Abstract
Background
Angioplasty and stent delivery are performed to treat atherosclerotic vascular disease, but often cause deleterious neointimal lesion formation. Previously, growth factor receptor-bound protein 2 (Grb2), an intracellular linker protein, was shown to be essential for neointima formation and for p38 mitogen-activated protein kinase (MAPK) activation in vascular smooth muscle cells (SMCs). In this study, the role of vascular SMC p38α MAPK in neointimal development was examined.
Methods and Results
Compound transgenic mice were generated with doxycycline-inducible, SMC-specific expression of dominant negative p38α MAPK (DN-p38α). Doxycycline treatment resulted in the expression of DN-p38α mRNA and protein in transgenic arteries. Doxycycline-treated compound transgenic mice were resistant to neointima formation 21 days after carotid injury and also showed reduced arterial p38 MAPK activation. To explore the mechanism by which p38α MAPK promotes neointima formation, an in vitro SMC culture system was employed. Inhibition of p38α MAPK in cultured SMCs by treatment with SB202190 or with short interfering RNA (siRNA) blocked platelet-derived growth factor (PDGF)-induced SMC proliferation, DNA replication, phosphorylation of the retinoblastoma protein (Rb), and induction of mini-chromosome maintenance protein 6 (MCM6).
Conclusions
SMC p38α MAPK activation is required for neointima formation perhaps because of its ability to promote Rb phosphorylation and MCM6 expression.
Keywords: restenosis, smooth muscle cell, signal transduction
The proliferation of arterial SMCs contributes to several pathological conditions, including restenosis after angioplasty, post-transplant coronary artery disease, and hypertensive vasculopathy. Elaboration of neointima comprised of extracellular matrix and SMCs that proliferate and migrate from the tunica media, are known to contribute to the development of restenosis.1,2
SMC proliferation may be triggered by the action of extracellular peptide growth factors and ligands that are released locally in blood vessels and that stimulate intracellular signaling cascades. In particular, PDGF and basic fibroblast growth factor (bFGF) are thought to be involved in the neointimal hyperplasia that develops after vascular injury.3 Other growth factors and ligands are released locally in vessels after injury including epidermal growth factor (EGF) and thrombin.4,5 These ligands bind to transmembrane receptors on the surface of SMCs that initiate intracellular signaling cascades.
In previous work, the role of the intracellular linker protein Grb2 in the development of neointima after vascular injury was evaluated.6 Grb2 is a Src homology 2 and Src homology 3 domain-containing protein that facilitates the activation of the small GTPase ras, and downstream MAPKs, by growth factor receptor tyrosine kinases. Haploinsufficiency for Grb2 rendered mice resistant to neointimal lesion development after carotid injury.6 Also, Grb2+/− cultured murine aortic SMCs were resistant to PDGF-induced cell proliferation. Reduction of Grb2 protein in cultured rat arterial SMCs caused defective p38 MAPK and c-jun N-terminal kinase (JNK) MAPK activation in response to PDGF stimulation. These findings suggest that p38 MAPK and/or JNK MAPK are critical intermediaries in growth factor signaling cascades, downstream of Grb2, responsible for SMC proliferation and neointima development.
The p38 MAPK family of serine/threonine kinases consists of four members, p38α, β, γ, and δ, and p38α MAPK is ubiquitously expressed in mammalian tissues.7 The activation and function of p38α MAPK has been studied in more detail than the other family members, and in some cell types, activation of p38α MAPK antagonizes cell cycle progression, promotes inflammatory responses, and may also contribute to apoptosis.7 In particular, p38α MAPK activity in cardiomyocytes blocks cell cycle progression and cytokinesis, and also induces the expression of inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6.8,9 Furthermore, in immature thymocytes, p38α MAPK activation by V(D)J-mediated double-strand DNA breaks induces a G2/M cell cycle checkpoint via the phosphorylation and accumulation of p53.10 However, the role of p38α MAPK in cell proliferation and cell survival is dependent on cell type and physiological context.7
The biological response of cultured mammalian SMCs to p38α MAPK activation contrasts with that observed in cardiomyocytes and immature thymocytes. In particular, recently published studies support the hypothesis that p38α MAPK plays an important role in growth factor-stimulated SMC proliferation. Work by Chen demonstrated that endothelin-dependent rat aortic SMC proliferation is dependent on p38α/β MAPK activity.11 Specifically, endothelin-1-stimulated p38α/β MAPK activation led to c-Myc and E2F gene expression that contributed to cell cycle progression. In another study, Jacob demonstrated that human cultured vascular SMC proliferation in response to injury is dependent on p38α/β MAPK activation.12 Furthermore, Kavurma and Khachigian, employing SB202190, showed that p38α/β MAPK is important for cultured SMC proliferation.13 However, the specific requirement for p38α MAPK in neointima formation in vivo, has not been determined. In this work, the role of p38α MAPK in the pathogenesis of neointima formation was analyzed by use of compound transgenic mice with inducible SMC-specific expression of DN-p38α.
Methods
Generation of SM/TRE-DN-p38α compound transgenic mice
SM22α-rtTA (SM) mice were originally generated in the C57Bl/6J × CBA mixed genetic background with DNA encoding the reverse tetracycline transactivator (rtTA) linked to a 455 base pair fragment of the SM22α promoter, as previously described.14 Over 10 original transgenic lines were generated, and one line that exhibited persistent arterial expression of rtTA in adult mice was used in these studies. SM mice were crossed into the C57Bl/6J background for >7 generations.
TRE-flag-DN-p38α (TRE-DN-p38α) mice were generated with DNA containing a dominant negative form of murine p38α MAPK where the TxY activation loop is mutated to AxF.15 In addition, the DNA is linked to an N-terminus flag tag sequence and tetracycline response element (TRE). A linearized form of this DNA construct was injected into the male pro-nucleus of one-cell murine pure C57Bl/6J embryos to generate TRE-DN-p38α mice by the Diabetes Research and Training Center Transgenic Core Facility at Washington University in St. Louis, School of Medicine. One founder was obtained and was bred with C57Bl/6J non-transgenic mice.
