Abstract
Astrocytes can release glutamate in a calcium-dependent manner and consequently signal to adjacent neurons. Whether this glutamate release pathway is used during physiological signaling or is recruited only under pathophysiological conditions is not well defined. One reason for this lack of understanding is the limited knowledge about the levels of calcium necessary to stimulate glutamate release from astrocytes and about how they compare with the range of physiological calcium levels in these cells. We used flash photolysis to raise internal calcium in astrocytes, while monitoring astrocytic calcium levels and glutamate, which evoked slow inward currents that were recorded electrophysiologically from single neurons grown on microislands of astrocytes. With this approach, we demonstrate that modest changes of astrocytic calcium, from 84 to 140 nM, evoke substantial glutamatergic currents in neighboring neurons (−391 pA), with a Hill coefficient of 2.1 to 2.7. Because the agonists glutamate, norepinephrine, and dopamine all raise calcium in astrocytes to levels exceeding 1.8 μM, these quantitative studies demonstrate that the astrocytic glutamate release pathway is engaged at physiological levels of internal calcium. Consequently, the calcium-dependent release of glutamate from astrocytes functions within an appropriate range of astrocytic calcium levels to be used as a signaling pathway within the functional nervous system.
Communication between astrocytes, a subtype of glial cells, and neurons is bidirectional. Astrocytes exhibit a form of excitability and communication based on changes in intercellular Ca2+ (1–3), which can be initiated by neuronal synaptic activity (4–6). These astrocytic Ca2+ variations can cause the release of the excitatory neurotransmitter glutamate, which then signals to adjacent neurons (7–10) and modulates synaptic transmission (6, 11, 12). By modulating synaptic transmission, astrocytes could play a role in information processing in the brain. However, whether the release of glutamate from astrocytes and the consequent signaling to neurons are used as a physiological signaling pathway or are recruited only under pathophysiological conditions is not well defined. One reason for this lack of understanding is that we have little knowledge about the levels of calcium necessary for glutamate release from astrocytes and about how they compare with the range of physiological calcium levels in astrocytes.
We have demonstrated previously that stimuli that elevated internal Ca2+ in astrocytes can induce a slow inward current (SIC) in adjacent neurons (11) that was mediated by both N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) ionotropic glutamate receptors. These receptors were activated by glutamate released from astrocytes with a calcium-dependent mechanism. In the present work, we studied the relationship between internal calcium levels of astrocytes and glutamate release by photolytically elevating calcium in astrocytes while recording glutamate-evoked SICs in adjacent neurons. We demonstrate that the astrocyte-to-neuron glutamate signaling pathway is activated even with modest changes in astrocytic calcium levels that are well within the physiological range of calcium in these cells. Additionally, these quantitative photolysis experiments demonstrate two different populations of astrocytes based on their ability to induce SICs: (i) astrocytes in which increasing calcium levels cause a graded release of glutamate and (ii) others in which, after calcium stimulates glutamate release, further elevations of calcium cause little change in the amplitude of the SIC. These studies demonstrate that the calcium-regulated release of glutamate from astrocytes is engaged at physiological calcium levels, suggesting that this recently identified signaling pathway has the potential to modulate synaptic transmission and neuronal excitability within the functional nervous system.
Materials and Methods
Culture Preparation.
A modified microculture method (13, 14) was used to grow single hippocampal neurons on microislands of substratum. Briefly, 12-mm round glass coverslips were coated with a thin layer of 0.15% agarose that prevents adhesion of cells. A permissible substratum (250 μg/ml collagen and 500 μg/ml polyethyleneimine) was sprayed onto the agarose layer forming randomly distributed microislands. Rat hippocampal cells, dissociated as described below, were plated onto these coverslips. Cells adhered only to collagen/polyethyleneimine islands.
