Abstract
The unique therapeutic value of dendritic cells (DCs) for the treatment of allergy, autoimmunity and transplant rejection is predicated upon our ability to selectively deliver antigens, drugs or nucleic acids to DCs in vivo. Here we describe a method for delivering whole protein antigens to DCs based on carbohydrate-mediated targeting of DC-expressed lectins. A series of synthetic carbohydrates was chemically-coupled to a model antigen, ovalbumin (OVA), and each conjugate was evaluated for its ability to increase the efficiency of antigen presentation by murine DCs to OVA-specific T cells (CD4+ and CD8+). In vitro data are presented that demonstrate that carbohydrate modification of OVA leads to a 50-fold enhancement of presentation of antigenic peptide to CD4+ T cells. A tenfold enhancement is observed for CD8+ T cells; this indicates that the targeted lectin(s) can mediate cross-presentation of antigens on MHC class I. Our data indicate that the observed enhancements in antigen presentation are unique to OVA that is conjugated to complex oligosaccharides, such as a high-mannose nonasaccharide, but not to monosaccharides. Taken together, our data suggest that a DC targeting strategy that is based upon carbohydrate-lectin interactions is a promising approach for enhancing antigen presentation via class I and class II molecules.
Keywords: carbohydrates, dendritic cells, glycoconjugates, targeting, tolerance
Introduction
In recent years the dual immunological function of dendritic cells (DCs) has become increasingly appreciated.[1,2] On the one hand, DCs are the most potent of the professional antigen-presenting cells (APCs) in initiating immune responses to pathogens. In the absence of infection, however, DCs playa second, crucial role in maintaining peripheral tolerance by regulating the numbers and states of self-reactive T cells. Given the importance of DCs in orchestrating the human immune system along these two paths, strategies that can selectively access these different functions will greatly advance immunotherapy and facilitate the design of more rational, mechanistic vaccines. The selective modulation of DC function in the steady state, however, will depend upon our ability to specifically target DCs/DC subsets.
One approach to accessing DCs in vivo consists of targeting DC-specific surface receptors with ligand–antigen or ligand mimetic–antigen conjugates that deliver targeted antigens to the antigen-processing/presentation machinery of DCs via receptor-mediated endocytosis. One such manifestation of this strategy involves chemically coupling an antibody against a defined DC surface receptor to an antigen of interest. Antibody targeting of the DC integrin CD11c, for example, has been reported to dramatically improve the kinetics and quality of antibody responses against a model antigen in mice.[3] At the other end of the immunological spectrum of responses, Steinman and colleagues targeted the C-type lectin DEC-205 with anti-DEC-205-antigen conjugates and demonstrated the DC-mediated induction of T cell tolerance.[4,5] These studies demonstrate that targeting DCs in vivo can lead to defined immunological outcomes, and serve to underscore the fact that targeting different receptors on the same cell type can produce dramatically different results.
While antibodies can offer unparalleled specificity for their cognate antigens, as therapeutic agents they have the distinct disadvantages of being expensive to produce and potentially immunogenic in many patients. In addition to these issues, there might be circumstances in which more versatile chemistries are required for the formation of an antigen conjugate than those that are offered by the antibody's protein sequence. Thus, a need exists for developing robust, therapeutically useful methods of accessing DCs in vivo.
Here we explore the use of synthetic carbohydrate structures as an alternative to the antibody-based targeting of DCs. DCs express a number of cell surface lectins of the C-type class that recognize carbohydrate ligands that are appended to glycoproteins, and in most cases that were studied, these lectins mediate the adsorptive uptake of bound ligand.[6,7] DC-expressed lectins represent a particularly interesting class of surface receptors to target, not only for their restricted expression patterns, but also in light of recent evidence that demonstrates the participation of lectins in certain cell-signalling events.[8-10] Thus, targeting DC-expressed lectins not only opens the possibility of accessing DC's uniquely efficient antigen-processing and presentation capabilities, but also offers the opportunity to modulate DC signalling networks. With carbohydrate chemistry at a stage where biologically useful quantities of complex oligosaccharide can be routinely prepared[11] and with glycan microarrays that can aid in the determination of highly specific lectin–ligand interactions,[12,13] the requisite tools are in place to pursue a DC-targeting strategy that is founded upon lectin–carbohydrate interactions.
Results and Discussion
Coupling of lectin-targeting carbohydrates to a model antigen
To examine the ability of high-mannose oligosaccharide–antigen conjugates to engage DC surface receptors such as lectins and lead to the presentation through the MHC class I and class II pathways, we prepared a series of ovalbumin (OVA) conjugates that bear structures 1−6 (Scheme 1). We chose OVA as a model antigen because it is known to be presented on H-2Kb MHC class I molecules to CD8+ T cells and I-Ab MHC class II molecules to CD4+ T cells, including TCR transgenic OT-I and OT-II T cells. The carbohydrate structures were chosen for DC targeting on the basis of their recognition by the DC lectin, DC-SIGN and the anticipated specificity of DC-SIGN's murine homologues.[14,15]
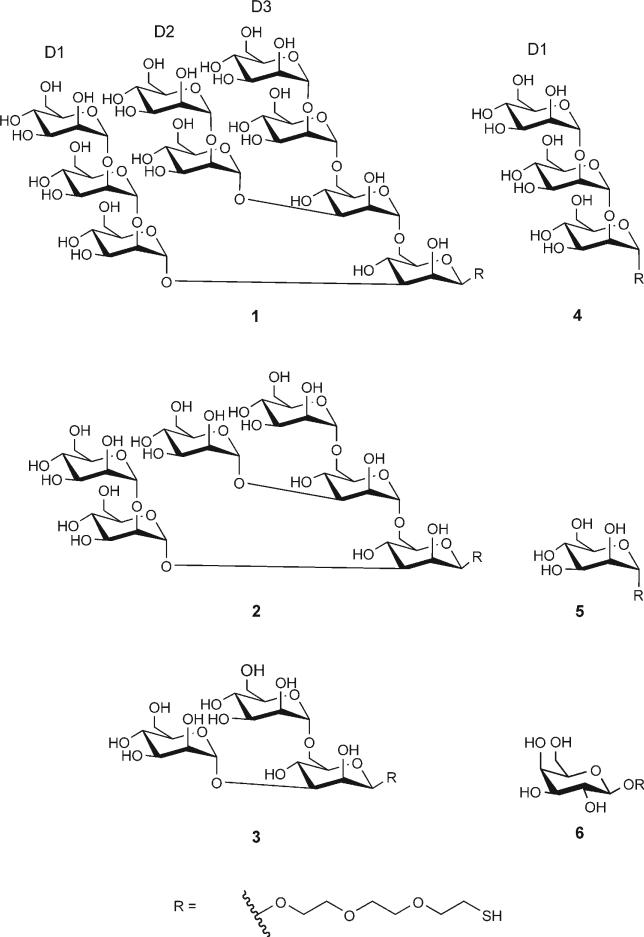
Scheme 1.
