Abstract
(RS)-2-cis, 4-trans-abscisic acid (ABA), a naturally occurring plant stress hormone, elicited rapid agonist-specific changes in myo-inositol hexakisphosphate (InsP6) measured in intact guard cells of Solanum tuberosum (n = 5); these changes were not reproduced by (RS)-2-trans, 4-trans-abscisic acid, an inactive stereoisomer of ABA (n = 4). The electrophysiological effects of InsP6 were assessed on both S. tuberosum (n = 14) and Vicia faba (n = 6) guard cell protoplasts. In both species, submicromolar concentrations of InsP6, delivered through the patch electrode, mimicked the inhibitory effects of ABA and internal calcium (Cai2+) on the inward rectifying K+ current, IK,in, in a dose-dependent manner. Steady state block of IK,in by InsP6 was reached much more quickly in Vicia (3 min at ≈1 μM) than Solanum (20–30 min). The effects of InsP6 on IK,in were specific to the myo-inositol isomer and were not elicited by other conformers of InsP6 (e.g., scyllo- or neo-). Chelation of Ca2+ by inclusion of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid or EGTA in the patch pipette together with InsP6 prevented the inhibition of IK,in, suggesting that the effect is Ca2+ dependent. InsP6 was ≈100-fold more potent than Ins(1,4,5)P3 in modulating IK,in. Thus ABA increases InsP6 in guard cells, and InsP6 is a potent Ca2+-dependent inhibitor of IK,in. Taken together, these results suggest that InsP6 may play a major role in the physiological response of guard cells to ABA.
Despite the fact that myo-inositol hexakisphosphate (InsP6) is the most abundant myo-inositol phosphate in nature (1) and is the most readily identified of this class of compounds, the cellular functions of InsP6 remain enigmatic. This is in part a consequence of an apparent lack of response of InsP6 to extracellular signals, with a notable exception in the response of Schizosaccharomyces pombe to hypertonic osmotic shock, which leads to rapid increases in the levels of InsP6 (2). A role for InsP6 in cell signaling is suggested by recent work in two other systems. In the first, three genes, whose products constitute a path linking Ins (1,4,5)P3 to InsP6, have been identified as members of a group that functionally complement lesions in mRNA export from the nucleus in yeast; the genes respectively encode phospholipase C, an Ins (1,4,5)P3 3-/Ins (1,3,4,5)P4 6-kinase and Ins (1,3,4,5,6)P5 2-kinase (3, 4). Secondly, in insulin-secreting pancreatic β cells (5), it was shown that 10 μM InsP6 in the patch pipette increased an L-type Ca2+ current; the effect may reflect the ability of InsP6 to inhibit protein phosphatases, types PP1, PP2A, and PP3, with consequent activation of the L-type Ca2+-channels. In this paper, we define a function for InsP6 in plants, as a signal, generated in guard cells in response to the stress hormone, (RS)-2-cis, 4-trans-abscisic acid (ABA). We have further defined an electrophysiological target for InsP6. In drought conditions, ABA is produced and induces changes in ion channel activity in both plasmalemma and tonoplast of guard cells. The ensuing net loss of K+ and associated anions from the vacuole and from the cell leads to reduction in turgor and closure of the stomatal pore. The mechanisms by which ABA leads to activation and inactivation of specific ion channels have been the subject of much study, but detailed description of the events and signaling chains remains very incomplete. Ca2+ has been identified as a signaling intermediate in some, but not all, of the ABA-induced changes in guard cell ion channels; ABA-induced inactivation of the plasmalemma inward K+ channel, IK,in (6–8), is Ca2+ mediated (8), whereas ABA-induced activation of the plasmalemma outward K+-channel, IK,out (6–9), is Ca2+ independent (8, 10). In neither case is the detailed signal transduction chain established.
Several of the signaling functions of specific inositol phosphates seem to be intimately associated with the second messenger function of internal calcium (Cai2+); thus the Ins (1,4,5)P3 receptor is well documented as a Ca2+-release channel in the endoplasmic reticulum in animal cells, and other studies have assigned a specific function to Ins (3,4,5,6)P4 as an uncoupler of Ca2+-activated Cl− secretion in T84 colonic epithelial cells (11). The role of InsP3 as a second messenger in plants is less well established, but it has been suggested to play a role in the signal transduction processes in the guard cells responsible for ABA-induced stomatal closure (12–14). In this paper, evidence is presented for involvement of InsP6 in the ABA response, with the demonstration that InsP6 regulates inward K+ current, probably the best characterized electrophysiological target of ABA in guard cells (15). We show that ABA increases the level of InsP6 in guard cells, that InsP6 in the patch pipette is a potent inhibitor of guard cell plasmalemma inward K+ current, and that InsP6-dependent inhibition of inward K+ current is manifest in a calcium-dependent manner.
