Abstract
The separate roles of inflammation and insulin resistance (IR) in the pathogenesis of cardiovascular disease (CVD) are well recognized. We investigated whether presence of inflammation would modify coronary artery disease (CAD) risk prediction in subjects with or without IR. Insulin, glucose, CRP and fibrinogen levels were determined in 317 Caucasians and 222 African Americans undergoing diagnostic coronary angiography. Extent of CAD was defined by a composite score (0–75). The overall prevalence of IR (HOMA-IR≥3.0) in Caucasians and African Americans was 32.5% and 22.9%, respectively (P<0.05). The degree of CAD (composite score) was higher in subjects with IR (20.7 vs. 14.5, P=0.014 and 20.1 vs. 13.1, P=0.031 for Caucasians and African Americans, respectively), and in a multiple regression model IR was an independent predictor for CAD in both groups. In both ethnic groups, subjects with a combination of IR and high CRP (≥3 mg/L) had significantly higher composite score compared to those with no IR and low CRP (<3 mg/L) (21.2 vs. 13.9, P<0.05 and 20.9 vs. 10.2, P<0.05 for Caucasians and African Americans respectively). Similarly, the composite score was significantly higher in subjects with IR and high fibrinogen (≥340 mg/dl) compared to those with no IR and low fibrinogen. In conclusion, elevated levels of inflammatory markers were positively associated with IR. Further, a combination of IR and inflammation resulted in a higher degree of CAD in both Caucasians and African Americans. The results suggest that inflammation may potentiate the cardiovascular risk factor role of IR.
Keywords: Insulin resistance, inflammation, CRP, fibrinogen, ethnicity
INTRODUCTION
Insulin resistance is a major risk factor for type 2 diabetes mellitus, and is associated with cardiovascular disease (CVD) risk factors including obesity, hypertension, and dyslipidemia [1,2]. Insulin resistance and related metabolic conditions are becoming increasingly common. Notably, a substantial proportion of apparently healthy people are reported to be insulin resistant [3]. So far, the association of insulin resistance with CVD independent of classic risk factors have been inconsistent. Further, insulin resistance is associated with several nontraditional CVD risk factors, such as markers of coagulation, systemic inflammation, oxidative stress, subclinical vascular disease and plasma adipokine levels [4–6].
The role of inflammation in the pathogenesis of CVD is well recognized. Inflammation contributes to all phases of atherosclerosis, from fatty streak initiation, growth, and complication of the atherosclerotic plaque to CVD events [7]. Clinical and epidemiological studies across different ethnic groups have substantiated an association between inflammation and insulin resistance [8–10]. In view of these results, a recent report from the Third National Health and Nutrition Examination Survey (NHANES) concluded that elevated levels of inflammatory markers such as CRP and fibrinogen were positively and independently associated with insulin resistance [11]. The underlying mechanisms for the risk factor role of insulin resistance are not fully known. Possible mechanisms include activation of pro-inflammatory signaling pathways, and subsequently insulin resistance has been recognized as a pro-inflammatory condition.
However, the issue whether cardiovascular risk prediction in subjects with or without insulin resistance would vary depending on the degree of inflammation across ethnicity is unresolved. Therefore, the purpose of this study was to investigate the potential synergistic effect of insulin resistance and inflammation in the assessment of coronary artery disease (CAD) risk in two ethnic groups with varying degree of cardiovascular risk factors.
MATERIALS AND METHODS
Subjects
Subjects were recruited from a patient population scheduled for diagnostic coronary arteriography either at Harlem Hospital Center in New York City or at the Mary Imogene Bassett Hospital in Cooperstown, NY. The clinical characteristics of the study population and the study design including inclusion and exclusion criteria have been described previously, and notably, exclusion criteria included use of lipid lowering drugs and hormone replacement treatment [12–14]. Briefly, a total of 648 patients, self-identified as Caucasians (n=344), African American (n=232) or Other (n=72) were enrolled. The present report is based on the findings in 539 of the 576 Caucasian and African American subjects (317 Caucasians, 222 African Americans); 37 subjects were excluded due to incomplete data. The study was approved by the Institutional Review Boards at Harlem Hospital, the Mary Imogene Bassett Hospital, Columbia University College of Physicians and Surgeons, and University of California Davis, and informed consent was obtained from all subjects.
