Abstract
OBJECTIVE
We investigated the regulation of adipose tissue gene expression during different phases of a dietary weight loss program and its relation with insulin sensitivity.
RESEARCH DESIGN AND METHODS
Twenty-two obese women followed a dietary intervention program composed of an energy restriction phase with a 4-week very-low-calorie diet and a weight stabilization period composed of a 2-month low-calorie diet followed by 3–4 months of a weight maintenance diet. At each time point, a euglycemic-hyperinsulinemic clamp and subcutaneous adipose tissue biopsies were performed. Adipose tissue gene expression profiling was performed using a DNA microarray in a subgroup of eight women. RT–quantitative PCR was used for determination of mRNA levels of 31 adipose tissue macrophage markers (n = 22).
RESULTS
Body weight, fat mass, and C-reactive protein level decreased and glucose disposal rate increased during the dietary intervention program. Transcriptome profiling revealed two main patterns of variations. The first involved 464 mostly adipocyte genes involved in metabolism that were downregulated during energy restriction, upregulated during weight stabilization, and unchanged during the dietary intervention. The second comprised 511 mainly macrophage genes involved in inflammatory pathways that were not changed or upregulated during energy restriction and downregulated during weight stabilization and dietary intervention. Accordingly, macrophage markers were upregulated during energy restriction and downregulated during weight stabilization and dietary intervention. The increase in glucose disposal rates in each dietary phase was associated with variation in expression of sets of 80–110 genes that differed among energy restriction, weight stabilization, and dietary intervention.
CONCLUSIONS
Adipose tissue macrophages and adipocytes show distinct patterns of gene regulation and association with insulin sensitivity during the various phases of a dietary weight loss program.
Obesity is a major risk factor for diabetes and cardiovascular disease. The excess of fat mass is linked to an impairment of insulin sensitivity through complex multifactorial and still poorly understood mechanisms. Adipose tissue dysfunctions have been recognized as essential in this link (1,2). Alterations of fatty acid metabolism leading to increased fatty acid flux cause metabolic disturbances in liver and skeletal muscle (3). Moreover, alteration of the immune system during chronic overnutrition can result in low-grade inflammation, which favors the development of insulin resistance (4,5). In situations of prolonged positive energy balance, macrophages and adipocytes are activated and show morphological as well as functional changes, such as infiltration of adipose tissue with macrophages and imbalance of fatty acid metabolism and adipokine production (6–9). In humans, the interconnections between metabolic and inflammatory pathways in adipocytes and macrophages and the impact on insulin sensitivity remain largely unknown.
Moderate weight loss improves insulin sensitivity and many of the concurrent medical complications associated with obesity such as type 2 diabetes (10,11). Diet-induced weight loss is associated with a reduction of systemic inflammation and specific metabolic adaptations, suggesting an interaction between nutrition, the immune system, and metabolism (12,13). Nevertheless, the adaptations occurring in adipose tissue during dietary weight management programs in humans are currently largely unknown. It is becoming clear that no single cause or molecule will explain the changes in insulin sensitivity during weight reduction (4). In clinical practice of weight management, dietary intervention is often combined with an initial calorie-restricted diet followed by a weight stabilization phase. However, the long-term outcome of dietary interventions remains poorly understood because of a lack of knowledge regarding the kinetics of complex adipose tissue adaptations during weight loss and weight maintenance and its relation with insulin sensitivity.
The general goal of this study was to identify the whole array of gene expression changes occurring in adipose tissue during the different steps of a dietary intervention program and to explore the links with insulin sensitivity. Because adipose tissue is composed of different cell types, the cell specificity of regulated genes was determined. A specific aim was to analyze the regulation of macrophage gene expression during the course of long-term dietary intervention. We demonstrate that the molecular adaptations occurring in adipose tissue are clearly different between the initial very-low-calorie diet (VLCD) and the following weight stabilization period. Global adipose tissue and macrophage profiling of gene expression showed the opposite regulation of genes expressed in adipocytes involved in metabolism and macrophage genes participating in immune pathways. Genes that may contribute to the improvement in insulin sensitivity clearly differed in the two phases.
RESEARCH DESIGN AND METHODS
Subjects, clinical investigation, and dietary intervention program.
Twenty-two obese premenopausal women were recruited. The study was approved by the ethical committee of the Third Faculty of Medicine of Charles University in Prague, Czech Republic. Written informed consent was obtained for all subjects. Exclusion criteria were weight changes of 3 kg within the 3 months before the start of the study, hypertension, diabetes, or hyperlipidemia treated by drugs, drug-treated obesity, pregnancy, participation in other trials, and alcohol or drug abuse. Obese patients followed a dietary intervention program composed of three successive periods: a 1-month VLCD, a 2-month low-calorie diet, and 3–4 months of a weight maintenance diet (Fig. 1A). During the VLCD phase, subjects received an energy-restricted diet of 800 kcal/day (liquid formula diet: Redita; Promil). During the low-calorie diet phase, the diet was designed to provide 600 kcal/day less than the individually estimated energy requirement based on an initial resting metabolic rate multiplied by 1.3. During the weight maintenance phase, subjects were instructed to consume an individual weight maintenance diet. Patients consulted a dietitian once a week during energy restriction and once a month during the weight maintenance phase. They provided a written 3-day dietary record at each dietary consultation during the weight stabilization phase. A complete clinical investigation in the fasting state was conducted before and at the end of each phase. A needle biopsy of abdominal subcutaneous adipose tissue 15 cm lateral of the umbilicus was performed under local anesthesia (1% lidocaine). Approximately 1 g of adipose tissue was washed in physiological saline, divided into aliquots, and frozen immediately in liquid nitrogen. Anthropometric measurements, blood sampling, and a euglycemic-hyperinsulinemic clamp were performed as described previously (14,15).
