick formalin-fixed paraffin embedded sections of human infant medulla were de-paraffinzed before antigen retrieval was performed by incubating the tissue in a 1× citrate buffer solution (pH 6.0) in a microwave oven at 195°C for 10 min. Sections were allowed to cool for 10 min at room temperature before being washed in running water for 10 min. Sections were then washed 4×15 min in 1× phosphate buffered saline (PBS) with 0.1% Triton-X and treated for 30 min with 3% hydrogen peroxide. The sections were then blocked in 4% bovine serum albumin (BSA) for 60 min before being incubated in primary antibody (1:100) overnight at 4°C. Sections were then washed 3×20 min in 1xPBS with 0.1% Triton-X before being incubated in a biotinylated secondary antibody (Vectastain Elite Goat ABC Kit) for 60 min, rinsed briefly in PBS, and incubated in avidin-horseradish peroxidase solution (Vectastain Elite Goat ABC Kit, Vector Laboratories, Burlingame, CA) for 10 min. Staining was then developed by the addition of di-amino benzadine substrate (Vector Laboratories, Burlingame, CA) to the section for 1–5 min. DAB Enhancer solution (Zymed Laboratories) was then applied for 1–2 min to enhance immunostaining. Sections were then washed in running water before dehydration in a series of alcohols (80%, 90%, and 100%) cleared in xylene and coverslipped with permount. To determine the specificity of staining with the antibody, control sections were processed as above with omission of the primary and/or secondary antibody, which resulted in no staining. Pre-immune absorption was also performed by incubating the primary antibody in solutions containing 2, 4 and 8μg/ml of blocking peptide (sc-15073 P, Santa Cruz Biotechnology Inc., Santa Cruz, CA) before processing tissue sections as normal. No immunostaining for 5-HT2A receptors was observed in these sections. Moreover, immunostaining was absent in brain tissue sections from 5-HT2A receptor knockout mice that do not express the 5-HT2A receptor protein. Each case had at least three positively stained sections at the selected mid and rostral medullary levels analyzed. Immunostained sections were visualized with an Olympus BX51 microscope (Olympus America Inc., Melville, NY) with image capture using an Optronics Microfire S99808 camera and Microfire 1.0 and Neurolucida 5.0 software (Microbrightfield, Colchester, VT). Images were captured as TIFF files and imported into Photoshop 6.0 (Adobe Systems, San Jose, CA) where they were scaled relative to each other and appropriate labels and scale bars were added and images were optimized to enhance clarity and contrast.
Double-label immunocytochemistry
Double-label immunofluorescence was performed to determine the distribution and cellular localization of 5-HT2A receptors relative to 5-HT neurons on 4 μm 4% paraformaldehyde fixed sections of medulla using the same 5-HT2A goat polyclonal antibody used for single labeling and mouse-antihuman PH8 antibody (MAB5278, Millipore International, Temecula, CA) against tryptophan hydroxylase (TPOH) to identify 5-HT neurons. Tryptophan hydroxylase is the rate limiting enzyme in 5-HT synthesis and is a specific marker for 5-HT neurons labeling 5-HT cell bodies, fibers, and terminals. We have used this antibody to identify medullary 5-HT neurons in previous studies (Kinney et al., 2007; Paterson et al., 2006b). Double-label immunofluorescence was performed following the protocol for single-label immunocytochemistry except that the hydrogen peroxide blocking step was omitted. Sections were incubated simultaneously in 5-HT2A goat polyclonal antibody (1:100) and PH8 mouse monoclonal antibody (1:8,000) overnight at 4 C. Sections were then washed 3×20 min in 1×PBS with 0.1% Triton-X before being incubated in Alex-Fluor 594 donkey anti-goat (A-11058, Invitrogen, Carlsbad CA) and Cy2 donkey anti-mouse (715-225-150, Jackson Immunoresearch Laboratories, West Grove, PA) fluorescent secondary antibodies for 1 hour at room temperature. Sections were then washed 3 × 20 min in 1XPBS with 0.1% Triton-X, and allowed to dry for 60 min at room temperature before being cover slipped in Fluoromount-G (Southern Bio-ctechnology). Omission of the primary antibody was used as a negative control. Immunofluorescence was visualized with an Olympus BX51 microscope (Olympus America Inc., Melville, NY) using FITC and TRITC filters with image capture using a Coolsnap fx camera (Photometrics, Tuscon, AZ) and MCID Elite 6.0 software (Imaging Analysis Inc., Ontario, Canada). Images were captured as TIFF files and imported into Photoshop 6.0 (Adobe Systems, San Jose, CA) where they were corrected for background immunofluorescence, scaled relative to each other, and appropriate labels and scale bars were added.
Analysis of the human infant medulla
The medullary 5-HT system, as defined by us, consists of the 5-HT neurons in the midline raphé (raphé obscurus, raphé pallidus, and raphé magnus), lateral extra-raphé (gigantocellularis, paragigantocellularis lateralis, intermediate reticular zone, subtrigeminalis, and lateral reticular nucleus), ventral surface (arcuate nucleus) and the regions in the medulla receiving major projections from these 5-HT neurons that are involved in autonomic and respiratory control including the hypoglossal nucleus (upper respiratory control), preBötC (respiratory rhythm genesis); human homologue in the PGCL (Gray et al., 1999; Rekling and Feldman, 1998; Smith et al., 1991), DMX (preganglionic parasympathetic output) and NTS (visceral sensory input). These nuclei are distributed from the caudal medulla (at the level of the area postrema) to rostral medulla (at the level of the paragigantocellularis lateralis), according to the standardized brainstem levels previously defined in our laboratory (Kinney et al., 2007; Kinney et al., 2001) based upon the human brainstem atlases of Olszewski and Baxter, (1954), Paxinos and Huang, (1995), and Tork and Hornung, (1990). These same standardized levels were analyzed in this study to determine the distribution of 5-HT2A receptors in the human infant medulla using autoradiography and immunocytochemistry.