Materials and Methods
Biochemistry.
Epidermal fragments isolated (16) from 5–6 g of leaves of Solanum tuberosum (Desirée) were labeled for 24 h in 10 ml of 10 mM Mes, pH 5.5 (KOH)/0.2 mM CaCl2, osmolality adjusted to 470 mOsm⋅kg−1 with sorbitol, containing 25 μCi⋅ml−1 myo-[2-3H]inositol (specific activity 16–18 Ci⋅mmol−1, Amersham Pharmacia). Tissue was split into approximately equal aliquots before challenge for 5 min with 30 μM ABA, (RS),-2-trans, 4-trans-abscisic acid (obtained from Sigma), or water. ABA was stored frozen in the dark and thawed immediately before use. Tissue was rapidly filtered on 200 μm nylon mesh, washed with 50 ml water, and frozen in liquid nitrogen. Inositol phosphates were extracted, mixed with standards obtained from previously defined sources (17, 18) and resolved on Partisphere SAX (Whatman) HPLC columns with gradients of NaH2PO4: 0 min 0-M; 5 min, 0-M; 75 min, 2.5-M.
Electrical Recording.
Guard cell protoplasts (GCPs) from Vicia faba were obtained as described (9). GCPs from S. tuberosum (Desirée) were prepared from epidermal fragments (as obtained in ref. 16). Enzymatic solution and protocol for isolation of GCPs are as described for V. faba (9). The bath medium contained 14 mM K+ glutamate, 0.5 mM CaCl2, 2 mM MgCl2, 10 mM Mes, pH 5.5 (KOH), osmolality adjusted to 480–500 mOsm⋅kg−1 with mannitol or sorbitol. Pipette solutions were 100 or 180 mM K+ glutamate/3.4 mM CaCl2 (or 3.21 mM at 180 mM K+ glutamate)/5 mM EGTA (giving ≈100 nM free Ca2+)/2 mM K2-ATP/2 mM MgCl2/10 mM Hepes, pH 7.5 (KOH), osmolality adjusted to 520–540 mOsm⋅kg−1 with mannitol or sorbitol. Electrical recordings and analysis were performed as described (9). All chemicals, including InsP3 and InsP6, were from Sigma. Scyllo- and neo-inositol hexakisphosphate were obtained from the laboratory of the late Dennis Cosgrove, Commonwealth Scientific and Industrial Research Organization, Plant Industry, Canberra, Australia. The structures of these inositol phosphates were confirmed by 31P NMR before use.
Results
Biochemical Studies.
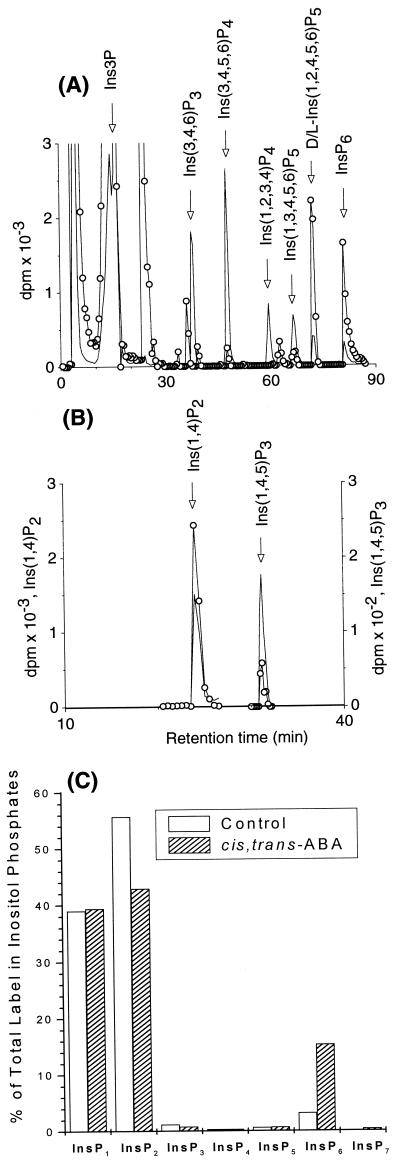
Guard cell preparations from S. tuberosum were prelabeled with [3H]inositol, and the distribution of label in different inositol phosphates and the effect of treatment of guard cells with 30 μM (RS)-2-cis, 4-trans abscisic acid on distribution of label between different inositol phosphates were determined. For identification of individual inositol phosphates, guard cell extracts were mixed with internal 14C standards (Fig. 1A) or 32P standards (Fig. 1B) and resolved by HPLC as shown. The extent of labeling of inositol phosphates was variable between different experiments, and the low levels of labeling precluded rigorous identification of stereoisomers. Inositol phosphates were identified as follows: InsP2 (coeluting with [Ins (1,4)P2]); three [InsP3] peaks, one of which coeluted with Ins (1,4,5)P3; InsP4; three InsP5 peaks; InsP6 and a peak eluting later than InsP6, which is probably a diphosphoinositol polyphosphate, perhaps a diphosphoinositol pentakisphosphate (colloquially known and referred hitherto in this manuscript as “InsP7”).
