Abstract
Background
High-dose therapy (HDT) with autologous stem cell transplantation (ASCT) is the standard treatment for patients with chemosensitive relapsed/refractory Hodgkin lymphoma (HL), but this therapy is commonly denied to patients with resistant disease. We explored the utility of HDT and ASCT for chemoresistant HL since there are few established therapies for these patients.
Patients and Methods
Sixty-four chemoresistant HL patients underwent HDT followed by ASCT at our center. Baseline characteristics included: median age = 35 years (range, 14–59 yrs), stage III/IV = 49 (77%), nodular sclerosis histology = 51 (80%), and prior radiation = 32 (50%). Twenty-six patients (41%) received total body irradiation (TBI)-based regimens and 38 (59%) underwent non-TBI conditioning.
Results
The estimated 5-year overall survival (OS) and progression-free survival (PFS) were 31% and 17%, respectively, (median follow-up = 4.2 years). Multivariable analysis only identified year of transplant as independently associated with improved OS (p=.008) and PFS (p=.04), with patients transplanted in later years having better outcome. The probabilities of 3-year PFS for patients transplanted between 1986–1989, 1990–July 1993, August 1993–1999, and 2000–2005 were 9%, 21%, 33%, and 31%, respectively.
Conclusions
These data suggest that HDT and ASCT may result in prolonged remissions and survival for a subset of chemoresistant HL pts, with improved outcomes in patients transplanted more recently.
Introduction
Over 7500 patients are diagnosed with Hodgkin’s lymphoma (HL) each year in the United States with 70–90% achieving cure with initial therapy1–3. Patients with early relapse or primary refractory disease have a much worse prognosis with long-term disease-free survival rates reported at 5–20% if they are treated with conventional salvage chemotherapy or radiotherapy alone 4, 5. Over the past two decades several phase II trials, numerous case series from bone marrow transplant registries, and two phase III trials have established high-dose therapy (HDT) followed by autologous stem cell transplant (ASCT) as the preferred treatment for these high-risk patients in chemosensitive relapse, with cure rates around 40–60%6–14.
Several series have identified chemoresistant disease as a poor prognostic factor for survival in HL patients undergoing HDT and ASCT, and many centers therefore deny this treatment to HL patients that do not achieve at least a partial response to salvage therapy 1, 12, 13, 15–19. The current study reviewed our experience treating patients who have chemoresistant HL with HDT and ASCT to better define the overall efficacy in this setting and to attempt to identify characteristics associated with improved overall survival (OS) and progression-free survival (PFS).
Patients and methods
Patients
Patients were identified from the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) computerized database. Clinical and research records of all HL patients were reviewed and only those with chemoresistant disease were included in this analysis. Patients were considered to have chemoresistant HL if they achieved less than a partial remission (less than 50% reduction in tumor bulk) with the salvage chemotherapy regimen administered immediately preceding conditioning for ASCT. All patients provided informed consent for treatment on transplant protocols approved by the appropriate institutional review board. In addition, separate institutional approval was obtained to retrospectively gather data from patient records and databases.
Study variables
The specific variables evaluated included: age, gender, stage, histology, tumor bulk at transplant, date of transplant, number of extranodal sites (ENS), number of prior chemotherapy regimens, prior radiotherapy (RT), use of total body irradiation (TBI) conditioning, and stem cell source. Tumor bulk was defined based on the greatest long-axis diameter of the largest tumor mass by computerized tomography noted following salvage therapy and before initiation of transplant conditioning. Flurodeoxyglucose (FDG) positron emission tomography (PET) results were gathered prior to transplant when performed but were not used to define chemosensitivity. A prior regimen was defined as one or more cycles of specific chemotherapy or radiotherapy.