Clinical and biochemical assessments
Blood pressure was measured with a random-zero mercury sphygmomanometer. Waist circumference was calculated as the average of 2 measurements taken after inspiration and expiration at the midpoint between the lowest rib and iliac crest. Participants were asked to fast for 12 hours, and blood samples were drawn in the morning approximately 2 to 4 hours before the catheterization procedure. Serum and plasma samples were separated and stored at −80°C prior to analysis. Concentrations of triglycerides (Sigma Diagnostics, St. Louis, MO), total and HDL cholesterol and glucose (Roche, Sommerville, NJ) were determined using standard enzymatic procedures. HDL cholesterol levels were measured after precipitation of apoB-containing lipoproteins with dextran sulfate. High-sensitivity CRP levels were measured using an enzyme-linked immunoabsorbent assay, standardized according to the World Health Organization First International Reference Standard [15,16]. Fibrinogen levels were measured by the clot-rate method of Clauss [17]. All biochemical assessments were made in duplicate. Homeostasis model assessment – insulin resistance (HOMA-IR) was calculated using the updated model available from the Oxford Centre for Endocrinology and Diabetes [18]. Insulin resistance defined as HOMA-IR ≥3.0.
Coronary angiography
The coronary angiograms were read by 2 experienced readers blinded to patient identity, the clinical diagnosis, and laboratory results. The readers recorded the location and extent of luminal narrowing for 15 segments of the major coronary arteries [19]. In the present study, patients were classified as having CAD if a stenosis of ≥50% was found in at least one of the segments. Patients without CAD were defined as having <50% stenosis in all of the segments. Of the patients without CAD, the majority (80.5%) had <25% stenosis, and of the patients with CAD, 81% had >75% stenosis. A composite cardiovascular score (0–75) was calculated based on determination of presence of stenosis on a scale of 0–5 of the 15 predetermined coronary artery segments.
Statistics
Analysis of data was done with SPSS statistical analysis software (SPSS Inc, Chicago, IL). Results were expressed as means ± SD. All variables were assessed for normality and were log (base e) transformed as appropriate. Thus, triglyceride, CRP, HOMA-IR levels and the cardiovascular score were logarithmically transformed to achieve normal distributions. Proportions were compared between groups using χ2 analysis, and Fisher exact test where appropriate. Group means were compared using Student's t-test. CRP and fibrinogen levels were dichotomized as high and low groups (CRP <3 vs. ≥3 mg/L, fibrinogen < 340 vs. ≥340 mg/dl, respectively) based on common practice from previous studies [20,21]. Age, gender and BMI adjusted Spearman partial correlation coefficients were calculated for markers of insulin resistance (fasting insulin and HOMA-IR) and inflammatory markers (CRP and fibrinogen) across ethnicity. Standardized HOMA-IR quartiles were calculated and HOMA-IR quartiles for Caucasians were defined as: ≤1.24; 1.25–1.99; 2.00–3.49; ≥3.50, and for African Americans were ≤1.19; 1.20–1.89; 1.90–2.89; ≥2.90, respectively. One-way ANOVA was used to compare mean levels of independent variables across HOMA-IR quartile groups, and post hoc analyses were performed by Tukey’ honest significant difference test. Multiple linear regression analyses were applied to assess the association of cardiovascular risk factors with the composite cardiovascular score, an integrated measure of the degree of stenosis of the 15 measured coronary artery segments. All analyses were two-tailed, and P-values less than 0.05 were considered as statistically significant.