TRE-DN-p38α mice were bred with SM mice to generate SM22α-rtTA X TRE-flag-DN-p38α (SM/TRE-DN-p38α) mice in the C57Bl/6J genetic background. SM/TRE-DN-p38α mice were obtained in the expected Mendelian ratio, appeared normal, and were fertile. SM/TRE-DN-p38α compound transgenic mice were compared to SM single transgenic littermates for all experiments described in this study. All studies involving the use of mice were performed in strict accordance with protocols approved by the Animal Studies Committee of Washington University in St. Louis, School of Medicine.
Statistical Analysis
All statistical analysis was performed with SigmaStat® 3.1 (Systat Software, Inc., San Jose, CA). For animal, lesion area data, Mann-Whitney Rank Sum tests were performed. For cell culture data, Kruskal-Wallis One Way Analysis of Variance on Ranks tests with All Pairwise Multiple Comparison Procedures, conducted via the Student-Newman-Keuls Method, were performed to assess statistical significance. PCNA-index data was analyzed with t-test. A value of p < 0.05 was considered to be statistically significant.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
For a description of other Methods used in this study, please see the “Online Data Supplement”.
Results
Generation of Compound Transgenic Mice with Inducible SMC-Specific Expression of DN-p38α
In previous work, Grb2+/− mice were demonstrated to be resistant to neointima formation in response to carotid injury and arterial p38 MAPK activation was found to be reduced in these animals.6 To directly test the role of p38α MAPK in neointima formation in vivo, we generated compound transgenic mice with doxycycline-inducible expression of dominant negative p38α MAPK in arterial SMCs. A DNA construct was generated that contained a tetracycline response element (TRE) linked to cDNA encoding DN-p38α where the TxY kinase activation loop amino acid sequence is mutated to AxF.15 This DNA construct was linearized and injected into the male pro-nucleus of one-cell murine C57Bl/6J embryos and used to generate TRE-DN-p38α founder mice. One line of TRE-DN-p38α transgenic mice in the C57Bl/6J genetic background was created by this method. TRE-DN-p38α transgenic mice were crossed with SM22α-rtTA transgenic mice (SM) that were previously demonstrated to exhibit arterial SMC-specific expression of the reverse tetracycline transactivator (rtTA).14
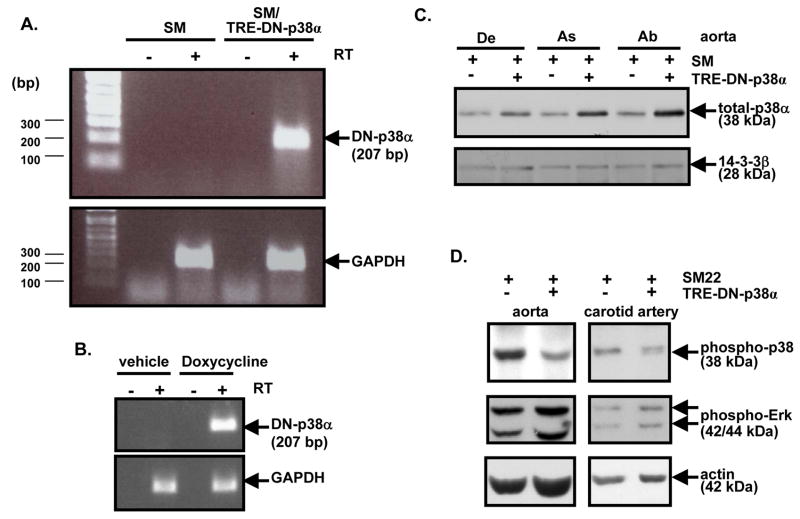
To induce expression of DN-p38α MAPK, SM22α-rtTA X TRE-DN-p38α compound transgenic mice (SM/TRE-DN-p38α) and control single transgenic SM22α-rtTA mice (SM) were treated with doxycycline. Following one week of doxycycline treatment, aortas were isolated for RT-PCR assays. RT-PCR with aortic RNA demonstrated that DN-p38α MAPK mRNA was expressed in the SM/TRE-DN-p38α compound transgenic background, but was not expressed in the SM single transgenic background (Figure 1A). Doxycyline-inducible expression of DN-p38α MAPK mRNA in the carotid arteries of SM/TRE-DN-p38α compound transgenic mice was also assessed. Following one week of doxycycline, induction of DN-p38α MAPK mRNA was observed (Figure 1B). Also, following two weeks of doxycycline treatment, carotid arteries, aortas, and other tissues were obtained for protein analysis. Western blot analysis of protein lysates revealed that DN-p38α protein was expressed in the aorta in the SM/TRE-DN-p38α compound transgenic background, but not in the control single transgenic, SM background (Figure 1C). Furthermore, two weeks of doxycycline-induction resulted in decreased activation of native p38 MAPK in the aorta and carotid arteries of SM/TRE-DN-p38α compound transgenic mice compared to arterial tissues from SM mice (Figure 1D). In contrast, DN-p38α induction did not block extracellular signal-regulated kinase (ERK) MAPK activation (Figure 1D).
Figure 1. Doxycycline-induced DN-p38α expression in SM/TRE-DN-p38α compound transgenic mice reduces activation of native p38 MAPK in arteries.
A, SM and SM/TRE-DN-p38α mice were administered doxycycline rodent chow and doxycycline drinking water ad libitum for one week. Then, semi-quantitative RT-PCR was conducted with aortic total RNA to assess expression of DN-p38α mRNA (RT, reverse transcriptase). B, SM/TRE-DN-p38α mice were administered doxycycline rodent chow and doxycycline drinking water or standard rodent chow and sucrose (vehicle control) drinking water ad libitum for one week as described. Then, RT-PCR was conducted as described for “A”. C, Mice were given doxycycline as described in “A” for two weeks. Next, aortic lysates were prepared and probed via Western blotting with anti-total p38α MAPK antibody. Re-probing with anti-14-3-3β antibody was conducted to control for protein loading (De, descending; As, ascending; Ab, abdominal). D, Mice were given doxycycline as described in “C”. Then, carotid artery and aortic lysates were prepared and probed via Western blotting with anti-phospho (Thr180/Tyr182) p38 MAPK antibody. Re-probing was conducted with anti-phospho (Thr202/Tyr204) ERK and anti-actin antibodies to control for the specificity of DN-p38α and protein loading, respectively.