Definition of endpoints and statistical analysis
OS and PFS were computed from the date of stem cell infusion to the date of death or progression using the method of Kaplan and Meier20. Events for the endpoint of OS included deaths from any cause, while events for PFS included death from any cause or progressive disease. Univariate and multivariable Cox regression models were fit to examine the association between various factors and the outcomes of OS and PFS. Reported two-sided p-values from regression models were obtained from the Wald test, and no adjustments were made for multiple comparisons.
Results
Baseline characteristics
Between November 1981 and May 2005, we treated 167 patients with HL using HDT and ASCT at our Center with 64 (38%) of these patients meeting the definition of chemoresistant disease. Baseline characteristics for this group are summarized in Table 1. In addition, front-line chemotherapy consisted of ABVD in 25 patients, an ABVD/MOPP hybrid in 22 patients, MOPP in 5 patients, Stanford V in 3 patients, other ABVD hybrids in 2 patients, and a combination of other therapies in 7 patients. Twenty-nine patients (45%) were refractory to their initial chemotherapy regimen. The median number of prior regimens was 2 (range 1–5). Thirty-two patients (50%) had received prior RT and all patients had a Southwest Oncology Group (SWOG) performance status of 0 or 1 prior to transplant. Eight patients had PET imaging prior to conditioning, of these 7 had FDG-avid foci of active disease.
Table 1.
Baseline Characteristics of the 64 patients with chemoresistant Hodgkin’s lymphoma who underwent autologous stem cell transplantation.
| Characteristic |
Number |
Percent |
|---|---|---|
| Median age at transplant | 35 years | Range 14–59 |
| Male sex | 37 | 58 |
| Histology | ||
| Nodular Sclerosis | 51 | 80 |
| Mixed Cellularity | 11 | 17 |
| Lymphocyte Predominant | 2 | 3 |
| Number of extranodal sites | ||
| 0 | 31 | 48 |
| 1 | 22 | 34 |
| 2 | 10 | 16 |
| 3 | 1 | 2 |
| Median number of prior regimens | 2 | Range 1–5 |
| Prior radiation therapy | 32 | 50 |
| Stage at transplant | ||
| I–II | 15 | 23 |
| III | 17 | 27 |
| IV | 32 | 50 |
| Bulk at transplant | ||
| < 5 cm | 42 | 66 |
| ≥ 5 cm | 22 | 34 |
| Preparatory regimens | ||
| Chemotherapy and TBI | 26 | 41 |
| Chemotherapy only | 38 | 59 |
| Stem cell source | ||
| Bone marrow | 24 | 38 |
| Peripheral blood | 39 | 61 |
| Both | 1 | 2 |
Regimens immediately prior to transplant to which the HL was deemed resistant included ICE in 14 patients, DHAP in 9 patients, MOPP/ABVD in 7 patients, CED in 7 patients, ABVD in 4 patients, MOPP in 3 patients, and COPP in 2 patients with the remaining 18 patients receiving 17 various other regimens. When all prior regimens were assessed for responses in each patient, 40 (63%) patients were found to be resistant to anthracyclines, 33 (52%) to high-dose alkylating agents (cyclophosphamide [CY] and ifosfamide), 33 (52%) to platinum (cisplatin and carboplatin), and 19 (30%) to high-dose cytarabine.
Conditioning regimens and stem cell sources
Twenty-six patients (41%) were treated with TBI-based preparatory regimens, including 23 receiving TBI/CY/etoposide (VP16) and three patients treated with CY/TBI. The remaining 38 patients (59%) were treated with chemotherapy-only regimens including: busulfan (Bu)/melphalan (Mel)/thiotepa (TT) (n=18), carmustine/CY/VP-16 (n=12), Bu/CY (n=3), carmustine/VP-16/cytararbine/Mel (n=3), Bu/TT (n=1), and Bu/CY/Lithium (n=1). Twenty-four patients received autologous bone marrow, 39 received autologous mobilized peripheral blood stem cells (PBSC), and 1 received both.
