Abstract
G protein-coupled receptor (GPCR) kinases (GRKs) are known as a family of serine/threonine kinases that function as key regulators of GPCRs, as well as other types of receptors. Extensive studies of GRKs at the cellular and organismal levels have led to a consensus that GRK-catalyzed phosphorylation of receptors is the primary mechanism underlying their physiological functions. Here, we report that down-regulation of GRK2 in zebrafish embryos with GRK2 morpholino results in developmental early arrest and, interestingly, that this arrest can be rescued by exogenous expression of a GRK2 kinase-dead mutant, K220R. A physical interaction between GRK2 and cyclin B1 regulator patched homolog 1 (PTCH1), stimulated by Hedgehog (Hh), rather than GRK2-mediated phosphorylation of downstream targets, appears as the underlying mechanism. We identify residues 262–379 as the PTCH1-binding region (BP). Interaction of GRK2, K220R, and BP with PTCH1 reduces the association of PTCH1 with cyclin B1 and disrupts PTCH1-mediated inhibition of cyclin B1 nuclear translocation, whereas the PTCH1-binding deficient GRK2 mutant (Δ312–379) does not. Cell cycle and cell proliferation assays show that overexpressing PTCH1 remarkably inhibited cell growth and this effect could be attenuated by GRK2, K220R, or BP, but not Δ312–379. In vivo studies show that BP, as well as the nuclear-localizing cyclin B1 mutant, is effective in rescuing the early arrest phenotype in GRK2 knockdown embryos, but Δ312–379 is not. Our data thus reveal a novel kinase activity-independent function for GRK and establish a role for GRK2 as a cell-cycle regulator during early embryonic development.
Keywords: cyclin B1, early arrest, embryonic development, G protein-coupled receptor kinase, PTCH1
G protein-coupled receptor (GPCR) kinases (GRKs) are known as a family of serine/threonine kinases that function as key regulators of signaling by 7-transmembrane receptors (7TMRs), as well as other types of receptors. Receptor phosphorylation catalyzed by GRKs plays a pivotal role in the initiation of homologous internalization and desensitization of 7TMRs, preventing overactivation of receptor signaling pathways (1–4). However, emerging evidence implicates that mechanisms independent of the kinase activity of GRKs also underlie their physiological functions. Carman et al. (5) reported that both GRK2 and its kinase dead mutant, K220R, interact with Gαq and thus inhibit PLC-β activity. Dhami et al. (6) showed that K220R could also interact with the second intracellular loop of mGluR1a and attenuate agonist-stimulated receptor signaling. Very recently, the interaction of GRK5 with IκBα and the inhibitory effect of GRK5 on NFκB activity were demonstrated (7). These studies indicate that the catalytic activity independent regulations of GRKs may also be an important mechanism underlying their physiological functions.
Important roles of GRKs in the nervous (1), immune (8–10), and cardiovascular systems (11–14) have been demonstrated in cells and organisms by using overexpression, knockdown, and knockout techniques. Jaber et al. (14) reported that GRK2 is the unique member of GRK family as ablation of GRK2 gene causes lethal effects in mice. Homozygous knockout of GRK2 gene in mice is embryonically lethal and GRK2−/− embryos do not survive beyond gestational day 15.5, but the underlying mechanisms are obscure. GRK2−/− mice exhibit heart failure, but cardiac-specific ablation of GRK2 is not lethal (15). Very recently, it has been demonstrated that GRK2 is involved in Smoothened (Smo) signaling and GRK2 morphants phenocopy Smo pathway mutants in zebrafish embryos (16–18). Retarded growth and developmental stall are also observed in GRK2−/− mouse embryos (18), suggesting a potential role of GRK in regulating cell cycle during early development.
Cell cycle progression is regulated by a variety of cell cycle-related proteins, including cyclins. Phosphorylation of histone H1 by cyclin B1 complex results in a relaxation in chromatin structure and the onset of DNA replication during mitosis (19). Dysregulation of cyclin B1 has drastic consequences, including aberrant growth, cell death, and embryonic early arrest (20–22). Nuclear translocation of cyclin B1 is essential for its function as a cell cycle checkpoint and is under stringent regulation. Patched 1 (PTCH1) is a 12-transmembrane receptor that functions as a cell cycle gatekeeper and a tumor suppressor (23–26). In addition to its classic functions in regulating Hedgehog (Hh)-Smo signaling, it has also been shown to interact with cyclin B1 to inhibit cyclin B1 nuclear accumulation and cyclin B1-mediated cell proliferation (27). In the present study, we demonstrate that GRK2 regulates cell cycle and early development in zebrafish by interacting with PTCH1 and promoting cyclin B1 function. Our findings reveal a novel, kinase activity-independent function of GRK2 in regulating cell cycle checkpoints during embryonic development.
Results
GRK2 Interacts with PTCH1.