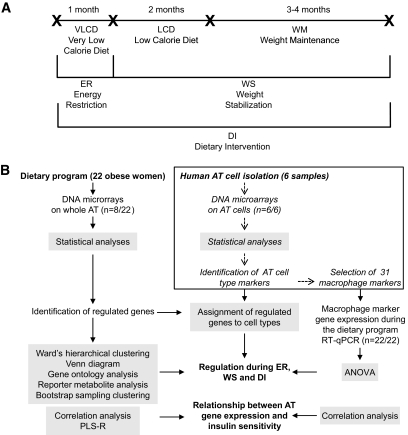
FIG. 1.
Study design of the dietary weight loss program (A) and adipose gene expression analyses (B). A: Upper line segments illustrate subsequent dietary periods and their time duration and cross-points indicate clinical investigation days (see research design and methods). The lower lines show three dietary phases investigated using DNA microarray analysis. B: Flowchart of adipose tissue (AT) gene expression analysis. Twenty-two obese women followed the dietary weight loss program. DNA microarray analysis was performed on adipose tissue from eight of them. Independently, gene expression profiling was performed on the cell types composing subcutaneous adipose tissue from six women to identify adipocyte and macrophage markers. RT-qPCR was used to study the regulation of 31 macrophage markers during the dietary weight loss program.
Total RNA preparation.
Total RNA was isolated from adipose tissue samples with an RNeasy Mini kit (Qiagen). RNA quantity and quality were checked with the Experion automated electrophoresis system (Bio-Rad Laboratories).
DNA microarrays.
DNA microarray experiments were performed on a subgroup of eight subjects (Fig. 1B). Targets were generated from 500 ng of total RNA with a low RNA input amplification kit (Agilent Technologies) and hybridized to whole genome 44K oligonucleotide arrays (Agilent Technologies). A whole transcriptome analysis was performed to compare three dietary periods (Fig. 1): 1) an energy restriction phase, i.e., before versus after the VLCD, 2) a weight stabilization phase, i.e., after the VLCD versus after weight maintenance, and 3) the entire dietary intervention, i.e., before the VLCD versus after weight maintenance. Each combination of samples was analyzed twice using a dye swap design (i.e., a total of 48 hybridizations). Data acquisition and image processing were done with a GenePix 4000B scanner (Axon Instruments) and Feature Extraction 8.5 (Agilent Technologies). Raw data were normalized with a global Lowess procedure and filtered with the LIMMA R package (Bioconductor).
Analysis of DNA microarray data.
Differentially expressed genes were identified with the significance analysis of microarray (SAM) procedure with an estimated false discovery rate of 5% (16). Ward's hierarchical two-way classification was done using differentially expressed genes and the subjects. Further clustering was done using bootstrap sampling to identify robust sets of genes with similar expression pattern using the pvclust R package. Gene functions and biological processes were investigated using the PANTHER classification system (17). Representation of biological processes was evaluated using binomial tests and the Bonferroni correction. Gene lists were also analyzed using Ingenuity Pathways Analysis software 6.0 (Ingenuity Systems) to identify networks and canonical pathways. Prediction of secreted proteins was performed using SignalP 3.0 (18). Two recently published high-confidence models of human metabolism were used to identify metabolites that were catabolized in a statistically significant number of reactions and which enzymes exhibited differential expression across the phases (19–21). Microarray data were deposited to Gene Expression Omnibus with GEO Series accession number GSE11975.
Selection of human adipose tissue macrophage-specific markers.
Adipocytes, macrophages, progenitor cells, endothelial cells, and a negative fraction were isolated from liposuction-obtained subcutaneous adipose tissue of six women by collagenase digestion and immunoselection and depletion (7,22). DNA microarray analysis of the cell types was performed as described above (N.V., B.R., J.G., A.B., D.L., unpublished data). Thirty-one human adipose tissue macrophage-specific markers were selected according to the following criteria: expressed in macrophage more than in any other cell types of adipose tissue (SAM multiclass and pairwise analyses with a false discovery rate of 5%), >10- and 2-fold higher expression in macrophages compared with that in adipocytes and other nonadipocyte cell types, respectively, and >1.5-fold higher expression in macrophages than in the stroma-vascular fraction.
Real-time RT–quantitative PCR.
In the entire group of 22 subjects, total RNA was reverse transcribed using random hexamers and SuperScript II (Invitrogen) or a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) when using microfluidic cards (Fig. 1B). Gene expression was assessed by RT-quantitative (q) PCR with an Applied Biosystems 7500 or 7900 real-time PCR system using SYBR Green–based detection (QuantiTect Primer Assay; Qiagen), TaqMan gene expression assay (Applied Biosystems), or TaqMan Custom arrays, i.e., 384-well microfluidic cards (Applied Biosystems). Ribosomal 18S was used as endogenous control.
Blood parameters.
Plasma glucose, HDL cholesterol, and total cholesterol levels were determined using routine laboratory procedures. Plasma nonesterified fatty acids and glycerol were determined with enzymatic (Wako, Unipath) and colorimetric (Sigma-Aldrich) techniques, respectively. Plasma insulin concentrations were measured using a chemiluminescent immunometric assay (Immulite 2000). C-reactive peptide levels was determined from serum samples by immunoturbidimetry using an ultrasensitive kit (Orion Diagnostica). Commercial ELISA assay kits were used to quantify leptin and adiponectin (BioVendor).
Statistical analyses.
Changes in gene expression and anthropometric and plasma parameters were analyzed by a Wilcoxon test (SPSS 15.0 software). Log-transformed data were analyzed by principal component analysis and partial least square-regression analysis (PLS-R) using SIMCA-P software (Umetrics). PLS-R is a recent technique that generalizes and combines features from principal component analysis and the multiple regression method (23). In this study, it was used to construct predictive models of genes explaining the variability in insulin sensitivity. The mean centroid of mRNA levels was calculated after normalization of gene expression levels to a mean of 0 and a variance of 1 across all individuals (24). A Spearman correlation coefficient was determined between mean centroid and clinical parameters.
RESULTS
Clinical parameters of obese subjects during a dietary weight loss program.
Anthropometric and plasma parameters were determined before and at the end of each dietary period (Fig. 1A, Table 1). Body weight and fat mass as well as plasma insulin, leptin, and C-reactive protein levels decreased during VLCD and remained low during subsequent periods. Lipid parameters (except total cholesterol) were improved at the end of the intervention. The glucose disposal rate (GDR) increased during VLCD and remained elevated in the latter phases.