Overall and progression-free survival
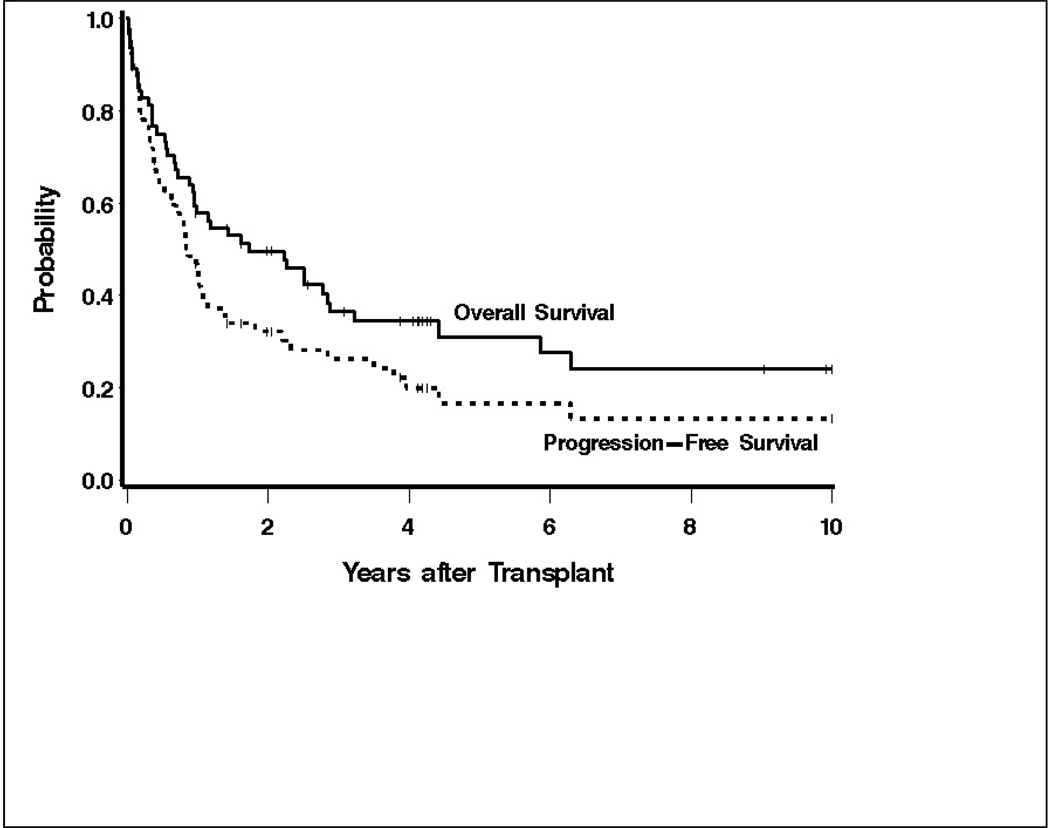
The 5-year OS and PFS were estimated to be 31% (95% confidence intervals [CI] 18–44%) and 17% (95% CI 6–27%) respectively, with median follow-up of 4.2 years (range 1–16.2 years) among surviving patients (Figure 1). At the last follow up, 21 patients (33%) were alive and 13 (20%) were alive and progression-free. Thirty of the 43 deaths during this study period followed relapse. Non-relapse causes of death included: respiratory failure (5 patients), infections (2 patients), AML/MDS (2 patients), hepatic toxicity (1 patient), renal failure (1 patient), and unknown causes (2 patients). Therapy for the 8 patients surviving following relapse included non-myeloablative allogeneic transplantation (3 patients), chemotherapy alone (2 patients), chemotherapy and radiation (1 patient), radiation alone (1 patient) and surgical resection (1 patient). Of the 7 patients with positive PET imaging pre-transplant, 4 are alive and progression-free from 5 months-2.1 years after transplant, 1 died of AML without relapse of HL at 4 yrs, and 2 suffered progression at 5 and 9 months, respectively. The one patient with a negative PET is alive and progression-free at 2.6 years.
Figure 1.