RESULTS
125I DOI binding to 5-HT2A receptors in the human infant medulla
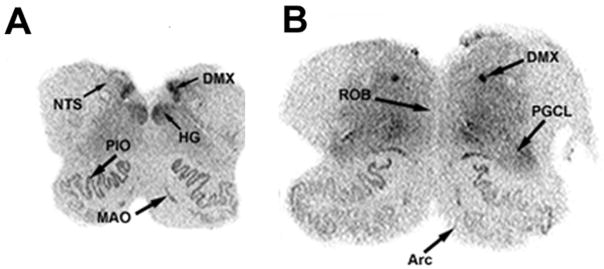
The highest density of 125I DOI binding was observed in the DMX (1.41 ± 0.25 fmol/mg) (Figs. 1 and 2). High binding was also present in the HG and the DAO. Intermediate levels of binding were observed in the NTS (0.70 ± 0.12 fmol/mg) and in the extra-raphé regions containing 5-HT neuron cell bodies including the GC, PGCL, and IRZ. In the olivo-cerebellar system, there was a differential distribution of binding intensity, with the highest level in the DAO (1.39 ± 0.20 fmol/mg), similar to that seen in the DMX (Figs. 1 and 2). There was intermediate binding in the MAO and PIO (Figs. 1 and 2). Negligible binding was observed in the Rob and in the Arc. Postmortem interval had no significant effect on binding.
Figure 1.
Autoradiographic images of 125I DOI binding to 5-HT2A receptors in transverse sections of the caudal (A) and rostral (B) human infant medulla. High 5-HT2A receptor binding density is present in the DMX and HG, moderate binding density in the NTS and PGCL, and relatively low binding density in the ROB and Arc.
Figure 2.
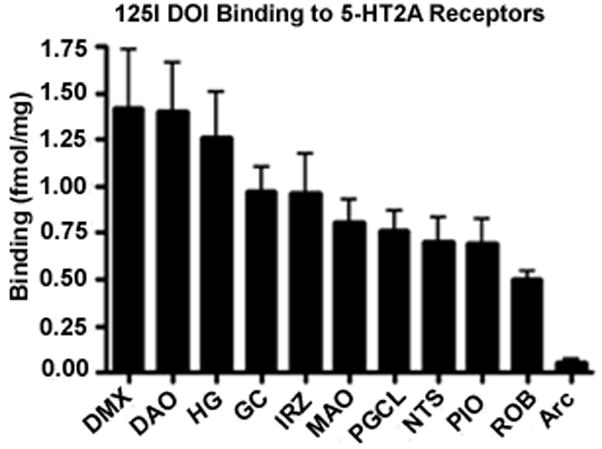
Bar graph displaying 5-HT2A receptor binding density in medullary nuclei presented from the highest to lowest. 5-HT2A receptor binding density is highest in medullary nuclei without 5-HT neurons (e.g., DMX, HG).
Distribution of 5-HT2A receptor Immunostaining in the human infant medulla
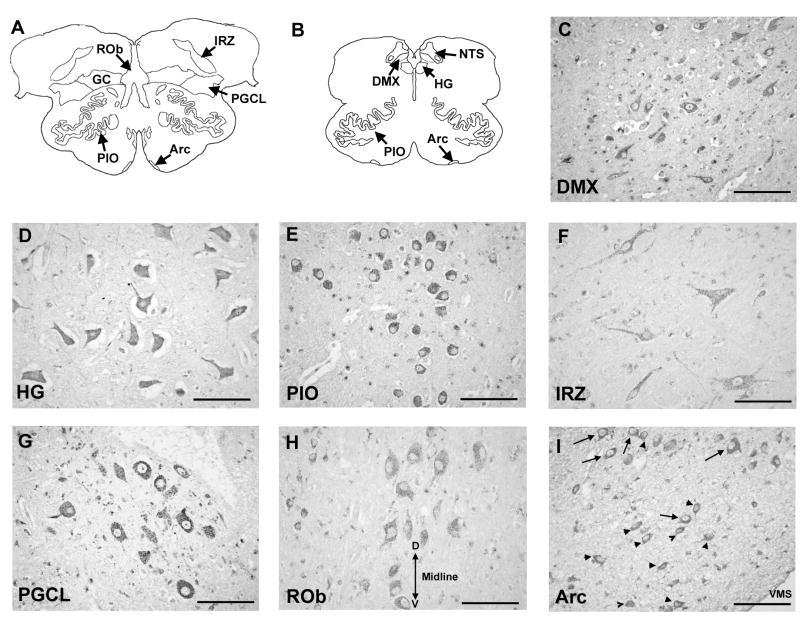
The pattern of 5-HT2A immunoreactivity in medullary nuclei in the infant cases generally paralleled the distribution of 5-HT2A receptor binding density observed with 125I DOI. Intensely stained immunoreactive neurons were observed in the DMX and in motor neurons in the HG (Fig 3C and D). Intensely stained large, round neurons, were also observed in all subdivisions of the inferior olivary system (i.e., PIO, MAO, DAO) (Fig 3E). Moderately stained neurons of heterogeneous size and morphology were observed in the NTS and scattered throughout the extra-raphé regions (GC, PGCL, IRZ) (Fig 3F). Moreover, a group of small spherical 5-HT2A immunoreactive neurons were frequently observed in the lateral extent of the PGCL (Fig 3G). 5-HT2A immunoreactive neurons were also observed in the raphé obscurus and in the arcuate nucleus, both sites in which receptor binding to the radioligand 125I-DOI was negligible. Immunostaining in the raphé obscurus was light and localized to spherical and fusiform neurons in the midline (Fig 3H). In the arcuate nucleus immunostaining was intense and localized to small, irregularly-shaped cells, consistent in morphology to astrocytes and to larger spherical neurons the major cell type in this region (Fig 3I). In virtually all immunopositive regions, the immunostaining was punctate and co-localized with cell bodies and/or processes, consistent with post-synaptic localization of the receptors.