RESULTS
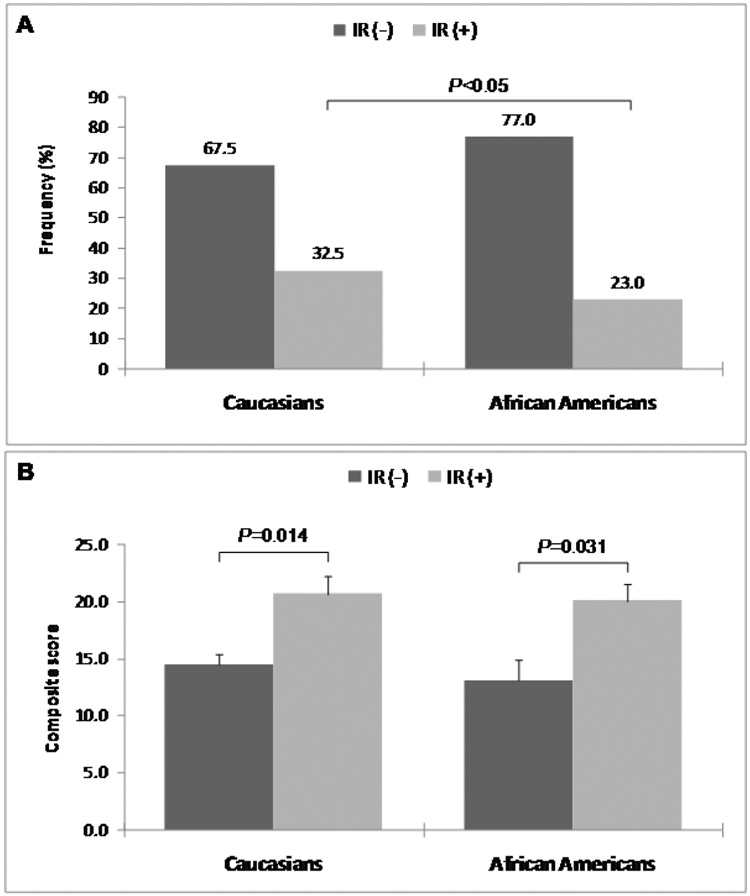
Characteristics of the subjects are shown in Table 1. Compared to Caucasians, African Americans were younger, less obese and had higher diastolic blood pressure. There was no difference in the levels of total and LDL cholesterol or the composite score between the two ethnic groups. African Americans had significantly higher levels of HDL cholesterol, CRP and fibrinogen, and lower levels of triglyceride, glucose, insulin, and HOMA-IR compared to Caucasians. The overall prevalence of smoking, alcohol consumption and postmenopausal women were 23 vs. 46%; 44 vs. 28% and 77 vs. 66%, for Caucasians and African Americans respectively. The frequency distribution curves of HOMA-IR were similar in Caucasians and African Americans (Supplemental Figure). However, the overall prevalence of insulin resistance, as defined by HOMA-IR≥3.0, was higher in Caucasians than in African Americans (32.5% vs. 23.0%, P<0.05) (Figure 1A). An increased degree of CAD (composite cardiovascular score) was seen in subjects with insulin resistance with a similar pattern in Caucasians and African Americans (Figure 1B).
TABLE 1.
Clinical characteristics of Caucasians and Africans Americans undergoing coronary angiography
| Characteristics | Caucasians (n=317) | African Americans (n=222) | P-value |
|---|---|---|---|
| Men/Women | 200/117 | 125/97 | NS |
| Hypertension (%) | 200(60%) | 165(74%) | 0.006 |
| Postmenopausal (%) | 90(77%) | 64(66%) | NS |
| Smoking (%) | 76(24%) | 102(46%) | <0.001 |
| Alcohol (%) | 137(43%) | 61(28%) | <0.001 |
| Anthropometric | |||
| Age (yrs) | 56.5±10.3 | 54.6±9.5 | 0.037 |
| BMI (kg/m2) | 29.6±6.0 | 28.5±6.1 | 0.047 |
| Systolic blood pressure (mm Hg) | 125±18 | 129±19 | NS |
| Diastolic blood pressure (mm Hg) | 75±10 | 78±13 | 0.005 |
| Metabolic | |||
| Total cholesterol (mg/dl) | 199±41 | 196±45 | NS |
| LDL cholesterol (mg/dl) | 124±35 | 124±42 | NS |
| HDL cholesterol (mg/dl) | 40±12 | 49±17 | <0.001 |
| Triglyceride (mg/dl) | 154(115–222) | 105(79–142) | <0.001 |
| Glucose (mg/dl) | 129±61 | 118±45 | 0.015 |
| Insulin (µU/ml) | 15.1(9.2–25.5) | 13.8(8.7–21.5) | 0.028 |
| HOMA-IR | 2.0(1.3–3.5) | 1.9(1.2–2.9) | 0.010 |
| CRP (mg/L) | 2.9(1.4–7.6) | 3.9(1.9–10.7) | 0.004 |
| Fibrinogen (mg/dl) | 328±89 | 387±115 | <0.001 |
| Composite score | 12.3(1.7–26.4) | 8.6(1.5–24.4) | NS |
NS, not significant. Data are means ± SD or for non-normally distributed variables as median (interquartile range). Group means were compared using t-test. Values for triglyceride, insulin, HOMA-IR, CRP and composite score, were logarithmically transformed before analyses.