Overall and progression-free survival of 64 patients with chemoresistant Hodgkin’s lymphoma following high-dose therapy and autologous stem cell transplantation.
Univariate and multivariable analyses
Hazard ratios (HR) for mortality from univariate analyses included: mixed cellularity (MC) histology, 0.42 (95% CI 0.16–1.07, p=.07 vs. all other); prior radiation, 2.05 (95% CI 1.11–3.79, p=.02); male gender, 1.05 (95% CI 0.57–1.92, p=.88); use of TBI, 0.65 (95% CI 0.34–1.21, p=.17); use of bone marrow-derived stem cells, 1.85 (95% CI 1.01–3.38, p=.05); ≥ 2 extranodal sites (ENS) of disease, 2.10 (95% CI 1.03–4.29, p=.04); and tumor bulk ≥ 5 cm, 1.39 (95% CI 0.75–2.56, p=.30). The HR for death for stage III disease was 0.94 (95% CI 0.39–2.27, p=.89) and for stage IV was 1.13 (95% CI 0.54–2.38, p=.75) when compared to patients with stage I/II disease. Age at transplant (p=.30) and number of cycles of previous chemotherapy (p=.79) were not statistically significantly associated with mortality in univariate models when evaluated as a continuous linear variable. In contrast, the year of transplant was correlated with mortality, with improved survival occurring following more recent transplants (p=.008). After adjusting for year of transplant, none of the other factors were statistically significantly associated with OS, while year of transplant remained associated with survival even after adjusting for the other factors (Table 2). Similar results were observed when PFS was evaluated, with no other factors statistically significantly associated with outcome after adjusting for year of transplant with later transplants being associated with improved PFS (p=.04). The presence of >1 ENS of disease approached statistical significance for PFS when adjusted for transplant year (HR=1.89 (95% CI 0.92–3.88), p=.08).
Table 2.
Multivariable analysis of associations of baseline factors with mortality adjusted for year of transplant in chemoresistant Hodgkin’s lymphoma patients undergoing high-dose therapy and autologous transplantation.
| Baseline Factor |
Year of transplant |
|||
|---|---|---|---|---|
| Factor | Hazard Ratio | 95% CI | p-value | p-value* |
| Stem cell source | ||||
| PBSC | 1 | |||
| BM | 0.77 | 0.28–2.17 | .63 | .05 |
| Histology | ||||
| Non-MC | 1 | .01 | ||
| MC | 0.44 | 0.17–1.13 | .09 | |
| Prior radiation | ||||
| No | 1 | .06 | ||
| Yes | 1.58 | 0.80–3.12 | .18 | |
| Gender | ||||
| Female | 1 | .007 | ||
| Male | 1.09 | 0.60–2.00 | .78 | |
| Use of TBI | ||||
| No | 1 | .02 | ||
| Yes | .76 | 0.40–1.44 | .40 | |
| Number of prior regimens* | -- | -- | .74 | .007 |
| Disease Bulk | ||||
| <5cm | 1 | .01 | ||
| ≥5cm | 0.95 | 0.49–1.86 | .88 | |
| Extranodal sites | ||||
| 0–1 | 1 | .02 | ||
| ≥2 | 1.73 | 0.84–3.58 | .14 | |
| Stage | ||||
| I–II | 1 | |||
| III | 1.82 | 0.64–5.14 | .26 | .006 |
| IV | 1.22 | 0.57–2.60 | .60 | |
| Age* | ||||
| -- | -- | .70 | .01 | |
P-value for year of transplant modeled as a continuous linear variable after adjusting for the corresponding factor.