Hippocampal enriched type-1 astrocytes were prepared as described (15). Cells were plated initially into culture flasks for 8–19 days, and then, after purification, astrocytes were replated onto agarose-coated glass coverslips containing microislands of permissible substratum. Cells adhered only to collagen/polyethyleneimine microislands and occupied the majority of these microislands. Astrocytes were then cultured for 1–4 days to allow their proliferation/growth until they covered the surface of microislands. At that time, dissociated neurons from hippocampi of 0- to 1-day-old postnatal rats (Sprague–Dawley) were prepared as described (7) and then seeded onto precultured coverslips containing astrocytic microislands. Cultures were maintained for 5–25 days after neuronal plating (20–39 days in culture from initial plating of purified type-1 astrocytes). Typically, we used microislands 60–90 μm in diameter containing between three and five astrocytes. Culture medium for proliferation of astrocytes consisted of Eagle's minimum essential medium (Earle's salts, phenol-free; GIBCO/BRL) supplemented with 10% (vol/vol) FCS (HyClone). At the time of neuronal plating, this medium was replaced completely by a fresh culture medium supplemented with 5% (vol/vol) FCS and Mito+ serum extender (Collaborative Biomedical Products, Bedford, MA). Mitotic inhibitor (5-fluoro-2′-deoxyuridine and uridine, 5 μM) was added after 2–3 days in culture (postneuronal plating) to suppress proliferation of nonneuronal cells. Cultures were fed once a week by exchanging 30% of the medium with fresh medium.
Immunocytochemistry.
In all studies, neurons were identified morphologically, and in some experiments, their identity was confirmed by labeling with fluorescein-conjugated C fragment of tetanus toxin (CFITC, List Biological Laboratories, Campbell, CA) by using a modification of a previously described procedure (16). Cells were incubated for 1 h in 10 μg/ml CFITC at 37°C. After washing, cells were either viewed live or, in other experiments, were exposed to Dent's fixative at room temperature for 30 min for parallel immunocytochemistry. A polyclonal antibody (Sigma; catalogue no. G9269; 1:100 dilution) was used to probe for glial fibrillary acidic protein, confirming a greater than 95% purity of astrocyte type-1 culture. Visualization of glial fibrillary acidic protein staining was accomplished by using rhodamine-conjugated secondary antibody and conventional epifluorescence microscopy.
Electrophysiology.
Whole-cell patch-clamp recordings were obtained from neurons with a PC-ONE patch-clamp amplifier (Dagan Corporation, Minneapolis) equipped with a whole-cell headstage (PC-ONE-30; 1 GΩ). Data acquisition was performed with an AT-MIO-16E-10 interface (National Instruments, Austin, TX) driven by vclamp software (Prairie Technologies, Middleton, WI). External control solution contained (in mM) NaCl 140, KCl 5, CaCl2 4, Hepes 10, glucose 10, and sucrose 6, as well as 10 μM glycine (pH 7.35). In some experiments, an Mg2+-containing solution composed of (in mM) NaCl 140, KCl 5, CaCl2 2, MgCl2 2, glucose 20, and Hepes 10 (pH 7.4) was used. Patch pipette solution contained (in mM) K-gluconate 140, EGTA 10, Mg-ATP 4, Tris-GTP 0.2, and Hepes 10 (pH 7.35). We used patch pipettes whose resistances were 3–5 MΩ. The membrane potential was held at −60 mV. In some cases, while assessing the presence of autaptic currents, the holding potential was held at −40 mV, where excitatory (glutamatergic) and inhibitory (γ-aminobutyratergic; GABAergic) autaptic currents appeared as inward and outward currents, respectively (14, 17). Autaptic currents were evoked by using a 4-ms step to 20 mV. We found that 13 of 21 tested neurons were autaptically connected (3 excitatory and 10 inhibitory). All experiments were performed at room temperature (20–23°C). Data are expressed as means ± SEM.
Calcium Measurements.
