Abstract
OBJECTIVE
The peroxisome proliferator–activated receptor-γ coactivator (PGC)-1 family of transcriptional coactivators controls hepatic function by modulating the expression of key metabolic enzymes. Hepatic gain of function and complete genetic ablation of PGC-1α show that this coactivator is important for activating the programs of gluconeogenesis, fatty acid oxidation, oxidative phosphorylation, and lipid secretion during times of nutrient deprivation. However, how moderate changes in PGC-1α activity affect metabolism and energy homeostasis has yet to be determined.
RESEARCH DESIGN AND METHODS
To identify key metabolic pathways that may be physiologically relevant in the context of reduced hepatic PGC-1α levels, we used the Cre/Lox system to create mice heterozygous for PGC-1α specifically within the liver (LH mice).
RESULTS
These mice showed fasting hepatic steatosis and diminished ketogenesis associated with decreased expression of genes involved in mitochondrial β-oxidation. LH mice also exhibited high circulating levels of triglyceride that correlated with increased expression of genes involved in triglyceride-rich lipoprotein assembly. Concomitant with defects in lipid metabolism, hepatic insulin resistance was observed both in LH mice fed a high-fat diet as well as in primary hepatocytes.
CONCLUSIONS
These data highlight both the dose-dependent and long-term effects of reducing hepatic PGC-1α levels, underlining the importance of tightly regulated PGC-1α expression in the maintenance of lipid homeostasis and glucose metabolism.
Imbalances in hepatic lipid metabolism, leading to accumulation of hepatic triglycerides, insulin resistance, inflammation, and apoptosis, are intimately related to diseases of energy imbalance; these include obesity, diabetes, hyperlipidemia, and atherosclerosis (1,2). Changes in hepatic energy balance are often modulated at the transcriptional level by hormonal signals acting on nuclear receptors and forkhead box O (FoxO) proteins (3). In addition to ligand-mediated receptor activation, physiological stimuli promote recruitment of coactivators to the transcriptional machinery, adding an additional layer of regulation by selective amplification of specific gene sets. Peroxisome proliferator–activated receptor (PPAR)-γ coactivator-1 (PGC-1α) is one such transcriptional coactivator shown to play a particularly important role in liver biology.
Hepatic PGC-1α binds to and activates multiple transcription factors, including FoxO1, glucocorticoid receptor, hepatic nuclear factor-4α, estrogen-related receptor-α, and PPAR-α, resulting in increased expression of genes important for gluconeogenesis, fatty acid oxidation, lipid transport, and oxidative phosphorylation (4,5). Glucagon increases hepatic PGC-1α expression during a fast, whereas insulin potently inhibits PGC-1α expression and activity (6–10). Thus, alterations in hormone activity, such as the insulin resistance or hyperglucagonemia associated with diabetes, may lead to dysregulation of PGC-1α. Hepatic PGC-1α expression levels are increased in multiple rodent models of diabetes and obesity, including liver insulin receptor knockout (11,12), high-fat–fed (13), leptin-deficient (ob/ob), and streptozotocin-administered mice (7). Given its role in promoting gluconeogenesis, inappropriately high levels of hepatic PGC-1α may exacerbate hyperglycemia. Therefore, reducing hepatic PGC-1α may be an attractive therapeutic strategy for improving hepatic insulin signaling and preventing inappropriate glucose production in diabetic patients.
Though highlighting the importance of PGC-1α within liver biology, previous studies have been limited to gain/loss-of-function strategies using adenoviral vectors or complete loss-of-function knockout mouse models. These models suggest that although complete loss of PGC-1α within the liver results in fasting-induced steatosis, it improves glucose tolerance concomitant with decreased gluconeogenesis and increased insulin sensitivity (11,14–17). However, PGC-1α knockout mice exhibit multiple metabolic abnormalities contributing to their overall phenotype because of loss of PGC-1α in other metabolically active tissues, including skeletal muscle, brain, brown fat, and heart (14,15,18). Additionally, adenoviral knockdown of PGC-1α can only address the acute effects of losing coactivator activity. Most importantly, the expression levels of PGC-1α and other coactivators are tightly regulated, often changing only mildly in response to physiological cues (16,19–21). Therefore, the above-mentioned models may not appropriately reflect the effects of physiological fluctuations of PGC-1α expression on tissue-specific target pathways.
We were interested in observing the consequences of moderate, long-term changes in hepatic PCG-1α expression. To do this, we used a tissue-specific gene-targeting approach to create mice with only one functional allele of PGC-1α within the liver. Using this mouse model, we have identified liver-specific PGC-1α–regulated pathways highly sensitive to quantitatively reduced coactivator expression. Loss of only one allele of hepatic PGC-1α was sufficient to cause significant dysfunctions in fasting lipid oxidation, ketogenesis, and the regulation of circulating triglyceride levels. Consistent with this, analysis of hepatic gene expression suggested that fatty acid oxidation and lipid processing pathways were most affected by loss of PGC-1α. More strikingly, chronic reduction of PGC-1α reduced hepatic insulin sensitivity, likely contributing to the alterations in hepatic glucose and lipid metabolism. These data underline the importance of PGC-1α expression to hepatic lipid metabolism and indicate that even moderate decreases in PGC-1α function may contribute to the development of liver disease.