Figure 3.
5-HT2A receptor immunostaining in the human infant medulla at 10 postnatal months. Panels (A) and (B) show diagrams of horizontal sections of rostral and mid-medulla, respectively, showing the location of the component nuclei of the medullary 5-HT system at each level. 5-HT2A receptor immunostaining is punctate and localized to neuronal cell bodies and processes, indicative of post-synaptic localization of the receptors. Figure shows intense immunostaining of neurons in the DMX (C), motor neurons in the HG (D), and spherical neurons in the PIO (E). Moderately stained neurons of heterogenous morphology were observed in the extra-raphé regions including the GC (F). Spherical 5-HT2A immunoreactive neurons were observed in the lateral extent of the PGCL (G) consistent in location and morphology with preBötC neurons in rodents. Lightly stained spherical and fusiform 5-HT2A receptor immunoreactive neurons were observed in the midline raphé (H). Midline of the medulla is labeled by double headed arrow; D, dorsal; V, ventral. Immunostaining to “classical” arcuate neurons (arrows) and astrocytes (arrow-heads) in the arcuate nucleus (I). VMS, ventral medullary surface. All images at ×40. Scale bar= 100 μm.
Distribution of 5-HT2A receptors relative to 5-HT neurons in the human infant medulla
We observed 5-HT neurons and fibers with TPOH immunofluorescence in the raphé, extra-raphé (GC, PGCL, and IRZ) and Arc at the ventral medullary surface, consistent with the distribution of 5-HT neurons previously described by us in the human infant medulla (Kinney et al., 2007; Paterson et al., 2006b). Double-label immunofluorescence revealed that 5-HT2A receptors co-localized to the soma and dendrites of a subset of 5-HT (TPOH) immunoreactive neurons in the raphé and extra-raphé regions of the human infant medulla (Fig 4). However, not all 5-HT neurons expressed 5-HT2A receptors, and not all neurons expressing 5-HT2A receptor immunoreactivity were 5-HT (Fig 4.)
Figure 4.
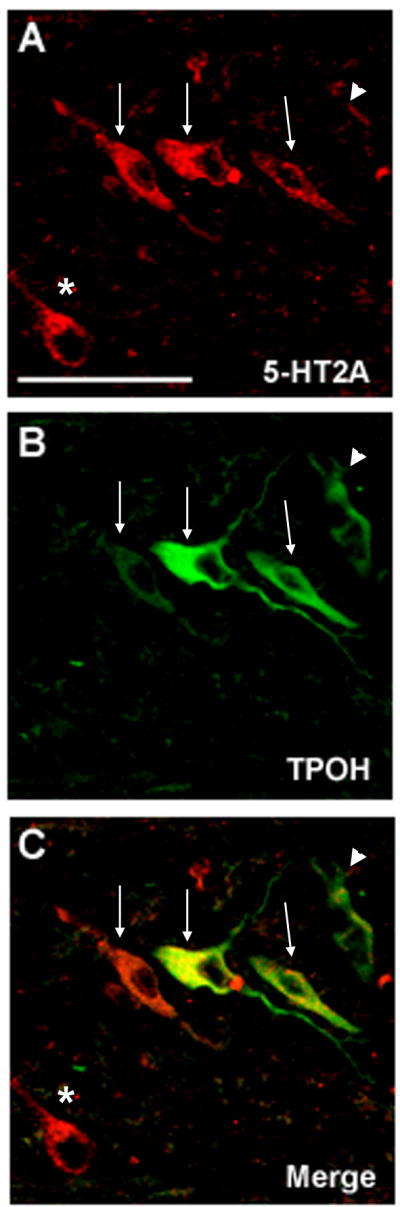
Double-label immunofluorescent images showing localization of 5-HT2A receptors and 5-HT neurons in the PGCL (×40). A. 5-HT2A immunofluorescent staining; B. TPOH immunofluorescent staining; C. Merged images. 5-HT2A receptors co-localized to the soma and dendrites of a subset of 5-HT immunoreactive neurons (arrows) in the medulla, but not all 5-HT neurons expressed 5-HT2A receptors (arrowheads), and not all neurons expressing 5-HT2A receptor immunoreactivity were 5-HT (*). ×40. Scale bar=100μm.