Synthetic analogues of the high-mannose oligosaccharide (Man)9(GlcNAc)2 that were used in the preparation of ovalbumin conjugates for dendritic cell targeting. These structures were chosen for DC targeting on the basis of their recognition by the DC lectin, DC-SIGN. The panel of structures consists of three branched oligosaccharides (1−3), a linear trisaccharide 4 that is derived from the D1 arm of the high-mannose nonasaccharide and two monosaccharides, mannose 5 and galactose 6. Conjugation of each structure was made possible by the incorporation of a thiol-bearing linker (shown as “R” here) The stereochemistry at the reducing end is as shown; it was β for structures 1−3 and 6, and it was α for structures 4 and 5.
To generate conjugates, OVA was modified with the hetero-bifunctional crosslinker SMCC to introduce maleimide functional groups; incubation with thiol-bearing saccharides 1−6 led to the formation of carbohydrate–OVA conjugates (Figure 1 A). In naturally occurring glycoproteins, high-mannose oligosaccharides are conjugated to the polypeptide chain via a β-manno-side linkage. Thus, in the high-mannose derivatives 1−3, a β-linkage is used to conjugate the synthetic oligosaccharide to OVA. For structures 4 (derived from the D1 arm of high-mannose 1) and 5, the stereochemistry at the reducing end is α. The stereochemistry of the control monosaccharide galactose 6 is in the β configuration at the anomeric carbon.
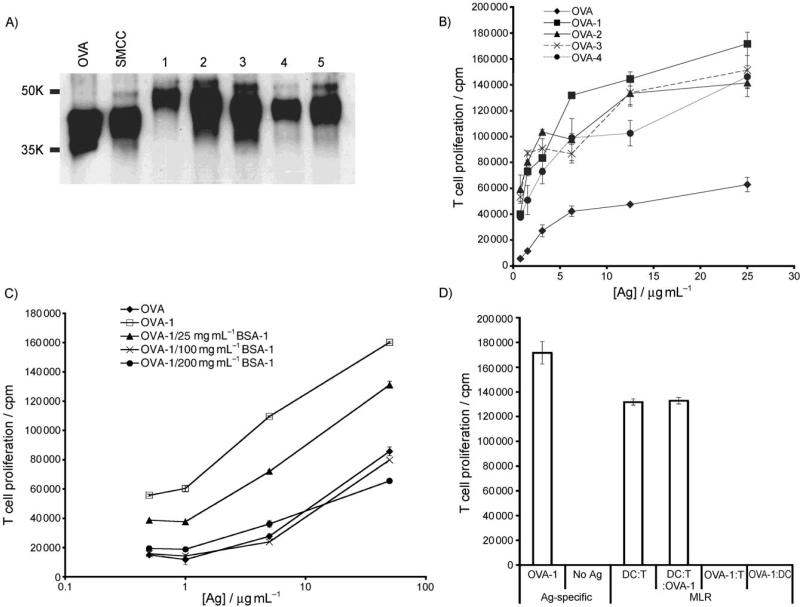
Figure 1.
Carbohydrate modification of OVA leads to enhanced presentation to antigen-specific T cells. A) OVA, SMCC-activated OVA and carbohydrate modified OVA were resolved by SDS-PAGE electrophoresis and immunoblotted with anti-OVA polyclonal antibody to reveal changes in molecular weight as a function of carbohydrate addition. B) Oligosaccharide modification of OVA elicits stronger presentation to OT-II T cells than unmodified OVA. Unfractionated OT-II splenocytes (3 × 105 per well) were incubated with graded doses of OVA or oligosaccharide-modified OVA for 84 h with [3H]thymidine (1 μCi) added for the last 12 h. Thereafter [3H]thymidine incorporation levels were measured and plotted. C) Observed enhancements in antigen presentation of (OVA)-1 to T cells is carbohydrate dependent. Splenocytes were incubated with antigen as in (B) ±(BSA)-1 at various doses; incubations were performed for 72 h, and [3H]thymidine (1 μCi) was added for the last 12 h. (D). Nonasaccharide 1 does not directly stimulate T cell proliferation. A mixed leukocyte reaction (MLR) was carried out with purified C57BL/6 CD11c+ DC and purified Balb/c CD4+ T cells at a 1:5 DC:T cell ratio (OVA)-1 (50 μg mL−1) for 72 h; [3H]thymidine (1 μCi) was added for the last 12 h. Control incubations were DCs + (OVA)-1 (50 μg mL−1) and T cells (–DCs) + (OVA)-1 (50 μg mL−1).
Carbohydrate modification of a model antigen leads to enhanced presentation to CD4+ and CD8+ T cells in vitro
We assessed the ability of these OVA-carbohydrate conjugates to enhance antigen uptake by DCs in comparison to unmodified OVA by incubating graded doses of conjugate or OVA with unfractionated splenocytes, which contain T cells, B cells, DCs and macrophages that were isolated from OT-II (CD4+ T cell) transgenic mice. The ability of DCs to present antigenic peptides that are derived from targeted proteins was measured as a function of OT-II T cell proliferation in response to those presented peptides, as quantified by [3H]-thymidine incorporation during T cell division. All of the conjugates that were tested resulted in a reproducible enhancement of antigen uptake and presentation to T cells when compared to unmodified OVA over the same dosage range (Figure 1 B). Among the structures that were tested, the nonasaccharide 1 led to the greatest enhancement of T cell proliferation compared to OVA.