RESEARCH DESIGN AND METHODS
Floxed PGC-1α alleles are previously described (14). To create liver-specific heterozygous (LH) animals, female mice with one floxed PGC-1α allele (PGC-1αfl/+) were crossed with mice transgenically expressing Cre recombinase under control of the rat albumin promoter (Jackson Laboratory). Control mice were a mixed population of PGC-1αfl/+ and PGC-1α+/+,alb-cre/+ littermates. All mice were on a mixed background of C57BL/129, which is similar to the background of other PGC-1α models (11,14,15). Animals were fed a regular chow diet (5008I; PharmaServ) or a high-fat diet (58% kcal fat, D12331; Research Diets). All experiments were performed in accordance with animal facility institutional animal care and use committee regulations.
Histology.
Liver tissue was frozen in OCT compound, sectioned, and stained with oil red O.
Body composition.
Percentage fat mass was determined by dual-energy X-ray absorptiometry scanning in anesthetized mice (Piximus II; Lunar).
Hepatic lipid levels.
Hepatic lipids were extracted as previously described (22). Triglycerides (Sigma), nonesterified free fatty acids (NEFAs; Wako), and cholesterol (Pointe Scientific) were measured by colorimetric assay and normalized to total protein content of initial homogenate.
RNA isolation and quantitative RT-PCR.
RNA was isolated from frozen tissue using TRIzol reagent (Invitrogen). A total of 1 μg of RNA was treated with DNase I and reverse-transcribed. cDNA was amplified and quantified with an Applied Biosystems real-time PCR system using SYBR Green PCR master mix and the ΔΔCt threshold cycle method. Gene expression levels were normalized to TATA binding protein (TBP) mRNA and expressed relative to control. Primer sequences are listed in supplementary Table A2, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1571/DC1.
Primary hepatocyte isolation.
Primary mouse hepatocytes from 10- to 12-week-old mice were isolated by collagen perfusion and percoll gradient purification. Cells were seeded on collagen-coated plates and maintained in Dulbecco's modified Eagle's medium supplemented with 0.2% BSA, 4.5 g/l glucose, 2 mmol/l sodium pyruvate, 0.1 μmol/l dexamethasone, and 1 nmol/l insulin (maintenance media).
Cell culture and treatment.
For overexpression and knockdown studies, hepatocytes were infected with adenovirus expressing either vector alone (green fluorescent protein), PGC-1α (7), scrambled small interfering RNA (siRNA), or PGC-1α siRNA (siPGC-1α), as indicated, for 48 h. To assess Akt signaling, cells were incubated overnight with media lacking dexamethasone and insulin (starvation media) and then treated with 100 nmol/l insulin or vehicle for the indicated times before isolating protein. For inhibition of gluconeogenic gene expression, cells were incubated in starvation media overnight and preincubated with 100 nmol/l insulin or vehicle for 10 min before the addition of media (control) or 25 nmol/l glucagon (Bachem) for 2 h at 37°C. For gene expression analysis, cells were harvested in TRIzol before quantification by real-time PCR. Endogenous hepatic PGC-1α protein levels were measured in freshly isolated hepatocytes or cultured hepatocytes treated for 4 h with vehicle or 1 μmol/l dexamethasone/10 μmol/l forskolin. Quantification of palmitate oxidation in cultured hepatocytes was performed as previously described (23).
Serum glucose, insulin, lipids, and ketones.
Plasma lipoproteins were fractionated by FPLC as described (24). Blood glucose was measured in tail blood using a standard glucometer. Serum insulin and β-hydroxybutyrate concentrations were determined by enzyme-linked immunosorbent assay. Triglyceride, NEFA, and cholesterol measurements were determined by colorimetric assay (Assay Core, Joslin Diabetes Center).
Solid-phase extraction of hepatic fatty acyl-CoAs and mass spectrometry.
Extraction of acyl-CoA was performed as previously described (25). The purified fraction was used for liquid chromatography/mass spectrometry/mass spectrometry analysis using an API 3000 mass spectrometer (Applied Biosystems).
Triglyceride secretion rate.
Mice were fasted 4 h before tail vein injection of 250 mg/kg tyloxapol (Sigma). Triglyceride concentration in blood, collected before and every hour for 4 h after injection, was measured by colorimetric assay.
Protein isolation and Western blotting.
Hepatocyte or liver protein was solubilized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors. PGC-1α was immunoprecipitated from 500 μg (dexamethasone/forskolin-treated) or 2 mg (fresh hepatocytes) of protein using an anti–PGC-1α antibody. Protein samples were resolved by SDS-PAGE, blotted, and incubated with anti–PGC-1α (gift from Dr. Thomas Gettys, Pennington Biomedical Research Center), phospho-Akt (Ser473), or total Akt antibodies (Cell Signaling). Equal loading was confirmed using anti-actin or 90-kDa heat shock protein.
In vivo insulin signaling and pyruvate tolerance tests.
Animals were fasted for 16 or 6 h before intraperitoneal injection of 2 g/kg sodium pyruvate or 0.8 units/kg insulin (Humulin; Eli Lilly), respectively. Glucose was measured in tail vein blood taken at 15-min intervals. For in vivo measurement of phospho-Akt, animals fed a high-fat diet for 16 weeks were fasted 4 h before intravenous injection of 0.5 units/kg insulin via the inferior vena cava. Liver was harvested 5 min after injection.
Statistical analysis.
Statistical significance (P < 0.05, P < 0.01, and P < 0.001) was assessed by ANOVA, Student's t test, or linear regression analysis using GraphPad Prism, as indicated.