Requirement of p38α MAPK for Response to Carotid Arterial Injury in Mice
To evaluate the role of SMC p38α MAPK activity in the pathogenesis of neointima formation, doxycycline-treated compound transgenic SM/TRE-DN-p38α and single transgenic, SM mice were subjected to carotid injury by the epoxy resin beaded-probe method. Both groups of mice tolerated the procedure well and no post-operation mortaility was observed. Evans blue dye and von Willebrand Factor staining demonstrated that our carotid injury method produces consistent endothelial denudation and that the degree of denudation is similar from mouse to mouse (Supplemental Figure I).
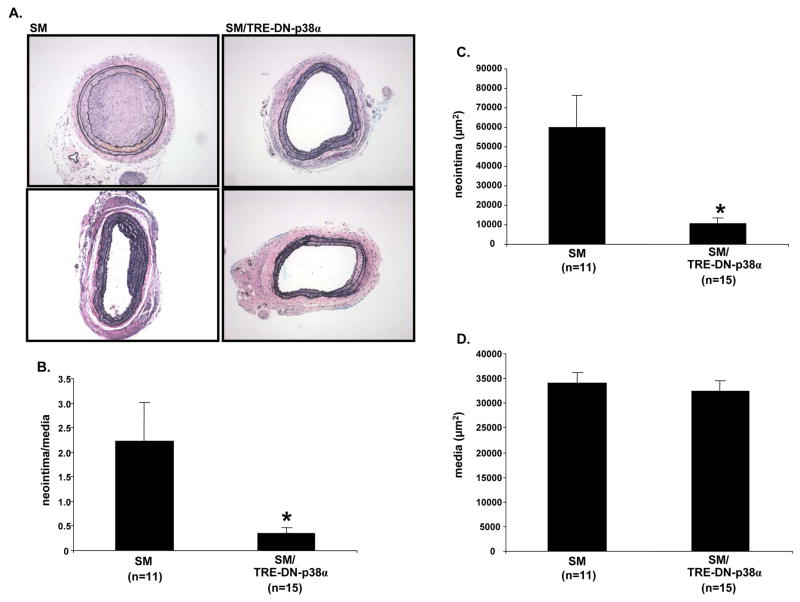
Histological examination of carotid cross-sections was performed 3 weeks after injury and revealed robust neointimal lesion formation in single transgenic SM mice (Figure 2A and 2B). In SM mice, the neointimal lesions were at least partially occlusive, and sometimes the lesions completely occluded the vessel lumen. In contrast, SM/TRE-DN-p38α compound transgenic mice exhibited markedly reduced lesion formation. There was a 84% reduction in the mean neointima/media ratio of SM/TRE-DN-p38α mice compared to SM mice (Figure 2B). Specifically, the mean neointima/media ratio of SM mice was 2.23 ± 0.80 (SE), but was only 0.36 ± 0.10 (SE) in SM/TRE-DN-p38α mice (Figure 2B, P=0.029). Also, the mean neointimal area was less for SM/TRE-DN-p38α mice compared to SM mice (Figure 2C, P=0.017). However, the mean medial area of SM and SM/TRE-DN-p38α mice was similar (Figure 2D).
Figure 2. SM/TRE-DN-p38α compound transgenic mice exhibit reduced carotid injury-induced neointimal lesion formation.
A, SM and SM/TRE-DN-p38α mice were given doxycycline and mechanical injury of the left carotid artery was performed. Three weeks post-mechanical injury, histological examination with hematoxylin and eosin, and Verhoeff’s von Giesen stain was performed to assess neointimal lesion formation. Representative sections are depicted for SM and SM/TRE-DN-p38α mice; n=2 mice per genotype. A 10x objective was used to collect the micrographs. B, Mechanical injury and histology was performed as described for “A”. Then, morphometry was performed to quantify neointima/media ratios (SM/TRE-DN-p38α mice (n=15), + doxycycline vs. SM mice (n=11), + doxycycline, *P=0.029). C, Neointimal area was quantified via morphometric analysis with the mice from “A” and “B” (SM/TRE-DN-p38α mice (n=15), + doxycycline vs. SM mice (n=11), + doxycycline, *P=0.017). D, Medial area was quantified via morphometric analysis with the mice from “A” and “B”. For the bar graphs in “B”, “C”, and “D”, results are expressed as mean ± SE.
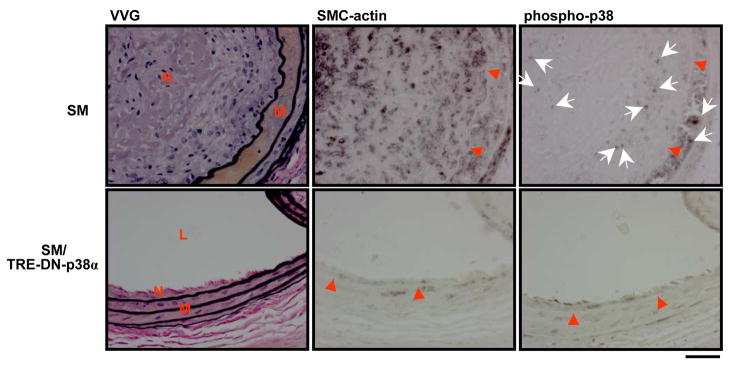
The SMC component of neointimal lesions was assessed by immunohistochemical staining with an anti-SMC-actin primary antibody. Immunohistochemical analysis revealed that SMCs were a major component of neointimal lesions in SM mice (Figure 3). Although SMCs were present in the tunica media and intima of SM/TRE-DN-p38α mice, there was markedly reduced SMC-actin staining compared to SM mice (Figure 3). Vascular signaling was also evaluated in carotid cross-sections from mice. Immunohistochemical staining with anti-phospho p38 MAPK antibody showed that p38 MAPK activation was present in both the tunica media and in the neointima of single transgenic SM mice (Figure 3). In contrast, p38 MAPK activation was dramatically reduced in the tunica media and intima of SM/TRE-DN-p38α mice (Figure 3).