Figure 1.
Activation of inositol phosphate metabolism in guard cells. Inositol phosphates in 3H-labeled guard cells (○), mixed with internal 14C (no symbol) standards (A) and 32P (no symbol) standards (B). The traces shown in A and B were from HPLC runs performed on different extracts of 3H-labeled guard cells. The bar graph represents the distribution of label in different inositol phosphates, as a percentage of the total label in InsP1 to InsP7, from control (transparent bars) or (RS)-2-cis, 4-trans-abscisic acid-treated (hashed bars) guard cells (C).
Marked differences were found in the pattern of labeling between ABA-treated cells and water-treated controls, with a large ABA-induced increase in the fraction of label associated with InsP6. The pattern of change was similar in different experiments even if the extent of labeling differed. Fig. 1C shows the pattern of labeling and the response to ABA in one such experiment, in which cells were treated with ABA for 5 min. There was little ABA-induced difference in the fraction of label in InsP1 to InsP5, but the label in InsP6 increased nearly 5-fold from 3% in the control to 15% in the ABA-treated cells, and that in InsP7 rose from 0.02% to 0.4%; the label in InsP2 fell by a much smaller fraction, from 56% to 43%. Thus InsP6, and perhaps InsP7, was the inositol phosphate, the labeling of which was most responsive to ABA. Table 1 summarizes the extent of the ABA-induced increases in labeling in InsP6 in five experiments, with treatments of 30 sec to 15 min. Although we were unable to quantify the chemical level of InsP6 in our system, even with the mass-sensitive metal dye detection HPLC system of Mayr (19) with which we can measure as little as 100 pmol InsP6 on-column, it is clear that ABA induces rapid changes in InsP6 metabolism.
Table 1.
ABA-induced increases in InsP6 in guard cells
| Period of treatment | dpm in InsP6
|
||
|---|---|---|---|
| Control | ABA | ||
| Expt. 1 | 30 sec | 2541 | 3533 |
| Expt. 2 | 1 min | n.d. | 575 |
| 5 min | 85 | 7444 | |
| Expt. 3 | 5 min | 2536 | 10608 |
| Expt. 4 | 5 min | 7100 | 40491 |
| Expt. 5 | 5 min | 312 | 4989 |
| 15 min | n.d. | 7204 | |
Epidermal fragments were labeled with myo-[2-3H]inositol for 24 hours before challenge with 30 μM (RS)-2-cis, 4-trans abscisic acid. Inositol phosphates were extracted and resolved by Partisphere SAX HPLC. Radioactivity in peak fractions was determined by scintillation counting. To account for differences in the amount of tissue extracted for control and ABA treatments, dpm recovered in the InsP6 peaks were normalized to the inositol peak. Expt., experiment.
By way of control, we have tested the effect of another naturally occurring isomer of ABA, 30 μM (RS)-2-trans, 4-trans-abscisic acid, which does not induce stomatal closure in stomata opened in the dark by CO2-free air (20). Treatment with 30 μM (RS)-2-trans, 4-trans-abscisic acid elicited within 5 min a rapid and dramatic reduction in the labeling of InsP6; in three experiments, the label in InsP6 was reduced to 5 ± 3% of that in the control. In one further experiment, after a 5-min treatment with the trans, trans isomer, labeling in InsP6 was reduced more than 3-fold. InsP7 was below the limit of detection in these experiments. These results show that the control by ABA of InsP6 metabolism in guard cells is stereospecific; the massive increase in label in InsP6 is effected only by the isomer that is active in promoting stomatal closure. The most likely explanation of the reduction in the level of InsP6 produced by the trans, trans isomer is that this reflects competition for an ABA receptor with endogenous cis, trans abscisic acid, which determines the resting level of InsP6 before external ABA is added.
Electrical Recordings.
The discovery of rapid stereospecific control of InsP6 metabolism by ABA isomers identifies InsP6 as a candidate signal in ABA-dependent processes in guard cells. It is important, therefore, to determine whether InsP6 regulates the activity of guard cell ion channels. We have used the patch-clamp technique in whole protoplast configuration to manipulate cytosolic composition to identify the cellular targets of InsP6. Specifically, we have studied the control of potassium channels in both S. tuberosum, on which the biochemistry described above was performed, and V. faba, which has been the subject of much electrophysiological work. Two K+ conductances have been characterized in V. faba, both showing time- and voltage-dependent characteristics. The first is an inward K+ current, open only at voltages more negative than about −120 mV, which is inhibited by cytoplasmic Ca2+ (21–23) and is down-regulated by ABA (6–8); the inhibition by ABA is prevented by the inclusion of Ca2+ chelator in the patch pipette (8). The second is an outward K+ channel, opening at voltages positive of the potassium equilibrium potential (EK), which is insensitive to Ca2+ (21) and is activated by ABA in a Ca2+-independent manner (8, 10). There are fewer studies of Solanum guard cell K+ channels (16, 24), but nevertheless Dietrich et al. (24) have shown that the K+ channels of this species are similar to those of Vicia with respect to voltage dependence, selectivity, and single-channel conductance.