RESULTS
Generation of liver-specific PGC-1α heterozygous mice.
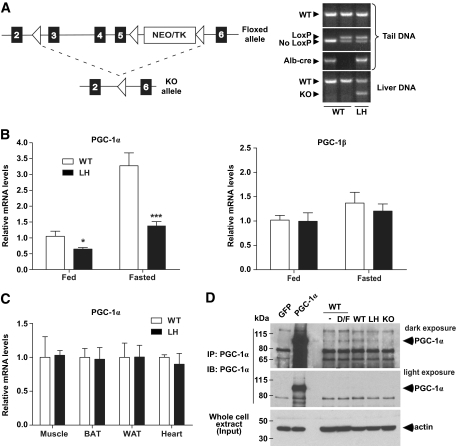
To investigate the effects of chronically reduced PGC-1α within the liver, we crossed mice harboring one floxed PGC-1α allele (PGC-1αfl/+) with transgenic mice expressing Cre recombinase under the control of the albumin promoter (alb-cre transgene) (Fig. 1A). The resulting mice possessed one functional and one disrupted PGC-1α allele within the liver (PGC-1α liver heterozygotes, LH mice) confirmed by PCR analysis of both tail and liver genomic DNA (Fig. 1A). mRNA levels of PGC-1α in LH livers were, on average, 57, 42, and 34% of wild-type levels in fed, overnight-fasted, and long-term–fasted mice, respectively (Fig. 1B and supplementary Fig. A1). The >50% reduction was expected in fasted LH mice given that PGC-1α acts in an autoregulatory positive feedback loop (26,27). Hepatic levels of the structurally related transcriptional coactivator PGC-1β remained unchanged (Fig. 1B). LH mice expressed wild-type levels of PGC-1α in brown fat, white fat, muscle, and heart (Fig. 1C), confirming tissue specificity of PGC-1α heterozygosity. PGC-1α protein was difficult to detect by Western blot of whole-liver extracts (not shown). To visualize hepatic PGC-1α protein levels, we immunoprecipitated endogenous PGC-1α from protein extracts of freshly isolated hepatocytes from wild-type, LH, and whole-body PGC-1α knockout mice (Fig. 1D). These data show that relative protein levels of PGC-1α within wild-type and LH hepatocytes correlated with hepatic mRNA levels.
FIG. 1.
Generation and characterization of liver-specific PGC-1α heterozygous (LH) mice. A: Mice were bred to carry one floxed PGC-1α allele and transgenically express Cre recombinase under control of the rat albumin promoter, leading to excision of exons 3–5 of PGC-1α within the liver. △, LoxP sites. PCR analysis detected the presence of the LoxP sites (floxed allele), alb-cre transgene, and knockout allele. B and C: Relative mRNA expression of either PGC-1α or PGC-1β in liver (B) and in muscle, BAT, WAT, and heart tissue (C) from LH versus control (wild-type [WT]) mice. D: Endogenous PGC-1α was immunoprecipitated from primary hepatocyte protein extracts isolated from wild-type, LH, and whole-body PGC-1α knockout animals (KO) to show relative protein expression. Immunoprecipitates from cultured hepatocytes treated with dexamethasone/forskolin (D/F) and adenovirally overexpressed PGC-1α demonstrate anti–PGC-1α antibody specificity. *P < 0.05; ***P < 0.001. GFP, green fluorescent protein.
LH mice were born in the expected Mendelian ratio, with no obvious growth abnormalities observed at birth. Although they were slightly lighter, this difference was not statistically significant. Importantly, they exhibited no differences in fat mass compared with control mice at 24 weeks of age (supplementary Fig. A2, panels A and B).
Levels of hepatic PGC-1α correlate inversely with fasting hepatosteatosis.
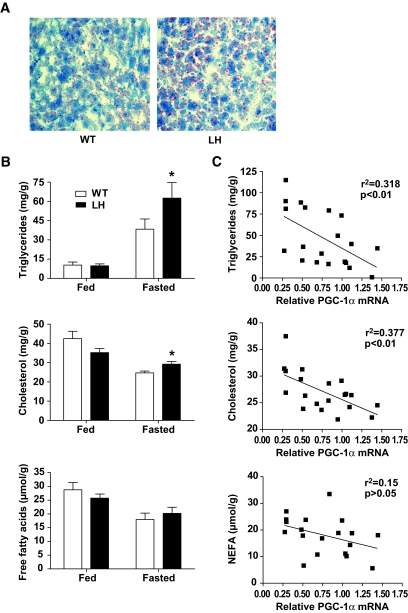
Because PGC-1α plays a key role in modulating the hepatic response to nutrient deprivation (7,16,17), we fasted wild-type and LH mice to identify potential differences in glucose and lipid metabolism. Although PGC-1α is known to play a significant role in the regulation of hepatic gluconeogenesis (7,9,17), mice lacking only one allele of PGC-1α in the liver did not show evidence of fasting hypoglycemia or impaired pyruvate metabolism on a regular chow diet (supplementary Fig. A2, panels C and D). However, oil red O staining of liver sections indicated that LH livers had a higher lipid content than wild-type controls after a 24-h fast (Fig. 2A). Quantitatively, fasted LH livers accumulated significantly more triglycerides and cholesterol than wild-type controls, whereas there was no difference in NEFA levels (Fig. 2B). Furthermore, the relative level of hepatic PGC-1α transcripts in fasted mice inversely correlated with the concentration of triglycerides and cholesterol, but not NEFA (Fig. 2C). Fed levels of hepatic triglycerides, cholesterol, and NEFA were similar between groups (Fig. 2B and supplementary Fig. A2, panel E). These data indicate that fasting lipid metabolism is highly sensitive to reductions in PGC-1α expression and that even modest reductions can cause significant defects in lipid processing, leading to fatty liver disease.