Figure 3. SM/TRE-DN-p38α compound transgenic mice exhibit reduced arterial SMC and phospho-p38 staining.
SM and SM/TRE-DN-p38α mice were given doxycycline and mechanical injury of the left carotid artery was performed. Three weeks after injury, immunohistochemical analysis of carotid artery sections was performed with anti-SMC-actin and anti-phospho (Thr180/Tyr182) p38 MAPK antibodies (VVG, Verhoeff’s von Giesen stain). Red arrow heads and white arrows depict the border between the intimal and medial areas and areas of intense phospho-p38 MAPK staining, respectively (N, neointima; L, lumen; M, media). Scale bar depicts 30 μM.
Role of Rb and MCM6 in p38α MAPK-Induced SMC Proliferation
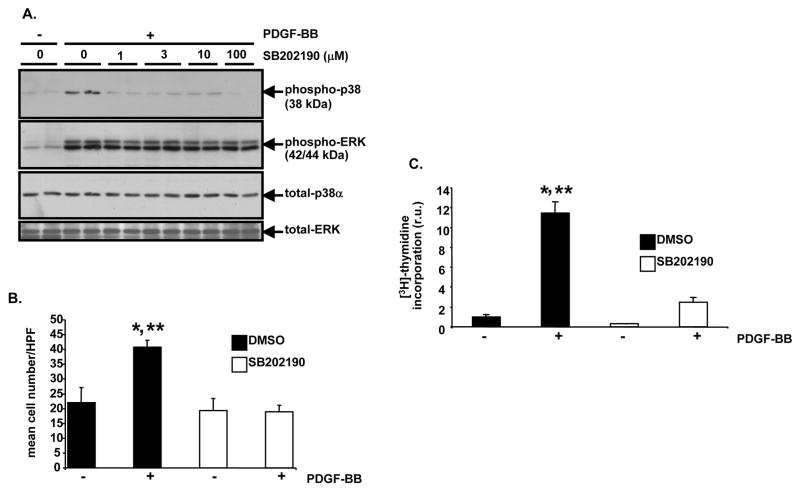
To explore the molecular mechanism(s) by which p38α MAPK promotes carotid artery neointima formation in response to injury, an in vitro model system was employed with a cultured rat vascular SMC-derived cell line. Sub-confluent A10 cells were pre-treated with a pharmacological inhibitor of both p38α and p38β MAPK, SB202190, or with control diluent. PDGF treatment of A10 cells caused robust phosphorylation of the p38 MAPK activation loop (Figure 4A). Activation of p38 MAPK, but not ERK MAPK, was blocked by SB202190 (Figure 4A). When quiescent A10 cells were pre-treated with control diluent and then stimulated with PDGF for 20 hours, a 1.85-fold increase in cell number was observed (Figure 4B, P<0.05). However, when A10 cells were pre-treated with SB202190, PDGF treatment did not result in increased cell number (Figure 4B). [3H]-thymidine incorporation assays were performed to determine whether p38 MAPK function is required for PDGF-induced DNA synthesis. PDGF treatment of A10 cells resulted in an 11.4-fold increase in [3H]-thymidine incorporation (Figure 4C, P<0.05), but SB202190 dramatically reduced [3H]-thymidine incorporation (Figure 4C). Similarly, SB202190 blocked PDGF-induced primary rat aortic smooth muscle cell (RAOSMC) proliferation and reduced [3H]-thymidine incorporation (Supplemental Figure IIA and IIB).
Figure 4. SB202190 blocks PDGF-induced p38 MAPK activation, cell proliferation, and DNA replication in A10 cells.
A, Analysis of p38 MAPK activation after SB202190 treatment. Serum-deprived A10 cells were treated with the indicated concentrations of SB202190 for 1 hour. Then, they were treated with or without PDGF-BB (60 ng/ml) for 5 minutes at 37 degrees C. Following stimulation, lysates were generated and Western blotting with anti-phospho (Thr180/Tyr182) p38 MAPK antibody was conducted. Subsequent re-blotting was conducted with anti-phospho (Thr202/Tyr204) ERK, anti-total p38α MAPK, and anti-total ERK antibodies. B, Evaluation of PDGF-stimulated cell proliferation after SB202190 treatment. Serum-deprived A10 cells were pre-treated with SB202190 (10 μM) or vehicle (DMSO) for 1 hour. Then, in triplicate wells, the monolayers were treated with PDGF-BB (60 ng/ml) or 0.1% BSA, as a vehicle control for PDGF-BB, in the continued presence of SB202190 or DMSO. After 20 hours cell number was determined. HPF, 400x high power field (DMSO, + PDGF-BB vs. DMSO, −PDGF-BB, *P<0.05; DMSO, + PDGF-BB vs. SB202190, − PDGF-BB, **P<0.05). C, Examination of PDGF-induced DNA replication after SB202190 treatment. Serum-deprived A10 cells were pre-treated with SB202190 (10 μM) or DMSO as in “B”. Then, the monolayers were treated with PDGF-BB (60 ng/ml) or 0.1% BSA for 15 hours in a tissue culture incubator in the continued presence of SB202190 or DMSO. Next, each well was pulsed with [6-3H]-thymidine (1 μCi/ml) for 2.5 hours and thymidine incorporation was determined; r.u., relative units (DMSO, + PDGF-BB vs. DMSO, −PDGF-BB, *P<0.05; DMSO, + PDGF-BB vs. SB202190, + PDGF-BB, **P<0.05). For bar graphs in “B” and “C”, results are expressed as mean ± SD of experiments conducted in triplicate.
