Abstract
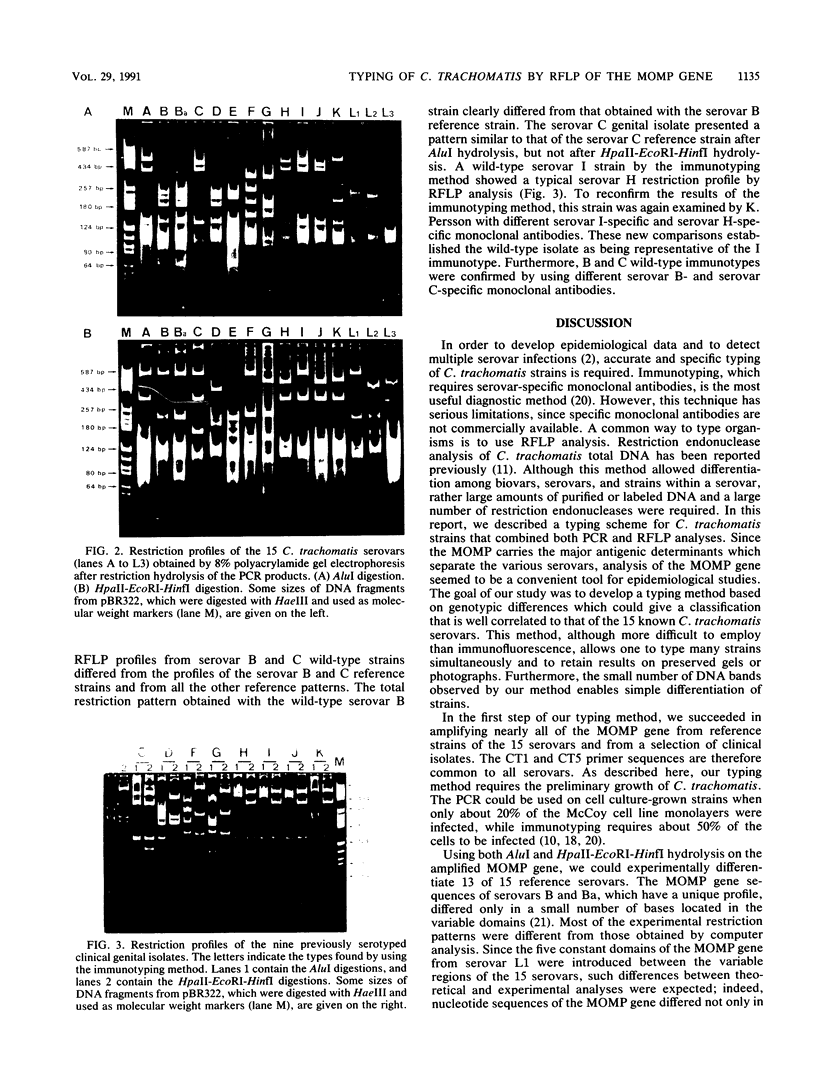
A procedure was developed for characterization of Chlamydia trachomatis strains by using restriction endonuclease analysis of amplified genes of the major outer membrane protein (MOMP). Reference strains of the 15 serovars (A through K and L1 through L3) and clinical isolates were tested. The nucleotide sequences of the MOMP genes of each of the 15 serovars were arbitrarily constructed by using the sequences of the four variable domains known for each serovar and the constant domains of serovar L1. Computer analysis of these sequences indicated that two restriction digestions performed in parallel, one with AluI and the other with IIpaII, followed by HinfI and EcoRI, would allow the theoretical differentiation of 13 serovars. Serovars Ba and L1 presented the same theoretical restriction profile. Our typing method consisted of polymerase chain reaction amplification of a fragment of about 1,200 bp of the MOMP gene, followed by restriction endonuclease digestion with the aforementioned enzymes. From the 15 serovars, we obtained 14 different patterns; 13 profiles were serovar specific, while serovars B and Ba presented the same pattern. Application of this typing method to C. trachomatis strains isolated from clinical material gave the same results as the immunotyping method for 14 of 17 strains. Furthermore, restriction endonuclease analysis detected differences within a serovar. This method seems to be promising for epidemiological studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. C., Rompalo A. M., Stamm W. E. Comparison of Chlamydia trachomatis serovars causing rectal and cervical infections. J Infect Dis. 1987 Dec;156(6):953–958. doi: 10.1093/infdis/156.6.953. [DOI] [PubMed] [Google Scholar]
- Barnes R. C., Suchland R. J., Wang S. P., Kuo C. C., Stamm W. E. Detection of multiple serovars of Chlamydia trachomatis in genital infections. J Infect Dis. 1985 Nov;152(5):985–989. doi: 10.1093/infdis/152.5.985. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh B., Bébéar C., Rodriguez P., Vekris A., Bonnet J., Garret M. Specific amplification of a DNA sequence common to all Chlamydia trachomatis serovars using the polymerase chain reaction. Res Microbiol. 1989 Jan;140(1):7–16. doi: 10.1016/0923-2508(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Griffais R., Thibon M. Detection of Chlamydia trachomatis by the polymerase chain reaction. Res Microbiol. 1989 Feb;140(2):139–141. doi: 10.1016/0923-2508(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Hamilton P. T., Malinowski D. P. Nucleotide sequence of the major outer membrane protein gene from Chlamydia trachomatis serovar H. Nucleic Acids Res. 1989 Oct 25;17(20):8366–8366. doi: 10.1093/nar/17.20.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Holmes K. K., Grayston J. T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983 Aug;41(2):865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncan T., Eb F., Orfila J. Monoclonal antibodies in serovar determination of 53 Chlamydia trachomatis isolates from Amiens, France. Res Microbiol. 1990 Jul-Aug;141(6):695–701. doi: 10.1016/0923-2508(90)90063-v. [DOI] [PubMed] [Google Scholar]
- Persson K., Osser S. Serovars of Chlamydia trachomatis causing postabortion salpingitis. Eur J Clin Microbiol Infect Dis. 1989 Sep;8(9):795–798. doi: 10.1007/BF02185848. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., de la Maza L. M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990 Jun 11;18(11):3414–3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Restriction endonuclease analysis of DNA from Chlamydia trachomatis biovars. J Clin Microbiol. 1988 Apr;26(4):625–629. doi: 10.1128/jcm.26.4.625-629.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Wang S. P. Chlamydia trachomatis immunotypes in Finland. Acta Pathol Microbiol Immunol Scand B. 1987 Apr;95(2):131–134. doi: 10.1111/j.1699-0463.1987.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]