Developmental profile of 5-HT2A expression in the human infant medullary 5-HT system
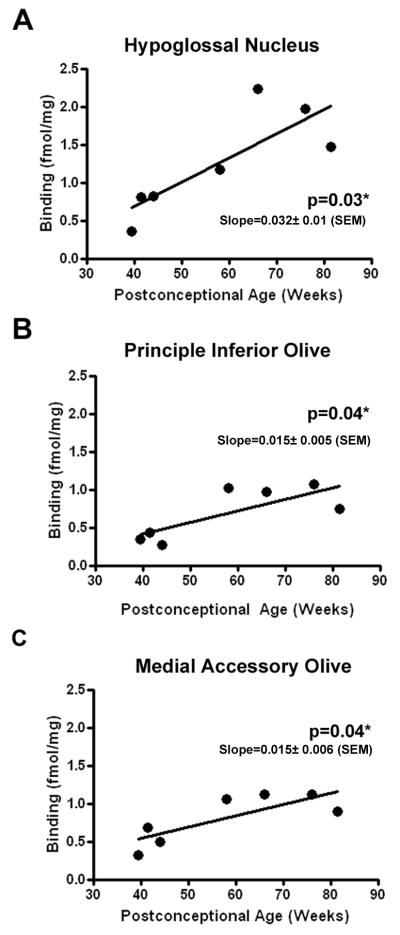
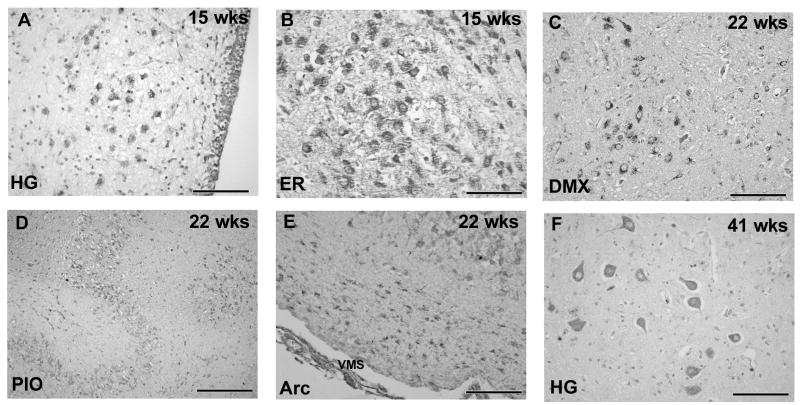
The developmental profile of 5-HT2A receptor binding was analyzed across infancy to determine if there were any age-related changes in binding density in the component nuclei of the medullary 5-HT system. A trend for 5-HT2A receptor binding density to increase with age was observed in all medullary nuclei examined, with statistically significant increases observed in the HG (p=0.027), PIO (p=0.044) and MAO (p=0.038) (Figs 5A-C). 5-HT2A receptor immunostaining was present in the developing medulla as early as 15 gestational weeks with the distribution of receptors already “set” in the mature configuration at this age. Immunostaining was most intense in the HG (Fig 6A) and in the lateral extra-raphé where 5-HT2A immunoreactive neurons were tightly clustered (Fig 6B). In all regions of the medulla at 15 weeks neurons were spherical and undifferentiated with few or no few immunoreactive processes observed (Fig 6A). At 22 weeks gestation 5-HT2A receptor immunoreactivity was observed in distinct nuclei including the DMX (Fig 6C), PIO (Fig 6D) and arcuate nucleus (Fig 6E). By term (41 gestational weeks), the neurons had increased in size, developed immunoreactive processes and differentiated into distinct morphological subtypes, e.g., motorneurons in the HG (Fig 6F). By 10 postnatal months 5-HT2A immunoreactive neurons of heterogeneous morphology were observed in different medullary regions, e.g., motorneurons in the HG (Fig 3B) and fusiform neurons in the in raphè and extra-raphé (Fig 3F). No obvious changes in the distribution or intensity of 5-HT2A receptor immunostaining were observed from late gestation through infancy.
Figure 5.
Linear regression plots of the relationship between 5-HT2A receptor binding density and postconceptional age (weeks) in HG (A), PIO (B) and MAO (C). In each nuclei, receptor binding density increases significantly with age. *p<0.05 linear regression analysis.
Figure 6.
Developmental expression of 5-HT2A receptor immunocytochemistry in the human medulla from 15 gestational weeks to term (41 gestational weeks). Immunostaining in the HG (A) and extra-raphé (B) (i.e., GC/PGCL) at 15 weeks gestation. Neurons in the extra-raphé were tightly clustered together with no obvious distinction between nuclei. Neurons are spherical and undifferentiated. At 22 weeks gestation 5-HT2A receptor immunoreactivity was observed in distinct nuclei including the DMX (C), PIO (D) and arcuate nucleus (E) with evidence of neuronal differentiation. Motor neurons in the HG expressing 5-HT2A receptor immunoreactivity at term (41 weeks gestation) (F). All images at ×40 (scale bar=100μm) except D at ×20 (scale bar=200μm). VMS, ventral medullary surface.