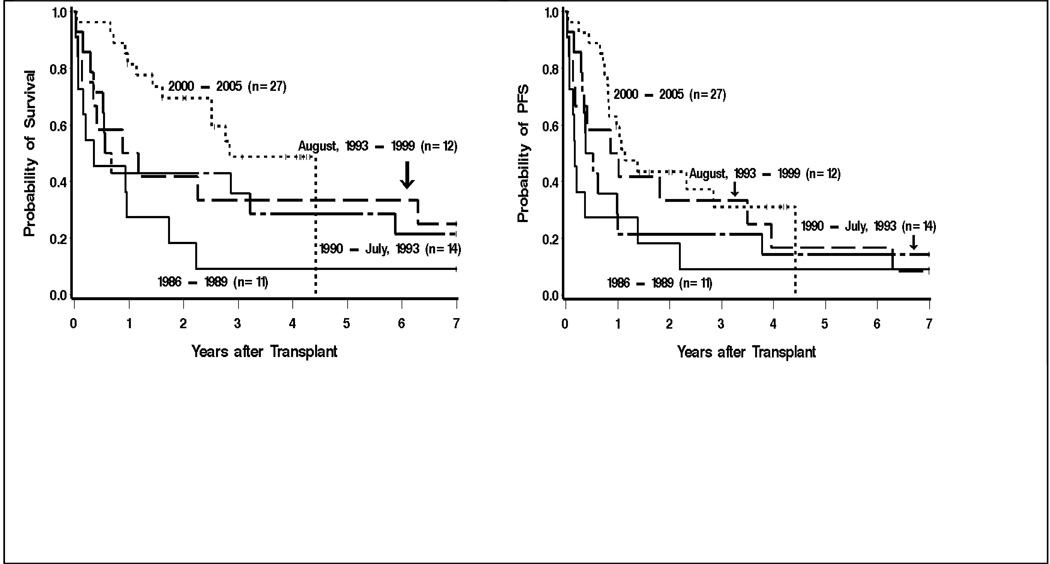
Because of the strong correlation between era of transplant and source of stem cells (all recent transplants were done with PBSC) we evaluated the impact of year of transplant within and between the PBSC (August 1993–2005) and bone marrow (1986-July 1993) periods (Table 3). Overall survival appeared to improve over time within both the bone marrow and peripheral blood stem cell source eras as did PFS though the only statistically significant differences were between the most recent and most distant time periods (OS p=.003, PFS p=.02). The absolute risk of relapse in general decreased over time, though demonstrating statistically significant differences were likely limited by the small number of events. The estimated probabilities of 3-year survival for patients transplanted between 1986–1989, 1990-July 1993, August 1993–1999, and 1999–2005 were 9%, 36%, 33%, and 49%, respectively (Figure 2). Likewise, the estimated 3-year PFS for patients transplanted between 1986–1989, 1990-July 1993, August 1993–1999, and 1999–2005 were 9%, 21%, 33%, and 31%, respectively (Figure 2).
Table 3.
Hazard ratios from a multivariable analysis of outcomes stratified by year of transplant and stem cell source.
| Hazard Ratios |
|||||
|---|---|---|---|---|---|
| Transplant era | Stem Cell Source | Mortality | Mortality or Relapse | Relapse | Non-relapse Mortality |
| 2000–2005 (n=27) | PBSC | 1 | 1 | 1 | 1 |
| July 1993–1999(n=12) | PBSC | 1.85 (p=.16) | 1.44 (p=.34) | 1.42 (p=.43) | 1.45 (p=.63) |
| 1990-August 1993(n=14) | BM* | 1.92 (p=.12) | 1.64 (p=.19) | 1.95 (p=.11) | 0.90 (p=.90) |
| 1986–1989(n=11) | BM | 3.56 (p=.003) | 2.46 (p=.02) | 2.17 (p=.11) | 2.98 (p=.13) |
One patient in this era received PBSC.
Figure 2.
Overall survival (left) and progression-free survival (right) of 64 chemoresistant Hodgkin’s lymphoma patients treated with high-dose therapy and autologous transplantation stratified by year of transplant.