Figure 1.
Frequency of insulin resistance (A) and composite cardiovascular score in subjects with and without insulin resistance (B) across Caucasian and African American ethnicity.
The clinical characteristics of the study population across HOMA-IR quartile groups are shown in Table 2. As seen in the table, there was a significant increase in BMI, glucose and insulin across HOMA-IR quartiles for both Caucasians and African Americans. Triglyceride levels were higher in Caucasians, whereas HDL-C levels was significantly lower in both ethnic groups across HOMA-IR quartiles. The composite cardiovascular score showed a gradual increase from lowest to highest HOMA-IR quartiles in both Caucasians (8.6–18.5) and African Americans (7.4–14.0), although the difference did not reach statistical significance in either ethnic group. A gradual increase in CRP (1.8–4.5 mg/L) and fibrinogen (308–352 mg/dl) levels across HOMA-IR quartiles were seen in Caucasians. In contrast, the distribution of CRP levels was relatively narrow (3.2–4.6 mg/L) across HOMA-IR quartiles among African Americans.
TABLE 2.
Clinical characteristics of study population across HOMA-IR quartile.
| Ethnicity | HOMA-IR quartile | P-value | |||
|---|---|---|---|---|---|
| Variables | 1 | 2 | 3 | 4 | for trend |
| Caucasians (n) | 79 | 74 | 80 | 84 | |
| Age (yrs) | 55.8±11.1 | 56.4±9.9 | 56.6±10.3 | 56.9±9.8 | NS |
| BMI (kg/m2) | 26.0±3.8 | 28.8±4.7 | 30.7±6.1 | 32.8±6.7 | <0.001 |
| Total cholesterol (mg/dl) | 196±40 | 201±43 | 198±42 | 201±40 | NS |
| LDL cholesterol (mg/dl) | 123±33 | 125±33 | 123±38 | 123±34 | NS |
| HDL cholesterol (mg/dl) | 43±13 | 42±11 | 41±13 | 36±10 | 0.002 |
| Triglyceride (mg/dl) | 128(100–192) | 143(115–205) | 162(112–206) | 202(134–305) | <0.001 |
| Glucose (mg/dl) | 96±10 | 108±26 | 130±46 | 177±87 | <0.001 |
| Insulin (µU/ml) | 7.3(6.5–8.6) | 11.7(10.5–13.0) | 17.4(15.8–20.2) | 38.4(29.9–61.9) | <0.001 |
| CRP (mg/L) | 1.8(0.9–3.7) | 3.0(1.2–6.8) | 2.9(1.6–8.4) | 4.5(2.3–13.8) | <0.001 |
| Fibrinogen (mg/dl) | 308±90 | 321±80 | 327±91 | 352±89 | 0.017 |
| Composite score | 8.6(1.4–22.8) | 10.3(0.0–23.3) | 12.9(3.1–30.4) | 18.5(4.3–32.6) | NS |
| African Americans (n) | 54 | 55 | 56 | 57 | |
| Age (yrs) | 53.1±10.3 | 54.8±8.9 | 54.2±9.7 | 56.3±9.1 | NS |
| BMI (kg/m2) | 24.4±4.7 | 27.5±6.0 | 30.4±5.5 | 31.2±5.9 | <0.001 |
| Total cholesterol (mg/dl) | 185±45 | 194±48 | 200±37 | 204±50 | NS |
| LDL cholesterol (mg/dl) | 111±36 | 124±46 | 130±31 | 133±49 | 0.032 |
| HDL cholesterol (mg/dl) | 55±23 | 48±15 | 47±15 | 47±13 | 0.038 |
| Triglyceride (mg/dl) | 87(72–118) | 105(85–134) | 106(83–146) | 119(83–163) | NS |
| Glucose (mg/dl) | 100±23 | 105±26 | 118±43 | 145±60 | <0.001 |
| Insulin (µU/ml) | 6.0(5.0–7.3) | 11.0(9.6–12.3) | 16.5(15.1–18.3) | 33.3(24.2–54.0) | <0.001 |
| CRP (mg/L) | 3.2(1.2–7.4) | 3.4(1.5–11.3) | 4.2(2.5–12.8) | 4.6(3.0–9.8) | NS |
| Fibrinogen (mg/dl) | 355±84 | 388±115 | 395±121 | 406±131 | NS |
| Composite score | 7.4(1.1–17.5) | 5.7(1.5–21.3) | 8.3(2.9–23.6) | 14.0(3.4–33.3) | NS |
NS, not significant. Data are means ± SD or for non-normally distributed variables as median (interquartile range). Group means were compared using Oneway ANOVA. Values for triglyceride, insulin, CRP and composite score, were logarithmically transformed before analyses. HOMA-IR quartiles for Caucasians were defined as: ≤1.24; 1.25–1.99; 2.00–3.49; ≥3.50, and for African Americans were ≤1.19; 1.20–1.89; 1.90–2.89; ≥2.90, respectively.