TABLE 1.
Anthropometric and plasma parameters of 22 obese women during the dietary weight loss program
| Basal | End of VLCD | End of LCD | End of WM | |
|---|---|---|---|---|
| BMI (kg/m2) | 35.3 ± 1.0 | 32.7 ± 1.0* | 31.5 ± 0.9* | 31.7 ± 0.9* |
| Weight (kg) | 96.8 ± 3.4 | 89.6 ± 3.2* | 86.6 ± 3.2* | 86.9 ± 3.2* |
| Waist circumference (cm) | 104.0 ± 2.7 | 98.3 ± 2.7* | 96.0 ± 2.7* | 95.7 ± 2.5* |
| Fat mass (%) | 40.1 ± 1.3 | 37.1 ± 1.3* | 34.4 ± 1.2* | 35.2 ± 1.5* |
| FFM (%) | 60.6 ± 1.4 | 62.9 ± 1.3* | 65.6 ± 1.2* | 64.8 ± 1.5* |
| Glucose (mmol/l) | 5.5 ± 0.1 | 5.30 ± 0.1* | 5.4 ± 0.1 | 5.3 ± 0.2 |
| Insulin (mIU/l) | 13.2 ± 1.7 | 6.8 ± 0.8* | 5.8 ± 0.5* | 6.9 ± 0.6* |
| NEFAs (μmol/l) | 671 ± 28 | 730 ± 27 | 575 ± 37* | 561 ± 38* |
| Glycerol (μmol/l) | 211 ± 16 | 157 ± 8* | 138 ± 12* | 147 ± 10* |
| Triacylglycerol (mmol/l) | 1.5 ± 0.2 | 1.1 ± 0.1* | 1.1 ± 0.1* | 1.1 ± 0.1* |
| HDL cholesterol (mmol/l) | 1.1 ± 0.1 | 1.0 ± 0.1* | 1.1 ± 0.1 | 1.3 ± 0.2* |
| Total cholesterol (mmol/l) | 4.8 ± 0.1 | 3.9 ± 0.2* | 4.3 ± 0.2* | 4.6 ± 0.7 |
| hs-CRP (mg/l) | 5.0 ± 1.1 | 2.3 ± 0.6* | 3.7 ± 1.0* | 2.5 ± 0.6* |
| Leptin (ng/ml) | 40 ± 3 | 20 ± 3* | 23 ± 3* | 28 ± 3* |
| Adiponectin (μg/ml) | 8.1 ± 0.9 | 7.8 ± 0.6 | 8.2 ± 0.7 | 8.8 ± 0.8 |
| GDR (mg · kg−1 · min−1) | 3.0 ± 0.3 | 3.7 ± 0.4* | 4.1 ± 0.4* | 4.1 ± 0.4* |
| GDR (mg · kg FFM−1 · min−1) | 5.0 ± 0.5 | 5.8 ± 0.5* | 6.2 ± 0.5* | 6.3 ± 0.5* |
Data are means ± SE.
*P < 0.05 vs. basal. FFM, fat-free mass; hs-CRP, high-sensitivity C-reactive protein; LCD, low-calorie diet; NEFA, nonesterified fatty acid; WM, weight maintenance diet.
Adipose tissue gene expression profiling during the dietary weight loss program.
Statistical analysis of DNA microarray data. The subset of eight subjects used for DNA microarray experiments did not show differences in clinical parameters compared with the entire group (data not shown). The SAM method showed that 1,535 genes were regulated during at least one of the phases of the dietary program (Fig. 1B and supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0033/DC1): 592 (41 up and 551 down) genes during energy restriction, 814 (244 up and 570 down) genes during weight stabilization, and 581 (89 up and 492 down) genes during dietary intervention. Microarray data were confirmed by RT-qPCR analysis for 45 genes with different expression profiles and cellular origins on 15–22 subjects (supplementary Table 2).
Ward's hierarchical cluster analysis and Venn diagram.
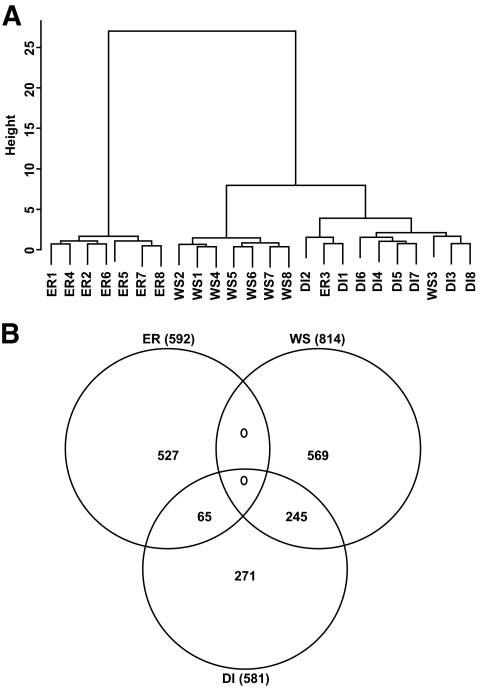
Cluster analysis based on gene expression in the different phases for the eight subjects revealed a clear distinction between the energy restriction and the weight stabilization phases (Fig. 2A). This was also shown by the Venn diagram, which displayed no gene regulated either during energy restriction or weight stabilization (Fig. 2B). However, 65 genes regulated during energy restriction were regulated during dietary intervention and 245 genes regulated during weight stabilization were regulated during dietary intervention.
FIG. 2.
DNA microarray analysis of regulated genes in eight subjects during the different phases of a dietary weight loss program. A: Ward's hierarchical clustering of differentially expressed genes. B: Venn diagram of differentially expressed genes. DI, dietary intervention; ER, energy restriction; WS, weight stabilization.
Gene ontology analysis.