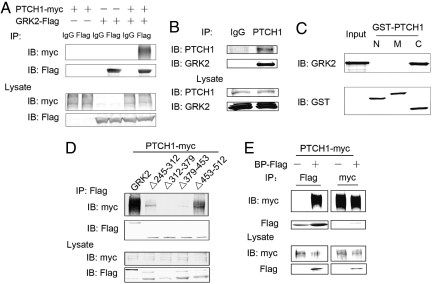
By using bovine GRK2 as bait in a yeast two-hybrid screen, we isolated cDNA clones encoding the carboxyl terminal sequences (residues 1267–1447) of human PTCH1 and thus identified PTCH1 as a potential interaction partner of GRK2. As shown in Fig. 1 A and B, association of PTCH1 with GRK2 was confirmed by coimmunoprecipitation of epitope-tagged PTCH1 and GRK2 or the endogenously expressed proteins from human embryonic kidney 293 (HEK293) cells. In vitro interaction assays revealed that PTCH1 interacts with GRK2 via its C-terminal (residues 1180–1447) sequences (Figs. S1A and 1C). As shown in Figs. S1B and S2B, mutation of lysine 220 in the GRK catalytic domain, which eliminates the kinase activity of GRK2, had no effect on the ability of GRK2 to bind PTCH1 whereas deletion of residues 186–514 (the GRK catalytic domain) abolished its binding to PTCH1. Furthermore, coimmunoprecipitation data showed that the GRK2 sequence flanked by residues 186 and 543 is not only required, but is also sufficient for PTCH1 binding (Fig. S2C). Coimmunoprecipitation screening of a series of GRK truncation mutants revealed that residues 312–379 are most critical for GRK2 and PTCH1 interactions (Fig. 1D) and a 118-aa fragment of GRK2 comprising residues 262–379 (BP) is sufficient for PTCH1 binding (Fig. 1E).
Fig. 1.
GRK2 interacts with PTCH1. (A) Interaction between PTCH1 and GRK2 was detected in HEK293 cells transfected with human myc-tagged Patched 1 (PTCH1-myc) and/or bovine Flag-tagged GRK2 (GRK2-Flag) cDNA. Lysates were immunoprecipitated with anti-Flag antibody (B). The immunocomplex (Upper) and the input cell lysate (Lower) were detected by Western blotting using the indicated antibodies. (B) Lysates of nontransfected HEK293 cells were immunoprecipitated with anti-PTCH1 antibody (Santa Cruz). (C) Direct interaction of GRK2-Flag with GST-tagged segments of PTCH1, GST-PTCH1-N (1–502 amino acids), GST-PTCH1-M (503–1121 amino acids), and GST-PTCH1-C (1180–1447 amino acids) was examined in GST-pull down assays. (D and E) Interactions between PTCH1 and GRK2 mutants Δ245–312, Δ312–379, Δ379–453, Δ453–512 (D) and 262–379 (BP-Flag) (E).
Interaction of GRK2 with PTCH1 Inhibits Cyclin B1-PTCH1 Interaction and Promotes Cyclin B1 Nuclear Accumulation.
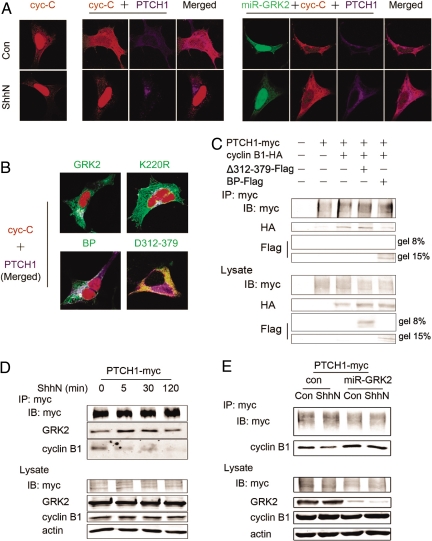
PTCH1 is a direct binding partner of cyclin B1, and it has been reported that interaction of PTCH1 with cyclin B1 inhibits nuclear accumulation of cyclin B1, causing cell cycle arrest (27). Therefore, we next examined the potential effect of GRK2–PTCH1 interaction on the accumulation of cyclin B1 in the nucleus. As shown in Figs. 2A and S3B, overexpression of PTCH1 in HEK293 cells caused 2 cyclin B1 analogs to accumulate in the cytoplasm: NLS-CRSGlu (also termed cyc-C), a constitutively phosphorylated mimic and nuclear-targeted derivative of cyclin B1 and NLS-B1-Glu, the full-length phosphorylated mimic of cyclin B1 (27). By contrast, activation of PTCH1 with the amino-terminal fragment of sonic hedgehog (ShhN) resulted in redistribution of cyclin B1 from the cytoplasm to the nucleus. Immunofluorescence and Western blot analyses show that knockdown of endogenous GRK2 expression by GRK2 RNAi inhibits ShhN-stimulated nuclear translocation of cyclin B1 (Figs. 2A and S3A). Furthermore, ShhN stimulation had no significant effect on the nuclear distribution of GRK2 (Fig. S4), but coexpressing either the wild-type GRK2 or its kinase-dead mutant K220R reversed the inhibitory effect of PTCH1 on cyclin B1 nuclear translocation (Fig. 2B). An increase of cyclin B1 was also detected in the nuclear fraction of HEK293 cells overexpressing GRK2 (Fig. S3B), whereas control experiments indicated that overexpression of GRK2 in the absence of PTCH1 has no effect on nuclear distribution of cyclin B1.