Spearman’s partial correlation coefficients between markers of insulin resistance (fasting insulin and HOMA-IR) and inflammatory markers (CRP and fibrinogen) adjusted for age, gender and BMI differed across ethnicity. In Caucasians, both CRP and fibrinogen were positively correlated with fasting insulin levels (r=0.206, P<0.001; and r=0.196, P=0.001, respectively) and with HOMA-IR (r=0.209, P<0.001; and r=0.193, P=0.001, respectively). No such association was seen among African Americans for either fasting insulin or HOMA-IR.
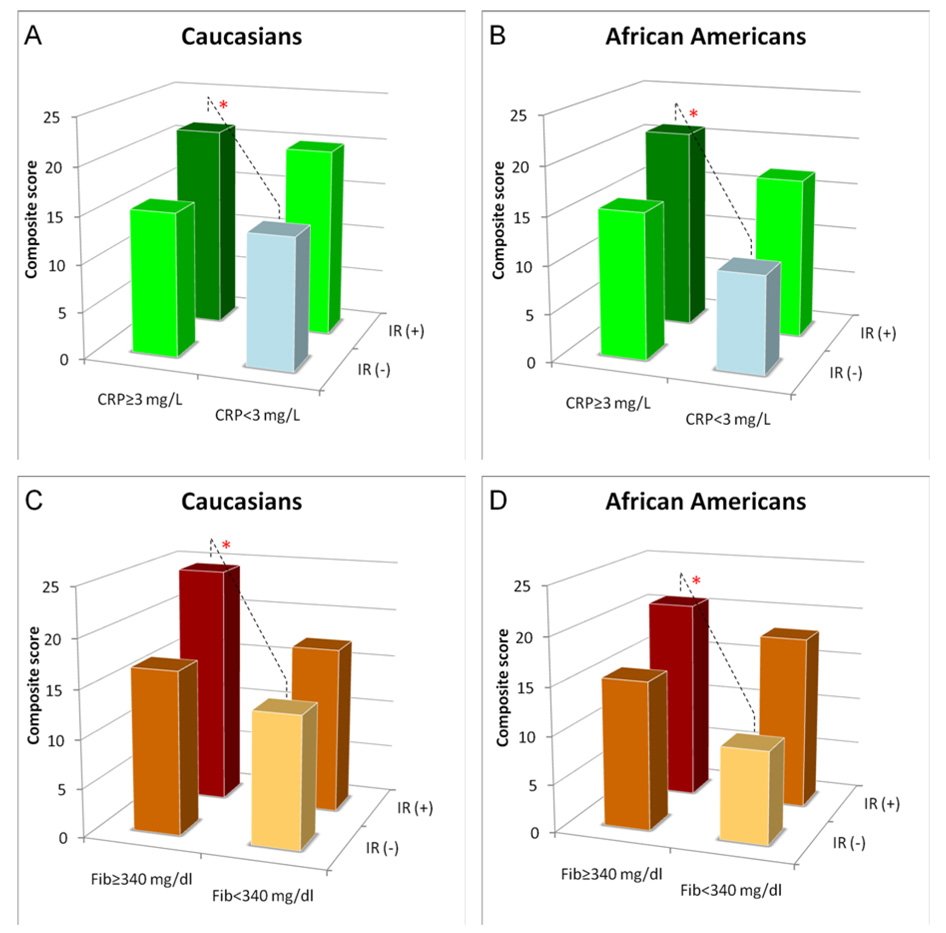
We next analyzed whether presence of inflammation would modulate CAD risk prediction in insulin resistance. In both ethnic groups, subjects with a combination of insulin resistance (HOMA-IR) and high CRP levels (≥3 mg/L) had significantly higher composite cardiovascular score compared to those with no insulin resistance and low CRP levels (<3 mg/L) (21±17 vs. 14±15, P<0.05 and 21±18 vs. 10±12, P<0.05 for Caucasians and African Americans respectively) (Fig 3A and B). Similarly, the composite cardiovascular score was significantly higher in subjects with insulin resistance and high fibrinogen levels (≥340 mg/dl) compared to those with no insulin resistance and low fibrinogen levels (24±19 vs. 14±14, P<0.05 and 21±17 vs. 10±11, P<0.05 for Caucasians and African Americans respectively) (Fig 3 C and D).