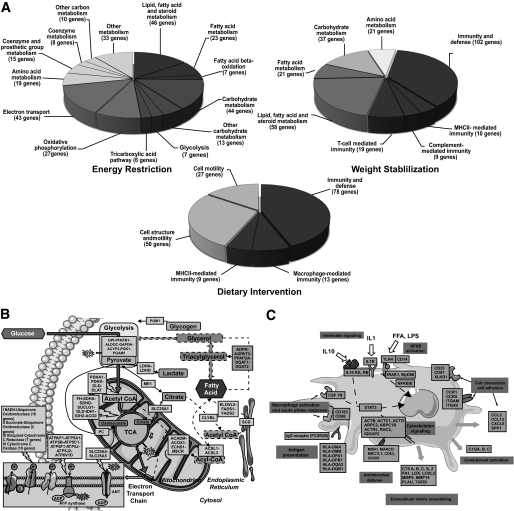
To get insight into the nature of the genes regulated during the different phases, we performed gene ontology analysis using the PANTHER classification system analyzed by binomial test statistics and the Bonferroni correction. Significantly enriched pathways in the different phases are shown in Fig. 3A. During energy restriction, the majority of the genes involved in lipid, carbohydrate, and mitochondrial energy pathways were downregulated (Fig. 3B). During weight stabilization, most of the genes related to lipid and carbohydrate metabolism were upregulated and expression of genes involved in immunity and defense was decreased. The overall dietary intervention was characterized by downregulation of genes involved in immunity and tissue remodeling (Fig. 3C).
FIG. 3.
Gene ontology analysis during the various phases of the dietary weight loss program. A: Significantly enriched pathways using the PANTHER classification system. B: Fatty acid, glucose, and energy metabolism pathways downregulated during energy restriction. The names of genes and cellular specificity are provided in supplementary Table 6. C: Inflammatory and cellular remodeling pathways downregulated during dietary intervention. The names of genes and cellular specificity are provided in supplementary Table 7.
Reporter metabolite analysis.
Coordinated changes in gene expression of enzymes have been shown to identify so-called reporter metabolites, i.e., metabolites that display differential expression in their associated enzymes (21). We identified acyl-CoA, CoA, acetyl-CoA, NADP+/NADPH, and ubiquinol/ubiquinone as reporter metabolites displaying coordinated downregulation of their associated enzymes during energy restriction and a reversed pattern during weight stabilization (supplementary Table 3).
Bootstrap sampling clustering.
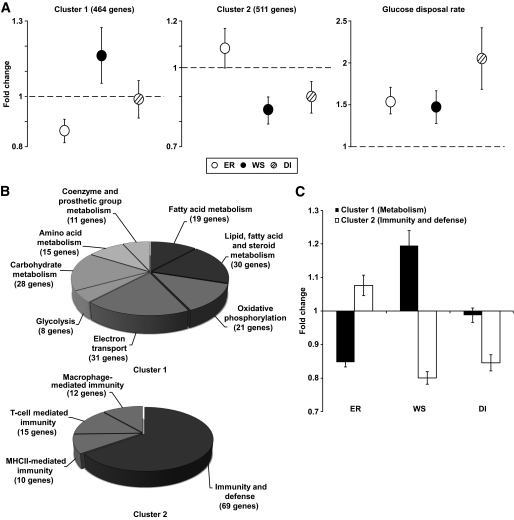
We also performed clustering using bootstrap sampling to determine the robustness of gene sets found to be differentially expressed during the phases of the dietary program. Two large clusters reached significance at P < 0.05 (Fig. 4A). The first was composed of 464 genes downregulated during energy restriction, upregulated during weight stabilization, and unchanged during dietary intervention. The second cluster comprised 511 genes not changed or upregulated during energy restriction and downregulated during weight stabilization and dietary intervention. Next, we performed analysis of gene ontology on the two clusters (Fig. 4B). In the first cluster, all enriched pathways belonged to metabolism. In the second cluster, all enriched pathways belonged to immunity and defense pathways. Strikingly, metabolism genes from cluster 1 and immunity and defense genes from cluster 2 showed opposite patterns during energy restriction and weight stabilization, respectively (Fig. 4C). During dietary intervention, there was marked downregulation of immunity and defense genes (Fig. 4C).
FIG. 4.
Analysis of the two main clusters of genes regulated during the dietary intervention program. A: Gene expression profiles in clusters significant at P < 0.05 identified by hierarchical clustering using bootstrap sampling. The number of transcripts in each cluster is shown in parentheses. For comparison, evolution of GDR is also shown. Mean ± SE fold changes in gene expression are shown on the y-axis. Open circles, energy restriction (ER); black circles, weight stabilization (WS); striped circles; dietary intervention (DI). B: Gene ontology analysis. Enriched pathways identified by bootstrap sampling using the PANTHER classification system. C: Mean ± SE fold changes of genes involved in metabolism from cluster 1 (■) and genes involved in immunity and defense from cluster 2 (□) during energy restriction (ER), weight stabilization (WS), and dietary intervention (DI).
Assignment of genes regulated during the dietary weight loss program to adipose tissue cell types.
Genes found to be regulated during the dietary weight loss program were assigned to adipocytes, macrophages, and other cell types based on DNA microarray analyses of subcutaneous adipose tissue cells performed in an independent experiment (see research design and methods and Fig. 1B). Genes expressed in adipocytes and macrophages represented the vast majority of genes modulated during the dietary program (Table 2). A clearly distinct pattern was observed between the phases. During energy restriction, adipocyte-specific genes were the predominantly regulated category. Less than 1% of genes showed macrophage specificity. During weight stabilization and dietary intervention, the proportion of regulated genes assigned to adipocytes was lower, whereas the proportion of regulated genes assigned to macrophages was higher. Interestingly, the shift from energy restriction to weight stabilization between adipocytes and macrophages was even more pronounced for genes encoding secreted factors (Table 2 and supplementary Table 4, available in an online appendix). Next, genes of the two clusters identified by bootstrap sampling (Fig. 4A) were analyzed in terms of cellular origin. In the first cluster, 47% of genes were adipocyte specific and only 1% were macrophage specific. In the second cluster, 17% were macrophage specific and 10% of genes were adipocyte specific.