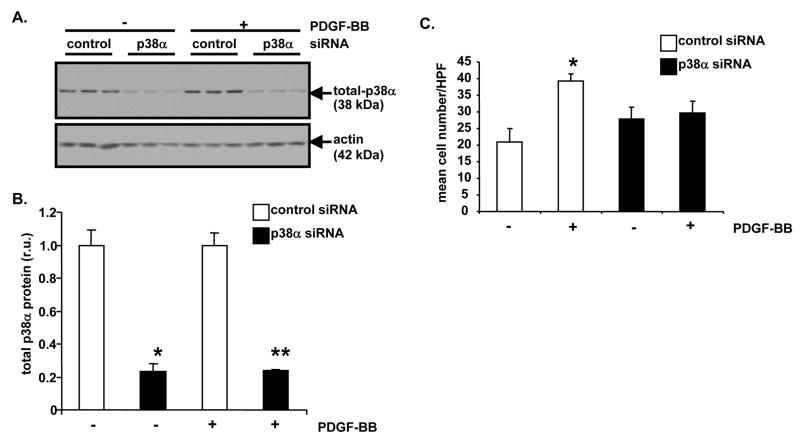
To specifically reduce p38α MAPK protein levels and activity in cultured cells, a siRNA approach was employed. Sub-confluent A10 cells were transfected with a rat p38α MAPK siRNA construct or with control, non-targeting siRNA. Following 24 hours of transfection in serum-free media, A10 cells were stimulated with PDGF or control diluent for 20 hours. Following stimulation, some A10 cells were used to generate lysates for Western blotting. Western blot analysis revealed that p38α MAPK protein levels were dramatically reduced in p38α MAPK siRNA-transfected A10 cells compared to control siRNA-transfected cells (Figure 5A). Specifically, densitometric analysis demonstrated approximately 76% reduction in total p38α MAPK protein (Figure 5B). When stimulated with PDGF for 20 hours, A10 cells transfected with p38α MAPK siRNA proliferated at a much slower rate than control siRNA-transfected cells (Figure 5C). A10 cell number increased by 1.9-fold after PDGF stimulation in control siRNA-transfected cells (P<0.05). Increase in A10 cell number was blocked with p38α MAPK siRNA. Following 20 hours of PDGF stimulation, [3H]-thymidine incorporation was reduced approximately 56% in p38α MAPK siRNA-transfected A10 cells compared to control siRNA-transfected cells (data not shown, P=0.007).
Figure 5. siRNA-mediated reduction of p38α MAPK protein inhibits PDGF-BB-induced A10 cell proliferation.
A, Analysis of p38α MAPK protein levels after siRNA treatment. Sub-confluent A10 monolayers were transfected with p38α MAPK-specific siRNA or siCONTROL™ Non-Targeting siRNA #2. Following 24 hours of transfection, in triplicate wells A10 cells were treated with PDGF-BB (60 ng/ml) or 0.1% BSA, as a vehicle control, for 20 hours. Then, lysates were generated and Western blotting was performed with anti-total p38α MAPK antibody. Re-probing with anti-actin antibody was conducted to control for protein loading. B, Densitometric analysis of Western blot from “A” was conducted (p38α MAPK siRNA, − PDGF-BB vs. control siRNA, − PDGF-BB, *P<0.05; p38α MAPK siRNA, + PDGF-BB vs. control siRNA, + PDGF-BB, **P<0.05). C, Analysis of PDGF-stimulated cell proliferation after p38α MAPK siRNA treatment. Sub-confluent A10 monolayers were transfected and stimulated as described in “A”. Then, determination of cell number was conducted (control siRNA, − PDGF-BB vs. control siRNA, + PDGF-BB, *P<0.05). For bar graphs in “B” and “C”, results are expressed as mean ± SD of experiments conducted in triplicate.
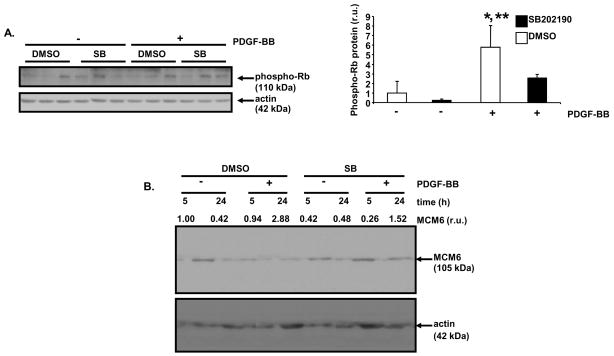
To address the downstream target(s) of p38α MAPK action responsible for growth factor-stimulated SMC proliferation, the phosphorylation status of a key regulator of mammalian cell cycle progression, Rb, was evaluated.16 In non-proliferating cells, Rb is hypo-phosphorylated and is able to bind to and sequester members of the E2F family of transcription factors. Activation of cyclin-dependent kinases results in the hyper-phosphorylation of Rb, the subsequent release of E2F proteins, and the transcription of genes involved in cell cycle progression.17,18 In previous work, inhibition of human vascular SMC proliferation by salicylate administration, correlated with reduced Rb hyper-phosphorylation.19 PDGF treatment of A10 cells for 20 hours resulted in a robust increase in Rb phosphorylation that was blocked by pre-treatment of cells with SB202190 (Figure 6A). Also, PDGF induced hyper-phosphorylation of Rb in RAOSMCs which was blocked by SB202190 pretreatment (Supplemental Figure III).
Figure 6. SB202190 blocks PDGF-induced Rb hyper-phosphorylation, and MCM6 induction in A10 cells.