DISCUSSION
In the present study, we demonstrated that 5-HT2A receptors are expressed in high density in medullary nuclei that are critical for autonomic and respiratory control. We identified high 5-HT2A receptor binding density in the DMX (preganglionic parasympathetic output), and HG (airway patency), and intermediate binding in the NTS (visceral sensory input), GC, PGCL and IRZ. In addition, 5-HT2A receptors are expressed in high density by neurons in the inferior olivary complex. The olivary complex is involved in somatomotor coordination (Azizi and Woodward, 1987; Ikeda et al., 1989; Nisimaru et al., 1991; Sugita et al., 1989; Urbano et al., 2006), but also has an underappreciated role in cardiorespiration via its connections with the cerebellum (Harper et al., 2000; Nisimaru et al., 1991; Nisimaru et al., 1998; Okahara and Nisimaru, 1991; Sugita et al., 1989). These observations suggest that, as in experimental animals, medullary 5-HT2A receptors play important roles in 5-HT mediated regulation of autonomic and respiratory function. We also found that 5-HT2A receptors are expressed in medullary cardiorespiratory-related nuclei as early as 15 gestational weeks, the earliest time-period studied by us, and that there are significant changes in 5-HT2A receptor binding in the HG and the inferior olivary complex after birth. These observations suggest that the medullary 5-HT system is not fully developed at birth, but continues to mature throughout the first postnatal year. Below, we discuss these observations in relation to the roles of medullary 5-HT2A receptors in putatively mediating respiratory and autonomic function in the human infant. We begin by considering the distribution of 5-HT2A receptors in the human infant medulla.
Distribution of 5-HT2A Receptors in Human Infant Medulla
In this study we observed that 5-HT2A receptors are concentrated in high density in the HG, DMX and NTS, moderate to low density in the lateral extra-raphé regions (i.e., GC and PGCL), the inferior olivary complex, and in the Arc and in negligible density in the Rob. This distribution is consistent with previous studies describing 5-HT2A receptors in the human brainstem (Burnet et al., 1995; Hall et al., 2000; Ozawa and Okado, 2002; Ozawa and Takashima, 2002) and with the distribution of 5-HT2A receptors in the medulla in rats (Brandes et al., 2007; Cornea-Hebert et al., 1999; Fonseca et al., 2001; Haan et al., 1987; Huang and Pickel, 2002; Huang and Pickel, 2003; Jansson et al., 1998; Okabe et al., 1997; Wright et al., 1995). These observations suggest, that as in rats, 5-HT2A receptors in the human infant medulla are concentrated in nuclei that receive significant innervation from 5-HT neurons in the caudal raphé and extra-raphé (Barnes and Sharp, 1999; Hamada et al., 1998; Mengod et al., 1996; Xu and Pandey, 2000), and thus, are predominantly postsynaptic to 5-HT axon terminals. This idea is supported by our previous demonstration of high density serotonin transporter (5-HTT) binding, a marker of 5-HT presynaptic terminals, in the DMX, NTS and HG (Paterson et al., 2004; Paterson et al., 2006b). The distribution of 5-HT2A receptors in the human infant medulla also contrasts distinctly to the distribution of 5-HT1A receptors: 5-HT1A binding density is highest in the Rob (Paterson et al., 2004; Paterson et al., 2006a), 5-HT2A binding is negligible in this region; similarly, 5-HT1A receptors co-localize extensively with 5-HT neuronal cell bodies in the human infant medulla (Paterson et al., 2006a); Paterson et al., unpublished observations), while 5-HT2A receptors localize predominantly to non-5-HT neurons. We propose that the differential distribution of 5-HT1A and 5-HT2A receptors underscores their different functional roles in the human infant medulla, i.e., 5-HT1A somato-dendritic autoreceptor modulating 5-HT neuron function versus 5-HT2A post-synaptic excitatory receptor mediating autonomic and respiratory function.
Developmental Changes in 5-HT2A Receptor Expression in the Human Infant Medulla
We observed a trend for 5-HT2A receptor binding density to increase with age across infancy in all medullary nuclei examined, consistent with widespread postnatal changes in 5-HT2A receptor expression observed in rat medulla (Liu and Wong-Riley, 2008). Significant age-related increases in binding were observed in the HG and component nuclei of the inferior olivary complex. While we did not observe any obvious change in the distribution or intensity of 5-HT2A immunostaining in the medulla from mid-gestation through infancy, we did observe an increase in size and complexity of 5-HT2A immunoreactive neurons, which was particularly evident in the HG. Literature reports indicate that the HG undergoes significant development in the postnatal period in both humans and rodents, increasing the size and complexity of its dendritic arborization with a concomitant increase in 5-HT immunoreactive fiber density (Nara et al., 1989; O’Kusky, 1998); (Berger et al., 1992)(Talley et al., 1997). In rats, hypoglossal neuron excitability and firing also change significantly during the postnatal period, with a progressive decrease in excitability observed with age (Bayliss et al., 1997; Berger et al., 1992; Berger et al., 1996). Developmental changes in neuronal receptor expression might be expected to accompany these functional changes and, assuming a similar developmental process in humans, may account for the age-related changes in 5-HT2A receptors observed in the HG in this study. We also observed significant age-related increases in 5-HT2A receptor expression in the inferior olivary complex. It receives input from 5-HT neurons in the caudal domain but the precise influence of 5-HT upon olivary function, especially via 5-HT2A receptors, is unknown. Similarly, little evidence is available on the developmental expression of 5-HT2A receptors in this nucleus, thus the significance of the developmental changes in 5-HT2A receptor binding observed in this study are also unknown.