Fig. 2.
GRK2 blocks PTCH1-mediated inhibition of cyclin B1 nuclear translocation by disrupting the interaction between PTCH1 and cyclin B1. (A) GRK2 is essential for ShhN-induced cyclin B1 nuclear translocation. HEK293 cells were transfected with the cyclin B1 derivative cyc-C (NLS-CRSGlu-HA) alone (Left), cyc-C and PTCH1-myc (Middle), or cyc-C, PTCH1-myc, and miR-GRK2-EGFP (Right). At 48 h posttransfection, the cells were treated with vehicle (con, control) or 5 nM ShhN for 2 h and then analyzed by immunofluorescence microscopy. (B) GRK2 promotes cyclin B1 nuclear translocation. Cells were cotransfected with cyc-C and PTCH1-myc, in combination with GRK2 or its mutants. (C) Effects of GRK2 BP-Flag and Δ312–379-Flag on the interaction between PTCH1 and cyclin B1. Cells were transfected with PTCH1-myc alone or in combination with cyclin B1-HA, or cyclin B1-HA and BP-Flag, or Δ312–379-Flag. Lysates were immunoprecipitated with anti-myc antibody. (D) ShhN stimulation induces changes in the PTCH1/GRK2/cyclin B1 complex. Cells were treated with ShhN for indicated times 48 h after transfection with PTCH1-myc and GRK2 plasmids. Immunoprecipitation was performed by using anti-myc antibody and proteins were detected in Western blots by using the indicated antibodies. (E) GRK2 is essential for the dissociation of cyclin B1 and PTCH1 under ShhN stimulation. Cells transfected with PTCH1-myc, cyclin B1-HA, and miR-GRK2 or control siRNA (con) were treated with ShhN for 2 h, and interactions were detected as described above.
We next examined the role of GRK2/PTCH1 binding in regulation of cyclin B1. As shown in Fig. S3 B and C, expression of Δ312–379, a GRK2 truncation mutant incapable of binding PTCH1, did not affect subcellular distribution of cyclin B1 but expression of BP, the PTCH1-binding fragment of GRK, showed an effect comparable to full-length GRK2 in its ability to block PTCH1-mediated inhibition of cyclin B1 nuclear accumulation. These data indicate that the association of GRK2 and PTCH1 blocks PTCH1-mediated inhibition of cyclin B1 translocation to the nucleus and raises the possibility that binding of GRK2 to PTCH1 may disrupt the interaction of latter with cyclin B1. Therefore, we tested the influence of GRK2-PTCH1 interaction on the association of PTCH1 and cyclin B1. Coimmunoprecipitation assays demonstrated that expression of BP, but not Δ312–379, reduces the amount of cyclin B1 detected in the PTCH1 immunoprecipitation complex (Fig. 2C). A previous study (27) showed that stimulation of PTCH1 with its ligand ShhN disrupts its association with cyclin B1 and allows cyclin B1 translocation to the nucleus, but the mechanism is not clear. As shown in Fig. 2 D and E, an association of PTCH1 with GRK2 and cyclin B1 could be detected in the absence of ShhN; ShhN stimulation brought about an increase of GRK2 and a decrease of cyclin B1 in PTCH1 immunocomplex, whereas the reduction of association of cyclin B1 with PTCH1 in response to PTCH1 activation was not detected in cells transfected with GRK2 miRNA. These data support the hypothesis that recruitment of GRK2 to the activated PTCH1 following exposure to ShhN induces the dissociation of cyclin B1 from PTCH1 complex, which results in the nuclear translocation of cyclin B1 and cycle progression.
Interaction of GRK2 with PTCH1 Is Essential for Regulation of Cyclin B1-Mediated Cell Proliferation.