To verify that this enhancement was due to the carbohydrate moiety that is attached to OVA, we incubated splenocytes with conjugate (OVA)-1 in the presence and absence of an unrelated protein, bovine serum albumin (BSA) that was also modified with 1. As peptides that are derived from processed BSA cannot be recognized by the transgenic OT-II cells, competitive inhibition of OVA-1 uptake by (BSA)-1 leads to the expected diminution of OT-II proliferation (Figure 1 C), and this indicates that the uptake of (OVA)-1 is due to the appended oligosaccharide. Co-incubation of unmodified BSA did not affect the (OVA)-1-promoted enhancements (not shown). To ensure that our (OVA)-1 conjugate was not directly activating T cells and inducing their proliferation, we added (OVA)-1 to purified T cells in the absence of APCs and to a mixed leukocyte reaction between DCs and allogeneic T cells (Figure 1 D). In both cases we observed that conjugate addition did not directly induce T cell proliferation or augment the mixed leukocyte reaction. These results demonstrate that (OVA)-1 promotes T cell proliferation in an antigen-specific fashion, and that the peptide antigen that drives this proliferation must be presented by DC (or other APC). Thus, the increase in T cell proliferation that was observed in DC:T cell cocultures that were incubated with (OVA)-1 vs. OVA (Figure 1 B) is due to increased presentation of antigenic peptides by DCs and not by direct stimulation of T cells by (OVA)-1.
Accessing the transporter of antigenic peptides (TAP) and thereby achieving cross-presentation of antigen by DCs to MHC class I-restricted CD8 T cells leads to efficient stimulation of these T cells, and in the steady state, their deletion from the T cell repertoire.[4] To determine if the receptor that promotes (OVA)-1 uptake can process proteins through the MHC class I pathway, we incubated (OVA)-1 or OVA with unfractionated splenocytes that were isolated from OT-I (CD8+ T cell) mice. The T cells from transgenic OT-I mice recognize an eight amino acid residue sequence (SIINFEKL) that is derived from OVA and can therefore be used to gauge the efficiency of DC presentation of MHC class I antigenic peptides. We observed a moderate (tenfold) increase in the efficiency of MHC class I presentation with (OVA)-1 compared to OVA alone (Figure 2 A). Inhibition of this enhancement is again achieved by co-incubation with (BSA)-1, and as shown for OT-II T cells, (OVA)-1 does not appear to achieve this enhancement via direct activation of T cells.
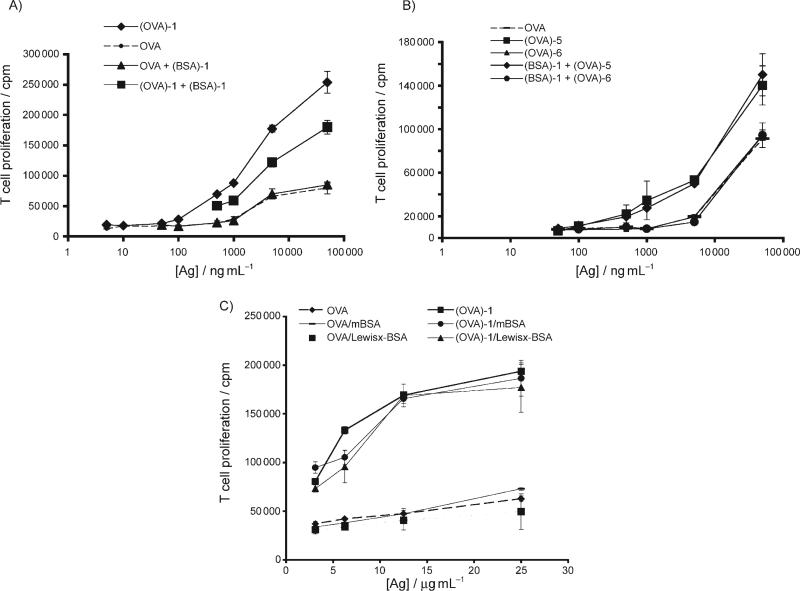
Figure 2.
Nonasaccharide 1-promoted presentation via MHC class I pathway for presentation to CD8+ T cells. A) (OVA)-1 enhances presentation of antigenic peptides to CD8+ T cells in a dose and carbohydrate-dependent fashion. Unfractionated OTI splenocytes (3 × 105 per well) were incubated with graded doses of OVA ±(OVA)-1 (50 ng ml−1) or (OVA)-1 ±(BSA)-1 (10 μg mL−1). Incubations were performed for 72 h, and [3H]thymidine (1 μCi) was added for the last 12 h. B) The monosaccharide mannose 5 targets a different receptor than 1. OT-II splenocytes were incubated with graded doses of monosaccharide-modified OVA ±(BSA)-1 and T cell proliferation was measured as in (A). C) The receptor-mediated uptake of (OVA)-1 has a different binding profile than that which was observed for DC-SIGN. Unfractionated splenocytes were incubated with OVA or (OVA)-1 ±mBSA or Lewisx–BSA (each at 100 μg mL−1). Incubations were performed for 72 h, and [3H]thymidine (1 μCi) was added for the last 12 h. All values reported are the mean of triplicate measurements; Ag: antigen.
Antigen presentation is not enhanced by monosaccharide conjugatesand isnot blocked by Lewisx conjugates
In an effort to determine if the increased efficiencies of antigen presentation obtained via (OVA)-1 could be attained via simple monosaccharide-antigen conjugates, we repeated our splenocyte incubations with (OVA)-5 and (OVA)-6 (Figure 2 B). We found that the monosaccharide mannose 5 only weakly enhanced presentation to CD4+ T cells while galactose 6 did not promote increased presentation at all compared to unmodified OVA. Interestingly, coincubation of (OVA)-5 with (BSA)-1 did not inhibit the uptake of (OVA)-5; this indicates that the monosaccharide 5 and the more complex 1 are likely to interact with different DC receptors.