TABLE 2.
Distribution of 1,535 differentially expressed genes among adipose tissue cell types
| Adipocytes | Macrophages | Other cell types | No cell specificity | Total | |
|---|---|---|---|---|---|
| Significantly regulated genes | |||||
| ER | 260 (43.9) | 5 (0.8) | 7 (1.2) | 320 (54.1) | 592 (100) |
| WS | 184 (22.6) | 94 (11.5) | 22 (2.7) | 514 (63.1) | 814 (100) |
| DI | 78 (13.4) | 80 (13.8) | 5 (0.9) | 418 (71.9) | 581 (100) |
| Secreted factors | |||||
| ER | 62 (53.0) | 3 (2.6) | 4 (3.4) | 48 (41.0) | 117 (100) |
| WS | 41 (16.9) | 57 (23.5) | 7 (2.9) | 138 (56.8) | 243 (100) |
| DI | 26 (16.2) | 44 (27.3) | 1 (0.6) | 90 (55.9) | 161 (100) |
| Genes identified by PLS-R | |||||
| ER | 35 (44.3) | 7 (8.9) | 2 (2.5) | 35 (44.3) | 79 (100) |
| WS | 20 (19.4) | 34 (33.0) | 0 (0) | 49 (47.6) | 103 (100) |
| DI | 13 (11.4) | 41 (36.0) | 0 (0) | 60 (52.6) | 114 (100) |
Data are n (%). DI, dietary intervention; ER, energy restriction; WS, weight stabilization.
Effect of the dietary weight loss program on adipose tissue macrophage marker gene expression.
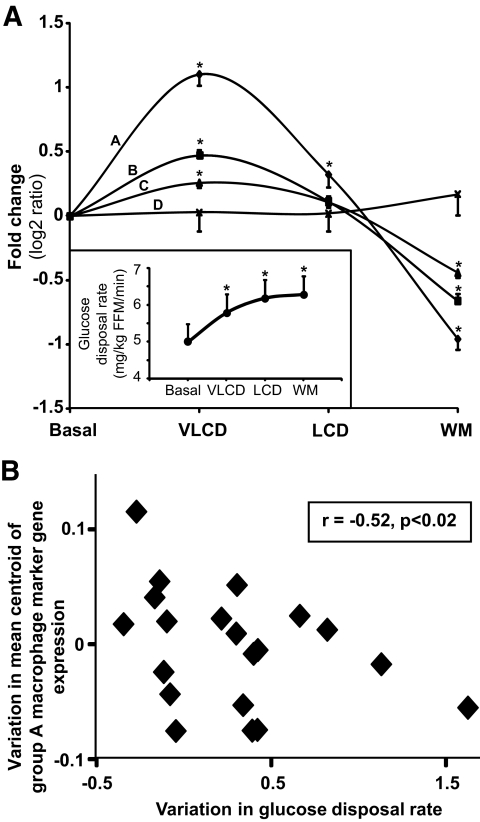
To further characterize the impact of the diet on macrophages, the expression of 31 macrophage markers identified in an independent experiment was analyzed by RT-qPCR in adipose tissue of the 22 subjects after the dietary program (Fig. 1B, Table 3). ANOVA analysis of the mRNA levels revealed four groups of markers labeled A to D, differing by the amplitude of variations at the four time points (Fig. 5A and supplementary Fig. 1). Consistent with microarray data, expression of 29 macrophage markers increased during the VLCD and decreased at subsequent time points (groups A–C). Two genes showed no variations (group D). Of note, the mRNA levels of these genes were significantly higher in obese than in lean subjects (unpublished data).
TABLE 3.
List of 31 macrophage-specific markers derived from DNA microarray analysis of human AT cell types
| Symbol cluster | Aliases | Gene name | Macrophage vs. adipocyte | Macrophage vs. SVF |
|---|---|---|---|---|
| ACP5 | TRAP | Acid phosphatase 5. tartrate resistant | 88.9 | 1.7 |
| CCRL2 | Chemokine (C-C motif) receptor-like 2 | 70.4 | 3.7 | |
| CD14 | CD14 molecule | 17.8 | 1.8 | |
| CD163 | CD163 molecule | 120.9 | 1.5 | |
| CD209 | DCSIGN | CD209 molecule | 25.3 | 1.6 |
| CD33 | SIGLEC3 | CD33 molecule | 28.5 | 2.8 |
| CD68 | CD68 molecule | 19.3 | 1.8 | |
| CENTA2 | Centaurin, alpha 2 | 61.6 | 1.7 | |
| CLEC10A | CD301/CLECSF13 | C-type lectin domain family 10, member A | 224.4 | 3.2 |
| CXCL3 | Chemokine (C-X-C motif) ligand 3 | 40.5 | 2.9 | |
| FCGBP | Fc fragment of IgG binding protein | 157.4 | 2.4 | |
| FCGR2B | CD32 | Fc fragment of IgG, low affinity IIb, receptor (CD32) | 169.3 | 2.4 |
| FCN1 | Ficolin 1 | 134.8 | 2.9 | |
| GATM | Glycine amidinotransferase | 12.7 | 1.6 | |
| HLA-DMA | Major histocompatibility complex, class II, DM alpha | 14.5 | 2.3 | |
| HLA-DRA | Major histocompatibility complex, class II, DR alpha | 45.6 | 2.0 | |
| IL10 | Interleukin 10 | 27.0 | 2.5 | |
| IRF5 | Interferon regulatory factor 5 | 32.5 | 2.2 | |
| KYNU | Kynureninase | 50.5 | 4.1 | |
| LIPA | Lipase A, lysosomal acid, cholesterol esterase | 17.9 | 1.7 | |
| MARCO | Macrophage receptor with collagenous structure | 36.3 | 1.6 | |
| MS4A4A | CD20L1 | Membrane-spanning 4-domains, subfamily A. member 4 | 86.1 | 1.8 |
| MS4A6A | CD20L3 | Membrane-spanning 4-domains, subfamily A, member 6A | 76.4 | 2.7 |
| MS4A7 | CD20L4 | Membrane-spanning 4-domains, subfamily A, member 7 | 15.0 | 5.6 |
| MSR1 | CD204 | Macrophage scavenger receptor 1 | 68.1 | 1.9 |
| PLA2G7 | phospholipase A2, group VII | 180.4 | 2.7 | |
| SIGLEC1 | CD169 | Sialic acid binding Ig-like lectin 1, sialoadhesin | 12.8 | 1.8 |
| SLCO2B1 | Solute carrier organic anion transporter family, member 2B1 | 45.0 | 1.5 | |
| SNCA | Synuclein, alpha | 48.1 | 2.5 | |
| SPP1 | Osteopontin | Secreted phosphoprotein 1 | 259.1 | 2.4 |
| TLR7 | CD187 | Toll-like receptor 7 | 33.3 | 1.8 |
Macrophage vs. adipocyte: ratio of expression in adipose tissue macrophages vs. adipocytes. Macrophage vs. stroma-vascular (SVF): ratio of expression in adipose tissue macrophages vs. SVF.