Discussion
Chemoresistant HL continues to represent a challenge for clinicians. Several series have repeatedly identified chemoresistance prior to ASCT as a major adverse prognostic factor for HL and, thus, many patients whose disease displays this feature are currently denied ASCT 1, 12, 13, 15–19. Our study sought to specifically evaluate the outcomes of these patients following ASCT and potentially define a cohort that could attain prolonged remission and survival. Despite chemoresistance, the estimated the 5-year OS and PFS were 31% and 17%, respectively, suggesting a meaningful clinical benefit may be afforded to about one in six patients.
Multivariable analysis identified the year of transplant as a major predictor of survival with patients transplanted more recently having improved outcomes. The estimated OS and PFS at 3 years was 49% and 31%, respectively, among patients treated in the last 5 years as compared to a 9% 3-year OS and PFS in the earliest time period. Assessment of the impact of both transplant era and stem cell source on outcome suggested that the improvement cannot be attributed solely to the use of PBSC, as the OS and PFS among BM vs. PBSC patients are similar in the two time windows that span the change in stem cell source (1989–1999). One could postulate that the etiology of these improvements is likely multifactorial including both more effective supportive care as well as refined patient selection, though the retrospective nature of this study was not able to confirm this hypothesis. For example, differentiating outcomes based on the exact percent response or progression in these chemoresistant cases following salvage therapy was not within the scope of this project, but may have had a major impact on outcome. It is possible that those transplanted in the most recent era had a larger proportion of patients with minor responses to cytoreductive chemotherapy than in earlier times. Improvements in OS could also arise from improved post-relapse therapies, as 3 individuals that attained prolonged survival after relapse in our series underwent non-myeloablative allogeneic transplantation and 3 others had their disease effectively controlled with gemcitabine-based chemotherapy.
Most importantly, our recent data can be more useful than more distant historical results in counseling those with chemoresistant HL about prognosis after ASCT as one-third of the patients in our series transplanted since August 1993 were expected to be alive and progression-free at 3 years. Similarly, these findings would also suggest that caution should be used when comparing outcomes from novel transplant regimens in HL to historical data, as superior results may not be solely due to a change in the intervention being studied. Along these lines, we were not able to directly compare transplant outcomes in this patient population to best available non-transplant therapies, leaving definitive conclusions to future prospective randomized comparisons.
Despite the fact that some of our patients with resistant HL attained prolonged PFS, new effective approaches are critically needed in this population as the vast majority will still relapse and succumb to their disease. Recent data suggest that gemcitabine-based regimens can induce remissions in patients that have not responded to more traditional salvage therapies 21, 22. Such secondary pretransplant responses could both improve outcomes via tumor debulking and better identify disease that may respond to high-dose therapies. Individuals with resistant disease could be considered for investigational strategies using novel agents such as anti-CD30 monoclonal antibodies or transplants via reduced intensity allografting or tandem ASCT 23–28. Future directions should also attempt to take advantage of the radiosensitivity of HL by means of radioimmunotherapy-based transplant-conditioning regimens, as has been successfully employed in non-Hodgkin’s lymphoma 29–32. Furthermore, optimized patient selection with the use of PET may also yield refined risk stratification and determine who may benefit the most from ASCT and who should proceed directly to other therapies. Prior series have suggested that lymphoma patients with CT-defined chemoresistant disease but PET negativity may have comparable outcomes to those with CT-defined responsive disease following ASCT 33, 34. Unfortunately, the small number of individuals that had pre-transplant PET imaging in our series limited our ability to test this hypothesis. Nevertheless, 7 of 8 patients did have PET scanning that revealed residual disease with only 2 of these 7 experiencing relapse thus far.
Until novel effective therapies are developed the outcome for individuals with chemoresistant HL will be continue to be limited. Our data suggest that high-dose therapy and ASCT could be considered for and will likely benefit a significant subset of patients with HL, with nearly 1 in 3 patients transplanted in the last decade achieving prolonged remissions and survival. Longer follow up of the recently transplanted patients and results from other similar cohorts of transplanted and non-transplanted patients will be needed to confirm these findings.