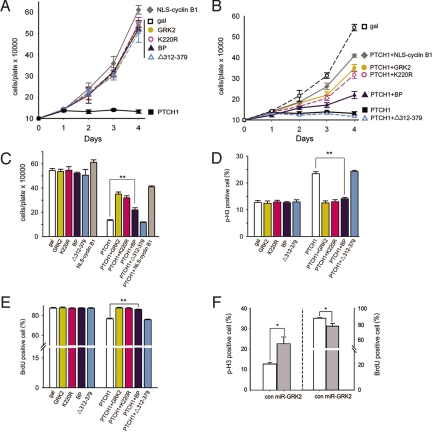
Nuclear accumulation of cyclin B1 is required for successful entry into mitosis and is thus critical for promoting cell proliferation (19), which is negatively regulated by interaction between cyclin B1 and PTCH1 in the cytoplasm (27). As shown in Fig. 3 A–C, overexpressing PTCH1 remarkably inhibited cell growth and this effect was attenuated by the wild-type GRK2, the kinase-dead GRK mutant K220R, the PTCH1-binding GRK fragment BP, and the constitutive nuclear-targeted form of cyclin B1, NLS-cyclin B1, but not by the PTCH1-binding deficient mutant of GRK2 Δ312–379 (Fig. 3 B and C). Moreover, overexpressing GRK2, K220R, BP, or Δ312–379 alone does not show obvious influence on cell proliferation (Fig. 3 A and C). These results indicate that through interaction with PTCH1, GRK2 participates in regulation of cyclin B1-mediated promotion of cell proliferation. Furthermore, cell cycle analysis by using phosphorylated histone H3 and BrdU as markers for cells in late G2- through M-phase and S-phase shows that overexpression of PTCH1 in HEK293 cells causes a 1-fold increase in G2/M-stage cells and a 13% decrease of the S-stage cells and that PTCH1-induced G2/M arrest in these cells can be rescued by GRK2, K220R, and BP but not Δ312–379 (Fig. 3 D and E). In addition, knockdown of GRK2 results in a 45% increase in G2/M-stage cells (Fig. 3F Left) and a 12% decrease in S-stage cells (Fig. 3F Right), suggesting that the interaction of GRK2 and PTCH1 is essential in promoting cell cycle to pass G2/M phase and move into S phase. Consistent with the results of cell proliferation, GRK2 or K220R alone did not have significant effect on the cell cycle (Fig. 3 D and E).
Fig. 3.
Interaction of GRK2 and PTCH1 is critical for cyclin B1-mediated cell proliferation. (A–C) Coexpression of GRK2, K220R, NLS-cyclin B1 and BP attenuates the inhibitory effect of PTCH1 on cyclin B1-mediated stimulation of cell proliferation. Growth curves (A–B) and day 4 cell counts (C) of HEK293 cells transfected with the indicated constructs. Distinct colors indicate different groups according to the symbol types of A. (D–E) Expression of GRK2 rescues G2/M arrest in HEK293 cells. Cells were incorporated with BrdU 40–48 h after transfection and labeled with anti-p-H3 and anti-BrdU antibodies. Numbers of p-H3-positive cells (D) or BrdU-positive cells (E) were counted and percentages relative to the total number of mitotic cells (Total p-H3-positive cells plus BrdU-positive cells) were calculated. (F) miR-GRK2 induces cell cycle arrest in G2/M stage in HEK293 cells. Cells were transfected with miR-GRK2 or control siRNA. The average value of 3 independent experiments is shown. Error bars represent the standard error of the mean. *, P < 0.05 in pair-wise Student's test; **, P < 0.01 in pair-wise Student's t test.
Interaction of GRK2 and PTCH1 Regulates Zebrafish Embryonic Early Development.
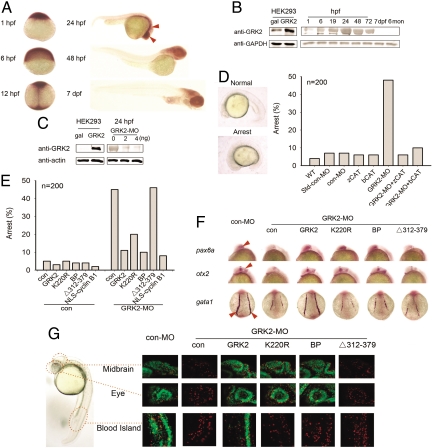
The potential function of GRK2 in embryonic development was examined in zebrafish. We first examined the temporal expression pattern of zebrafish GRK2 (18), a homolog of mammalian GRK2/3. In situ hybridization using a riboprobe targeting the N-terminal and catalytic domain of zebrafish GRK2 revealed a ubiquitous distribution of the GRK2 transcript before the onset of somitogenesis (Fig. 4A). During somitogenesis, GRK2 expression first became enriched along the midline and then in the anterior part of the body, with highest expression levels in the eyes, the forebrain, the midbrain, and the central midline by 24 h postfertilization (hpf). Western analysis demonstrated the presence of maternally derived GRK2 protein at low levels at the 1-cell stage and that the expression of GRK2 protein gradually increases to reach a peak level between 24 and 48 hpf, but remains at low levels in adult zebrafish (Fig. 4B). In situ hybridization assays showed that the expression pattern of PTCH1 is similar to that of GRK2 and PTCH1 transcript is present at high levels in the eyes and the brain (Fig. S5A).