K+ Currents in Solanum GCPs.
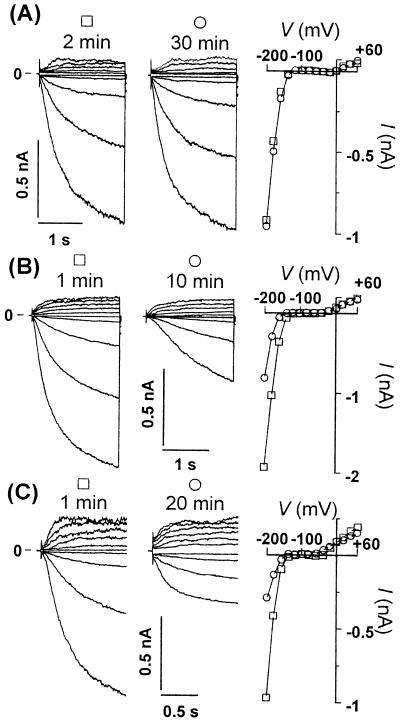
Fig. 2A shows typical current traces of potassium inward and outward rectifier (IK,in and IK,out, respectively) recorded from a GCP isolated from Solanum after going from protoplast-attached to whole-protoplast configuration. This experiment is included here to show that when the standard internal medium was used, with ≈100 nM free Ca2+ in the patch pipette, time- and voltage-dependent IK,in and IK,out currents were present and stable for as long as the gigaohm seal resistance between the cell and the pipette was maintained (n = 6). The current-voltage (I/V) plot shows two I/V scans made at 2 and 30 min with little or no variation between them. Thus, the effects of specific inositol phosphates described in the following are not a consequence of “run down” of currents because of depletion of undefined cytosolic components.
Figure 2.
InsP6 and increased Cai2+ inactivate IK,in but not IK,out in Solanum GCPs. Current traces of IK,in and IK,out (Left) and corresponding I/V curves (Right) were measured at the indicated times shown above each set of current traces (referring to the time after achieving whole-protoplast configuration). Control experiment carried out with ≈0.1 μM free Ca2+ in the patch pipette (A). Typical experiments with pipette solutions containing ≈1.3 μM free Ca2+ (B) and those containing 20 μM InsP6 (with ≈0.1 μM free Ca2+) (C).
Previous studies have noted that IK,in measured from Solanum protoplasts was largely insensitive to external calcium (16, 24), a result in contrast to that obtained with Vicia (8, 25). The sensitivity of the Solanum channel to internal Ca2+ (Cai2+) has not been defined, and this represents an issue of some importance to the regulation of IK,in. We show here that, as for Vicia (21–23), IK,in in Solanum is sensitive to Cai2+. Thus, in contrast to the temporally stable currents observed with low- (≈100 nM) pipette Ca2+ (Fig. 2A), inclusion of high Ca2+ (1.3 μM) caused a time-dependent reduction in currents consequent on equilibration of the cytoplasm with high-pipette Ca2+ (Fig. 2B). In all of three separate experiments, elevation of Ca2+ reduced the current carried at all tested voltages in the range within which IK,in is activated, with no or little effect on IK,out.
InsP6 Modulates IK,in in Solanum GCPs.
With confidence in the stability of K+ currents in GCPs from Solanum and in our ability to manipulate the cytosolic composition successfully, we tested the effect on both IK,in and IK,out of 20 μM InsP6 in the pipette, a concentration within the range quoted for this inositol phosphate in animal cells (26). An example is shown in Fig. 2C in which currents were measured 1 and 20 min after achieving whole-protoplast mode. A substantial decrease in the current carried by IK,in at any tested voltage was observed with no change in the amplitude of the current carried by IK,out. The sensitivity of IK,in to lower concentrations of InsP6 was also determined in Solanum (see Figs. 3A and 4A, with 1 μM and 0.1 μM InsP6 in the pipette, respectively). At lower concentrations, the inhibition developed more slowly, but up to 90% block was observed when gigaohm seals lasted for 30 to 60 min or more. Pipette concentrations as low as 0.1 μM were sufficient to trigger inactivation of IK,in by more than 70% at the steady state, with no effect on IK,out (n = 3).
Figure 3.