Our previous microarray analysis of glycan binding by DC-SIGN revealed that DC-SIGN can recognize both complex, branched mannans like 1 and 2 as well as dense arrays of linear oligosaccharides and simple monosaccharides. In addition, carbohydrate profiling by another group has revealed that DC-SIGN can recognize non-sialylated Lewis blood group antigens (e.g., Lewisx).[16] Five murine homologues of DC-SIGN have been described; one homologue, CIRE is expressed exclusively in CD8α−CD4+ and CD8α−CD4− DCs,[17] and another homologue, mSIGNR1, is expressed on marginal zone macrophages and is capable of recognizing both high-mannose oligosaccharides and Lewis antigens.[18] Although recent studies have demonstrated that murine CIRE is not a functional homologue of DC-SIGN,[19] we wished to address the possibility that a lectin with a DC-SIGN-like binding profile might be mediating the recognition and uptake of (OVA)-1. To this end, we incubated splenocytes with (OVA)-1 in the presence or absence of mannose-derivatized BSA (mBSA) or Lewisx-modified BSA (Lewisx-BSA) and measured the ability of these conjugates to inhibit the uptake of (OVA)-1 (Figure 2 C). Neither mBSA nor Lewisx-BSA appeared to significantly impair endocytosis and presentation of (OVA)-1 to OT-II T cells; this suggests that neither of these conjugates can serve as a ligand for the receptor-mediated uptake of (OVA)-1, and that the binding specificity of the (OVA)-1 receptor is likely to be distinct from that of human DC-SIGN. To directly examine CIRE's ability to recognize high-mannose oligosaccharides specifically, we performed cell-based endocytosis experiments with Chinese hamster ovary cells that stably express CIRE. When incubated with fluorescently-labelled OVA-1 and analyzed by flow cytometry, we were not able to demonstrate binding or endocytosis of OVA-1 by CIRE (not shown). Another candidate lectin that we examined was the mannose receptor; using commercially available antibodies against this receptor, we could not demonstrate expression of this receptor on purified ex vivo splenic or lymph node DCs, or on in-vitro-generated bone-marrow DCs (not shown). This is consistent with what has been reported by others.[20] Together, these data suggest that the mannose receptor, at least in vitro, is not responsible for recognition of OVA-1.
Enhanced T cell proliferation is due to conjugate uptake by CD11c+ DCs exclusively
To confirm that the increased T cell proliferation that is observed with (OVA)-1 was due to the selective uptake of this conjugate by DCs and not B cells or macrophages, we prepared purified CD11c+ DCs from wild-type C57BL/6 mice and the OVA-specific T cells from transgenic OT-II mice, and repeated the in vitro antigen presentation assays as described above. We did not pursue the response of CD8+ OT-I T cells due to the less dramatic enhancement by conjugates when compared to CD4+ T cells). A parallel titration series of (OVA)-1 and unmodified OVA revealed that CD11c+ DCs are highly efficient at capturing and presenting (OVA)-1 compared to soluble OVA, even at concentrations as low as 16 nm (Figure 3 A). By comparison, it required > 50-fold more unmodified OVA to obtain similar levels of T cell proliferation. When B cells and macrophages were used as APCs in place of purified DCs, the resulting T cell responses were negligible (Figure 3 C); this confirms that OVA-1 does not directly activate T cells, and demonstrates that CD11c+ DCs are the main APC that mediates the uptake and presentation of (OVA)-1 to OT-II T cells.
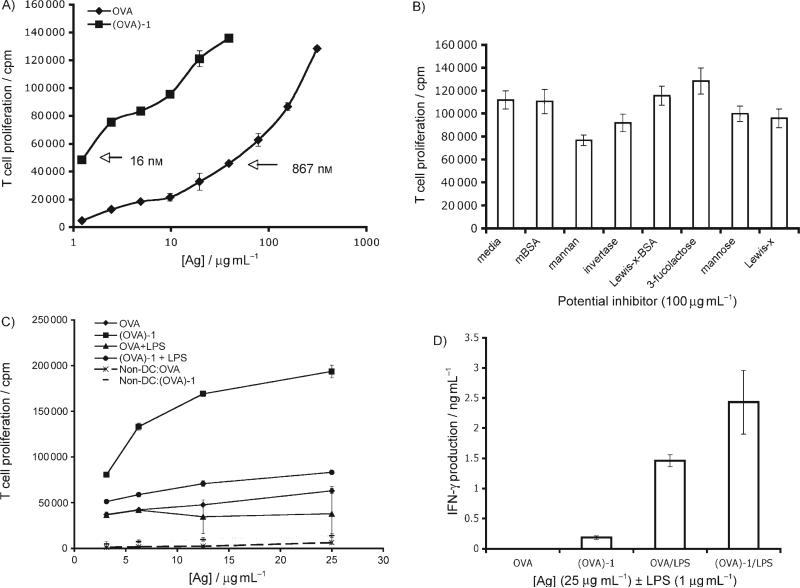
Figure 3.
CD11c+ DCs are the main APC-presenting nonasaccharide 1-targeted antigen. A) Purified CD11c+ DCs efficiently present (OVA)-1 to purified CD4+ T cells. 1.5 × 104 Splenic CD11c+ DCs that were purified from C57BL/6 mice and 3.0 × 104 purified OT-II T cells were incubated with OVA (starting concentration 300 μg mL−1) or (OVA)-1 (starting concentration 25 μg mL−1) in graded doses for 84 h, [3H]thymidine (1 μCi) was added for the last 12 h. B) The receptor-mediating uptake of (OVA)-1 preferentially binds complex mannans. Purified CD11c+ DCs and OT-II T cells were incubated with (OVA)-1 (25 μg mL−1) ±each potential inhibitor (100 μg mL−1). T cell proliferation was determined as in (A). Note: OVA incubation alone gave cpm counts of 30 000. C) Toll-like-receptor-induced DC maturation significantly decreases uptake and presentation of (OVA)-1 to T cells. CD11c+ DCs and OT-II T cells were incubated with OVA or (OVA)-1 ±lipopolysaccharide (1 μg mL−1). Also included are all “non-DCs” (macrophages and B cells) that were obtained during the purification of the DCs. T cell proliferation was determined as in (A). D) Targeting (OVA)-1 to DCs leads to a more vigorous T cell response to pro-inflammatory conditions. Supernatants from (C) were collected at 48 and 72 h and were measured for IFN-γ by ELISA; the values presented are from the 72 h time point. All measurements were performed in triplicate.