A, Analysis of PDGF-induced Rb phosphorylation after SB202190 treatment. Serum-deprived A10 cells were pre-treated with SB202190 (10 μM) or vehicle (DMSO) for 1 hour, and then monolayers were treated with PDGF-BB (60 ng/ml) or 0.1% BSA in the continued presence of SB202190 or DMSO. After 20 hours, cells were lysed and immunoblotting was performed with an anti-phospho (Ser 807/811) Rb antibody. Re-probing was conducted with anti-actin antibody to control for protein loading. Densitometric analysis of the Western blot was conducted (DMSO, + PDGF-BB vs. DMSO, − PDGF-BB, *P<0.05; DMSO, + PDGF-BB vs. SB202190, + PDGF-BB, **P<0.05 (SB, SB202190). B, Analysis of PDGF-stimulated MCM6 induction after SB202190 treatment. Serum-deprived A10 cells were pre-treated with SB202190 (10 μM) or vehicle (DMSO) for 1 hour. Monolayers were treated with PDGF-BB (60 ng/ml) or 0.1% BSA in the continued presence of SB202190 or DMSO for 5 or 24 hours. Cell lysates were analyzed by Western blotting with an anti-MCM6 antibody. Reprobing was conducted with anti-actin antibody. Densitometric analysis was performed for the Western blot. The amount of MCM6 signal in each lane was normalized to the amount of actin for that lane. The normalized MCM6 signal for each lane is listed in relative units (r.u.). Results for “A” are expressed as mean ± SD of triplicate culture wells.
One target of E2F activity during cell cycle progression is MCM6. MCM6 is a regulator of DNA replication and DNA helicase activity.20 In previous work, liver X receptor ligand inhibition of human vascular SMC proliferation correlated with reduced expression of MCM6.21 PDGF treatment of sub-confluent A10 cells for 24 hours resulted in a robust increase of MCM6 protein levels that was blocked by pre-treatment of cells with SB202190 (Figure 6B).
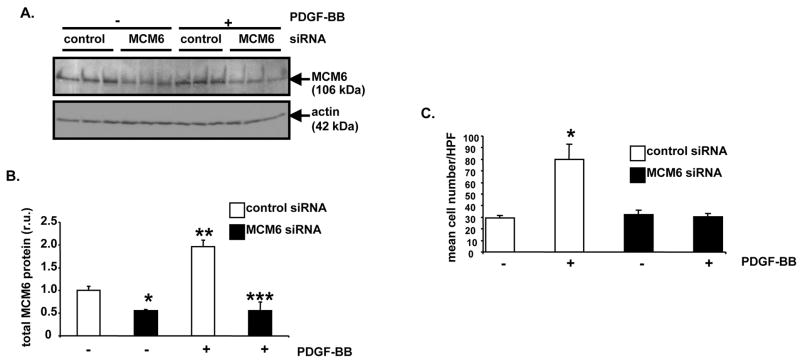
We assessed requirement for MCM6 in RAOSMC proliferation and DNA replication by transfecting RAOSMCs with MCM6-specific siRNA. Sub-confluent RAOSMCs cells were transfected with a rat MCM6 siRNA construct or with control, non-targeting siRNA. Following two days of transfection in serum-free media, RAOSMCs cells were stimulated with PDGF or control diluent for 15 hours. Following stimulation, some RAOSMC lysate was generated for Western blotting. Western blot analysis revealed that MCM6 protein levels were dramatically reduced in MCM6 siRNA-transfected RAOSMCs compared to control siRNA-transfected cells (Figure 7A and 7B). In RAOSMCs transfected with control siRNA, overnight-stimulation with PDGF induced a 2-fold increase in MCM6 protein (Figure 7A and 7B, P<0.05). MCM6-specific siRNA reduced baseline MCM6 protein to approximately 55% compared to control siRNA-transfected RAOSMCs (P<0.05). Also, MCM6-specific siRNA blocked PDGF-induction of MCM6 protein (Figure 7A and 7B, P<0.05). Moreover, MCM6-specific siRNA reduced both PDGF-induced proliferation (Figure 7C) and thymidine incorporation (data not shown). Specifically, in control siRNA-transfected RAOSMCs, cell number increased by 2.5-fold after 40 hours of PDGF stimulation (Figure 7C, P<0.05). However, increase in RAOSMC number was blocked with MCM6 siRNA.
Figure 7. siRNA-mediated reduction of MCM6 protein inhibits PDGF-BB-induced RAOSMC proliferation.
A, Analysis of MCM6 protein levels after siRNA treatment. Sub-confluent RAOSMC monolayers were transfected with MCM6-specific siRNA or siCONTROL™ Non-Targeting siRNA #2. Two days after transfection, in triplicate wells the cells were treated with PDGF-BB (5 ng/ml) or 0.1% BSA, as a vehicle control, for 15 hours. Lysates were generated and Western blotting was performed with anti-MCM6 antibody. Re-probing with anti-actin antibody was conducted to control for protein loading. B, Densitometric analysis of Western blot from “A” was conducted (MCM6 siRNA, − PDGF-BB vs. control siRNA, − PDGF-BB, *P<0.05; MCM6 siRNA, + PDGF-BB vs. MCM6 siRNA, − PDGF-BB, **P<0.05; MCM6 siRNA, + PDGF-BB vs. control siRNA, + PDGF-BB, ***P<0.05). C, Analysis of PDGF-stimulated cell proliferation after MCM6 siRNA transfection. Sub-confluent RAOSMC monolayers were transfected and stimulated as described in “A” for 40 hours. Then, determination of cell number was conducted (control siRNA, − PDGF-BB vs. control siRNA, + PDGF-BB, *P<0.05). For bar graphs in “B” and “C”, results are expressed as mean ± SD of experiments conducted in triplicate.