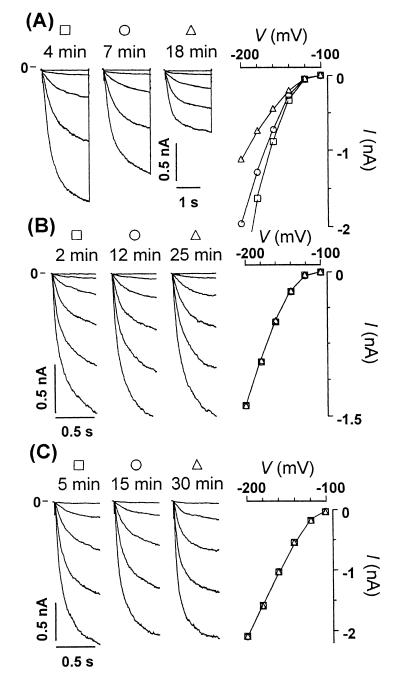
Stereospecific inactivation of IK,in by InsP6 conformers is relieved by buffering Cai2+ in Solanum GCPs. Current traces of IK,in and corresponding I/V curve obtained with pipette solutions containing 1 μM myo-InsP6 (A), 1 μM neo-InsP6 (B), and 1 μM myo-InsP6 together with 20 mM BAPTA (C).
Figure 4.
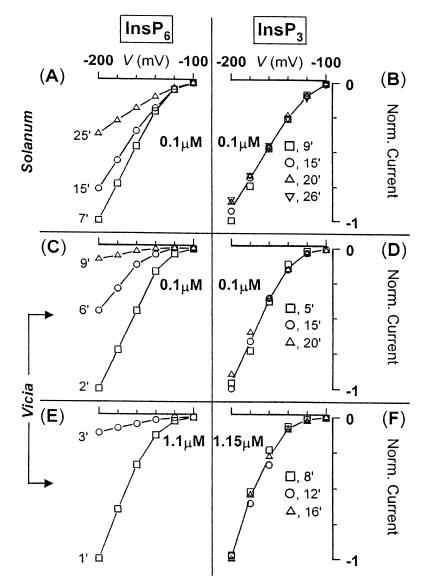
At the submicromolar range, InsP6 is more potent in the inhibition of IK,in than Ins (1,4,5)P3 in both Solanum (A and B) and Vicia GCPs (C–F). In A–F, I/V scans were taken at different times, as indicated in the graph; the amount of inositol polyphosphate used is also indicated. A, C, and E are experiments carried out with InsP6 in the patch pipette. B, D, and F are experiments carried out with Ins (1,4,5)P3 in the patch pipette. Note the remarkable lack of effect of Ins (1,4,5)P3 compared with InsP6-induced large inhibition of IK,in at either concentration. This figure also highlights the speed at which GCPs from Vicia respond to InsP6 [at −200 mV, 90% block in only 9 min (C)] when compared with GCPs from Solanum (at −200 mV, 60% block in 25 min; A).
InsP6 Modulation of IK,in Is Faster in Vicia than in Solanum.
We have further extended our observation of InsP6-dependent inhibition of IK,in to guard cell protoplasts of Vicia, which is the only species in which the electrophysiological consequences of release of Ins (1,4,5)P3 have been determined (13). Our results show that InsP6-mediated inhibition of IK,in is evident in both species. Thus, InsP6 gave a response in 11 of 14 Solanum protoplasts tested and in all of 6 Vicia protoplasts tested. Solanum and Vicia differed markedly in the speed of response to InsP6 with the response developing more rapidly in Vicia. Measured at −180 mV, inhibition of IK,in by 1 μM InsP6 reached 43% in 18 min for Solanum (Fig. 3A) compared with greater than 85% inhibition achieved in 3 min for Vicia (Fig. 4E). Also compare Fig. 4 A and C, where 0.1 μM InsP6 was used (at −200 mV: 60% inhibition in 25 min for Solanum vs. 90% inhibition in just 9 min for Vicia).
InsP6-Induced Inhibition of IK,in in Solanum GCPs Is Stereo Specific.
Having defined IK,in as a specific downstream target of the elevation of InsP6 observed in ABA-stimulated Solanum guard cells, we sought to define the stereo specificity of the interaction of InsP6 with its effector(s) or target ion channels. For this purpose, we used two conformers of InsP6, scyllo-InsP6 and neo-InsP6. These molecules differ from myo-InsP6 in the orientation of phosphate ester substituents relative to the plane of the cyclohexane ring (1). In scyllo-InsP6, all phosphates are equatorial. In myo-InsP6, all phosphates with the exception of that in the 2-position are equatorial (2- is axial), whereas in neo-InsP6, the 2- and 5-phosphates are both axial. Fig. 3 A and B show that when 1 μM neo-InsP6 (Fig. 3B) was substituted for myo-InsP6 (Fig. 3A), no effect could be seen 12 or even 25 min after achieving whole-protoplast mode. Likewise, by using 1 μM scyllo-InsP6, no inhibition could be detected after even 23 min (data not shown). No effect on potassium currents was seen in seven of all eight protoplasts challenged with these two conformers. These results unequivocally discount one of the potential criticisms of the interpretation of InsP6 action in cell signaling, namely that InsP6 effects may be unspecific and merely a function of the high negative charge density and the potential to chelate internal Mg2+. Our standard patch-pipette medium contains 2 mM MgCl2.