The ability of stimuli to elevate internal Ca2+ in astrocytes was monitored by fluorescence microscopy by using the Ca2+ indicator fluo-3. Cultures were incubated at 37°C for 45 min with the acetoxymethyl (AM) ester of fluo-3 (fluo-3 AM, 10 μg/ml; Molecular Probes). To aid solubilization of fluo-3 AM in aqueous medium, we added Pluronic F-127 (0.025%; Molecular Probes). After washing, the dye was allowed to deesterify for 20–45 min. Coverslips containing fluo-3-loaded cells were visualized with a ×60 Plan Apo objective and wide-field epifluorescence illumination that was achieved by using a xenon arc lamp with a standard fluorescein filter set. Fluo-3 emission was detected with an IC-300 intensified charge-coupled device camera (Photon Technology International, Monmouth Junction, NJ) and a photomultiplier tube (P30CW5-04; Electron Tubes, Middlesex, England), both mounted onto a dual photo/CCTV adapter (25-70-44; Optem International, Fairport, NY) that was attached to a Nikon Diaphot inverted microscope. In experiments with a photomultiplier tube, the fluo-3 emission was collected only from the area corresponding to a single astrocytic microisland by positioning an aperture in the emission pathway. To achieve spatial (x and y) control of the aperture, we used a pinhole turret diaphragm cassette (Nikon), which contains different sizes of apertures at the secondary image plane. All experiments were performed at room temperature (20–23°C) on cells bathed in the external solutions described in Electrophysiology above. Data are expressed as means ± SEM.
Calcium levels were estimated either by using standard calibration curves for fluo-3 in vitro (18) or after in situ calibration (19) by using the Ca2+-ionophore 4-bromo-A23187 (10 μM; Molecular Probes). To use standard calibration curves in vitro, we first determined the resting calcium level of astrocytes (87 ± 4 nM; n = 70) by using the ratiometric indicator fura-2 as we have described in detail elsewhere (17). Background-subtracted ratio images (350/380 nm) of fura-2 loaded astrocytes were used to calculate [Ca2+]i according to equation 5 of Grynkiewicz et al. (20). Calibration of fura-2 was performed in situ with 4-bromo-A23187 (10 μM). In our system, Rmin was 0.28–0.45; Rmax was 4.00–5.79; and Fo/Fs was 7.94–13.03. A Kd of 224 nM was used as reported (20). This estimated resting calcium level (87 nM) was used to assign the resting fluorescence level of fluo-3 (Fo) on a standard calibration curve that had been obtained in vitro (18). Using the resting calcium concentration and corresponding ΔF/Fo values, we established the relationship (r = 0.99) that can be formulated as [Ca2+]i = 87 nM × EXP(0.0094 × ΔF/Fo). Using this relationship and ΔF/Fo from astrocytes loaded with fluo-3, we estimated [Ca2+]i in our experiments. In parallel, we also performed, in some experiments, an in situ calibration of fluo-3 with 4-bromo-A23187 (10 μM) as described (19) by using a Kd of 385 nM (21). Data obtained with these two calibration approaches were in good agreement with one another.
Photolysis of Caged Calcium.
Cells were coloaded with the calcium cage, o-nitrophenyl (NP)-EGTA AM ester (10 μg/ml, Molecular Probes), fluo-3 AM (10 μg/ml), and Pluronic F-127 (0.025%) by incubation for 45 min. For photolysis of NP-EGTA, we used a nitrogen-pulsed laser (VSL-337ND; Laser Science, Newton, MA) as a UV source (337 nm principal line) as described (22, 23). The 3-ns duration pulses generated by this laser were launched into a UV-transmitting optical fiber (105-μm core diameter) by using a single plano-convex synthetic fused silica lens. To indicate the location where the UV pulses were delivered for photolysis, we also coupled a diode laser (635 nm) into the same optical fiber. This red light acted as a searchlight to guide the positioning of the optical fiber above the sample. We positioned the fiber with a standard micromanipulator. The size of the photolysis area, controlled by the diameter of an optical fiber and the distance between the tip of the fiber and microislands of interest, was sufficiently large to allow successful photolysis of NP-EGTA in all astrocytes within a single microisland on the delivery of a single UV pulse. In control experiments, we confirmed that a single UV pulse reliably elevates calcium levels within each astrocyte of a microisland (14 of 14 tested astrocytes within three different microislands) by measuring fluo-3 intensity with a camera. As a criterion for successful photolysis, we used calcium increases that were greater than three standard deviations from the resting calcium level. By using this criterion, all astrocytes within each microisland exhibited similar elevations of internal calcium level caused by a single UV pulse delivery. In these controls experiments, the calcium level increased from a resting level of 87 ± 1 nM to 112 ± 4 nM (n = 14; paired t test; P < 0.01). Because the entire population of astrocytes loaded with NP-EGTA within each microisland responded to a single UV pulse with a significant and homogenous increase in internal calcium, we performed fast acquisition experiments by using a photomultiplier tube, without jeopardizing the determination of absolute changes in astrocytic intercellular calcium levels.