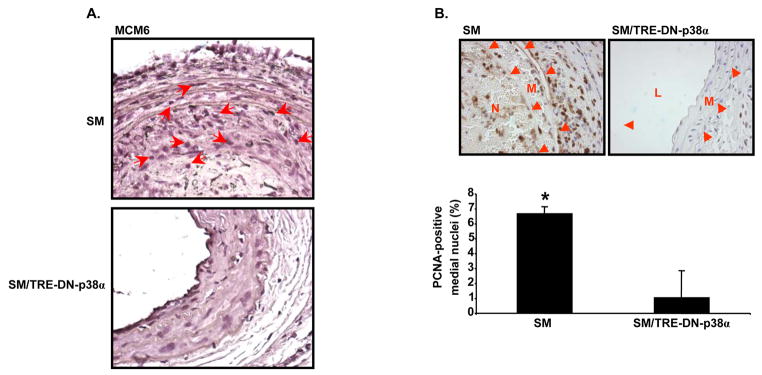
MCM6 expression was evaluated in carotid cross-sections of SM and SM/TRE-DN-p38α mice by immunohistochemical staining. Immunohistochemical analysis revealed that carotid cross-sections from SM mice, isolated three weeks after injury, had increased MCM6 expression compared to sections from compound transgenic, SM/TRE-DN-p38α mice (Figure 8A). Sections were also stained for Proliferating Cell Nuclear Antigen (PCNA). Carotid cross-sections from SM mice had increased medial PCNA staining compared to SM/TRE-DN-p38α mice (Figure 8B). Specifically, 6.69 ± 0.45% of SM medial nuclei were PCNA-positive. However, this value was reduced to 1.04 ± 1.80% for SM/TRE-DN-p38α mice (Figure 8B, P=0.006).
Figure 8. SM/TRE-DN-p38α compound transgenic mice exhibit reduced arterial MCM6 and PCNA staining.
A, SM and SM/TRE-DN-p38α mice were given doxycycline and mechanical injury of the left carotid artery was performed. Three weeks after injury, immunohistochemical analysis of carotid cross-sections was performed with anti-MCM6 antibody. The sections were counterstained with hematoxylin. Micrographs were collected with a 63x objective. Red arrows depict areas exhibiting enriched MCM6 staining. B, Mice were given doxycycline and injured as in “A”. Three weeks after injury, immunohistochemical analysis of carotid cross-sections was performed with anti-PCNA antibody. The sections were counterstained with hematoxylin. Micrographs were collected with a 40x objective. The PCNA-positive nuclei are depicted with DAB (brown) staining. The red arrows highlight the media-adventitia and media-neointima borders (N, neointima; L, lumen; M, media). The bar graph shows the mean ± SD percentage of PCNA-positive medial nuclei per group (SM mice (n=3), SM/TRE-DN-p38α mice (n=3), *P=0.006).
To evaluate whether increased apoptosis was responsible for the phenotype observed in SM/TRE-DN-p38α mice 3 weeks after carotid injury, TUNEL was performed on carotid cross-sections. Infrequent TUNEL-positive cells were detected in the media and neointima of SM carotid arteries (n=3), but no TUNEL-positive cells were detected in the media or neointima of SM/TRE-DN-p38α carotid arteries (n=3, data not shown).
Discussion
The MAPKs are important effectors of growth factor and ligand action in eukaryotic cells. The p38 family of MAPKs consists of 4 proteins encoded by separate genes that regulate important aspects of cell physiology, including cell growth, proliferation, survival, and differentiation.7 In this work, the role of p38α MAPK in the formation of neointima after arterial injury was analyzed. In previous work, the role of the ubiquitously expressed intracellular linker protein, Grb2, which links growth factor receptor activation to MAPK activation, was analyzed.6 Grb2 haploinsufficient mice were resistant to neointima formation after carotid injury. Furthermore, Grb2 haploinsufficient cultured SMCs exhibited reduced p38 and JNK MAPK activation in response to PDGF stimulation.
To address the specific role of p38α MAPK in neointima formation, SM/TRE-DN-p38α mice were generated in the present study. Doxycycline treatment of these animals resulted in the expression of DN-p38α mRNA and protein in aortae and carotid arteries. Next, doxycycline-treated SM/TRE-DN-p38α compound transgenic mice were found to be resistant to carotid injury-induced neointima formation compared to control SM mice. Furthermore, immunohistochemical analysis revealed that p38 MAPK activation was dramatically reduced in the tunica media and intima of SM/TRE-DN-p38α mice after carotid injury. These results strongly suggest that p38α MAPK activity is required for neointima formation after arterial injury.
To determine the molecular mechanism(s) that contribute to p38α MAPK-mediated neoinitima formation, an in vitro SMC culture system was employed. PDGF-induced cell proliferation was blocked in cultured SMCs after treatment with SB202190, an inhibitor of p38α/β MAPK. To more specifically examine the role of p38α MAPK in SMC proliferation in vitro, a siRNA approach was used. PDGF-induced SMC proliferation was blocked in cells treated with p38α MAPK siRNA.
One potential effector of p38α MAPK action that is involved in SMC proliferation is Rb, a master regulator of cell cycle progression.16 PDGF-induced hyper-phosphorylation of Rb was blocked in SMCs after SB202190 treatment. Previous work by Marra showed that the anti-proliferative effect of salicylates on growth factor-stimulated SMCs was correlated with reduced Rb hyper-phosphorylation.19 It is unclear whether p38α MAPK directly phosphorylates Rb in PDGF-stimulated SMCs or if p38α MAPK indirectly affects Rb phosphorylation via activation of a cyclin-dependent kinase or other protein kinase.
Another potential effector of p38α MAPK in SMCs is MCM6, a protein required for DNA synthesis during S phase of the cell cycle.20 MCM6 gene expression in SMCs is induced by the E2F transcription factor after it is released from hyper-phosphorylated Rb.21 PDGF stimulation of control SMCs promoted the expression of MCM6, but this response was blocked in cells treated with SB202190. Furthermore, siRNA reduction of MCM6 blocked PDGF-induced SMC proliferation and DNA replication.