InsP6-Induced Inhibition of IK,in Is Relieved by Ca2 + Chelators.
Our results show that InsP6 is competent to regulate the Ca2+-dependent inward K+ channel in guard cells. Because of the precedent of inactivation of IK,in by increasing [Ca2+]i, we have investigated whether InsP6-driven inhibition of the inward K+ current is similarly a calcium-dependent process. This was tested by the inclusion of 20 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) with 1 μM myo-InsP6 in the patch pipette. As shown in Fig. 3C, buffering of cytoplasmic Ca2+, by inclusion of BAPTA in the pipette, completely relieved the inactivation of IK,in by InsP6, and this effect was persistent up to 30 min. The effect was confirmed in three further experiments by using 20 mM BAPTA and in one by using 40 mM EGTA.
InsP6 Is Much More Potent than Ins (1,4,5)P3 in Modulating IK,in.
Because our results with InsP6 are reminiscent of the inhibition of IK,in observed on photolytic release of 1–10 μM Ins (1,4,5)P3 into intact guard cells (13), we have compared the effectiveness of InsP6 and Ins (1,4,5)P3 in modulating IKin. We now show that InsP6 is considerably more potent in the inactivation of IK,in in GCPs than is Ins (1,4,5)P3. Fig. 4 A–D compares the effects of InsP6 and Ins (1,4,5)P3, each at 100 nM, in both Vicia and Solanum, and it is clear that Ins (1,4,5)P3 is without effect at this concentration. Ins (1,4,5)P3 was also without effect at 1 μM in Vicia (Fig. 4F). Only when added at supraphysiological concentrations (10 to 22 μM; n = 5) did Ins (1,4,5)P3 produce a strong inhibition (up to 87% block at 22 μM; data not shown). Thus InsP6 is some 100 times more potent than Ins (1,4,5)P3 for inactivation of IK,in. This observation is important as it demonstrates that the potent effects of InsP6 are not a consequence of its metabolism to Ins (1,4,5)P3; note also the absence of consistent and detectable change of InsP3 in Fig. 1C.
Discussion
The work described above establishes that ABA produces rapid changes in InsP6 in guard cells and that InsP6, delivered at submicromolar concentrations through the patch pipette, mimicked the inhibitory effects of ABA and Cai2+ on the inward K+ channel, and that this inhibition was abolished by the inclusion of Ca2+ chelator in the pipette. The changes in InsP6 induced by ABA are rapid, detectable within a minute of application, and comparable with the time scale for ABA-induced electrical changes in intact guard cells (6, 7). The response of the inward current to diffusion of InsP6 from the patch pipette is similarly rapid, within a few minutes, especially in Vicia GCPs [at 1.1 μM InsP6, maximum inhibition of IK,in was reached in 3 min (Fig. 4E)]. InsP6 is also much more efficient than Ins (1,4,5)P3 in the inhibition of IK,in at submicromolar concentrations. Thus, the results are consistent with a dominant role for InsP6 in a Ca2+-dependent signaling chain by which ABA inhibits the inward K+ current in guard cells.
Various explanations of the calcium dependency of InsP6 results are possible, and two seem prominent. The first is that InsP6 may elicit Ca2+ increases either by entry of Ca2+ from the outside, as seen for example in the activation of L-type Ca2+ channels by InsP6 in animal cells (5), or may release Ca2+ from internal stores, perhaps by analogy to Ins (1,4,5)P3 induced-calcium release. An alternative possibility is that InsP6 acts downstream of Ca2+ to modulate the interactions with Ca2+-sensitive targets. Indeed, Luan and coworkers (27) have shown that Ca2+-dependent inhibition of IK,in is abolished by immunosuppressants but induced by the inclusion of a constitutively active fragment of bovine brain calcineurin in the patch pipette, arguing perhaps that calcineurin-like mediated protein dephosphorylation may be a consequence of elevation of Ca2+ in guard cells. Future measurements of the effect of InsP6 on cytoplasmic Ca2+ will allow the two possibilities to be distinguished.