Agonists.
Glutamate, dopamine, and norepinephrine (all at 50 μM) were applied to astrocytes by a 30-s pressure ejection from glass pipettes.
Antagonists.
The AMPA glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM; Sigma) and an NMDA glutamate receptor antagonist d-2-amino-5-phosphonopentanoic acid (D-AP5; 50 μM; Research Biochemicals, Natick, MA) were applied to the bath.
Statistical Analysis.
The effects of UV photolysis on astrocytic internal calcium and neuronal currents were determined either by paired t test or by one-way ANOVA followed by Fisher's least significant difference test.
Results and Discussion
The most common type of microislands that we used in our experiments contained both astrocytes and single neurons as shown in Fig. 1. Here, a single neuron (Fig. 1 Left) is growing on top of an astrocytic microisland (Fig. 1 Right). Cell types were identified based on their morphology, and in some experiments, we used cell-type-specific fluorescent tags to confirm their morphological identification. Hence, neurons were identified with FITC-conjugated C fragment of tetanus toxin, and astrocytes were identified with antibody against glial fibrillary acidic protein.
Figure 1.
Single neurons were grown on microislands consisting of purified astrocytes. Neurons were identified by labeling with the fluorescein-conjugated C fragment of tetanus toxin (CFITC; Left), and anti-glial fibrillary acidic protein (GFAP) antibody was used to identify astrocytes (Right).
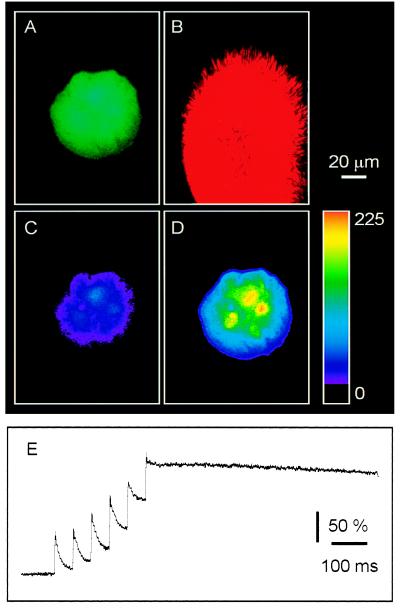
To monitor and control the astrocytic intracellular calcium levels quantitatively, cells were coloaded with the calcium indicator fluo-3 and the calcium cage NP-EGTA. Once an astrocytic microisland had been located (Fig. 2A), the optical fiber was brought into the field of view. To control the position of UV photolysis, the diode laser, which acted as a searchlight, was enabled (Fig. 2B). The red emission from the laser exited the fiber and was used to identify the x, y position for photolysis (Fig. 2B). A train of six 3-ns, 337-nm pulses (at 20 Hz) significantly elevated calcium within the desired microisland (n = 13; ΔF/Fo = 186 ± 29%; P < 0.01; Fig. 2 C and D). Delivery of a train of UV pulses onto an astrocytic microisland evoked transient accumulating increases in intracellular calcium (Fig. 2E), indicating that multiple pulses of UV light can be used to control the level of intracellular calcium experimentally. The change in fluorescence emission of fluo-3 was not due to damage caused by UV light, because application of the UV stimuli to cells loaded with fluo-3 alone did not lead to elevated calcium (n = 4; ΔF/Fo = 13 ± 1%; P > 0.1; see also refs. 22 and 23).
Figure 2.
Photolysis of NP-EGTA increased [Ca2+]i in all astrocytes within single microislands. Cells were coloaded with the calcium indicator fluo-3 and the calcium cage NP-EGTA. (A) The astrocytes of interest were localized by using the fluorescence of fluo-3. (B) After locating the astrocytic microisland of interest, the optical fiber was brought into the field of view. To control the position of UV photolysis, a diode laser, which acted as a searchlight, was enabled. The position of UV pulses corresponds with the position of the red illumination that is shown here. Exposure of the astrocytic microisland to UV pulses delivered through the optical fiber elevated internal calcium level because of photolysis of NP-EGTA. (C) A pseudocolor representation of the resting calcium level. (D) The calcium level after photolysis. Color scale indicates linear pseudocolor representation of fluorescence intensity ranging from 0 to 255 units. (E) The change in [Ca2+]i was also monitored, in a separate experiment, with a photomultiplier tube. Exposure to a train of UV pulses evoked a staircase-like increase in [Ca2+]i, indicating that multiple pulses of UV light can be used to control the level of [Ca2+]i experimentally. Changes in calcium are represented as percentage of ΔF/Fo.