Taken together, our results show that p38α MAPK activity is required for SMC proliferation in vitro and neointima formation in vivo. This activity of p38α MAPK contrasts with its role in cardiomyocytes, where in certain situations p38α MAPK blocks cell proliferation, promotes apoptosis, and antagonizes hypertrophic growth.8,22–25 However, one of the hallmarks of MAPKs is that the biological effects of kinase activation are highly dependent on the cell type and the physiological context.7 One molecular mechanism of p38α MAPK action in SMCs may be to promote the hyper-phosphorylation of Rb, the subsequent activation of E2F family members, and the stimulation of MCM6 expression that results in cell proliferation and arterial neointima formation.
The results of this study provide preliminary evidence that p38α MAPK inhibitors may be useful for the treatment of arterial restenosis and transplant vasculopathy. Our previous work and the work of other groups also support the use of p38α MAPK inhibitors to limit pathological cardiac remodeling after myocardial infarction.24–26 Although numerous p38α MAPK inhibitors have been developed, none are currently approved for use in human patients, and clinical trials evaluating the efficacy of these agents in cardiovascular disease have not been published.27
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by grants from the Burroughs Wellcome Fund, the American Heart Association, and the National Institutes of Health (HL061567, HL076770) (A.J.M.). The authors acknowledge the assistance of the Diabetes Research and Training Center Transgenic Core Facility at Washington University in St. Louis, School of Medicine, directed by Dr. Burton Wice (DRTC P60 DK020579). The authors also acknowledge the assistance of the DDRCC Morphology Core Facility at Washington University in St. Louis, School of Medicine (DDRCC P30 DK52574). B.M.P. is a recipient of an American Heart Association, Heartland Affiliate Predoctoral Fellowship.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Pauletto P, Puato M, Faggin E, Santipolo N, Pagliara V, Zoleo M, Deriu GP, Grego F, Plebani M, Sartore S, Bon GB, Heymes C, Samuel JL, Pessina AC. Specific cellular features of atheroma associated with development of neointima after carotid endarterectomy: the carotid atherosclerosis and restenosis study. Circulation. 2000;102:771–778. doi: 10.1161/01.cir.102.7.771. [DOI] [PubMed] [Google Scholar]
- 2.Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol. 2000;86 (suppl):6H–11H. doi: 10.1016/s0002-9149(00)01094-8. [DOI] [PubMed] [Google Scholar]
- 3.Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci USA. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley ST, Ray CJ, Nawaz D, Majack RA, Horwitz LD. Multiple growth factors are released from mechanically injured vascular smooth muscle cells. Am J Physiol. 1995;269(5 Pt 2):H1641–H1647. doi: 10.1152/ajpheart.1995.269.5.H1641. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox JN. Molecular biology: insight into the causes and prevention of restenosis after arterial intervention. Am J Cardiol. 1995;72:88E–95E. doi: 10.1016/0002-9149(93)91043-h. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Ren J, Khan MF, Cheng AM, Abendschein D, Muslin AJ. Grb2 is required for the development of neointima in response to vascular injury. Arterioscler Thromb Vasc. 2003;23:1788–1793. doi: 10.1161/01.ATV.0000085015.49110.85. [DOI] [PubMed] [Google Scholar]
- 7.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 10.Pedraza-Alva G, Koulnis M, Charland C, Thornton T, Clements JL, Schlissel MS, Rincon M. Activation of p38 MAP kinase by DNA double-strand breaks in V(D)J recombination induces a G2/M cell cycle checkpoint. EMBO J. 2006;25:763–773. doi: 10.1038/sj.emboj.7600972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Qiong Y, Gardner DG. A role for p38 mitogen-activated protein kinase and c-myc in endothelin-dependent rat aortic smooth muscle cell proliferation. Hypertension. 2006;47:252–258. doi: 10.1161/01.HYP.0000198424.93598.6b. [DOI] [PubMed] [Google Scholar]
- 12.Jacob T, Ascher E, Alapat D, Olevskaia Y, Hingorani A. Activation of p38 MAPK signaling cascade in a VSMC injury model: role of p38 MAPK inhibitors in limiting VSMC proliferation. Eur J Endovasc Surg. 2005;29:470–478. doi: 10.1016/j.ejvs.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Kavurma MM, Khachigian LM. ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J Cell Biochem. 2003;89:289–300. doi: 10.1002/jcb.10497. [DOI] [PubMed] [Google Scholar]
- 14.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435:502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 15.Xing H, Zhang S, Weinheimer C, Kovacs A, Muslin AJ. 14-3-3 proteins inhibit apoptosis and differentially regulate MAPK cascades. EMBO J. 2000;19:349–358. doi: 10.1093/emboj/19.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 17.La Thangue NB. E2F and the molecular mechanisms of early cell-cycle control. Biochem Soc Trans. 1996;24:54–59. doi: 10.1042/bst0240054. [DOI] [PubMed] [Google Scholar]
- 18.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 19.Marra DE, Simoncini T, Liao JK. Inhibition of vascular smooth muscle cell proliferation by sodium salicylate mediated by upregulation of p21(Waf1) and p27(Kip1) Circulation. 2000;102:2124–2130. doi: 10.1161/01.cir.102.17.2124. [DOI] [PubMed] [Google Scholar]
- 20.You Z, Komamura Y, Ishimi Y. Biochemical analysis of the intrinsic Mcm4-Mcm6-mcm7 DNA helicase activity. Mol Cell Biol. 1999;19:8003–8015. doi: 10.1128/mcb.19.12.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaschke E, Leppanen O, Takata Y, Caglayan E, Liu J, Fishbein MC, Kappert K, Nakayama KI, Collins AR, Fleck E, Hsueh WA, Law RE, Bruemmer D. Liver X receptor agonists suppress vascular smooth muscle cell proliferation and inhibit neointima formation in balloon-injured rat carotid arteries. Circ Res. 2004;95:e110–e123. doi: 10.1161/01.RES.0000150368.56660.4f. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 25.See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, Itescu S, Krum H. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 27.Kerkela R, Force T. p38 mitogen-activated protein kinase: A future target for heart failure therapy? J Am Coll Card. 2006;48:556–558. doi: 10.1016/j.jacc.2006.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.