In a previous study investigating the developmental expression of 5-HT receptors in the human infant medulla, using 3H lysergic acid diethylamide (3H-LSD) autoradiography, we observed no significant age-related changes in receptor binding density (Paterson et al., 2004). However, 3H-LSD binds to multiple 5-HT receptor subtypes (5-HT1A-1D and 5-HT2), and thus, age-related changes in individual 5-HT receptor subtypes may have been masked. Indeed, in the same study, we observed an age-related increase in 5-HT1A receptor binding in the HG in the same cases using 3H 8-OH-DPAT autoradiography (Paterson et al., 2004). This observation parallels the postnatal increase in 5-HT2A receptor binding identified in the HG in this study. Moreover, we have also identified subtle changes in the distribution and morphology of 5-HT neurons in the medulla during development (Kinney et al., 2007). The developmental changes in 5-HT2A receptors observed in this study, taken together with the studies described above, therefore, support the idea that the structure and neurochemistry of the medullary 5-HT system, and thus, 5-HT mediated homeostatic function, continue to develop during the first year of postnatal life.
Potential Limitations of the Study
A potential limitation of this study is the small sample size of fetal and infant medullae. “Normal” tissue specimens from human infants, as used in this study, are particularly hard to accrue due to the rarity of autopsies in this age group. The small sample size is particularly problematic when determining the effects of age on 5-HT2A receptor binding levels in the medulla, as individual cases can have a disproportionate effect on statistical observations. The significant age-related changes in binding observed in the HG and PIO in this study therefore need to be confirmed in a larger dataset. Ongoing accrual of the cases needed for confirmation is in progress. However, the age-related increases in 5-HT2A receptor binding observed in this study are supported by previous observations in our laboratory describing subtle developmental changes in 5-HT neurons (Kinney et al., 2007) and 5-HT receptors (Paterson et al., 2004) across infancy. Moreover, in the same cases analyzed in this study, we have previously observed statistically significant age-related reductions in nicotinic receptor binding in the medulla (Duncan et al., 2008), that we believe underscore the robustness of the observations made in this study, despite the small sample size. We therefore propose that the changes in 5-HT2A receptor binding observed in this study are consistent with a pattern of developmental changes that occur in the medulla during the postnatal period.
6. Conclusions
In conclusion, the spatial distribution of 5-HT2A receptors in the human fetal and infant brainstem is consistent with a role in cardiorespiratory regulation. The ongoing changes in their binding density in the hypoglossal nucleus and inferior olivary complex across infancy indicate that the medullary 5-HT system is not fully mature at birth, but it continues to develop postnatally. This extended maturation period suggests that the system is vulnerable to developmental insults both before and after birth, especially through the end of infancy. Consequently, different insults at single or multiple pre- and/or postnatal time-points will likely have differential effects upon the structural and/or neurochemical development of the system, and ultimately upon different functional outcomes, not only in early life but beyond. This study provides baseline information for the analysis of the 5-HT2A receptors in pediatric brainstems disorders in early life.
Acknowledgments
We thank Mr. Richard A. Belliveau for technical assistance in the performance of the study and in the preparation of the manuscript, and Dr. Felicia L. Trachtenberg for statistical analysis. We also thank the Office of the Chief Medical Examiner, San Diego, CA, for help in the accrual of human infant tissue specimens.
The study was supported by the Scottish Cot Death Foundation, CJ Foundation for SIDS, CJ Murphy Foundation and R37-HD20991.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azizi SA, Woodward DJ. Inferior olivary nuclear complex of the rat: morphology and comments on the principles of organization within the olivocerebellar system. J Comp Neurol. 1987;263(4):467–484. doi: 10.1002/cne.902630402. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21(2 Suppl):33S–45S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104(2–3):251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1):48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the afterhyperpolarization. Journal of Neurophysiology. 1997;77(3):1362–1374. doi: 10.1152/jn.1997.77.3.1362. [DOI] [PubMed] [Google Scholar]
- Belzile O, Gulemetova R, Kinkead R. Role of 5-HT2A/C receptors in serotonergic modulation of respiratory motor output during tadpole development. Respir Physiol Neurobiol. 2002;133(3):277–282. doi: 10.1016/s1569-9048(02)00169-6. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143(1–2):164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81(3):1039–1048. doi: 10.1152/jappl.1996.81.3.1039. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Hilaire G. 5-Hydroxytryptamine(2A) and 5-hydroxytryptamine(1B) receptors are differently affected by the monoamine oxidase A-deficiency in the Tg8 transgenic mouse. Neurosci Lett. 2000;296(2–3):141–144. doi: 10.1016/s0304-3940(00)01653-0. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Dean C, Hopp FA, Jakovcevic D, Stuth EA. Retrograde labeling reveals extensive distribution of genioglossal motoneurons possessing 5-HT2A receptors throughout the hypoglossal nucleus of adult dogs. Brain Res. 2007;1132(1):110–119. doi: 10.1016/j.brainres.2006.10.099. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res. 1995;676(1):157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. Postnatal changes in the respiratory response of the conscious rat to serotonin 2A/2C receptor activation are reflected in the developmental pattern of fos expression in the brainstem. Brain Res. 2002;942(1–2):51–57. doi: 10.1016/s0006-8993(02)02690-2. [DOI] [PubMed] [Google Scholar]