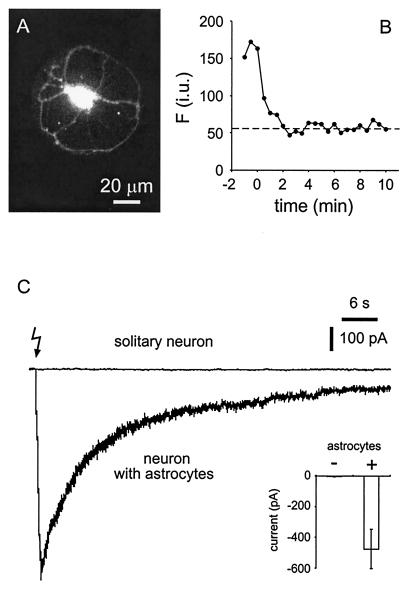
To examine the relationship between astrocytic internal calcium levels and calcium-dependent glutamate release from astrocytes, we photolysed NP-EGTA within astrocytes while recording glutamate-dependent currents from single neurons on astrocytic microislands. After the establishment of a whole-cell patch-clamp recording, neurons were dialyzed for 10 min before initiating the experiment, a period that we found to be sufficient to remove fluo-3 and presumably NP-EGTA from the neuron (Fig. 3 A and B). Delivery of UV stimuli reliably caused an increase of astrocytic internal calcium, as monitored by fluo-3 emission (n = 8; ΔF/Fo = 196 ± 33%; P < 0.01), and evoked an SIC in cocultured neurons (n = 8; peak current of −475 ± 128 pA; P < 0.01; Fig. 3C). In control experiments, cultures were loaded with only the calcium indicator fluo-3. In this condition, UV pulses caused neither a calcium elevation nor an SIC, indicating that these responses require the presence of NP-EGTA (n = 4). In conditions where cells were incubated initially in NP-EGTA AM, we controlled for the possibility that UV light causes currents in neurons as a result of the photolysis of NP-EGTA that had not been dialyzed out of the neuron by repeating these experiments on microislands that contained only neurons. After a 10-min dialysis period, UV pulses failed to evoke an SIC in neurons cultured in the absence of astrocytes (n = 5; −2 ± 2 pA; P > 0.9; Fig. 3C). Taken together, these data demonstrate that the flash-photolysis-evoked neuronal SIC requires the presence of a calcium elevation in astrocytes.
Figure 3.
UV photolysis of NP-EGTA evoked an SIC in neurons cultured on astrocytic microislands but not in solitary neurons. Cells were coloaded with fluo-3 and NP-EGTA by bath incubation in the AM esters. To determine the period of dialysis necessary to remove fluo-3 and NP-EGTA from neurons, the fluorescent intensity of fluo-3 was monitored after establishing a whole-cell recording from a solitary neuron (A). In B, the intensity of fluorescence is plotted against time after establishing a whole-cell recording (t = 0). Note that fluorescence signal subsides until it reaches a level corresponding to autofluorescence (dashed line) well within the 10-min period that we adopted as a standard to dialyze NP-EGTA and fluo-3 from neurons in this study. i.u., intensity unit. (C) Neuronal currents recorded after UV illumination in the presence and absence of astrocytes. UV illumination, to photolyse NP-EGTA, caused an SIC in neurons that were cocultured with astrocytes (neuron with astrocytes) but not in solitary neurons. The lightning bolt indicates the onset of the UV stimuli (train of six UV pulses at 20 Hz). Inset summarizes all experiments (n = 8 for neurons with astrocytes; n = 5 for solitary neurons). Bars indicate means ± SEM.
