Abstract
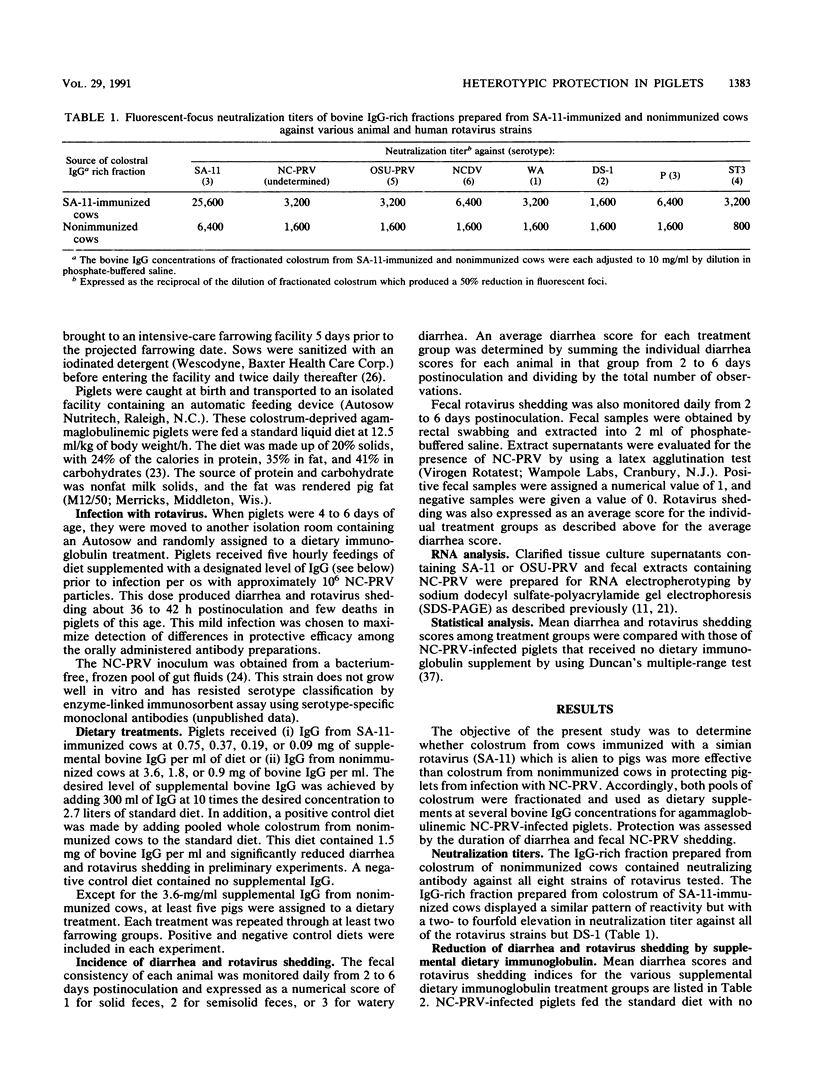
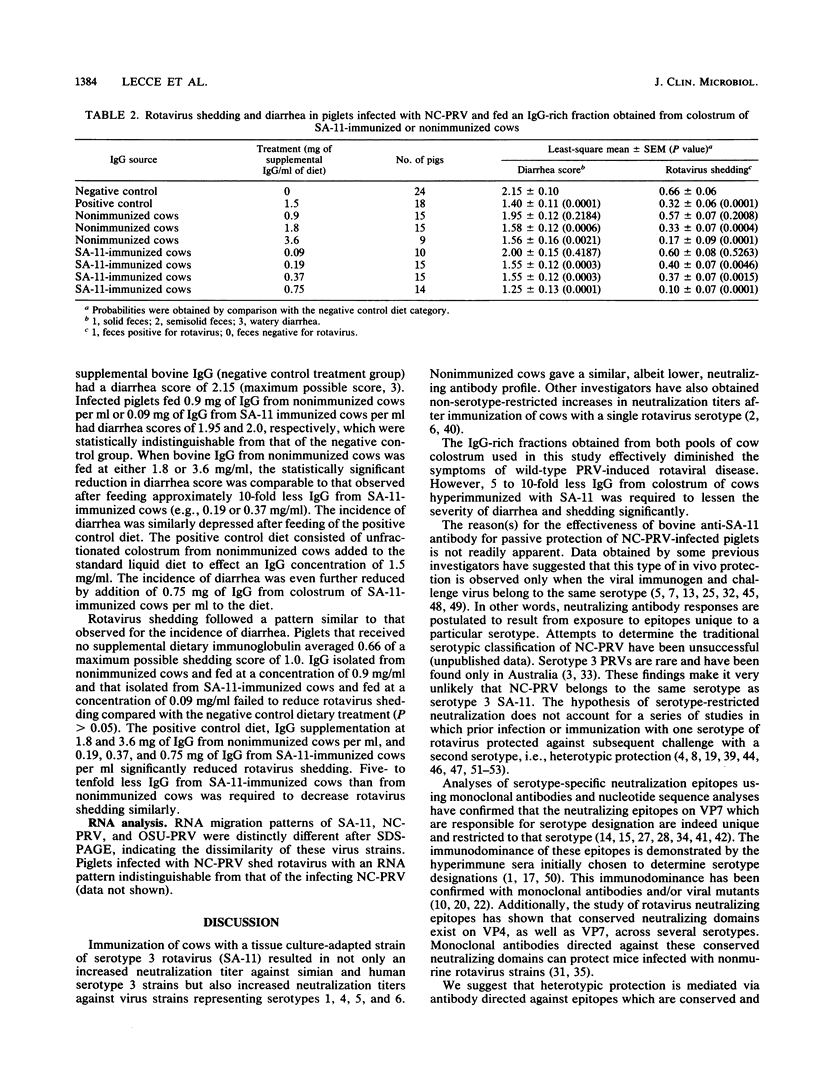
Rotavirus, a double-stranded RNA virus, has been implicated as a diarrhea-provoking agent in a variety of animal species. Several previous reports have shown that immunization with a single serotype may result in increased in vitro neutralization titers against serotypes not represented in the immunogen. This study was undertaken to determine whether antibody from cows immunized against simian rotavirus strain SA-11 (which is alien to pigs) could protect neonatal piglets from infection with a North Carolina isolate of porcine rotavirus. Accordingly, cows were immunized with SA-11 and an immunoglobulin G (IgG)-rich fraction was isolated from their colostrum. An IgG-rich fraction was similarly isolated from colostrum of nonimmunized cows. At equal concentrations, IgG from SA-11-immunized cows had two- to fourfold higher neutralization titers to seven of eight test strains of rotavirus, including SA-11 (serotype 3); human rotavirus serotypes 1, 3, and 4; North Carolina porcine rotavirus (serotype undetermined); Ohio State porcine rotavirus (serotype 5); and bovine rotavirus (serotype 6). The IgG-rich fractions were fed as dietary supplements to agammaglobulinemic piglets infected with the North Carolina porcine rotavirus. IgG from the SA-11-immunized cows was about eightfold more effective in protecting piglets than was IgG from nonimmunized cows.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Unicomb L. E., Tzipori S. R., Bishop R. F. Isolation and serotyping of animal rotaviruses and antigenic comparison with human rotaviruses. Brief report. Arch Virol. 1987;93(1-2):123–130. doi: 10.1007/BF01313898. [DOI] [PubMed] [Google Scholar]
- Bellinzoni R. B., Mattion N. M., Matson D. O., Blackhall J., La Torre J. L., Scodeller E. A., Urasawa S., Taniguchi K., Estes M. K. Porcine rotaviruses antigenically related to human rotavirus serotypes 1 and 2. J Clin Microbiol. 1990 Mar;28(3):633–636. doi: 10.1128/jcm.28.3.633-636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. C., Blackhall J., Baro N., Auza N., Mattion N., Casaro A., La Torre J. L., Scodeller E. A. Efficacy of an inactivated oil-adjuvanted rotavirus vaccine in the control of calf diarrhoea in beef herds in Argentina. Vaccine. 1989 Jun;7(3):263–268. doi: 10.1016/0264-410X(89)90241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R. F., Tzipori S. R., Coulson B. S., Unicomb L. E., Albert M. J., Barnes G. L. Heterologous protection against rotavirus-induced disease in gnotobiotic piglets. J Clin Microbiol. 1986 Dec;24(6):1023–1028. doi: 10.1128/jcm.24.6.1023-1028.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Walther I., Fryder V., Sidoti J., Bruttin A. Cross-neutralizing antibodies induced by single serotype vaccination of cows with rotavirus. J Gen Virol. 1988 Jul;69(Pt 7):1647–1658. doi: 10.1099/0022-1317-69-7-1647. [DOI] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Tursi J. M., McAdam W. J., Bishop R. F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986 Oct 30;154(2):302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Dolan K. T., Twist E. M., Horton-Slight P., Forrer C., Bell L. M., Jr, Plotkin S. A., Clark H. F. Epidemiology of rotavirus electropherotypes determined by a simplified diagnostic technique with RNA analysis. J Clin Microbiol. 