FIG. 2.
Accumulation of hepatic lipids in fasted LH mice. A: Oil red O staining of liver sections isolated from 24-h–fasted mice. B: Average triglyceride, cholesterol, and NEFA levels in fed or 24-h–fasted wild-type (WT) and LH livers after chloroform/methanol lipid extraction, n = 6 (fed) or 9–10 (fasted) mice per group. C: Individual fasted lipid concentrations were plotted against the relative PGC-1α mRNA value for each mouse and subjected to linear regression analysis. *P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
Genetic reduction of hepatic PGC-1α diminished the gene program of fatty acid oxidation.
Next, we investigated the effects of having reduced PGC-1α on the expression of mRNAs encoding key metabolic enzymes in livers of fed and fasted mice. We observed modest yet significant reductions in the expression of select genes involved in fatty acid oxidation, including lipin-1 (LPIN-1), very-long-chain acyl-CoA dehydrogenase (VLCAD), long-chain acyl-CoA dehydrogenase (LCAD), and short-chain acyl-CoA dehydrogenase (SCAD) (Fig. 3A and supplementary Table A1). Similarly, reduced levels of VLCAD, LCAD, and SCAD and increased hepatic lipid content were also observed after long-term fasting of 24–72 h (supplementary Fig. A3). Interestingly, the levels of medium-chain acyl-CoA dehydrogenase, carnitine palmitoyl transferase-1α, and PPAR-α, known PGC-1α target fatty acid oxidation genes, were not significantly affected by chronic knockdown of hepatic PGC-1α (Fig. 3B and supplementary Fig. A3). To confirm that the regulation of these genes by PGC-1α was cell autonomous, we overexpressed PGC-1α using adenovirus in primary hepatocytes and measured the levels of gene transcripts by quantitative RT-PCR. PGC-1α overexpression significantly increased the mRNA levels of many genes specifically involved in mitochondrial β-oxidation of fatty acids (Fig. 3C).
FIG. 3.
Reduced fatty acid oxidation gene expression and function in LH mice. A and B: Affected (A) and unaffected (B) genes. Hepatic mRNA levels were quantified by RT-PCR in mice fed ad libitum or fasted overnight. Bars represent the means ± SE (n = 6) and are expressed relative to wild-type (WT) fed values. C: mRNA expression levels, expressed relative to green fluorescent protein (GFP)-infected control, from primary hepatocytes overexpressing PGC-1α. Values are the means ± SD of triplicate values, representative of three individual experiments. D: 14C-palmitate oxidation in wild-type and LH primary hepatocytes. Values are the means ± SD of quadruplicate values. E: Serum ketones. The concentration of β-hydroxybutyrate in serum of fed or 24-h–fasted mice is shown. Data are the means ± SE (n = 11). F: Concentrations of individual acyl-CoA metabolites isolated from 24-h–fasted livers, as determined by mass spectrometry. Data are the means ± SE (n = 9–10). *P < 0.05; **P < 0.01; ***P < 0.001. AOX, acyl-CoA oxidase; SBCAD, short/branched-chain acyl-CoA dehydrogenase.
Consistent with a hepatic defect in hepatic fatty acid oxidation, 14C-palmitate oxidation was significantly lower in LH primary hepatocytes (Fig. 3D). Moreover, LH mice had lower levels of circulating β-hydroxybutyrate in both the fed and fasted state, suggesting a deficiency in ketogenesis (Fig. 3E). Analysis of liver samples by mass spectrometry revealed that LH livers accumulated significantly higher levels of medium- to long-chain fatty acyl-CoAs, specifically C12:2, C12:1, C14:2, C14:1, and C18:2, after a 24-h fast (Fig. 3F). This pattern is similar to that found in mice lacking LCAD or VLCAD enzymes (28–30).
Dysregulation of hepatic PGC-1α leads to hypertriglyceridemia.
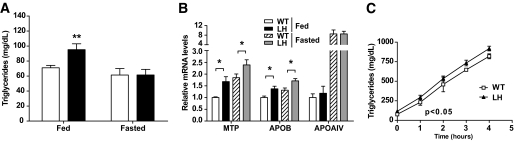
Defects in hepatic lipid catabolism can directly affect levels of circulating lipids. Circulating levels of triglycerides were significantly higher in fed LH mice, whereas fasting levels showed no differences (Fig. 4A). There were no differences in the fed or fasted concentrations of circulating free fatty acids, and levels of cholesterol in the VLDL, LDL, or HDL fractions were similar between wild-type and LH mice (supplementary Fig. A4).
FIG. 4.
LH mice exhibit increased circulating triglycerides and altered expression of genes involved in VLDL production. A: Concentrations of circulating triglycerides in fed or 24-h–fasted mice. Data are the means ± SE (n = 11). □, Wild-type; ■, LH. B: Lipid transport genes. Hepatic mRNA levels were quantified by RT-PCR in mice fed or fasted overnight. Data are the means ± SE (n = 6). Values are expressed relative to wild-type (WT) fed levels. C: Time–course of serum triglyceride accumulation after inhibition of lipolysis with intravenously injected Tyloxapol. Values are the means ± SE (n = 3) and are representative of two independent experiments. P < 0.05 by two-way ANOVA. *P < 0.05; **P < 0.01.