To identify the independent contributions of each variable we performed multiple regression analyses. As seen in Table 3, in the overall population, age, gender, smoking, LDL cholesterol, triglyceride, insulin resistance, and diabetes, but not ethnicity or inflammation (CRP and fibrinogen) were associated with the cardiovascular score (r2=0.251, F=13.6, P<0.001). As diabetes status independently predicted CAD, we next did a separate analysis for diabetic and non-diabetic subjects. Among non-diabetic subjects, triglyceride levels and insulin resistance were no longer associated with CAD, while in subjects with diabetes insulin resistance remained an independent predictor of composite cardiovascular score.
TABLE 3.
Multiple regression model of CAD on IR, inflammation and established cardiovascular risk factors in Caucasians and African Americans.
| Ethnicity | Predictors | R2 | β | P-value |
|---|---|---|---|---|
| All | 0.251 | <0.001 | ||
| Ethnicity | −0.01 | NS | ||
| Age, yrs | 0.37 | <0.001 | ||
| Gender, M/F | −0.21 | <0.001 | ||
| Hypertension, ≥130/85 mmHg | 0.05 | NS | ||
| Smoking, Y/N | 0.13 | 0.002 | ||
| Drink alcohol, Y/N | −0.05 | NS | ||
| Waist, cm | −0.04 | NS | ||
| LDL cholesterol, mg/dl | 0.12 | 0.003 | ||
| Triglyceride, mg/dl | 0.10 | 0.019 | ||
| CRP, mg/L | 0.02 | NS | ||
| Fibrinogen, mg/dl | 0.004 | NS | ||
| HOMA-IR | 0.10 | 0.035 | ||
| NIDDM | 0.09 | 0.040 | ||
| Non-NIDDM | 0.251 | <0.001 | ||
| Ethnicity | −0.03 | NS | ||
| Age, yrs | 0.39 | <0.001 | ||
| Gender, M/F | −0.20 | <0.001 | ||
| Hypertension, ≥130/85 mmHg | 0.06 | NS | ||
| Smoking, Y/N | 0.14 | 0.010 | ||
| Drink alcohol, Y/N | 0.002 | NS | ||
| Waist, cm | 0.04 | NS | ||
| LDL cholesterol, mg/dl | 0.11 | 0.039 | ||
| Triglyceride, mg/dl | 0.10 | NS | ||
| CRP, mg/L | 0.03 | NS | ||
| Fibrinogen, mg/dl | −0.009 | NS | ||
| HOMA-IR | −0.02 | NS | ||
| NIDDM | 0.289 | <0.001 | ||
| Ethnicity | 0.02 | NS | ||
| Age, yrs | 0.29 | <0.001 | ||
| Gender, M/F | −0.27 | <0.001 | ||
| Hypertension, ≥130/85 mmHg | −0.01 | NS | ||
| Smoking, Y/N | 0.14 | 0.050 | ||
| Drink alcohol, Y/N | −0.18 | 0.016 | ||
| Waist, cm | −0.17 | 0.028 | ||
| LDL cholesterol, mg/dl | 0.16 | 0.022 | ||
| Triglyceride, mg/dl | 0.14 | NS | ||
| CRP, mg/L | 0.05 | NS | ||
| Fibrinogen, mg/dl | 0.01 | NS | ||
| HOMA-IR | 0.18 | 0.011 | ||
Analyses were performed for logarithmically transformed values for triglyceride, CRP and HOMA-IR.