Fig. 4.
Dysregulation of PTCH1-cyclin B1 pathway is involved in early arrest of GRK2 knockdown embryos. (A) Expression pattern of GRK2 during zebrafish developmental process by using whole-mount in situ hybridization (WISH) with a zebrafish GRK2-specific antisense riboprobe on embryos at the indicated stages. Areas with high intensity hybridization signal are indicated by arrows. (B) Expression levels of GRK2 during early developmental stages. Zebrafish embryos (15–20) were collected at each time point, and lysates were analyzed in Western blots by using rabbit anti-GRK2 antibody (Santa Cruz). Lysates from HEK293 cells transfected with beta-galactosidase (gal) or bovine GRK2 expression plasmids were taken as controls. GAPDH was used as a loading control (hpf, hours postfertilization; dpf, days postfertilization). (C) Effect of GRK2-MOs on GRK expression levels. Zebrafish embryos were injected at the 1- or 2-cell stage with 2- or 4-ng GRK2 splicing morpholino (GRK2-MO). GRK2 levels in embryo lysates were examined by Western blot analysis. (D) Somite stage arrest in GRK2 morphants, could be rescued by both zebrafish and bovine GRK2 catalytic domain. Each embryo was injected at the 1- or 2-cell stage with 2-ng con-MO, or 2-ng GRK2-MO alone, or with 0.15ng zebrafish or bovine GRK2 catalytic domain mRNA. Embryos were categorized, counted, and photographed 19 hpf. The percentages of arrest embryos are shown (200 embryos were counted for each bar). (E) GRK2, K220R, BP, or NLS-cyclin B1 rescues the arrest phenotype of GRK2 knockdown embryos. (F) GRK2, K220R, BP, or NLS-cyclin B1 rescues the deficiency in zebrafish eye and midbrain development. WISH was performed on 15–20 somite stage embryos injected with GRK2-MO and mRNA of indicated constructs, using pax6a, otx2 and gata1 riboprobes. Images of pax6a and otx2 were taken from a lateral view and those of gata1 from a dorsal view. (G) Effects of GRK2 on cell cycle progression in eye, midbrain, and hematopietic system were examined using p-H3 and BrdU assays. Twenty-four hpf injected embryos were collected and stained for p-H3 and BrdU. The images were taken from a lateral viewpoint, showing the eye, the midbrain, and the blood island.
Functions of GRK2 in the regulation of early development were explored by using an antisense morpholino that knocks down endogenous GRK2 by blocking the splicing of grk2 premRNA transcript (GRK2-MO). As shown in Fig. 4C, microinjection of GRK2-MO at 2 ng/embryo reduced GRK2 protein expression by >70%. Figs. 4D and S7A demonstrate that GRK2 morphants display a novel developmental early arrest in addition to abnormalities previously reported in GRK2 knockdown animals such as heart tube malformation, reported by Jaber et al. (14) (Fig. S6), abnormal somites and Ptch1 down-regulation (Fig. S5B), reported by Philipp et al. (18). At 6 hpf, over 90% embryos injected with the 5-bp mismatched control morpholino (con-MO) or standard control morpholino (Std-con MO) developed into gastrulation stage, with “germ ring” formation. In contrast, injection of 2 ng GRK2-MO resulted in 39% of embryos remaining in the high-cell stage, indicating a slower rate of cell proliferation in GRK morphants (Fig. S7A). At 19 hpf, almost all con-MO embryos reached the 20–25-somite stage (containing a characteristic elongated yolk), whereas 45%–65% of the GRK2-morphants remained at the 15-somite stage (Fig. 4D). Only 10% of the GRK2 MO embryos that arrested at the 15-somite stage survived 24 hpf. These embryos exhibited dysplastic changes, including coiled tail, reduced length, smaller eyes, relatively larger head, pericardial edema, circulation deficiency, and loss of pigment. As shown in the bar graphs in Figs. S7A and 4D, coinjection of mRNA encoding the catalytic domain of zebrafish or bovine GRK2 significantly reduced the percentage of GRK2 morphants suffering early arrest; compared with the full length GRK2 (Figs. S7B and 4E), the catalytic domain of GRK2 has equal or slightly better rescue effect, probably because removal of the N- and C-terminal domains makes the binding site in the GRK2 catalytic domain more accessible to PTCH1. These data suggest that the early arrest phenotype is specifically caused by down-regulation of GRK2, and is not the result of nonspecific GRK2-MO toxicity. These results also suggest that the regulatory functions of GRK2 during early embryonic development are conserved among vertebrates.