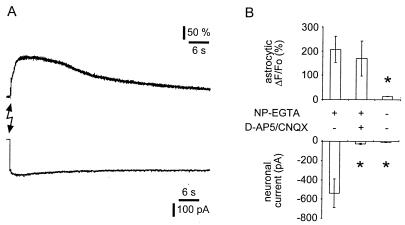
To test the hypothesis that the flash-photolysis-dependent SIC is caused by glutamate that is released from astrocytes, we performed pharmacological studies. The presence of D-AP5, an NMDA receptor antagonist, and CNQX, an AMPA receptor antagonist, significantly attenuated (n = 4; P < 0.05) the amplitude of the flash-photolysis-evoked SIC amplitude, without affecting the elevation of internal calcium in the astrocytes (Fig. 4). Although these data support a role for glutamate in mediating the SIC, it is remotely possible that a calcium elevation in the astrocyte modulates autaptic glutamate release from neurons to cause the SIC. However, when the single neuron on the astrocyte microisland formed inhibitory outward autaptic connections and was presumably γ-aminobutyratergic (GABAergic), photorelease of calcium in the astrocyte still caused an SIC (n = 2). Thus, we conclude that flash photolysis causes the calcium-dependent release of glutamate from astrocytes that leads to an ionotropic glutamate receptor-mediated SIC in the adjacent neuron.
Figure 4.
Photolytic elevation of astrocytic calcium causes the NMDA and AMPA receptor-dependent neuronal SIC. Simultaneous recordings of astrocytic calcium levels and neuronal currents indicate that the increase in astrocytic calcium is sufficient to cause glutamate-mediated SIC in neurons. Cells were coloaded with the calcium indicator fluo-3 and the calcium cage NP-EGTA. Both fluo-3 and NP-EGTA were dialyzed out from single neurons on the top of astrocytic islands for 10 min by using a pipette in a whole-cell patch-clamp configuration as indicated by a loss of fluo-3 fluorescence. Astrocytes remained loaded with NP-EGTA that was photolysed by using six UV pulses (20 Hz) delivered through a UV transmitting optical fiber (A, lightning bolt) to cause an increase in astrocytic [Ca2+]i (A, upper trace). Whole-cell recordings from single neurons on top of the astrocytic island indicate that photolysis reliably evoked an SIC (A, lower trace). (B) In contrast, UV stimulation of unloaded astrocytes neither elevated astrocytic calcium nor caused a neuronal SIC. Additionally, D-AP5/CNQX significantly attenuated the ability of photolytic Ca2+ elevations in astrocytes to cause neuronal SICs. Changes in astrocytic calcium are represented as percentage of ΔF/Fo. Asterisks indicate significant reduction of measurements as compared with the control group (NP-EGTA +, D-AP5/CNQX −; one-way ANOVA, followed by post hoc Fisher's least significant difference test; P < 0.05).
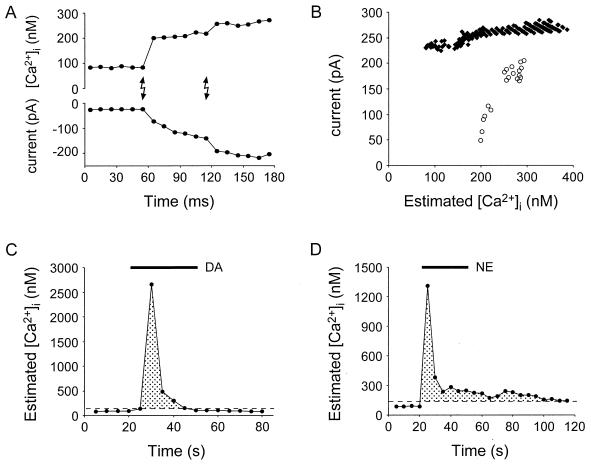
To make predictions about the potential roles for the calcium-dependent release of glutamate from astrocytes, it is important to determine what calcium levels are necessary for this release to occur and how these levels compare with those normally seen in astrocytes. Toward these goals, we quantitatively examined the relation between internal astrocytic calcium level and the neuronal SIC by making simultaneous calcium and patch recordings from astrocytes and neurons, respectively. Fig. 5A shows data from one typical experiment in which two UV flashes were provided to the sample. In this experiment, the initial flash elevated calcium from about 100 to 200 nM and caused an SIC. A second UV flash, which caused a further increase in calcium, led to an augmentation of the SIC recorded in the neuron. On average, the first UV flash elevated the internal calcium level from a resting level of 84 ± 1 nM to 140 ± 15 nM and caused an SIC of −391 ± 139 pA (n = 7). Therefore, astrocytes are able to release sufficient quantities of glutamate to have prolonged actions on neighboring neurons even with modest changes of their internal calcium level.