Increases in serum triglycerides may arise because of dysregulation of hepatic lipid assembly, secretion, or catabolism. Because PGC-1α regulates key genes involved in lipid transport (31), we investigated whether hepatic PGC-1α heterozygosity affected lipid secretory pathways. We saw no differences in the expression of APOAIV, APOAV, or APOCIII (Fig. 4B and supplementary Table A1), previously characterized PGC-1α target genes. However, in both fed and fasted mice, there was significantly increased expression of microsomal triglyceride transfer protein (MTP), a protein essential for LDL assembly (Fig. 4B and supplementary Table A1). We also observed increased APOB expression levels in LH mice (Fig. 4B and supplementary Table A1), which, taken together, may suggest an increase in lipoprotein synthesis.
To assess whether high triglyceride levels were caused by increased triglyceride secretion, we inhibited lipoprotein lipase using tyloxapol and measured the rate of triglyceride accumulation in the serum. Consistent with previous findings, LH mice exhibited higher levels of circulating triglycerides before and at all points after tyloxapol injection (Fig. 4C). However, the rate of triglyceride accumulation in the serum of wild-type and LH mice was similar (Fig. 4C), suggesting that the higher levels of circulating triglycerides were not caused by increased hepatic lipoprotein secretion.
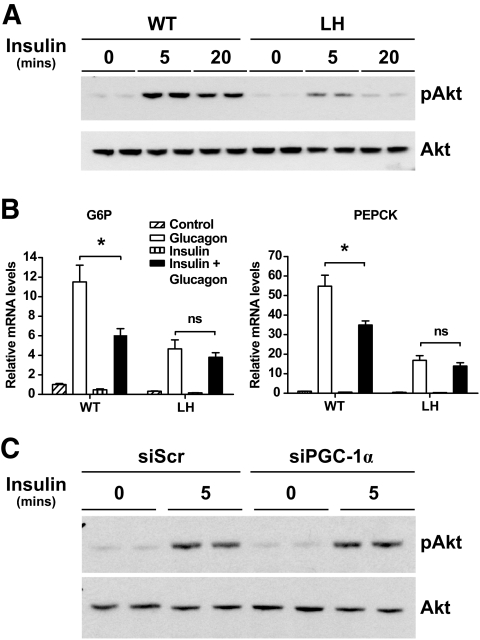
LH hepatocytes exhibit defects in insulin signaling.
Defects in fatty acid oxidation and high hepatic triglycerides have been linked to hepatic insulin resistance (32). Isolated primary hepatocytes from LH mice showed markedly reduced levels of phosphorylated Akt after incubation with insulin (Fig. 5A). Furthermore, insulin pretreatment did not suppress the induction of PEPCK or glucose-6-phosphatase (G6P) by glucagon in primary LH hepatocytes (Fig. 5B). Acute knockdown of PGC-1α in primary hepatocytes with siRNA did not affect insulin signaling in primary hepatocytes (Fig. 5C and supplementary Fig. A5). Thus, it appeared that only chronic reduction of hepatic PGC-1α diminished the ability of liver cells to respond to insulin.
FIG. 5.
Decreased insulin signaling in primary LH hepatocytes. Levels of phosphorylated Akt (pAkt) and total Akt were measured in protein extracts from primary wild-type (WT) or LH hepatocytes (A) or wild-type hepatocytes infected with either siPGC-1α or control virus (C) treated with media alone (control) or 100 nmol/l insulin for the indicated time points. Western blots show biological duplicates and are representative of two individual experiments. B: Gluconeogenic gene expression in wild-type and LH hepatocytes after pretreatment with media (control) or 100 nmol/l insulin for 10 min before addition of vehicle or 25 nmol/l glucagon, as indicated. Values are the means ± SD and representative of two individual experiments. *P < 0.05. ns, nonsignificant; siScr, scrambled siRNA.
High-fat feeding exacerbates defects in hepatic insulin signaling in LH mice.
We next investigated whether mice with chronically reduced levels of PGC-1α exhibited alterations in insulin sensitivity in vivo. Differences in insulin action were not immediately evident in LH mice fed a chow diet (supplementary Fig. A6). Because hepatic insulin insensitivity is a hallmark of metabolic syndrome linked to the consumption of a diet high in fat, we challenged the LH mice with a diet consisting of 58% fat for up to 16 weeks and monitored hepatic metabolic function. Consistent with previous reports (13), high-fat feeding resulted in increased expression of hepatic PGC-1α in both wild-type and LH mice (Fig. 6A). Importantly, no significant differences in body weight, body composition, growth, or food intake were noted between the groups (Fig. 6B and data not shown). We observed reduced expression levels of select fatty acid oxidation genes in fasted and refed high-fat–fed LH mice (supplementary Fig. A7). Levels of hepatic lipids were extremely high in both fed and fasted mice, and although a trend toward higher triglycerides in LH mice was noted, it did not reach statistical significance (supplementary Fig. A7, panels C and D).
FIG. 6.