DISCUSSION
The main novel finding in our study was that for both Caucasians and African Americans, the degree of inflammation impacted on CAD risk prediction in subjects with insulin resistance. For both ethnic groups, compared to subjects without insulin resistance and inflammation, we found a significant difference in CAD degree for subjects with presence of both insulin resistance and inflammation.
Insulin resistance is a major risk factor for type 2 diabetes, and is associated with CVD risk factors such as obesity, hypertension, hypertriglyceridemia and low HDL cholesterol [22]. However, findings from recent prospective studies have questioned whether insulin resistance is involved in the pathogenesis of CVD independently of established risk factors [18,23]. The association of insulin resistance and CVD might be attenuated or potentially confounded by other risk factors. In this study we performed analyses to assess the independent contribution of insulin resistance and inflammation in association with CAD as measured by a composite cardiovascular score. We found higher levels of many established cardiovascular risk factors in both African American and Caucasian subjects with presence of insulin resistance. Notably, in both ethnic groups, the more pronounced increase in such levels was seen for the highest HOMA-IR quartile. Further, both African American and Caucasian subjects with insulin resistance had significantly higher degree of CAD as measured by the composite cardiovascular score.
Studies have shown that inflammatory factors such as CRP and fibrinogen play an important role in the pathogenesis of cardiovascular disease [24–26]. Further, a number of prospective and clinical studies have demonstrated that inflammation is associated with insulin resistance [8,9,27–29]. Results from NHANES indicate that elevated levels of inflammatory markers are positively and independently associated with insulin resistance among the US general population [11,30]. Studies from Europe [31,32] and Asia [8,9] have also demonstrated a positive association of insulin resistance and inflammation. Thus, WOSCOPS (West of Scotland Coronary Prevention Study) showed that CRP predicted the development of type 2 diabetes in middle-aged men independently of established risk factors [31]. Further, a cross-sectional study from France demonstrated that fibrinogen was independently related to insulin resistance [32,33]. Insulin resistance is well recognized as a pro-inflammatory state and the activation of pro-inflammatory pathways contribute to development of insulin resistance. This suggests that a combination of an active, ongoing inflammatory process and presence of insulin resistance might act synergistically in promoting coronary risk. Although many studies have investigated associations of either insulin resistance or inflammation with CVD, it remains to be established whether the degree of inflammation associated with insulin resistance would modify cardiovascular risk prediction or extent of cardiovascular disease across ethnicity. In our study, we found that the presence of inflammation, as detected by increased levels of CRP and fibrinogen, was associated with insulin resistance in Caucasians, but not in African Americans. The reasons for lack of an association of insulin resistance with inflammation among African Americans is unknown and further studies using more in-depth measure are warranted. However, multiple regression analysis showed that insulin resistance was an independent predictor of CAD in both groups. Further, we have demonstrated that a combination of insulin resistance and inflammation resulted in a high risk of CAD in both African Americans and Caucasians. Our results therefore suggest that a pro-inflammatory stimulus may potentiate the risk factor role of insulin resistance, and underscore the importance of a high risk metabolic environment in promoting CAD. In support of this, we recently reported a synergistic risk factor role of CRP and the metabolic syndrome [34].
We acknowledge some of the limitations of this study. Subjects in our study were recruited from patients scheduled for coronary angiography and are likely more typical of a high-risk patient group than the healthy population at large. This may explain the relatively high levels of CRP and fibrinogen among our subjects. However, none of the patients had a history of acute coronary symptoms or surgical intervention within 6 months, arguing against any secondary increase in inflammatory parameters due to an acute CAD. Further, clinical and laboratory parameters were in agreement with differences generally observed between healthy African American and Caucasian populations from other studies. However, additional studies are needed to verify these results other populations, notably, potential confounding effects of life style e.g. exercise, smoking, alcohol consumption and menopausal status.
In conclusion, for both African Americans and Caucasians elevated levels of inflammatory markers were positively associated with insulin resistance. Further, a combination of insulin resistance and inflammation resulted in a high risk of CAD in both ethnic groups. The results suggest that inflammation may potentiate the risk factor role of insulin resistance.
Supplementary Material
Supplemental Figure. HOMA-IR distribution in Caucasians and African Americans.
Figure 2.