The changes in InsP6 metabolism detected here are among the most rapid of the biochemical responses to ABA detected in guard cells. The size and the speed of the changes of InsP6 to a stimulus, in this case ABA, are more dramatic than responses reported in animals (26) and are similar to that reported in yeast (2). The response to ABA observed here is also fundamentally different from the developmentally programmed accumulation of InsP6 in turions (specialized mesophyll cells) of the duckweed, Spirodela polyrhiza, which incidentally also occurs in response to ABA (17). The latter study reported a pathway proceeding from Ins ⇒ Ins3P ⇒ Ins (3,4)P2 ⇒ Ins (3,4,6)P3 ⇒ Ins (3,4,5,6)P4 ⇒ Ins (1,3,4,5,6)P5 ⇒ InsP6. We have further confirmed the presence of Ins (3,4,5,6)P4 1-kinase activity in mesophyll cells (28). It is not possible to speculate at present about the route of activated InsP6 synthesis in guard cells. The lack of significant changes in the unidentified InsP3 coeluting with [32P]Ins (1,4,5)P3 might argue that inositol phospholipid turnover does not contribute to the production of InsP6 observed here. However, in contrast, Michell and coworkers (2) have characterized an enzyme activity that converts Ins (1,4,5)P3 to InsP6 in S. pombe, an experimental system in which InsP6 increases in response to hyperosmotic shock. It will be important in future work to establish the pathway for synthesis of InsP6 in guard cells.
The present results raise, but do not answer, the question of the relation between the InsP6-mediated signaling pathway and other pathways previously postulated in guard cells, those involving Ins (1,4,5)P3 and cyclic adenosine diphosphate-ribose cADPR. There is clear evidence that guard cells are competent to respond to Ins (1,4,5)P3 by increase in cytoplasmic calcium and inhibition of IK,in (12, 13) and to cADPR by increase in cytoplasmic calcium (29), but the evidence that these are major contributors to the ABA response in intact guard cells is not compelling. There is no evidence for stimulus-dependent changes in cADPR in guard cells, and indeed the case for significant stimulus-dependent change in Ins (1,4,5)P3 (30) in guard cells is, to our mind, still lacking (31). The relatively small effects of inhibitors of phospholipase C, or of synthesis of cADPR, on ABA-induced changes in aperture does not identify a predominant role for either pathway (29, 32, 33). Thus the relative contributions of the three potential signaling intermediates, Ins (1,4,5)P3, cADPR, and InsP6, in the physiological response to ABA are not yet established, and this remains an important goal for future work.
It is remarkable that InsP6 and Ins (1,4,5)P3 share common effects on a common target, IK,in, and that inhibition of IK,in by both of these agents is manifest in a calcium-dependent manner. Although it is widely held that the calcium dependence of inactivation of IK,in by Ins (1,4,5)P3 resides with an Ins (1,4,5)P3-triggered release of Ca2+ from internal stores, the evidence now attributes ABA-dependent inactivation of IK,in rather more potently to InsP6 than to Ins (1,4,5)P3. An intriguing possibility raised by our experiments is that the reported effects of release of Ins (1,4,5)P3 in guard cells is a consequence of conversion to InsP6. Although we find no evidence to support this suggestion in the present study, the work of Michell and coworkers (2) indicates that InsP6 production observed in response to hyperosmotic shock in S. pombe may derive from Ins (1,4,5)P3.
Returning to guard cells, the exact nature of the calcium dependence of InsP6 action on IK,in is not defined. Activation of plasma membrane channels that are permeable to calcium, either selectively [witness activation of L-type calcium channels by InsP6 (4)] or nonselectively, could represent a mechanism by which InsP6 might regulate IK,in. Indeed, Blatt and coworkers (34, 35) have characterized a hyperpolarization-activated plasma membrane calcium influx channel that is activated by ABA and that contributes significantly to ABA-dependent increase in cytoplasmic Ca2+ and inactivation of IK,in.
Consequently, in light of the ABA-driven changes in InsP6, the potency of inhibition of inward-K+ current by InsP6, and the calcium dependency of inhibition, irrespective of the exact mechanism by which InsP6 effects are manifested, the evidence suggests that InsP6 may play a major, if not dominant, role in ABA signaling to IK,in. In a much more general sense, our work defines a physiological target of InsP6 action in plants. That this target, the inward K+ current, has a well-defined function in control of guard cells by ABA further serves to highlight an important and emerging osmoregulatory context (2) for the function of this, the most enigmatic of inositol phosphates.
Acknowledgments
We thank Ingrid Wesley for preparation of guard cells and protoplasts. We are indebted to Max Tate, University of Adelaide, for the generous gift of a selection of inositol phosphates. Some inositol phosphate conformers were provided by Alan Richardson, from the former laboratory of the late Dennis J. Cosgrove at the Commonwealth Scientific and Industrial Research Organization, Canberra, Australia. C.B. is a Biotechnology and Biological Sciences Research Council (BBSRC) Advanced Research Fellow. F.L.-C. was supported during the course of this work by BBSRC Grant P05730 to E.M.