We next examined the role of the interaction of GRK2 with PTCH1/cyclin B1 pathway in the development of zebrafish early embryos. Bovine GRK2 and K220R, BP, and NLS-cyclin B1, but not Δ312–379, can rescue the early arrest phenotype of GRK2 morphants (Figs. S7B and 4E). In situ hybridization using probes for region- and tissue-specific marker mRNAs was carried out to further examine the effects of knockdown of GRK2 expression. Riboprobes against pax6a and otx2, markers for the eyes and the midbrain, where GRK2 and PTCH1 express at high levels, were used. Gata1 for the hematopoietic system was used as a control. As shown in Fig. 4F, expression of pax6a, otx2, and gata1 was significantly reduced in GRK2-MO injected embryos. When bovine GRK mRNA was coinjected with GRK2-MO, expression of pax6a, otx2, and gata1 was restored to wild-type levels. When mRNA encoding the GRK2 kinase-dead mutant K220R was coinjected, expression of pax6a, and otx2 was also rescued. In contrast, coinjection of the GRK2 kinase-dead mRNA did not rescue gata1 expression. Similar to the wild-type GRK and K220R, the BP peptide could also rescue the dysplastic phenotypes in the eyes and the midbrain, but could not rescue the developmental deficiencies in the blood island as marked by gata1. These data suggest that the physical interaction of GRK2 with PTCH1, but not the catalytic function of GRK, is essential for regulation of cyclin B1-mediated cell proliferation during early development. Mitotic assays to examine cell cycle patterns in the eyes, the midbrain, and the blood island of 24 hpf zebrafish embryos were also performed. An increase in p-H3 positive cells and a decrease in BrdU positive cells have also been observed in GRK2 morphants, but the extent of the increase of p-H3-positive cells was lower than those in GRK2 knockdown HEK293 cells. Taken together with tissue necrosis observed in GRK2 morphants, we infer that the smaller increases in p-H3-positive cells could be a result of increased apoptosis. Consistently, a remarkable reduction of BrdU-positive cells was also noticed in GRK2 morphants. It is also possible that in addition to regulating cyclin B1, other mechanisms may also underlie GRK2-mediated regulation of the cell cycle (Fig. 4G). The G2/M arrest in the eyes and the midbrain can be rescued by overexpression of GRK2, K220R, or BP; however, the cell cycle deficit in the blood island could only be rescued by GRK2, not K220R or BP. GRK2 Δ312–379 showed no rescue effect on the maldevelopment and cell cycle arrest in GRK2 morphants.
Discussion
The essential role of GRK-catalyzed receptor phosphorylation in the regulation of receptor internalization and receptor-mediated signaling and physiological functions has been demonstrated in numerous receptor systems. However, it has also been shown that not all regulatory functions of GRKs depend on their kinase activity. For example, endocytosis of the glucose-dependent insulinotropic polypeptide receptor was shown to be independent of GRK2-mediated receptor phosphorylation (32). Accumulating evidence also indicates that additional GRK subtypes possess kinase activity-independent functions. A variety of nonsubstrate binding partners for GRKs have been reported, including calmodulin, clathrin, actin, and GIT (33). Cellular experiments have shown that GRK2 and GRK3 interact with Gαq/11 through their RGS domain in an AlF4−-dependent manner and may thus serve as weak GTPase activating proteins for Gαq/11 and direct G protein inhibitory regulators (5). Subsequent research showed that interaction of GRK2 with Gαq/11 negatively regulates insulin-stimulated glucose transport in 3T3-L1 cells (34). Dhami et al. (6) reported that both GRK2 and K220R attenuate agonist-stimulated mGluR1a signaling via interactions with the second intracellular loop of the receptor. It was shown in bovine aorta endothelial cells that GRK5 interacts with IκB and thus regulates NFκB transcriptional activity (7). Recently, Penela et al. (35) reported that K220R showed an effect similar to wild-type GRK2 in regulation of cell morphology and promoting cell migration in COS7 cells. In this study, we demonstrate that GRK directly interacts with PTCH1 and that this interaction, but not the catalytic function of GRK, is essential for regulation of cyclin B1-mediated cell proliferation during early development. Our current study thus provides additional evidence for kinase activity-independent functions of GRK and demonstrated that GRK kinase-independent regulation of PTCH1/cyclin B1 pathway is essential for early development in zebrafish. Our data suggest that GRK regulates early development through both catalytic activity-dependent and -independent mechanisms.