In our previous attempts to inhibit (OVA)-1 internalization with mBSA and Lewisx–BSA (Figure 2 C) we might have missed the inhibitory effect of these molecules due to their potential effects on other non-DC cell types that are present in the splenocyte population. To address this possibility and to try other potential inhibitors of (OVA)-1 internalization, we repeated the inhibition experiment with purified DCs and T cells (Figure 3 B). As in our previous experiments, neither mBSA nor Lewisx–BSA were inhibitory. Soluble Lewisx had marginal effects on (OVA)-1 presentation by DCs, even when it was used at a > 300-fold excess over (OVA)-1. Likewise, a 1000-fold excess of soluble mannose only reduced conjugate presentation by 10%. More inhibitory were the complex mannan that was derived from Saccharomyces cerevisiae (31%), and the high-mannose-bearing glycoprotein invertase (18%). It is of interest that the Saccharomyces-derived mannan was not more inhibitory in this proliferation assay than mannan, which consists of many branched mannose-based oligomers. This could be due to the heterogeneity of structures in the preparation or differences in spacing of individual oligosaccharides that are appended to OVA vs. those that are present in mannan. The inhibition of (OVA)-1 presentation with an invertase concentration of 0.5 μm compared to the 0.55 μm mannose concentration that was required to achieve similar levels of inhibition further underscores the specificity that is exhibited by the high-mannose oligosaccharide receptor on DCs. Incubation with the common milk oligosaccharide, 3-fucosyllactose (3-FL) had no inhibitory action, but rather a weak stimulatory effect on T cell proliferation (14%) was observed.
No effect of OVA-1 on activation of DC and T cell inflammatory cytokines
To determine whether (OVA)-1 could modulate inflammatory pathways in DCs and T cells we compared the effects of adding lipopolysaccharide (LPS) to OVA-1 on the production of cytokines by T cells that had been exposed to DCs that present antigen. An in vitro presentation assay was performed in which lipopolysaccharide (LPS), a potent agonist of the toll-like receptor 4 signalling pathway,[21] was added to graded doses of OVA or (OVA)-1 (Figure 3 C). These experiments demonstrated that (OVA)-1 does not modify production of IL-10, IL-6 or IFN-γ in DC-T cultures. In agreement with what has been reported for the macrophage mannose receptor[22] and the DC-SIGN murine homologue CIRE, wherein TLR agonists led to dramatically decreased mRNA production for each lectin, we observed a significant decrease (60%) in the presentation of (OVA)-1 to T cells as a result of the TLR-mediated DC maturation. In the case of unmodified OVA, TLR activation led to a 30% decrease in antigen presentation to OT-II T cells (Figure 3 C). Despite the significant diminution of (OVA)-1 presentation by DCs upon LPS stimulation, targeting with nonasaccharide 1 was still better than unmodified OVA. This implies that antigen capture of (OVA)-1 by DCs prior to full maturation is considerably more efficient than unmodified OVA. This fact is further strengthened by analysis of pro-inflammatory IFN-γ production by responding OT-II T cells (Figure 3 D), where we observed an average of 40% less IFN-γ production by T cells that were responding to OVA vs. (OVA)-1.
Both CD8α+ and CD8α− DC subsets can present carbohydrate-modified antigens
Having established that DCs are the main APC that are capable of capture, processing, and presentation of (OVA)-1, our next objective was to establish if any particular subset of DCs was responsible for this activity. In the mouse spleen, there are at least three subsets of conventional DCs that are defined by their expression of the cellular antigens CD8 and CD4, namely CD8α+CD4−, CD8α−CD4+, and CD8α−CD4−.[23,24] Many functional differences among these subsets have been described, and it has been argued that the CD8α+ subset might be solely responsible for maintaining peripheral tolerance, while the CD8α− subset induces immunity to captured antigen.[25] To determine if the high-mannose receptor was restricted to a particular subset, we used a fluorescence-activated cell sorter to separate DCs into their respective CD8α+ and CD8α− subsets, and tested each subset for its ability to present OVA and (OVA)-1 to OT-II T cells (Figure 4 A). We were surprised to learn that while OVA is only weakly presented to T cells by the individual subsets, (OVA)-1 is presented by each subset, and the CD8α+ subset is approximately twofold more efficient than the CD8α− subset. Thus, we conclude that both DC subsets express a receptor that is capable of binding nonasaccharide 1 and mediating uptake of of antigens that have been modified with this oligosaccharide.
Figure 4.