- Comet MA, Bernard JF, Hamon M, Laguzzi R, Sevoz-Couche C. Activation of nucleus tractus solitarius 5-HT2A but not other 5-HT2 receptor subtypes inhibits the sympathetic activity in rats. Eur J Neurosci. 2007;26(2):345–354. doi: 10.1111/j.1460-9568.2007.05673.x. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409(2):187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM. Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: implications for sudden infant death syndrome. J Neurosci. 2005;25(36):8322–8332. doi: 10.1523/JNEUROSCI.1770-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT(2) receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2007;149(3):696–705. doi: 10.1016/j.neuroscience.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Auton Neurosci. 2008;144(1–2):61–75. doi: 10.1016/j.autneu.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MI, Ni YG, Dunning DD, Miledi R. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Brain Res Mol Brain Res. 2001;89(1–2):11–19. doi: 10.1016/s0169-328x(01)00049-3. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286(5444):1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan EA, Jennings IG, Cuello AC, Nakata H, Fujisawa H, Chow CW, Kushinsky R, Brittingham J, Cotton RG. Identification of serotonergic neurons in human brain by a monoclonal antibody binding to all three aromatic amino acid hydroxylases. Brain Res. 1987;426(1):19–27. doi: 10.1016/0006-8993(87)90420-3. [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse. 2000;38(4):421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54(2):199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Harper RM, Woo MA, Alger JR. Visualization of sleep influences on cerebellar and brainstem cardiac and respiratory control mechanisms. Brain Res Bull. 2000;53(1):125–131. doi: 10.1016/s0361-9230(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullaryneurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. Journal of Comparative Neurology. 1997;379(2):261–270. [PubMed] [Google Scholar]
- Helke CJ, McDonald CH, Phillips ET. Hypotensive effects of 5-HT1A receptor activation: ventral medullary sites and mechanisms of action in the rat. Journal of the Autonomic Nervous System. 1993;42(2):177–188. doi: 10.1016/0165-1838(93)90048-y. [DOI] [PubMed] [Google Scholar]
- Huang J, Pickel VM. Differential distribution of 5HT2A and NMDA receptors in single cells within the rat medial nucleus of the solitary tract. Synapse. 2002;44(2):64–75. doi: 10.1002/syn.10056. [DOI] [PubMed] [Google Scholar]
- Huang J, Pickel VM. Ultrastructural localization of serotonin 2A and N-methyl-D-aspartate receptors in somata and dendrites of single neurons within rat dorsal motor nucleus of the vagus. J Comp Neurol. 2003;455(2):270–280. doi: 10.1002/cne.10497. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Noda H, Sugita S. Olivocerebellar and cerebelloolivary connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1989;284(3):463–488. doi: 10.1002/cne.902840311. [DOI] [PubMed] [Google Scholar]
- Jansson A, Tinner B, Steinbusch HW, Agnati LF, Fuxe K. On the relationship of 5-hydroxytryptamine neurons to 5-hydroxytryptamine 2A receptor-immunoreactive neuronal processes in the brain stem of rats. A double immunolabelling analysis. Neuroreport. 1998;9(11):2505–2511. doi: 10.1097/00001756-199808030-00015. [DOI] [PubMed] [Google Scholar]
- King KA, McCall RB. The effects of 8-OH-DPAT on medullary 5-HT neurons and sympathetic activity in baroreceptor-denervated animals. European Journal of Pharmacology. 1991;200(2–3):357–360. doi: 10.1016/0014-2999(91)90596-i. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Belzile O, Gulemetova R. Serotonergic modulation of respiratory motor output during tadpole development. J Appl Physiol. 2002;93(3):936–946. doi: 10.1152/japplphysiol.00104.2002. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Belliveau RA, Trachtenberg FL, Rava LA, Paterson DS. The development of the medullary serotonergic system in early human life. Auton Neurosci. 2007;132(1–2):81–102. doi: 10.1016/j.autneu.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60(3):228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol. 2005;64(8):689–694. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic Brainstem Abnormalities in Northern Plains Indians with the Sudden Infant Death Syndrome. Journal of Neuropathology and Experimental Neurology. 2003;62(11):1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Crews FT. Brain 5-HT1A receptor autoradiography and hypothermic responses in rats bred for differences in 8-OH-DPAT sensitivity. Brain Res. 1998;782(1–2):1–10. doi: 10.1016/s0006-8993(97)01127-x. [DOI] [PubMed] [Google Scholar]
- Kubo T, Taguchi K, Ozaki S, Amano M, Ishizuka T. 8-OH-DPAT-induced hypotensive action and sympathoexcitatory neurons in the rostral ventrolateral medulla of the rat. Brain Research Bulletin. 1995;36(4):405–411. doi: 10.1016/0361-9230(94)00221-l. [DOI] [PubMed] [Google Scholar]
- Lalley PM. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N, dimethyltryptamine. Brain Res. 1994;648(1):87–98. doi: 10.1016/0006-8993(94)91909-7. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291(3):999–1007. [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21(14):5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J Appl Physiol. 2008;104(6):1801–1808. doi: 10.1152/japplphysiol.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Tecott LH, Palacios JM, Mengod G, Vilaro MT. Serotonin 2C receptor knockout mice: Autoradiographic analysis of multiple serotonin receptors. J Neurosci Res. 2002;67(1):69–85. doi: 10.1002/jnr.10072. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Systemic and regional haemodynamic responses to microinjection of 5-HT agonists in the rostral ventrolateral medulla in the rat. Neuroscience Letters. 1989;107(1–3):157–161. doi: 10.1016/0304-3940(89)90809-4. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp Physiol. 1997;82(1):31–41. doi: 10.1113/expphysiol.1997.sp004013. [DOI] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2008 doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Mengod G, Vilaro MT, Raurich A, Lopez-Gimenez JF, Cortes R, Palacios JM. 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem J. 1996;28(11):747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90(6):2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential regulation of brown adipose and splanchnic sympathetic outflows in rat: roles of raphe and rostral ventrolateral medulla neurons. Clin Exp Pharmacol Physiol. 2001;28(1–2):138–143. doi: 10.1046/j.1440-1681.2001.03406.x. [DOI] [PubMed] [Google Scholar]