Figure 5.
Simultaneous recordings of astrocytic calcium levels and neuronal currents can be used to elucidate the relationship between astrocytic calcium levels and consequent glutamate release. UV pulses (A, lighting bolts) caused a step-like increase in astrocytic internal calcium (A, upper trace) and an increase in the inward current of adjacent neurons (A, lower trace). (B) The relationships between astrocytic calcium and SIC (and underlying glutamate release from astrocytes) for “all-or-none” (black diamonds) and “graded” (open circles) astrocytes are depicted. These photolysis experiments demonstrate that low levels of calcium are sufficient to induce a neuronal SIC. (C and D) The calcium responses of astrocytes to the application of dopamine (DA) and norepinephrine (NE) (both at 50 μM) demonstrate that the threshold calcium levels necessary to evoke neuronal SICs (dashed lines) are well within the range of the calcium levels that occur in astrocytes. Shaded regions represent astrocytic calcium levels that we have demonstrated are capable of stimulating glutamate release from these nonneuronal cells.
To evaluate the relationship between internal calcium and the SIC further, we plotted the astrocytic calcium level against the change in current in the adjacent neuron (Fig. 5B). We found two types of responses to calcium elevations in astrocytes: either an essentially all-or-none response (n = 4 of 7; Fig. 5B, closed diamonds) or a response whose magnitude was graded with astrocytic internal calcium level (n = 3 of 7; Fig. 5B, open circles). The reason for the all-or-none response is unclear, although it could result from saturation of postsynaptic receptors or from low levels of calcium stimulating a maximum rate of glutamate release from astrocytes. However, it is not indicative of damage to the cell, because subsequent UV illumination, applied after the calcium level had returned to baseline, evoked an additional SIC (n = 3 of 3 tested). When the amplitude of the graded responses (n = 3 of 7) was plotted on a log–log plot, Hill coefficients of 2.1 to 2.7 were obtained, suggesting multiple binding sites for calcium in stimulating glutamate release from astrocytes.
Having determined the calcium levels that are necessary for glutamate release from astrocytes, we asked whether these calcium levels are physiologically relevant. To address this issue, we monitored astrocytic calcium while applying the agonists (each at 50 μM) glutamate, norepinephrine, and dopamine, three major endogenous agonists within the hippocampus. These ligands significantly elevated calcium above 140 nM, a level that we have shown to cause an SIC reliably in adjacent neurons. On average, calcium was increased by glutamate to 2.0 μM (n = 7), by dopamine to 3.5 μM (n = 7; Fig. 5C), and by norepinephrine to 1.8 μM (n = 8; Fig. 5D). Furthermore, studies performed with cerebellar slices have demonstrated that afferent stimulation elevates the calcium level of Bergmann's glia to values greater than 200 nM (24). Because astrocytes have a low threshold for calcium-induced glutamate release and because calcium levels that range up to 3.2 μM can cause glutamate release, as reported by the presence of an SIC, the entire calcium signal that is evoked by neuroligands has the ability to regulate the release of glutamate from astrocytes. This high sensitivity for release suggests that this calcium-dependent release pathway is a physiologically relevant process that can be used in the nervous system for information transfer and neuronal modulation.
One potential concern with these studies is whether cell-cultured astrocytes express abnormal properties compared with those present in the nervous system. Several lines of evidence demonstrate, however, that astrocytes within acutely isolated preparations are also capable of releasing the neurotransmitter glutamate. For example, addition of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) to hippocampal slices causes an elevation of calcium levels in astrocytes, which is followed by a 6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione (NBQX)- and D-AP5-sensitive delayed elevation in neuronal calcium (9). Stimuli that elevate astrocytic calcium can cause a D-AP5-sensitive increase in frequency of miniature synaptic current frequency (6). These data, together with those obtained from enzymatic assays (10), are consistent with the notion that in situ astrocytes release glutamate in response to elevated internal calcium.