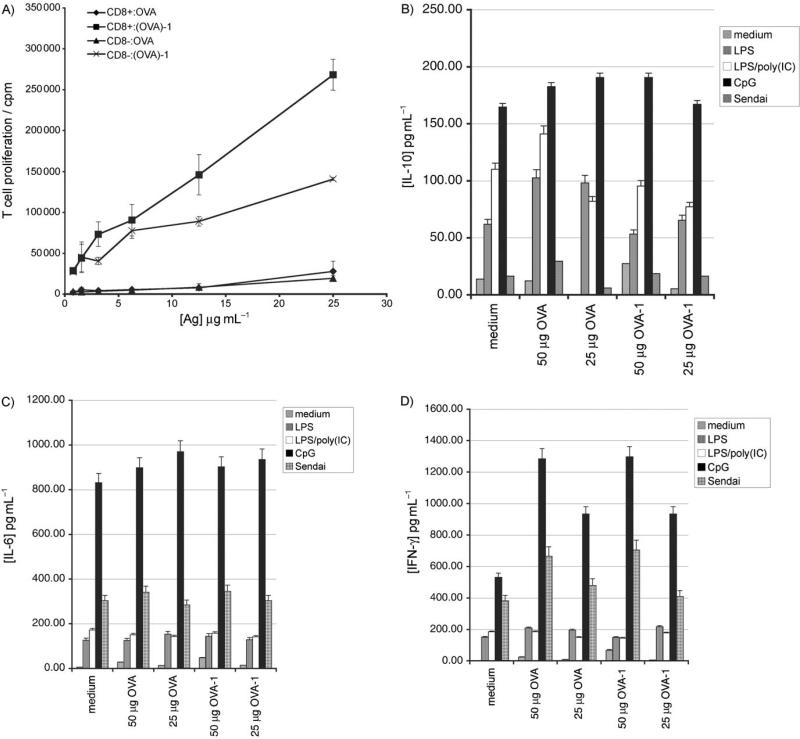
A) Both DC subsets can present (OVA)-1. CD11c+ DCs were stained with antibody against CD8α and I-Ab MHC class II molecules and sorted into their respective CD8α+ and CD8α− populations. 2 × 104 Cells of each subtype were incubated with 1 × 105 purified OT-II T cells with graded doses of OVA or (OVA)-1 for 84 h; [3H]thymidine (1 μCi) was added for the last 12 h. All data points were performed in triplicate. B) Nonsaccharide 1 does not inhibit or potentiate pro-inflammatory signalling networks relative to unmodified OVA. 105 Purified CD11c+ splenic DCs were incubated with media, OVA or (OVA)-1 at two different doses ±various TLR stimuli for 24 h. Supernatants were collected and analyzed for IL-10. LPS was used at 1 μg mL−1; poly(IC), 25 μg mL−1; Sendai Virus, 40 hemagglutination units (H.U.) mL−1; CpG1826, 2 mm. C) IL-6, as in (B). D) IFN-γ, as in (B). Values represent the mean of duplicate measurements.
Targeted Receptor engagement does not contribute to nor diminish pro-inflammatory pathways
Both DC-SIGN[9] and Dectin-1[10] have recently been described as having an immunomodulatory role in certain inflammatory situations, primarily through the upregulation of the anti-inflammatory cytokine IL-10. We wished to determine if the receptor that is targeted by OVA-1 had similar effects on initiated pro-inflammatory pathways, as this would suggest that successful targeting of this receptor could enable in vivo manipulation of the DC phenotype. Purified CD11c+ splenic DCs were incubated in the presence or absence of various pro-inflammatory stimuli with either medium, two doses of non-modified ovalbumin or two doses of OVA-1 and cytokine levels were measured at 7 and 24 h (Figure 4 B–D). We did not see any evidence for increased IL-10 production in cells that were pulsed with OVA-1 relative to umodified OVA, nor did we see any augmentation of any of the pro-inflammatory cytokines that were analyzed; this suggests that the receptor that is targeted by nonasaccharide 1 mediates efficient absorptive uptake, but is not likely to participate in pro-inflammatory signalling networks.
Conclusions
In the present study we have investigated the use of synthetic carbohydrates as a means of delivering antigens to DCs with the aim of broadening the scope of methods that are available for targeting DCs in vivo. Based on the established specificity of the C-type lectin, DC-SIGN, and the anticipated specificity of its murine homologues, we designed and synthesized a series of structures that are derived from the high-mannose oligosaccharide (Man)9(GlcNAc)2, wherein the (GlcNAc)2 moiety was replaced by a thiol-terminated ethylene glycol linker for ease of chemical conjugation to the antigens of interest. The synthetic installation of the thiol linker allowed for facile conjugation of the saccharides to a model antigen, ovalbumin. By taking advantage of antigen-specific transgenic T cells that are capable of recognizing defined amino acid sequences that are derived from ovalbumin, we were able to successfully demonstrate that these synthetic oligosaccharides target both CD8α+ and CD8α− DCs in vitro, and lead to significant enhancements in antigen-specific CD4+ T cell responses relative to non-modified ovalbumin. In addition, we found moderate enhancement of CD8+ T cell activation by conjugates by using unfractionated splenocytes that contain DCs and other antigen-presenting cells. The addition of these conjugates directly to T cell cultures (in the absence of DCs or other APCs) does not promote T cell proliferation, nor do these conjugates appear to enhance or inhibit the mixed leukocyte reaction. Furthermore, co-incubation of the nonasaccharide-ovalbumin conjugate, (OVA)-1, with various Toll-like receptor stimuli had no discernible effect on the pro-inflammatory pathways that are induced by these agents. Taken together, our results suggest that the synthetic oligosaccharide–protein conjugates target a carbohydrate-specific receptor(s) that are present on murine DCs and lead to enhanced antigen presentation to T cells by accessing the antigen-processing/presenting pathways in DCs more efficiently than non-targeted antigen, and not by directly inducing pro-inflammatory pathways in DCs or the responding T cells.