Regulation by GRK2 of immune and epithelial cell migration and differentiation, processes involved in embryonic development, has been previously reported (10, 35). Moreover, the somite abnormalities in GRK2 morphants have been shown to be related to GRK2 kinase -dependent phosphorylation of Smo (18). PTCH1 associates with Smo and inhibits Smo activation (24). Exposure to Hh stimulates GRK2-mediated Smo phosphorylation and β-arrestin recruitment, both of which facilitate Smo signaling (16, 17). The Smo signaling pathway plays important roles in embryonic development, especially in regulating the formation of the neural tube and slow muscles (36–38). Phenotypes of Hh pathway mutants were mimicked by β-arrestin 2 morphants (39). Philipp et al. (18) reported that GRK2 knockdown in zebrafish embryos results in somite malformation, similar to that observed in Smo signaling mutants, suggesting a role of GRK2 in the Smo pathway. Cyclin B1 is a cell cycle regulator, whose subcellular distribution is regulated by PTCH1. Cyclin B1 shuttles into and out of the nucleus, and its direct association with PTCH1 inhibits its nuclear accumulation and regulation on cell cycle (27). In the current study, we show that Hh stimulation induces an increase of GRK2 and a decrease of cyclin B1 in PTCH1 immunocomplex. In contrast, a reduction of the association of cyclin B1 with PTCH1 in response to PTCH1 activation was not detected in cells deficient in GRK2. It is possible that Hh binding to PTCH1 induces a conformation change in PTCH1 that favors its association with GRK2, which facilitates dissociation of cyclin B1 and subsequent accumulation of cyclin B1 in the nucleus. These data suggest that GRK2 may be present in a multiple-component complex, and its interaction with PTCH1, or possibly with other components in the complex, may play an important role in regulation of cellular functions. GRK2 may regulate PTCH1 mediated developmental signals through both Smo and cyclin B1 pathways.
The kinase-independent regulation is likely a conserved function of GRK. Both GRK2 and PTCH1 are highly conservative proteins among vertebrates. PTCH1 has been identified in zebrafish, chicken, mice, and bovines, as well as humans. Zebrafish and human PTCH1 share 75% homology. The zebrafish GRK2 gene was recently cloned and the protein sequence was shown 85% homologous to human GRK2 (18). It has been shown in both zebrafish and mice that knockout of PTCH1 results in similar abnormalities in the development of the neural system, especially in neural tube formation (40, 41). Black et al. (42) showed that PTCH1 lacZ+/− mice exhibit abnormal cell cycle regulation and display symptoms of Basal Cell Naevus syndrome similar to those observed in human. These studies suggest that PTCH1 has conservative functions in vertebrates. In this study, we showed that GRK2 knockdown in zebrafish results in a developmental arrest phenotype similar with those observed in embryos of GRK2 deficient mice and that the regulation of GRK2 on PTCH1/cyclin B1 pathway was essential in both human cells and zebrafish embryos. Moreover, the developmental early arrest and cell cycle arrest in GRK2 morphants could be rescued by both zebrafish and mammalian GRK2. These results suggest that regulation of GRK2 on PTCH1 may be conservative among multiple vertebrates and this regulation is likely also important for mammalian embryonic development and tumor formation.
Materials and Methods
Zebrafish Embryos and Microinjection.
Information of zebrafish culture, morpholino design, microinjection and whole-mount in situ hydridization is shown in SI Experimental Procedures.
Cell Transfection, Immunoprecipitation, and in Vitro Interaction Assay.
HEK293 cells were used as the host cell for coimmunoprecipitation assays. The procedures are performed as described previously (43). Details of in vitro interaction assay procedures are provided in SI Experimental Procedures.
Immunofluorescent Imaging.
Transfected HEK293 cells were treated with 5 nM ShhN (Sigma) in MEM or simply MEM for indicated times. After fixation and incubation with anti-myc or anti-HA antibodies, cells were stained with Cy5- or Cy3-conjugated antibody, and visualized by using a confocal microscope (Zeiss). Details of experimental procedures for phospho-histone H3 (P-H3) and BrdU assays are shown in SI Experimental Procedures.
Cell Proliferation Assay.
HEK293 cells were seeded in 60-mm plates at a density of 1.0 × 105/dish and transfected with 2.5 μg GRK2 or its mutants and/or 2.5- or 4-μg PTCH1. Cells were trypsinized and counted every 24 h after transfection as described previously (27).
Supplementary Material
Acknowledgments.
We thank Drs. G. Peng (Fudan University) and G. Pei (Chinese Academy of Sciences Shanghai Institutes of Biological Sciences) for encouragement and valuable comments, Drs. J.-L. Du (Chinese Academy of Sciences Shanghai Institutes of Biological Sciences and the National Zebrafish Resources of China) and J.-X. Gu (Fudan University) and their colleagues for technical support, Dr. D. J. Donoghue (University of California at San Diego, La Jolla, CA) for providing PTCH1 plasmid, and Dr. D. Saffen (Fudan University) for critical reading of the manuscript. This research was supported by grants from Ministry of Science and Technology (2005CB522406 and 2009CB522006) and Natural Science Foundation of China (30830042 and 30821002), Shanghai Municipal Commission for Education (B119), and National Science and Technology Major Project for Drug Discovery.