FIG. 5.
Gene expression of macrophage markers in 22 subjects determined by RT-qPCR at each time point of the dietary weight loss program. A: Mean ± SE fold changes of 31 genes represented as log2 ratio are shown on the y-axis. ANOVA revealed four groups of genes, labeled A–D according to profiles of changes. Inset, evolution of GDR during the different periods of the program. *P < 0.05, compared with basal. B: Relation between changes in GDR and mean centroid of the seven macrophage marker (group A) gene expression during the energy restriction phase. Spearman correlation coefficient and P value are indicated. LCD, low-calorie diet; WM, weigh maintenance.
Relation between adipose tissue gene expression and insulin sensitivity.
Adipose tissue dysfunction is thought to link obesity to insulin resistance. This theory prompted us to analyze the contribution of the diet-induced variations in adipose tissue gene expression to variations in insulin sensitivity (Fig. 1B). We first searched, using DNA microarray data, for genes with kinetic profiles of expression similar to the evolution in GDR in successive dietary phases. Ward's hierarchical clustering of significantly regulated genes revealed no gene that would parallel the pattern of GDR evolution during energy restriction, weight stabilization, and dietary intervention (Fig. 4A). Therefore, we analyzed the relation between variations of gene expression and of GDR independently in each phase. Spearman correlation analysis of the relation between variations of GDR and the mean centroids of genes involved in metabolism (cluster 1) and genes involved in immunity and defense (cluster 2) during each of the dietary phases did not show a significant correlation with insulin sensitivity. Further, the potential contribution of gene expression changes to the improvement in insulin sensitivity was evaluated using PLS-R. The analysis gave a comparable number of genes explaining the variability in GDR in each phase, i.e., 79 for energy restriction, 103 for weight stabilization, and 114 for dietary intervention (Table 2 and supplementary Table 5). During energy restriction, adipocyte genes involved in polyunsaturated fatty acid synthesis and elongation were part of the model (supplementary Fig. 2A). These genes are known to be regulated by the transcription factor sterol regulatory element binding transcription factor 1, which was also part of the model. During weight stabilization and dietary intervention, the models comprised macrophage receptors, antigens, and macrophage-secreted products. Ingenuity pathway analysis revealed a dense network of interactions between genes involved in chemokine signaling, the acute-phase response, interleukin (IL)-10 signaling, and leukocyte extravasation (supplementary Fig. 2B and C). Next, we looked specifically at the relation between macrophage markers and insulin sensitivity. The 31 markers had a profile that was clearly distinct from that of GDR (Fig. 5A). Spearman correlation analysis of the relation between macrophage marker gene expression and insulin sensitivity revealed a negative correlation for centroids of group A and B (r = −0.52 and r = −0.49, P < 0.02) during energy restriction: the higher the upregulation of macrophage genes, the lower the improvement in insulin sensitivity (Fig. 5B).
DISCUSSION
In this work, we investigated the molecular adaptations occurring within human adipose tissue during a dietary program including an initial severe calorie restriction followed by a weight stabilization phase and studied the relationship with insulin sensitivity in the different phases. We used transcriptomic techniques to perform a complete survey of kinetics of gene expression changes in subcutaneous adipose tissue, including the regulation of macrophage markers. Several studies have investigated changes in adipose tissue mRNA levels during hypocaloric diets (25–29). However, the clinically relevant distinct phases of dietary programs, i.e., the rapid weight loss phase associated with an energy deficit and the progressive weight stabilization leading to weight maintenance, have not been investigated. Moreover, no study has disclosed the behavior of macrophages and adipocytes in response to nutritional changes. Our work reveals that molecular adaptations in adipose tissue and their relation to insulin sensitivity vary strikingly between different dietary periods. Therefore, in the search for the regulatory mechanisms of adipose tissue functions during nutritional interventions, it is important to evaluate the impact of individual dietary phases.