- Nara T, Goto N, Yamaguchi K. Development of the human hypoglossal nucleus: a morphometric study. Dev Neurosci. 1989;11(3):212–220. doi: 10.1159/000111900. [DOI] [PubMed] [Google Scholar]
- Nisimaru N, Okahara K, Nagao S. Olivocerebellar projection to the cardiovascular zone of rabbit cerebellum. Neurosci Res. 1991;12(1):240–250. doi: 10.1016/0168-0102(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Nisimaru N, Okahara K, Yanai S. Cerebellar control of the cardiovascular responses during postural changes in conscious rabbits. Neuroscience Research. 1998;32(3):267–271. doi: 10.1016/s0168-0102(98)00094-7. [DOI] [PubMed] [Google Scholar]
- Nosjean A, Guyenet PG. Role of ventrolateral medulla in sympatholytic effect of 8-OHDPAT in rats. American Journal of Physiology. 1991;260(3 Pt 2):R600–609. doi: 10.1152/ajpregu.1991.260.3.R600. [DOI] [PubMed] [Google Scholar]
- O’Kusky JR. Postnatal changes in the numerical density and total number of asymmetric and symmetric synapses in the hypoglossal nucleus of the rat. Brain Res Dev Brain Res. 1998;108(1–2):179–191. doi: 10.1016/s0165-3806(98)00048-0. [DOI] [PubMed] [Google Scholar]
- Ogasa T, Ray AD, Michlin CP, Farkas GA, Grant BJ, Magalang UJ. Systemic administration of serotonin 2A/2C agonist improves upper airway stability in Zucker rats. Am J Respir Crit Care Med. 2004;170(7):804–810. doi: 10.1164/rccm.200312-1674OC. [DOI] [PubMed] [Google Scholar]
- Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110(2–3):151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- Okahara K, Nisimaru N. Climbing fiber responses evoked in lobule VII of the posterior cerebellum from a vagal nerve in rabbits. Neurosci Res. 1991;12(1):232–239. doi: 10.1016/0168-0102(91)90113-d. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitechture of The Human Brain Stem. Basel: S. Karger; 1954. [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435(4):485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33(3):142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Takashima S. Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic Sci Int. 2002;130(Suppl):S53–59. doi: 10.1016/s0379-0738(02)00139-1. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59(5):377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Belliveau RA, Trachtenberg F, Kinney HC. Differential development of 5-HT receptor and the serotonin transporter binding in the human infant medulla. J Comp Neurol. 2004;472(2):221–231. doi: 10.1002/cne.20105. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Thompson EG, Belliveau RA, Antalffy BA, Trachtenberg FL, Armstrong DD, Kinney HC. Serotonin transporter abnormality in the dorsal motor nucleus of the vagus in Rett syndrome: potential implications for clinical autonomic dysfunction. J Neuropathol Exp Neurol. 2005;64(11):1018–1027. doi: 10.1097/01.jnen.0000187054.59018.f2. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Thompson EG, Kinney HC. Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: Observations relevant to central chemosensitivity in early human life. Auton Neurosci. 2006a;124(1–2):112–124. doi: 10.1016/j.autneu.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama. 2006b;296(17):2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF. Atlas of the Human Brainstem. New York: Academic Press; 1995. [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22(24):11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14(6):665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23(4–5):709–726. doi: 10.1023/a:1025096718559. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rose D, Khater-Boidin J, Toussaint P, Duron B. Central effects of 5-HT on respiratory and hypoglossal activities in the adult cat. Respir Physiol. 1995;101(1):59–69. doi: 10.1016/0034-5687(95)00008-2. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115(4):1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Shen FM, Wang J, Ni CR, Yu JG, Wang WZ, Su DF. Ketanserin-induced baroreflex enhancement in spontaneously hypertensive rats depends on central 5-HT(2A) receptors. Clin Exp Pharmacol Physiol. 2007;34(8):702–707. doi: 10.1111/j.1440-1681.2007.04626.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Paallysaho J, Noda H. Topographical organization of the olivocerebellar projection upon the posterior vermis in the rat. Neurosci Res. 1989;7(2):87–102. doi: 10.1016/0168-0102(89)90050-3. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17(11):4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Bingaman M, Taveira-DaSilva AM, Pace PP, Gillis RA, Wrathall JR. Serotonin 1A receptor agonists reverse respiratory abnormalities in spinal cord-injured rats. J Neurosci. 2003;23(10):4182–4189. doi: 10.1523/JNEUROSCI.23-10-04182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tork I, Hornung JP. Raphe nuclei and the serotonergic system. In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press, Inc; 1990. pp. 1001–1022. [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26(10):2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Simpson JI, Llinas RR. Somatomotor and oculomotor inferior olivary neurons have distinct electrophysiological phenotypes. Proc Natl Acad Sci U S A. 2006;103(44):16550–16555. doi: 10.1073/pnas.0607888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman P, Edvinsson L. Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur J Neurol. 2006;13(12):1324–1332. doi: 10.1111/j.1468-1331.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Villalon CM, Centurion D. Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol. 2007;376(1–2):45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J Pediatr. 1997;130(1):89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351(3):357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull. 2000;51(6):499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]
- Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113(1):145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]