High-fat–fed LH mice exhibit hepatic insulin resistance. A: Relative levels of PGC-1α mRNA isolated from livers of wild-type (WT) and LH mice fed either a regular chow or high-fat diet (HFD) for 16 weeks. Bars represent the means ± SE (n = 6) and are expressed relative to values for wild-type mice fed regular chow. B: Average weight of wild-type and LH mice on high-fat diet for 6 weeks. Data are the means ± SE (n = 10). C: Western blot of phosphorylated Akt (pAkt) and total Akt in hepatic protein extracts from high-fat diet–fed wild-type or LH mice after intravenous injection of PBS (−) or insulin (+). D and E: Circulating insulin (D) and hepatic mRNA expression (E) levels in high-fat diet–fed mice fasted overnight or fasted mice refed for 2 h. Data are the means ± SE expressed relative to wild-type fasted levels (n = 6–14). F: Hepatic mRNA levels in high-fat–fed wild-type and LH mice. Data are the means ± SE (n = 6), expressed relative to wild-type levels. *P < 0.05; **P < 0.01. Hsp90, 90-kDa heat shock protein.
Levels of phosphorylated Akt in response to exogenous insulin administration were lower in LH mice after 16 weeks on a high-fat diet (Fig. 6C), suggesting a mild decrease in hepatic insulin signaling. Interestingly, although circulating insulin concentrations were similar in 24-h fasted mice, refeeding produced higher insulin levels in LH mice (Fig. 6D). Regardless, refed LH mice did not decrease hepatic PEPCK mRNA levels to the same extent as wild-type controls (Fig. 6E), suggesting a defect in the ability of endogenous insulin to shut down fasting-induced gluconeogenesis. A similar trend was also observed for G6P mRNA, though not reaching statistical significance (P = 0.055). Consistent with decreased hepatic insulin sensitivity, high-fat–fed LH livers had inappropriately high levels of gluconeogenic gene expression (Fig. 6F). Thus, hepatic insulin resistance was apparent at the level of both insulin signaling and target gene expression. Taken together, these data show a significant defect in hepatic insulin signaling that manifests physiologically in response to the metabolic stress of high-fat feeding.
Our data are in striking contrast to a previous study showing that acute knockdown of PGC-1α within the liver enhances insulin-mediated Akt phosphorylation through decreased expression of tribbles-3 (TRB-3), an inhibitor of Akt (11). To address this, we measured TRB-3 mRNA expression in our mouse model and found no differences between mice on either regular chow or high-fat diet (Fig. 6F and 7B). Thus, our study clearly illustrates the potential differences of chronic versus acute reduction of PGC-1α expression on hepatic metabolic function.
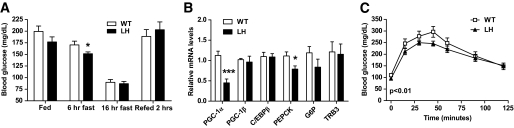
FIG. 7.
Fasting hypoglycemia and deficient gluconeogenesis in LH mice fed a high-fat diet. A: Blood glucose levels in fed, fasted (6 or 16 h), or refed (fasted overnight and refed 2 h) wild-type (WT) or LH mice maintained on a high-fat diet for 6 weeks. Data are the means ± SE (n = 4–14). B: Hepatic mRNA levels in high-fat diet–fed wild-type and LH mice after a 24-h fast. Data are the means ± SE (n = 9, expressed relative to wild-type levels). C: Pyruvate tolerance test. Blood glucose levels were measured in 16-week high-fat diet–fed wild-type or LH mice after an overnight fast (time 0) and at the indicated times after an intraperitoneal injection of pyruvate (pyruvate tolerance test). Values represent the means ± SEM (n = 10), P < 0.01 by two-way ANOVA, indicating a significance difference between wild-type versus LH curves. *P < 0.05; ***P < 0.001.
High-fat feeding of LH mice unmasks defects in gluconeogenesis.
Although PGC-1α is known to increase the expression of the gluconeogenic program (7,9,31), mice with a global knockout of the PGC-1α gene have constitutively increased PEPCK and G6P gene expression likely caused by increased CCAAT/enhancer binding protein-β (C/EBP-β) expression (14). Interestingly, these same PGC-1α knockout mice exhibit defects in the ability to convert pyruvate to glucose, demonstrating that gluconeogenesis remains impaired. Although LH mice fed a regular chow diet exhibited normal gluconeogenic gene expression and pyruvate tolerance (supplementary Table A1 and supplementary Fig. A2), we addressed whether a high-fat diet could reveal defects in hepatic gluconeogenesis within LH mice.
Under these dietary conditions, we observed a mild, yet significant, reduction in fasting glycemia in LH mice after short-term food deprivation (Fig. 7A). In contrast to LH mice fed ad libitum (Fig. 6F), fasted LH mice exhibited decreased PEPCK gene expression, consistent with the observed fasting hypoglycemia and dependence on PGC-1α to potentiate the gluconeogenic response during a fast (Fig. 7B). We observed no differences in the expression of C/EBP-β (Fig. 6F and 7B, supplementary Table A1).
To directly assess hepatic gluconeogenesis, we monitored the appearance of glucose in the blood after injection of pyruvate after 12 weeks of high-fat feeding. Fasted LH mice had significantly reduced area under the curve in the pyruvate tolerance test, further suggesting a defect in hepatic gluconeogenesis (Fig. 7C). Taken together, it is apparent that reduced levels of PGC-1α can affect hepatic glucose metabolism at the level of both insulin signaling in the fed state and glucose production in the fasted state.