During energy restriction, a salient feature was the downregulation of genes involved in adipocyte metabolism. Numerous genes involved in unsaturated fatty acid and triacylglycerol syntheses showed decreased expression. The glycolytic pathway was also affected negatively. Moreover, energy restriction had a profound suppressive impact on mitochondrial metabolism, especially on genes of the citrate cycle and oxidative phosphorylation. These changes during energy restriction promote a decrease in fat accretion capacity and energy consumption by the adipocytes. The reporter metabolite analysis supports this conclusion, as levels of acyl-CoA, acetyl-CoA, and metabolites of the mitochondrial respiratory chain were predicted to be diminished. Another feature of energy restriction was the upregulation of many genes expressed in macrophages and involved in innate immunity. Indeed, the measurement of adipose tissue macrophage markers showed that, although with a difference in amplitude, there was a coordinated increase in gene expression during energy restriction. Interestingly, IL-10, a typical anti-inflammatory marker, was among the upregulated genes. At the same time, there was a significant decrease in plasma C-reactive protein levels, indicating a diminution of systemic inflammation (30). This finding suggests that adipose tissue and systemic inflammatory processes are differently regulated during the severe calorie restriction phase. It can be hypothesized that during the severe calorie restriction phase that activates the immune system, either adipose tissue does not contribute to the control of systemic inflammation or the induction of anti-inflammatory genes by activated macrophages favors the diminution of systemic low-grade inflammation. When energy restriction and weight stabilization were compared, an opposite pattern of regulation was observed between adipocyte metabolism genes and genes belonging to pathways chiefly operating in macrophages. This finding suggests the presence of cross-regulatory mechanisms between these cells. During energy restriction, the diminution of metabolism in adipocytes could trigger the increase in macrophage activity, whereas during weight stabilization, the restoration of adipocyte metabolic capacity could promote the attenuation in the expression of macrophage genes. The clear upregulation of macrophage gene expression during energy restriction and downregulation during weight stabilization were also seen when analyzing the adipose tissue macrophage-specific markers. Early during energy restriction, lipolysis is increased because of a change in acute signals such as a drop in the levels of insulin, which is the main antilipolytic hormone (31,32). The decreased expression of cognate metabolic genes during energy restriction suggests a diminution in capacities of fat storage and energy utilization. These adaptations contribute to an increase in fatty acid net release by fat cells. One possible scenario of the reciprocal expression of adipocyte- and macrophage-related genes during energy restriction could be that fatty acids released by adipocytes during energy restriction can activate macrophages and lead to the upregulation of expression of macrophage-related genes (33). During weight stabilization, upregulation of metabolic gene expression suggests that fatty acids are stored and used within the fat cells. Their net release was, therefore, presumably decreased. This decrease may result in deactivation of macrophages and downregulation of macrophage gene expression. Another putative mechanism may involve the adipocyte fatty acid binding protein, FABP4. Indeed, it has recently been shown that deletion of FABP4 in vivo in mouse adipocytes resulted in reduced activation of macrophages (34). The upregulation of FABP4 found in the present study during energy restriction and downregulation during weight stabilization indicate a role for this lipid chaperone in the cross-talk between fat cells and macrophages. Conversely, it is possible that macrophage activity may influence adipocyte metabolism, notably through suppression of fatty acid storage and activation of lipolysis (33,35–37). During weight stabilization and dietary intervention, processes of immune response such as antigen presentation with regulation of major histocompatibility complex genes, macrophage activation, complement activation, and IL-1 and IL-10 signaling were downregulated. Interestingly, components of the receptor system for lipopolysaccharide including CD14, Toll-like receptor 4, Myd88, and MD-2/LY96 were downregulated. This system mediates the activation of proinflammatory pathways in macrophages and controls the production of cytokines such as IL-6 and tumor necrosis factor-α. It has also recently been shown to mediate the effect of fatty acids both in vitro and in vivo and to be part of the link between lipid, innate immunity, and insulin resistance (38,39). Adipose tissue remodeling was another modified pathway during dietary intervention because actin cytoskeleton signaling and extracellular matrix pathways were regulated as shown previously after weight loss induced by bariatric surgery (40).
Study of the transcriptome allows genome-wide analysis with no a priori hypothesis of the relation between adipose tissue gene expression and GDR. PLS-R gave the best models of prediction in each phase, with 80–110 genes associated with the variability in GDR. This finding clearly indicates that epistatic interactions among a set of genes rather than individual genes contribute to the association between adipose tissue and insulin sensitivity. The genes participating in insulin sensitivity models differed during dietary phases. During energy restriction, synthesis of unsaturated fatty acids was associated with the changes in GDR. In support of this finding, stearoyl-CoA desaturase 1 activity and fatty acid desaturation indexes in adipose tissue have recently been shown to be associated with insulin sensitivity (41). During weight stabilization and dietary intervention, pathways related to innate immunity were markedly represented in insulin sensitivity models. Accordingly, the proportion of macrophage genes increased in the PLS-R models of insulin sensitivity with metalloproteinase-9, osteopontin/secreted phosphoprotein 1, and tartrate-resistant acid phosphatase 5 as important contributors. Of note, these secreted factors have an important local role in adipose tissue, modulating tissue remodeling and inflammation (42–44). The analysis of correlation between expression of macrophage markers and GDR revealed an inverse relation during energy restriction. These data suggest that the activation of some macrophage gene expression may counteract the alleviation of insulin resistance during this phase.
To conclude, macrophage and fat cell gene expressions in adipose tissue are differentially regulated during the calorie restriction and weight maintenance phases of a weight loss program. The diet-induced improvement of insulin sensitivity is associated with changes in clusters of genes rather than in single genes, with the sets of genes being different in each phase of the program. The kinetics of changes within adipose tissue and the interactions between the metabolic state of adipocytes and the activation state of macrophages appear critical for the understanding of the beneficial effects on health resulting from a long-term dietary weight loss program in obese subjects.
Supplementary Material
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), by grant IGA NR 9161-3-2007 from the Ministry of Health and project MSM 0021620814 from the Ministry of Education of the Czech Republic, by the French National Research Agency program on Cardiovascular Disease, Diabetes and Obesity (RIOMA and FAIR projects), and by the Midi-Pyrénées Region and the Commission of the European Communities (Integrated Project HEPADIP [http://www.hepadip.org], contract LSHM-CT-2005-018734, Integrated Project MOLPAGE [http://www.molpage.org], contract LSH-2003-1.1.3-1, and collaborative project ADAPT [http://www.adapt-eu.net], contract HEALTH-F2-2008-2011 00).
No potential conflicts of interest relevant to this article were reported.