Given that astrocytes exhibit calcium-dependent glutamate release, what is the physiological role of this regulated transmitter release pathway? Cell culture and slice studies suggest that calcium-dependent glutamate release can modulate synaptic transmission (6, 11, 12), raising the interesting possibility that astrocytes may serve to modulate synaptic plasticity. However, because it is very difficult to suppress the astrocyte glutamate release pathway selectively, exact determinations of the role of this signaling pathway have yet to be completed. The recent demonstrations that glutamate release from astrocytes is sensitive to clostridial toxins (10, 25, 26), however, suggest that it should become possible to engineer selective manipulations to the release apparatus of astrocytes to test the functional role for this glutamate-dependent release pathway in modulating synaptic transmission and neuronal excitability.
Acknowledgments
We thank Dr. Mary Mazzanti for comments on the manuscript. This work was supported by Whitehall Foundation Grants AA98-38 and 2000-05-17 (to V.P.) and by National Institutes of Health Grant NS37585 (to P.G.H.).
Abbreviations
- NMDA
N-methyl-d-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionate
- D-AP5
d-2-amino-5-phosphonopentanoic acid
- NP-EGTA
o-nitrophenyl-EGTA
- SIC
slow inward current
- AM
acetoxymethyl
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 8196.
References
- 1.Cornell-Bell A H, Finkbeiner S M, Cooper M S, Smith S J. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 2.Cornell-Bell A H, Finkbeiner S M. Cell Calcium. 1991;2:185–204. doi: 10.1016/0143-4160(91)90020-f. [DOI] [PubMed] [Google Scholar]
- 3.Duffy S, MacVicar B A. J Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dani J W, Chernjavsky A, Smith S J. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 5.Porter J T, McCarthy K D. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang J, Jiang L, Goldman S A, Nedergaard M. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 7.Parpura V, Basarsky T A, Liu F, Jeftinija K, Jeftinija S, Haydon P G. Nature (London) 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 8.Hassinger T D, Atkinson P B, Strecker G J, Whalen L R, Dudek F E, Koseel A H, Kater S B. J Neurobiol. 1995;28:159–170. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- 9.Pasti L, Volterra A, Pozzan T, Carmignoto G. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 11.Araque A, Parpura V, Sanzgiri R P, Haydon P G. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 12.Araque A, Sanzgiri R P, Parpura V, Haydon P G. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furshpan E J, Landis S C, Matsumoto S G, Potter D D. J Neurosci. 1986;6:1061–1079. doi: 10.1523/JNEUROSCI.06-04-01061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekkers J M, Stevens C F. Proc Natl Acad Sci USA. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parpura V, Fang Y, Basarsky T A, Jahn R, Haydon P G. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 16.Charles A C. Dev Neurosci. 1994;16:196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- 17.Basarsky T A, Parpura V, Haydon P G. J Neurosci. 1994;14:6402–6411. doi: 10.1523/JNEUROSCI.14-11-06402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minta A, Kao J P, Tsien R Y. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 19.Thomas A P, Delaville F. In: Cellular Calcium. McCormack J G, Cobbold P H, editors. Oxford: Oxford Univ. Press; 1991. pp. 1–54. [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21.Kao J P Y, Harootunian A T, Tsien R Y. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- 22.Parpura V, Haydon P G. J Neurosci Methods. 1999;87:25–34. doi: 10.1016/s0165-0270(98)00155-1. [DOI] [PubMed] [Google Scholar]
- 23.Parpura V, Haydon P G. Croatian Med J. 1999;40:340–345. [PubMed] [Google Scholar]
- 24.Kulik A, Haentzsch A, Luckermann M, Reichelt W, Ballanyi K. J Neurosci. 1999;19:8401–8408. doi: 10.1523/JNEUROSCI.19-19-08401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeftinija S D, Jeftinija K V, Stefanovic G. Brain Res. 1997;750:41–47. doi: 10.1016/s0006-8993(96)00610-5. [DOI] [PubMed] [Google Scholar]
- 26.Araque A, Li N, Doyle R T, Haydon P G. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]