Acknowledgments
Grant Support: P01CA44991, K23CA85479, K08CA095448, SCOR grant 7040 from the Leukemia and Lymphoma Society, The Lymphoma Research Foundation, a Damon Runyon Career Development Award (JMP), and The Westlund Foundation.
References
- 1.Moskowitz C. An update on the management of relapsed and primary refractory Hodgkin's disease. Semin Oncol. 2004;31(2 Suppl 4):54–59. doi: 10.1053/j.seminoncol.2004.02.016. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15124135. [DOI] [PubMed] [Google Scholar]
- 2.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. Available from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.nejm.org/content/scripts/search/page.asp%3fvolume=339&page=1506. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15661684. [DOI] [PubMed] [Google Scholar]
- 4.Longo DL, Duffey PL, Young RC, Hubbard SM, Ihde DC, Glatstein E, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin's disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10(2):210–218. doi: 10.1200/JCO.1992.10.2.210. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1732422. [DOI] [PubMed] [Google Scholar]
- 5.Santaro A, Bonfante V, Bonadonna G. Salvage Chemotherapy with ABVD in MOPP-Resistant Hodgkin's disease. Ann Intern Med. 1982;96(2):139–143. doi: 10.7326/0003-4819-96-2-139. [DOI] [PubMed] [Google Scholar]
- 6.Yahalom J, Gulati S. Autologous bone marrow transplantation for refractory or relapsed Hodgkin's disease: the Memorial Sloan-Kettering Cancer Center experience using high-dose chemotherapy with or without hyperfractionated accelerated total lymphoid irradiation. Ann Oncol. 1991;2 Suppl 2:67–71. doi: 10.1007/978-1-4899-7305-4_11. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2049322. [DOI] [PubMed] [Google Scholar]
- 7.Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin's disease: importance of disease status at transplant. J Clin Oncol. 1993;11(4):704–711. doi: 10.1200/JCO.1993.11.4.704. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8478664. [DOI] [PubMed] [Google Scholar]
- 8.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. doi: 10.1016/0140-6736(93)92411-l. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8096958. [DOI] [PubMed] [Google Scholar]
- 9.Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin's disease. Blood. 1997;89(3):814–822. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9028312. [PubMed] [Google Scholar]
- 10.Josting A, Katay I, Rueffer U, Winter S, Tesch H, Engert A, et al. Favorable outcome of patients with relapsed or refractory Hodgkin's disease treated with high-dose chemotherapy and stem cell rescue at the time of maximal response to conventional salvage therapy (Dex-BEAM) Ann Oncol. 1998;9(3):289–295. doi: 10.1023/a:1008283909959. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9602263. [DOI] [PubMed] [Google Scholar]
- 11.Nademanee A, O'Donnell MR, Snyder DS, Schmidt GM, Parker PM, Stein AS, et al. High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin's disease: results in 85 patients with analysis of prognostic factors. Blood. 1995;85(5):1381–1390. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7858268. [PubMed] [Google Scholar]
- 12.Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89(3):801–813. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9028311. [PubMed] [Google Scholar]
- 13.Sweetenham JW, Taghipour G, Milligan D, Blystad AK, Caballero D, Fassas A, et al. High-dose therapy and autologous stem cell rescue for patients with Hodgkin's disease in first relapse after chemotherapy: results from the EBMT. Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997;20(9):745–752. doi: 10.1038/sj.bmt.1700963. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9384476. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12086759. [DOI] [PubMed] [Google Scholar]
- 15.Voillant LDE, Flesch M, et al. Role of chemoresistance prior to autologous bone marrow transplantation for Hodgkin's disease. Nouv Rev Fr Hematol. 1994;36(6):423–430. [PubMed] [Google Scholar]
- 16.Argiris A, Seropian S, Cooper DL. High-dose BEAM chemotherapy with autologous peripheral blood progenitor-cell transplantation for unselected patients with primary refractory or relapsed Hodgkin's disease. Ann Oncol. 2000;11(6):665–672. doi: 10.1023/a:1008396525292. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10942053. [DOI] [PubMed] [Google Scholar]