Combined effect of insulin resistance and the markers of inflammation - CRP (A, B) and fibrinogen (C, D) on cardiovascular score in Caucasians and African Americans. *: P<0.05 comparison between subjects with IR (HOMA-IR≥3.0)/inflammation (CRP≥3mg/L, Fib≥340 mg/dl) and subjects without IR (HOMA-IR≤3.0)/inflammation (CRP<3mg/L, Fib<340 mg/dl).
ACKNOWLEDGEMENTS
The project was supported by grants 49735 (Pearson, TA, PI) and 62705 (Berglund, L, PI) from National Heart, Lung and Blood Institute. This work was supported in part by the UC Davis CTSC (RR 024146), and Dr. E. Anuurad is a recipient of an American Heart Association Postdoctoral Fellowship (0725125Y).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Martin BC, Warram JH, Krolewski AS, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavaroni I, Bonora E, Pagliara M, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–706. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Pols HA, Stehouwer CD, et al. Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab. 2001;86:4398–4405. doi: 10.1210/jcem.86.9.7873. [DOI] [PubMed] [Google Scholar]
- 5.Carantoni M, Abbasi F, Warmerdam F, et al. Relationship between insulin resistance and partially oxidized LDL particles in healthy, nondiabetic volunteers. Arterioscler Thromb Vasc Biol. 1998;18:762–767. doi: 10.1161/01.atv.18.5.762. [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi N, Shiraishi T, Wada M. Association between C-reactive protein and insulin resistance in a Japanese population: the Minoh Study. Intern Med. 2005;44:542–547. doi: 10.2169/internalmedicine.44.542. [DOI] [PubMed] [Google Scholar]
- 9.Lee IT, Lee WJ, Huang CN, H-H Sheu W. The association of low-grade inflammation, urinary albumin, and insulin resistance with metabolic syndrome in nondiabetic Taiwanese. Metabolism. 2007;56:1708–1713. doi: 10.1016/j.metabol.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Festa A, Hanley AJ, Tracy RP, D'Agostino R, Jr, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–2965. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 12.Paultre F, Pearson TA, Weil HF, et al. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol. 2000;20:2619–2624. doi: 10.1161/01.atv.20.12.2619. [DOI] [PubMed] [Google Scholar]
- 13.Anuurad E, Rubin J, Lu G, et al. Protective effect of apolipoprotein E2 on coronary artery disease in African Americans is mediated through lipoprotein cholesterol. J Lipid Res. 2006;47:2475–2481. doi: 10.1194/jlr.M600288-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Philbin EF, Weil HF, Francis CA, et al. Race-related differences among patients with left ventricular dysfunction: observations from a biracial angiographic cohort. Harlem-Bassett LP(A) Investigators. J Card Fail. 2000;6:187–193. doi: 10.1054/jcaf.2000.9677. [DOI] [PubMed] [Google Scholar]
- 15.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 16.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 17.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 18.Adler AI, Levy JC, Matthews DR, et al. Insulin sensitivity at diagnosis of Type 2 diabetes is not associated with subsequent cardiovascular disease (UKPDS 67) Diabet Med. 2005;22:306–311. doi: 10.1111/j.1464-5491.2004.01418.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller M, Mead LA, Kwiterovich PO, Jr, Pearson TA. Dyslipidemias with desirable plasma total cholesterol levels and angiographically demonstrated coronary artery disease. Am J Cardiol. 1990;65:1–5. doi: 10.1016/0002-9149(90)90017-u. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 23.Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 24.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. [PubMed] [Google Scholar]
- 27.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 29.Festa A, D'Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 30.Meng YX, Ford ES, Li C, et al. Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic US from National Health and Nutrition Examination Survey 1999–2002. Clin Chem. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- 31.Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 32.Mennen LI, Balkau B, Charles MA, D'Hour A, le Mauff JM. Gender differences in the relation between fibrinogen, tissue-type plasminogen activator antigen and markers of insulin resistance: effects of smoking. D.E.S.I.R. Study Group. Data from an Epidemiological Study on Insulin Resistance Syndrome. Thromb Haemost. 1999;82:1106–1111. [PubMed] [Google Scholar]
- 33.Schenk S, Saberi M, Olefsky J. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anuurad E, Tracy R, Pearson T, Berglund L. Comparison of C-Reactive Protein and Metabolic Syndrome as Cardiovascular Risk Factors in African-Americans and European-Americans. Am J Cardiol. 2008 doi: 10.1016/j.amjcard.2008.10.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. HOMA-IR distribution in Caucasians and African Americans.