Abbreviations:
- cyc-C
cyclin B1 cytoplasmic retention signal domain containing 4 site mutations and a nuclear location sequence
- NLS-B1-Glu
full-length cyclin B1 containing 4 site mutations and a nuclear location sequence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812105106/DCSupplemental.
References
- 1.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 2.Premont RT, Inglese J, Lefkowitz RJ. Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 4.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 5.Carman CV, et al. Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem. 1999;274:34483–34492. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 6.Dhami GK, Babwah AV, Sterne-Marr R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor 1 signaling requires g protein-coupled receptor kinase 2 binding to the second intracellular loop. J Biol Chem. 2005;280:24420–24427. doi: 10.1074/jbc.M501650200. [DOI] [PubMed] [Google Scholar]
- 7.Sorriento D, et al. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proc Natl Acad Sci USA. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong AM, et al. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavelaars A, et al. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J Immunol. 2003;171:6128–6134. doi: 10.4049/jimmunol.171.11.6128. [DOI] [PubMed] [Google Scholar]
- 10.Vroon A, et al. Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. J Leukocyte Biol. 2004;75:901–909. doi: 10.1189/jlb.0403136. [DOI] [PubMed] [Google Scholar]
- 11.Koch WJ, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 12.Koch WJ. Genetic and phenotypic targeting of beta-adrenergic signaling in heart failure. Mol Cell Biochem. 2004;263:5–9. doi: 10.1023/B:MCBI.0000041843.64809.48. [DOI] [PubMed] [Google Scholar]
- 13.Martini JS, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci USA. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaber M, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matkovich SJ, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, et al. Activity-dependent internalization of smoothened mediated by β-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 17.Meloni AR, et al. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26:7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipp M, et al. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol Biol Cell. 2008;19:5478–5489. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Meyer AN, Donoghue DJ. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc Natl Acad Sci USA. 1997;94:502–507. doi: 10.1073/pnas.94.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doree M, Galas S. The cyclin-dependent protein kinases and the control of cell division. FASEB J. 1994;8:1114–1121. doi: 10.1096/fasebj.8.14.7958616. [DOI] [PubMed] [Google Scholar]
- 21.Russo AJ, et al. E2F-1 overexpression in U2OS cells increases cyclin B1 levels and cdc2 kinase activity and sensitizes cells to antimitotic agents. Cancer Res. 2006;66:7253–7260. doi: 10.1158/0008-5472.CAN-05-3725. [DOI] [PubMed] [Google Scholar]
- 22.Wehman AM, Staub W, Baier H. The anaphase-promoting complex is required in both dividing and quiescent cells during zebrafish development. Dev Biol. 2007;303:144–156. doi: 10.1016/j.ydbio.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res. 2006;66:2081–2088. doi: 10.1158/0008-5472.CAN-05-2146. [DOI] [PubMed] [Google Scholar]
- 24.Toftgard R. Hedgehog signalling in cancer. Cell Mol Life Sci. 2000;57:1720–1731. doi: 10.1007/PL00000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gailani MR, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 26.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20:2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/s1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- 30.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Wada Y, Sugiyama J, Okano T, Fukada Y. GRK1 and GRK7: Unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J Neurochem. 2006;98:824–837. doi: 10.1111/j.1471-4159.2006.03920.x. [DOI] [PubMed] [Google Scholar]
- 32.Tseng CC, Zhang XY. Role of G protein-coupled receptor kinases in glucose-dependent insulinotropic polypeptide receptor signaling. Endocrinology. 2000;141:947–952. doi: 10.1210/endo.141.3.7365. [DOI] [PubMed] [Google Scholar]
- 33.Ribas C, et al. The G protein-coupled receptor kinase (GRK) interactome: Role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Usui I, et al. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004;23:2821–2829. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penela P, et al. G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga ZM, et al. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- 37.Ingham PW, Kim HR. Hedgehog signalling and the specification of muscle cell identity in the zebrafish embryo. Exp Cell Res. 2005;306:336–342. doi: 10.1016/j.yexcr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Ochi H, Pearson BJ, Chuang PT, Hammerschmidt M, Westerfield M. Hhip regulates zebrafish muscle development by both sequestering Hedgehog and modulating localization of Smoothened. Dev Biol. 2006;297:127–140. doi: 10.1016/j.ydbio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Wilbanks AM, et al. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 40.Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Dev Biol. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 41.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 42.Black GC, et al. Abnormalities of the vitreoretinal interface caused by dysregulated Hedgehog signaling during retinal development. Hum Mol Genet. 2003;12:3269–3276. doi: 10.1093/hmg/ddg356. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Long H, Wu Z, Jiang X, Ma L. EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol Biol Cell. 2008;19:2973–2983. doi: 10.1091/mbc.E07-10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.