1985 May;21(5):753–758. doi: 10.1128/jcm.21.5.753-758.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Flores J., Kapikian A. Z. Immunity to rotaviruses. Curr Top Microbiol Immunol. 1989;146:123–136. doi: 10.1007/978-3-642-74529-4_14. [DOI] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Hoshino Y., Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989 Feb;168(2):429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Midthun K., Gorziglia M., Hoshino Y., Kapikian A. Z., Chanock R. M., Flores J. Comparison of the amino acid sequences of the major neutralization protein of four human rotavirus serotypes. Virology. 1987 Nov;161(1):153–159. doi: 10.1016/0042-6822(87)90181-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Taniguchi K., Mackow E. R., Kapikian A. Z. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990 Apr;161(4):667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Levine M. M., Yolken R. H., VanKirk D. H., Dolin R., Greenberg H. B., Chanock R. M. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983 Jan;147(1):95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- Knowlton D. R., Ward R. L. Effect of mutation in immunodominant neutralization epitopes on the antigenicity of rotavirus SA-11. J Gen Virol. 1985 Nov;66(Pt 11):2375–2381. doi: 10.1099/0022-1317-66-11-2375. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Sonza S., Dyall-Smith M. L., Coulson B. S., Holmes I. H. Demonstration of an immunodominant neutralization site by analysis of antigenic variants of SA11 rotavirus. J Virol. 1985 Oct;56(1):317–319. doi: 10.1128/jvi.56.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce J. G., Coalson J. A. Diets for rearing colostrum-free piglets with an automatic feeding device. J Anim Sci. 1976 Mar;42(3):622–629. doi: 10.2527/jas1976.423622x. [DOI] [PubMed] [Google Scholar]
- Lecce J. G., King M. W. The calf reo-like virus (rotavirus) vaccine: an ineffective immunization agent for rotaviral diarrhea of piglets. Can J Comp Med. 1979 Jan;43(1):90–93. [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Benfield D. A., Greenberg H. B. Characterization of homotypic and heterotypic VP7 neutralization sites of rhesus rotavirus. Virology. 1988 Aug;165(2):511–517. doi: 10.1016/0042-6822(88)90595-8. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Lee S. H., Faulkner R. S., Ethier J., Young C. H. Rotavirus infection in a geriatric population. Arch Intern Med. 1982 Feb;142(2):313–316. [PubMed] [Google Scholar]