- 17.Sureda A, Arranz R, Iriondo A, Carreras E, Lahuerta JJ, Garcia-Conde J, et al. Autologous stem-cell transplantation for Hodgkin's disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395–1404. doi: 10.1200/JCO.2001.19.5.1395. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11230484. [DOI] [PubMed] [Google Scholar]
- 18.Czyz J, Dziadziuszko R, Knopinska-Postuszuy W, Hellmann A, Kachel L, Holowiecki J, et al. Outcome and prognostic factors in advanced Hodgkin's disease treated with high-dose chemotherapy and autologous stem cell transplantation: a study of 341 patients. Ann Oncol. 2004;15(8):1222–1230. doi: 10.1093/annonc/mdh304. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15277262. [DOI] [PubMed] [Google Scholar]
- 19.Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. doi: 10.1093/annonc/mdi119. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15737986. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–1079. doi: 10.1093/annonc/mdm090. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17426059. [DOI] [PubMed] [Google Scholar]
- 22.Santoro A, Bredenfeld H, Devizzi L, Tesch H, Bonfante V, Viviani S, et al. Gemcitabine in the treatment of refractory Hodgkin's disease: results of a multicenter phase II study. J Clin Oncol. 2000;18(13):2615–2619. doi: 10.1200/JCO.2000.18.13.2615. Available from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.jco.org/cgi/content/abstract/18/13/2615. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Yao Z, Zhang Z, Garmestani K, Goldman CK, Ravetch JV, et al. Effective therapy for a murine model of human anaplastic large-cell lymphoma with the anti-CD30 monoclonal antibody, HeFi-1, does not require activating Fc receptors. Blood. 2006;108(2):705–710. doi: 10.1182/blood-2005-11-4607. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16551968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderlini P, Saliba R, Acholonu S, Okoroji GJ, Donato M, Giralt S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin's disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35(10):943–951. doi: 10.1038/sj.bmt.1704942. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15806128. [DOI] [PubMed] [Google Scholar]
- 25.Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365(9475):1934–1941. doi: 10.1016/S0140-6736(05)66659-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15936420. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12(2):172–183. doi: 10.1016/j.bbmt.2005.09.009. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16443515. [DOI] [PubMed] [Google Scholar]
- 27.Brice P, Divine M, Simon D, Coiffier B, Leblond V, Simon M, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin's disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10(12):1485–1488. doi: 10.1023/a:1008343823292. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10643540. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111(4):1848–1854. doi: 10.1182/blood-2008-01-127118. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18079362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopal AK, Gooley TA, Maloney DG, Petersdorf SH, Eary JF, Rajendran JG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102(7):2351–2357. doi: 10.1182/blood-2003-02-0622. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12750161. [DOI] [PubMed] [Google Scholar]
- 30.Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106(8):2896–2902. doi: 10.1182/blood-2005-03-1310. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(3):461–467. doi: 10.1200/JCO.2005.05.117. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15534357. [DOI] [PubMed] [Google Scholar]
- 32.Schnell R, Dietlein M, Staak JO, Borchmann P, Schomaecker K, Fischer T, et al. Treatment of refractory Hodgkin's lymphoma patients with an iodine-131-labeled murine anti-CD30 monoclonal antibody. J Clin Oncol. 2005;23(21):4669–4678. doi: 10.1200/JCO.2005.09.098. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16034043. [DOI] [PubMed] [Google Scholar]
- 33.Svoboda J, Andreadis C, Elstrom R, Chong EA, Downs LH, Berkowitz A, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38(3):211–216. doi: 10.1038/sj.bmt.1705416. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16770314. [DOI] [PubMed] [Google Scholar]
- 34.Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102(1):53–59. doi: 10.1182/blood-2002-12-3842. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12609836. [DOI] [PubMed] [Google Scholar]