Lectin–carbohydrate interactions have long been appreciated as a means of achieving cell and tissue-specific delivery of drugs, nucleic acids and proteins. Targeting the asialoglycoprotein receptor and the mannose receptor in the liver, for example, are early examples of this approach.[26,27] With the recent identification/enumeration of the C-type lectins of the immune system,[6] and the finding that some of these newly identified lectins appear capable of modulating cytokine production by DCs in certain settings, targeting lectins of the innate immune system, and DCs in particular, appears to offer a novel way of shaping the ensuing adaptive immune response. The aforementioned DEC-205 antibody-targeting work is a powerful example of how targeting DC-expressed lectins in vivo gives one fine control over the polarization (Th1, Th2 or Treg) and strength of the T cell response.[4,5]
Here we have presented our initial attempts at achieving a non-antibody based approach to targeting DC-expressed lectins. Carbohydrates offer an attractive alternative to antibody-based targeting methods for a number of reasons. Not only is it possible to generate large amounts of synthetic structure (gram to kilogram quantities) but one also has complete control over the composition of the end structure such that one can vary the conjugation chemistries, or vary the individual carbohydrate monomers to achieve improved binding by the receptor of interest or increased resistance to enzymatic degradation. Furthermore, carbohydrates have the distinct advantage of being non-immunogenic and are therefore unlikely to elicit an immune response during the course of a clinical regimen. One distinct disadvantage of such an approach, however, stems from the fact that lectin–carbohydrate interactions tend to be rather weak, with Kds are in the μm to mm range, and usually require multivalent clustering of carbohydrates to attain higher affinities (e.g., Kds in the nm range).[28] Another potential drawback of a carbohydrate–lectin-targeting approach is the potential overlap of carbohydrate specificities among the many lectins that are expressed in the immune system and tissues of the body. Overlapping carbohydrate specificities among different non-DC lectins will decrease the overall efficiency of DC targeting by limiting the amount of antigen that can reach DCs in vivo. Indeed, in our preliminary attempts to extend the present work to an in vivo setting we found that the enhancements in antigen-specific T cell activation/proliferation we observed in vitro were significantly diminished in vivo. Under certain conditions, however, co-administration of a molar excess of the neoglycoprotein mannose–BSA along with (OVA)-1 led to increased OVA-specific T cell responses; this suggests that the mannose-BSA—by saturating mannose-specific lectins that are expressed by cells other than DCs, such as macrophages and endothelial cells—could enable more efficient capture of (OVA)-1 by DCs.
Based upon our work here, it is our opinion that success in manipulating carbohydrate–lectin interactions for DC-specific targeting can be predicated from the biochemical identification of the DC lectin to be targeted, and an iterative process of optimizing the targeting carbohydrate ligand's structure to achieve high-affinity and specific interactions with the receptor, which is similar to how one would perform a structure–activity study of a small-molecule enzyme inhibitor, for example. By doing this, one can increase the likelihood of successfully targeting that receptor in a complex in vivo setting. Here, using a limited set of structures, we have shown that it is possible to achieve significant enhancements in antigen delivery to DCs, and we believe that further refinements/derivatives of these structures would lead to improvements in in vivo efficacy.
Experimental Section
Mice
6−12-Week-old females were used in all experiments and were maintained under specific pathogen-free conditions. C57BL/6 mice were purchased from Taconic Farms (Albany, NY, USA) and B6.Ly5.2/Cr mice were purchased from NCI-Frederick (Frederick, MD, USA). OT-I and OT-II mice were bred in an in-house facility; OT-I mice were genotyped by analyzing the expression of Vα2Vβ5.1/5.2 by FACS. All animal procedures were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
Reagents
Antibodies to CD45.2 (104) and all other surface markers (Vα2/B20.1, CD8α/53−6.7, I-Ab/AF6−120.1) were purchased from BD Biosciences (San Jose, CA, USA), as was streptavidin–APC. Horseradish peroxidase (HRP)-conjugated rabbit anti-ovalbumin was purchased from Research Diagnostics (Concord, MA, USA). Mannose, invertase, mannan, and mannose-BSA, BSA, ovalbumin and lipopolysaccharide (LPS) were all purchased from Sigma Chemical. 3-Fucosyllactose, Lewisx and Lewisx-BSA were purchased from Dextra Laboratories (Reading, UK). Immunostimulatory CpG1826 oligonucleotide was purchased from Invivogen (San Diego, CA, USA). Poly(IC) was purchased from Amersham Biosciences. Sendai virus was purchased from Charles River Laboratories (Wilmington, MA, USA). Synthetic OT-II OVA peptide [KISQAVHAAHAEINEAG] was prepared by the Peptide Core Facility at Massachusetts General Hospital (Charlestown, MA, USA) and purified by HPLC to > 95% purity. CHO cells that stably express the lectin CIRE were the kind gift of Irene Caminschi (Walter and Eliza Hall Institute, Australia).
Preparation of carbohydrate structures
Sulfhydryl-containing ethylene-glycol-derivatized oligosaccharides were prepared by analogous methods to those described in the literature.[29] Structural confirmation was achieved with NMR, ESI mass spectrometry, and MALDI-TOF mass spectrometry.
Carbohydrate modification of ovalbumin
Sulfosuccinimidyl 4-[maleimidomethyl]-cyclohexane-1-carboxylate (1.9 mg, Sulfo-SMCC, Pierce Endogen) was dissolved in dimethylformamide (88 μL) and added to ovalbumin (5 mg) in PBS (315 mL). The reaction solution was mixed for 1 h at room temperature. Maleimide-activated OVA was purified from nonreacted Sulfo-SMCC by gel filtration on a NAP-25 desalting column (Amersham) that had been preequilibrated in PBS. The OVA fractions were collected and mixed with thiol-containing oligosaccharides (0.6 μmol per structure) that had been previously reduced with tris-(carboxyethyl)phosphine hydrochloride (1 equiv). This reaction proceeded for 12 h at room temperature with constant mixing. Modified OVA was purified from excess oligosaccharide by multiple rounds of centrifugal ultrafiltration with Vivaspin 10 000 MW cut-off cartridges (Vivascience, Edgewood, NY, USA). Purified protein was lyophilized and stored at −20 °C until use.
Confirmation of conjugate formation was achieved by SDS-PAGE gel electrophoresis (8% gel) followed by Western blot with HRP-conjugated rabbit anti-ovalbumin polyclonal antibody.