We are indebted to Zuzana Parizkova (Department of Sports Medicine, Third Faculty of Medicine), Marie-Adeline Marques, Marion Combes, Carine Valle, Pauline Decaunes, and Jason Iacovoni (INSERM U858) for technical expertise.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP: Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9: 367– 377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS: Inflammation and metabolic disorders. Nature 2006; 444: 860– 867 [DOI] [PubMed] [Google Scholar]
- 3.Shulman GI: Cellular mechanisms of insulin resistance. J Clin Invest 2000; 106: 171– 176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenk S, Saberi M, Olefsky JM: Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008; 118: 2992– 3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolowczuk I, Verwaerde C, Viltart O, Delanoye A, Delacre M, Pot B, Grangette C: Feeding our immune system: impact on metabolism. Clin Dev Immunol 2008: 639803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54: 2277– 2286 [DOI] [PubMed] [Google Scholar]
- 7.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A: From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes 2004; 53: 1285– 1292 [DOI] [PubMed] [Google Scholar]
- 8.Qatanani M, Lazar MA: Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 2007; 21: 1443– 1455 [DOI] [PubMed] [Google Scholar]
- 9.Tilg H, Moschen AR: Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6: 772– 783 [DOI] [PubMed] [Google Scholar]
- 10.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D: Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 1987; 147: 1749– 1753 [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343– 1350 [DOI] [PubMed] [Google Scholar]
- 12.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E: Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006; 295: 1539– 1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You T, Nicklas BJ: Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr Diabetes Rev 2006; 2: 29– 37 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 15.Klimcakova E, Polak J, Moro C, Hejnova J, Majercik M, Viguerie N, Berlan M, Langin D, Stich V: Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab 2006; 91: 5107– 5112 [DOI] [PubMed] [Google Scholar]
- 16.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001; 98: 5116– 5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A: PANTHER: a library of protein families and subfamilies indexed by function. Genome Res 2003; 13: 2129– 2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendtsen JD, Nielsen H, von Heijne G, Brunak S: Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340: 783– 795 [DOI] [PubMed] [Google Scholar]
- 19.Duarte NC, Becker SA, Jamshidi N, Thiele I, Mo ML, Vo TD, Srivas R, Palsson BO: Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc Natl Acad Sci USA 2007; 104: 1777– 1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Sorokin A, Mazein A, Selkov A, Selkov E, Demin O, Goryanin I: The Edinburgh human metabolic network reconstruction and its functional analysis. Mol Syst Biol 2007; 3: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil KR, Nielsen J: Uncovering transcriptional regulation of metabolism by using metabolic network topology. Proc Natl Acad Sci USA 2005; 102: 2685– 2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A: Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004; 110: 349– 355 [DOI] [PubMed] [Google Scholar]
- 23.Wold H: Partial least squares. In Encyclopedia of Statistical Sciences, New York, Wiley, 1985, p. 581– 591 [Google Scholar]
- 24.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately down-regulated in human diabetes. Nat Genet 2003; 34: 267– 273 [DOI] [PubMed] [Google Scholar]
- 25.Bruun JM, Pedersen SB, Kristensen K, Richelsen B: Opposite regulation of interleukin-8 and tumor necrosis factor α by weight loss. Obes Res 2002; 10: 499– 506 [DOI] [PubMed] [Google Scholar]
- 26.Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Stich V, Cancello R, Langin D: Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J 2004; 18: 1657– 1669 [DOI] [PubMed] [Google Scholar]
- 27.Dahlman I, Linder K, Arvidsson Nordstrom E, Andersson I, Liden J, Verdich C, Sorensen TI, Arner P: Changes in adipose tissue gene expression with energy-restricted diets in obese women. Am J Clin Nutr 2005; 81: 1275– 1285 [DOI] [PubMed] [Google Scholar]
- 28.Kolehmainen M, Salopuro T, Schwab US, Kekalainen J, Kallio P, Laaksonen DE, Pulkkinen L, Lindi VI, Sivenius K, Mager U, Siitonen N, Niskanen L, Gylling H, Rauramaa R, Uusitupa M: Weight reduction modulates expression of genes involved in extracellular matrix and cell death: the GENOBIN study. Int J Obes (Lond) 2008; 32: 292– 303 [DOI] [PubMed] [Google Scholar]
- 29.Viguerie N, Vidal H, Arner P, Holst C, Avizou S, Astrup A, Saris WHM, MacDonald IA, Klimcakova E, Clément K, Martinez A, Hoffstedt J, Sorensen TIA, Langin D: Nutrient-Gene Interactions in Human Obesity—Implications for Dietary Guideline (NUGENOB) project: Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 2005; 48: 123– 131 [DOI] [PubMed] [Google Scholar]
- 30.Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT: Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol 2004; 24: 124– 128 [DOI] [PubMed] [Google Scholar]
- 31.Langin D: Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharm Res 2006; 53: 482– 491 [DOI] [PubMed] [Google Scholar]
- 32.Vernon RG: Effects of diet on lipolysis and its regulation. Proc Nutr Soc 1992; 51: 397– 408 [DOI] [PubMed] [Google Scholar]
- 33.Suganami T, Nishida J, Ogawa Y: A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vasc Biol 2005; 25: 2062– 2068 [DOI] [PubMed] [Google Scholar]
- 34.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS: Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest 2008; 118: 2640– 2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami M, Pekala PH, Lane MD, Cerami A: Lipoprotein lipase suppression in 3T3–L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci USA 1982; 79: 912– 916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langin D, Arner P: Importance of TNFα and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 2006; 17: 314– 320 [DOI] [PubMed] [Google Scholar]
- 37.Pekala P, Kawakami M, Vine W, Lane MD, Cerami A: Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med 1983; 157: 1360– 1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015– 3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y: Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007; 27: 84– 91 [DOI] [PubMed] [Google Scholar]
- 40.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K: Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 2008; 9: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjögren P, Sierra-Johnson J, Gertow K, Rosell M, Vessby B, de Faire U, Hamsten A, Hellenius ML, Fisher RM: Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia 2008; 51: 328– 335 [DOI] [PubMed] [Google Scholar]
- 42.Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A, Dahman M, Tordjman J, Clement K, McQuaid SE, Frayn KN, Huet PM, Gugenheim J, Lotersztajn S, Le Marchand-Brustel Y, Tran A, Gual P: Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes 2009; 58: 125– 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumie A: Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008; 117: 806– 815 [DOI] [PubMed] [Google Scholar]
- 44.Lang P, van Harmelen V, Ryden M, Kaaman M, Parini P, Carneheim C, Cassady AI, Hume DA, Andersson G, Arner P: Monomeric tartrate resistant acid phosphatase induces insulin sensitive obesity. PLoS ONE 2008; 3: e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.