- Matsui S. M., Offit P. A., Vo P. T., Mackow E. R., Benfield D. A., Shaw R. D., Padilla-Noriega L., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989 Apr;27(4):780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Nishioka N., Watanabe T., Kuniyasu C. Prolonged excretion and failure of cross-protection between distinct serotypes of bovine rotavirus. Vet Microbiol. 1986 Jun;12(1):7–14. doi: 10.1016/0378-1135(86)90036-2. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Hoshino Y., Taniguchi K., Green K. Y., Greenberg H. B., Kapikian A. Z., Chanock R. M., Gorziglia M. Rotavirus VP7 neutralization epitopes of serotype 3 strains. Virology. 1989 Aug;171(2):503–515. doi: 10.1016/0042-6822(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I., Blanco M., Vilar M., Garcia D., White L., Gonzalez R., Kapikian A. Z., Flores J. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. J Clin Microbiol. 1990 Mar;28(3):553–558. doi: 10.1128/jcm.28.3.553-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsbury F. T., Winkelstein J. A., Yolken R. H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980 Jul;97(1):61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Smith C. C., Manak M. M., Aurelian L. Prevention of rotavirus-induced diarrhea in neonatal mice born to dams immunized with empty capsids of simian rotavirus SA-11. J Infect Dis. 1984 Mar;149(3):434–438. doi: 10.1093/infdis/149.3.434. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Fitzgerald T., Campbell I., Scott F. M., Browning G. F., Miller D. L., Herring A. J., Greenberg H. B. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990 Mar;28(3):504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Hoshino Y., Nishikawa K., Green K. Y., Maloy W. L., Morita Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J Virol. 1988 Jun;62(6):1870–1874. doi: 10.1128/jvi.62.6.1870-1874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Torres A., Ji-Huang L. Diarrheal response of gnotobiotic pigs after fetal infection and neonatal challenge with homologous and heterologous human rotavirus strains. J Virol. 1986 Dec;60(3):1107–1112. doi: 10.1128/jvi.60.3.1107-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S. R., Makin T. J., Smith M. L. The clinical response of gnotobiotic calves, pigs and lambs to inoculation with human, calf, pig and foal rotavirus isolates. Aust J Exp Biol Med Sci. 1980 Jun;58(3):309–318. doi: 10.1038/icb.1980.31. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Rautanen T., Varis T., Beards G. M., Kapikian A. Z. Rhesus Rotavirus candidate vaccine. Clinical trial in children vaccinated between 2 and 5 months of age. Am J Dis Child. 1990 Mar;144(3):285–289. [PubMed] [Google Scholar]
- Woode G. N., Bew M. E., Dennis M. J. Studies on cross protection induced in calves by rotaviruses of calves, children and foals. Vet Rec. 1978 Jul 8;103(2):32–34. doi: 10.1136/vr.103.2.32. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Mebus C. A., Yolken R. H., Kalica A. R., James H. D., Jr, Kapikian A. Z., Chanock R. M. Rotaviral immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979 Feb 9;203(4380):548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P., Marbehant P., Marissens D., Lobmann M., Charlier P., Delem A., Zygraich N. Protection studies in colostrum-deprived piglets of a bovine rotavirus vaccine candidate using human rotavirus strains for challenge. J Infect Dis. 1983 Dec;148(6):1061–1068. doi: 10.1093/infdis/148.6.1061. [DOI] [PubMed] [Google Scholar]