Cell culture and proliferation assays
Pooled inguinal lymph nodes were dissociated in 10% Fetal Bovine Serum RPMI (supplemented with 2 μm l-glutamine) and either mechanically dissociated between two glass slides, or to improve the overall DC yield, incubated in the presence of collagenase (Boehringer) and EDTA (25 min at 37 °C/5% CO2) to further dissociate the tissue. For antigen presentation assays with whole splenocytes, isolated spleens were injected with collagenase/EDTA, gentlyteased apart and incubated as described above. Splenocytes were cultured in 96-well round-bottom plates (3 × 105 cells/0.2 mL). In antigen presentation assays that used purified DCs and T cells, DCs were isolated from the spleens of C57BL/6 mice after collagenase/EDTA treatment by labeling the DCs with Miltenyi anti-CD11c+ microbeads (according to the Miltenyi Biotec protocol), and by separating labeled DCs from non-DCs by the application of a magnetic field. Similarly, purified CD4+ T cells were obtained from the spleens of OT-II transgenic mice by using Dynal anti-CD4 magnetic beads. Purified DCs and T cells were seeded onto 96-well plates round-bottom plates at a 1:2 DC/T cell ratio unless stated otherwise in the Figures.
Cell sorting
To obtain sufficient numbers of DC subsets for antigen presentation and cytokine analysis, we injected C57BL/6 mice with a Flt3-ligand-secreting tumor cell line[30] to promote the expansion of DCs; 14 days after administration the mice were sacrificed, and the spleens were isolated. DCs were purified from the spleens by positive selection with anti-CD11c microbeads as described above, and stained with FITC-conjugated anti-I-Ab and Cy-chrome-conjugated anti-CD8α. The cells were then sorted on a MoFlo fluorescence-activated cell sorter (Dakocytomaton, Fort Collins, CO, USA) into CD8α+I-Ab+ and CD8α−I-Ab+ populations. DCs from each subset were used in antigen proliferation assays as described above.
Cytokine analyses
Aliquots of supernatant were sampled from antigen presentation assays at 24, 48, and 72 h (75−150 μL). Aliquots (75 μL) were removed from the antigen rechallenge assays at 14 h, prior to the addition of [3H]thymidine. Supernatants (25 μL) were diluted 1:1 with PBS/1% BSA and analyzed in triplicate for IFN-γ, IL-10, and IL-4 by using DuoSet ELISA reagents from R&D Systems. The lower limit of cytokine detection in these ELISAs was approximately 0.3 ng μL 1. IL-6, IL-10, MCP-1, TNF-α, IFN-γ, IL-10 and IL-12−70 were simultaneously measured by using BD Pharmingen's Mouse Inflammation Cytokine Bead Array on a BD FACScaliber.
Acknowledgements
EWA would like to thank L. Moita, C. Moita, J. Saramago and Bul Locks for technical assistance at different points throughout this work and Dr. Irene Caminschi for her generous gift of the CHOCIRE cell line. This work was supported by National Institutes of Health (NIH) Grant AI063 081 (to N.H.) E.W.A. was supported by a NDSEG graduate fellowship, and D.M.R. was supported by an NIH Biotechnology Training grant.
References
- 1.Steinman RM, Nussenzweig MC. Proc. Natl. Acad. Sci. USA. 2002;99:351. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Annu. Rev. Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Griffiths MN, Burton DR, Ghazal P. Proc. Natl. Acad. Sci. USA. 2000;97:847. doi: 10.1073/pnas.97.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. J. Exp. Med. 2002;196:1627. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Rav-etch JV, Steinman RM, Nussenzweig MC. J. Exp. Med. 2001;194:769. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figdor CG, van Kooyk Y, Adema GJ. Nat. Rev. Immunol. 2002;2:77. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 7.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. J. Cell Biol. 2000;151:673. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atochina O, Daly-Engel T, Piskorska D, McGuire E, Harn DA. J. Immunol. 2001;167:4293. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TBH, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CMJE, Appelmelk B, van Kooyk Y. J. Exp. Med. 2002;197:7. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers N, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweigh-offer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Immunity. 2005;22:507. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 12.Ratner DM, Adams EW, Disney MD, Seeberger PH. ChemBioChem. 2004;5:1375. doi: 10.1002/cbic.200400106. [DOI] [PubMed] [Google Scholar]
- 13.Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, Steinman RM, Feizi T. Int. Immunol. 2004;16:853. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Science. 2001;294:2163. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 15.Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Int. Immunol. 2001;13:1283. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- 16.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CMJE, Geijtenbeek TBH, van Kooyk Y. J. Immunol. 2003;170:1635. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 17.Caminschi I, Lucas K, O'Keefe M, Hochrein H, Laabi Y, Brodnicki TC, Lew A, Shortman K, Wright M. Mol. Immunol. 2001;38:365. doi: 10.1016/s0161-5890(01)00067-0. [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GC, Kraal G, van Oosterhout AJ, van Kooyk Y. Blood. 2002;100:2908. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 19.Caminschi I, Corbett AJ, Zahra C, Lahoud M, Lucas KM, Sofi M, Vremec D, Gramberg T, Pöhlmann S, Curtis J, Handman E, van Dommelen SLH, Fleming P, Degli-Esposit MA, Shortman K, Wright MD. Int. Immunol. 2006;18:741. doi: 10.1093/intimm/dxl011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linehan S, Martinez-Pomares L, Stahl PD, Gordan S. J. Exp. Med. 1999;189:1961. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp E, Medzhitov R. Curr. Opin. Immunol. 2003;15:396. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J. Exp. Med. 1995;182:389. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Immunol. Rev. 2004;199:9. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado-López R, Moser M. Semin. Immunol. 2001;13:275. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. J. Exp. Med. 1997;186:665. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki N, Kojima S, Bovin NV, Andre S, Gabius S, Gabius HJ. Adv. Drug Delivery Rev. 2000;43:225. doi: 10.1016/s0169-409x(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 27.van Bergen J, Ossendorp F, Jordens R, Mommaas AM, Drijhout JW, Koning F. Immunol. Rev. 1999;172:87. doi: 10.1111/j.1600-065x.1999.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor ME, Drickamer K. J. Biol. Chem. 1993;268:399. [PubMed] [Google Scholar]
- 29.Ratner DM, Plante OJ, Seeberger PH. Eur. J. Org. Chem. 2002:826. [Google Scholar]
- 30.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Cancer Res. 2000;60:3239. [PubMed] [Google Scholar]