DISCUSSION
It is clear that the PGC-1 coactivators play important roles in various aspects of energy homeostasis. Using gain-of-function and complete-loss-of-function studies, these proteins were shown to be dominant regulators of oxidative metabolism, particularly mitochondrial biogenesis, skeletal muscle biology, brown fat thermogenesis, and the hepatic fasting response (4,5,7,11,14,16). However, what remained unclear from these studies were the metabolic consequences of modulating PGC-1α within physiological levels.
Importantly, the expression of PGC-1α is quantitatively dysregulated in a variety of disease states. Increased PGC-1α expression has been shown in livers of diabetic mice (7,13), reduced levels are found in the muscle of insulin-resistant humans (33), and hepatic PGC-1α levels are inversely correlated with insulin resistance in humans (34). We show here that hepatic heterozygosity for PGC-1α, leading to a corresponding reduction in PGC-1α mRNA and protein, caused a substantial change in hepatic metabolism that is manifested primarily as hepatic steatosis and insulin resistance.
Our data demonstrate for the first time that chronically and mildly reduced hepatic PGC-1α causes hepatic insulin resistance. Primary LH hepatocytes and high-fat–fed LH livers exhibited decreased insulin-stimulated Akt activation (Figs. 5 and 6). Moreover, LH mice on a high-fat diet had increased fed gluconeogenic gene expression that could not be efficiently reduced after fasting/refeeding (Fig. 6). Interestingly, the decrease in hepatic insulin sensitivity shown here is in contrast to results from Koo et al. (11), who demonstrated that a sharp, adenoviral-mediated reduction of hepatic PGC-1α reduced TRB-3 mRNA expression and increased insulin sensitivity in vivo. In contrast, we observed no differences in TRB-3 mRNA expression in fed or fasted LH mice (Fig. 6F and 7B, supplementary Table A1). These differences may be attributable to the degree of PGC-1α loss in these two sets of experiments, the method of knockdown, or the differential effects of chronic versus transient decreases in PGC-1α expression.
Chronic reductions in hepatic PGC-1α affected triglyceride assembly and/or production (Fig. 4), which can also be attributed to hepatic insulin resistance. Insulin reduces the amount of circulating VLDL particles by directly suppressing hepatic VLDL production (2), and hepatic insulin resistance contributes to both increased hepatic VLDL production and decreased VLDL uptake in patients with type 2 diabetes (rev. in (35). Insulin has been shown to inhibit the expression of MTP, a protein that initiates the production of VLDL (36). Consistent with decreased insulin action, we observed increased expression of MTP in LH mice (Fig. 4B). We also detected increased expression of apoB, the major protein constituent of VLDL, which, along with high serum triglycerides, is associated with coronary artery disease (35,37). Thus, it is likely that hepatic insulin resistance contributed to the hypertriglyceridemia in fed LH mice. However, there remains the possibility that long-term reduction of hepatic PGC-1α has extrahepatic effects on triglyceride lipolysis or absorption.
Interestingly, we observed increased circulating insulin levels in LH mice after refeeding. In contrast to the muscle-specific PGC-1α knockout mice, we observed no difference in gross islet morphology (38) (data not shown). Thus, it is likely that chronically reduced hepatic PGC-1α has effects on peripheral tissue metabolism through currently unidentified mechanisms.
The most striking and clear-cut consequence of quantitatively decreasing hepatic PGC-1α was impairment of the fatty acid oxidation gene program. Decreased hepatic fatty acid oxidation and concomitant lipid accumulation have been shown to negatively affect insulin signaling (39). Our study showed that the fatty acid oxidation genes VLCAD, LCAD, and SCAD are highly sensitive to changes in PGC-1α expression levels. Other studies have shown that mice deficient in these fatty acid oxidation genes show marked hepatosteatosis and hepatic insulin resistance (30). Studies also suggest there is a synergistic effect of having reduced function in two or more of the acyl-CoA dehydrogenases (40). Given that PGC-1α is crucial for maintaining the expression levels of multiple enzymes within this family, it is likely that long-term dysregulation of lipid metabolism in LH mice contributes to the development of hepatic insulin resistance, particularly under the challenge of a high-fat diet. Interestingly, hepatic steatosis was not significantly worse in high-fat–fed LH mice. However, because insulin directly downregulates fatty acid oxidation (41), insulin resistance may mask the effects of reduced PGC-1α on fatty acid oxidation in these mice.
Our study clearly demonstrates that modest changes in hepatic PGC-1α expression can have significant effects on energy homeostasis. Furthermore, although chronic reduction of hepatic PGC-1α had only a modest effect on reducing gluconeogenesis, multiple aspects of hepatic metabolism were significantly disrupted by loss of the transcriptional coactivator. Because there is growing interest in the therapeutic potential of targeting this transcriptional coactivator during the development of metabolic diseases, it will be of interest to investigate how chemical modulators of PGC-1α activity affect liver function in diabetic and obese patients.
Supplementary Material
Acknowledgments
J.L.E. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research and the H.L. Holmes Award for Postdoctoral Studies from the National Research Council of Canada. National Institutes of Health Grant DK061562 (to B.M.S.) supported the work described in this report.
No potential conflicts of interest relevant to this article were reported.