Abbreviations
- InsP6
myo-inositol hexakisphosphate
- ABA
(RS)-2-cis, 4-trans-abscisic acid
- GCP
guard cell protoplast
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate or -tetraacetic acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140217497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140217497
References
- 1.Cosgrove D J. Inositol Phosphates, Their Chemistry, Biochemistry and Physiology. Amsterdam: Elsevier; 1980. [Google Scholar]
- 2.Ongusaha P P, Hughes P J, Davey J, Michell RH. Biochem J. 1998;335:671–679. doi: 10.1042/bj3350671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.York J D, Odom A R, Murphy R, Ives E B, Wente S R. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 4.Odom R A, Stahlberg A, Wente S R, York J D. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- 5.Larsson O, Barker C J, Sj-oholm A, Carlqvist H, Michell R H, Bertorello A, Nilsson T, Honkanen R E, Mayr G W, Zwiller J, et al. Science. 1997;278:471–474. doi: 10.1126/science.278.5337.471. [DOI] [PubMed] [Google Scholar]
- 6.Blatt M R. Planta. 1990;180:445–455. doi: 10.1007/BF00198799. [DOI] [PubMed] [Google Scholar]
- 7.Thiel G, MacRobbie E A C, Blatt M R. J Membr Biol. 1992;126:1–18. doi: 10.1007/BF00233456. [DOI] [PubMed] [Google Scholar]
- 8.Lemtiri-Chlieh F, MacRobbie E A C. J Membr Biol. 1994;137:99–107. doi: 10.1007/BF00233479. [DOI] [PubMed] [Google Scholar]
- 9.Lemtiri-Chlieh F. J Membr Biol. 1996;153:105–116. doi: 10.1007/s002329900114. [DOI] [PubMed] [Google Scholar]
- 10.Blatt M R, Armstrong F. Planta. 1993;191:330–341. [Google Scholar]
- 11.Vajanaphanich M, Schultz C, Rudolf M T, Wasserman M, Enyedi P, Craxton A, Shears S B, Tsien R Y, Barrett K E, Traynor-Kaplan A. Nature (London) 1994;371:711–714. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- 12.Gilroy S, Read N D, Trewavas A J. Nature (London) 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- 13.Blatt M R, Thiel G, Trentham D R. Nature (London) 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- 14.Allen G J, Muir S R, Sanders D. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- 15.Blatt M R, Grabov A. Physiol Plant. 1997;100:481–490. [Google Scholar]
- 16.Muller-Rober B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brearley C A, Hanke D E. Biochem J. 1996;314:227–233. doi: 10.1042/bj3140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brearley C A, Hanke D E. Biochem J. 1996;318:279–286. doi: 10.1042/bj3180279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayr G W. Biochem J. 1988;254:585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriedemann P E, Loveys B R, Fuller G L, Leopold A C. Plant Physiol. 1972;49:842–847. doi: 10.1104/pp.49.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder J I, Hagiwara S. Nature (London) 1989;338:427–430. [Google Scholar]
- 22.Grabov A, Blatt M R. Planta. 1997;201:84–95. [Google Scholar]
- 23.Grabov A, Blatt M R. Plant Physiol. 1999;119:277–287. doi: 10.1104/pp.119.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich P, Dreyer I, Wiesner P, Hedrich R. Planta. 1998;205:277–287. [Google Scholar]
- 25.Fairley-Grenot K A, Assmann S M. J Membr Biol. 1992;128:103–113. doi: 10.1007/BF00231883. [DOI] [PubMed] [Google Scholar]
- 26.Shears S B. Biochim Biophys Acta. 1998;1436:49–67. doi: 10.1016/s0005-2760(98)00131-3. [DOI] [PubMed] [Google Scholar]
- 27.Luan S, Li W, Rusnak F, Assmann S M, Schreiber S L. Proc Natl Acad Sci USA. 1993;91:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brearley C A, Hanke D E. Plant Physiol. 2000;122:1209–1216. doi: 10.1104/pp.122.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leckie C P, McAinsh M R, Allen G J, Sanders D, Hetherington A M. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y, Choi Y B, Suh S, Lee J, Assmann S M, Joe C O, Kelleher J F, Crain R C. Plant Physiol. 1996;110:987–996. doi: 10.1104/pp.110.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brearley C A, Parmar P N, Hanke D E. Biochem J. 1997;324:123–131. doi: 10.1042/bj3240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob T, Ritchie S, Assmann S M, Gilroy S. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staxen I, Pical C, Mongomery L T, Gray J E, Hetherington A M, McAinsh M R. Proc Natl Acad Sci USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabov A, Blatt M R. Proc Natl Acad Sci USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton D W A, Hills A, Kohler B, Blatt M R. Proc Natl Acad Sci USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]