The authors thank Marc Montminy for the siPGC-1α adenovirus and Thomas Gettys for the anti–PGC-1α antibody. We also thank Christoph Handschin and Sherry Chin for helpful discussion and technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Yeh MM, Brunt EM: Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol 2007; 128: 837– 847 [DOI] [PubMed] [Google Scholar]
- 2.Adiels M, Taskinen MR, Boren J: Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep 2008; 8: 60– 64 [DOI] [PubMed] [Google Scholar]
- 3.Staudinger JL, Lichti K: Cell signaling and nuclear receptors: new opportunities for molecular pharmaceuticals in liver disease. Mol Pharm 2008; 5: 17– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J, Handschin C, Spiegelman BM: Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005; 1: 361– 370 [DOI] [PubMed] [Google Scholar]
- 5.Finck BN, Kelly DP: PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 2006; 116: 615– 622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M: CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001; 413: 179– 183 [DOI] [PubMed] [Google Scholar]
- 7.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM: Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001; 413: 131– 138 [DOI] [PubMed] [Google Scholar]
- 8.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A: Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 2003; 52: 642– 649 [DOI] [PubMed] [Google Scholar]
- 9.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM: Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003; 423: 550– 555 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Monks B, Ge Q, Birnbaum MJ: Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 2007; 447: 1012– 1016 [DOI] [PubMed] [Google Scholar]
- 11.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M: PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 2004; 10: 530– 534 [DOI] [PubMed] [Google Scholar]
- 12.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR: Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 2008; 7: 125– 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR: Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 2005; 54: 1314– 1323 [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM: Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 2004; 119: 121– 135 [DOI] [PubMed] [Google Scholar]
- 15.Leone T, Lehman J, Finck B, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP: PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005; 3: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM: Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 2005; 122: 505– 515 [DOI] [PubMed] [Google Scholar]
- 17.Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN: Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J Biol Chem 2006; 281: 19000– 19008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handschin C, Spiegelman BM: Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006; 27: 728– 735 [DOI] [PubMed] [Google Scholar]
- 19.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM: A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829– 839 [DOI] [PubMed] [Google Scholar]
- 20.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T: cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 2000; 274: 350– 354 [DOI] [PubMed] [Google Scholar]
- 21.Pilegaard H, Saltin B, Neufer PD: Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 2003; 546: 851– 858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM: Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 2005; 120: 261– 273 [DOI] [PubMed] [Google Scholar]
- 23.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP: C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A 2002; 99: 9498– 9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanPatten S, Ranginani N, Shefer S, Nguyen LB, Rossetti L, Cohen DE: Impaired biliary lipid secretion in obese Zucker rats: leptin promotes hepatic cholesterol clearance. Am J Physiol Gastrointest Liver Physiol 2001; 281: G393– G404 [DOI] [PubMed] [Google Scholar]
- 25.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI: Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2005; 2: 55– 65 [DOI] [PubMed] [Google Scholar]
- 26.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM: An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A 2003; 100: 7111– 7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hondares E, Mora O, Yubero P, Rodriguez de la Concepcion M, Iglesias R, Giralt M, Villarroya F: Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology 2006; 147: 2829– 2838 [DOI] [PubMed] [Google Scholar]
- 28.Kurtz DM, Rinaldo P, Rhead WJ, Tian L, Millington DS, Vockley J, Hamm DA, Brix AE, Lindsey JR, Pinkert CA, O'Brien WE, Wood PA: Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci U S A 1998; 95: 15592– 15597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox KB, Hamm DA, Millington DS, Matern D, Vockley J, Rinaldo P, Pinkert CA, Rhead WJ, Lindsey JR, Wood PA: Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet 2001; 10: 2069– 2077 [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI: Mitochondrial dysfunction due to long-chain acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci U S A 2007; 104: 17075– 17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee J, Ge H, Yang W, Fan M, Handschin C, Cooper M, Lin J, Li C, Spiegelman BM: Partnership of PGC-1alpha and HNF4alpha in the regulation of lipoprotein metabolism. J Biol Chem 2006; 281: 14683– 14690 [DOI] [PubMed] [Google Scholar]
- 32.Petersen KF, Shulman GI: Etiology of insulin resistance. Am J Med 2006; 119: S10– S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003; 100: 8466– 8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croce MA, Eagon JC, LaRiviere LL, Korenblat KM, Klein S, Finck BN: Hepatic lipin 1beta expression is diminished in insulin-resistant obese subjects and is reactivated by marked weight loss. Diabetes 2007; 56: 2395– 2399 [DOI] [PubMed] [Google Scholar]
- 35.Adiels M, Olofsson SO, Taskinen MR, Boren J: Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28: 1225– 1236 [DOI] [PubMed] [Google Scholar]
- 36.Allister EM, Borradaile NM, Edwards JY, Huff MW: Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 2005; 54: 1676– 1683 [DOI] [PubMed] [Google Scholar]
- 37.Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, Hamsten A, Taskinen MR: Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS). Circulation 2003; 107: 1733– 1737 [DOI] [PubMed] [Google Scholar]
- 38.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM: Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 2007; 117: 3463– 3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage DB, Petersen KF, Shulman GI: Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007; 87: 507– 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuler AM, Gower BA, Matern D, Rinaldo P, Vockley J, Wood PA: Synergistic heterozygosity in mice with inherited enzyme deficiencies of mitochondrial fatty acid beta-oxidation. Mol Genet Metab 2005; 85: 7– 11 [DOI] [PubMed] [Google Scholar]
- 41.Wolf G: Role of fatty acids in the development of insulin resistance and type 2 diabetes mellitus. Nutr Rev 2008; 66: 597– 600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.