Abstract
By the year 2010, it is estimated that 18.1 million people worldwide will die annually because of cardiovascular diseases and stroke. “Global vascular risk” more broadly includes the multiple overlapping disease silos of stroke, myocardial infarction, peripheral arterial disease, and vascular death. Estimation of global vascular risk requires consideration of a variety of variables including demographics, environmental behaviors, and risk factors. Data from multiple studies suggest continuous linear relationships between the physiological vascular risk modulators of blood pressure, lipids, and blood glucose rather than treating these conditions as categorical risk factors. Constellations of risk factors may be more relevant than individual categorical components.
Exciting work with novel risk factors may also have predictive value in estimates of global vascular risk. Advances in imaging have led to the measurement of subclinical conditions such as carotid intima-media thickness and subclinical brain conditions such as white matter hyperintensities and silent infarcts. These subclinical measurements may be intermediate stages in the transition from asymptomatic to symptomatic vascular events, appear to be associated with the fundamental vascular risk factors, and represent opportunities to more precisely quantitate disease progression. The expansion of studies in molecular epidemiology and detection of genetic markers underlying vascular risks also promises to extend our precision of global vascular risk estimation.
Global vascular risk estimation will require quantitative methods that bundle these multi-dimensional data into more precise estimates of future risk. The power of genetic information coupled with data on demographics, risk-inducing behaviors, vascular risk modulators, biomarkers, and measures of subclinical conditions should provide the most realistic approximation of an individual's future global vascular risk. The ultimate public health benefit, however, will depend on not only identification of global vascular risk but also the realization that we can modify this risk and prove the prediction models wrong.
Keywords: cerebrovascular disease, epidemiology, prevention, risk factors
I am very grateful to the stroke council, to the members of the nominating committee, and to all those that have gone before me, to receive the William Feinberg Award. It is a special honor to me in many ways because I also knew and worked with Bill Feinberg. I had the opportunity to work with Bill on the risk factor panel.1 Bill was a warm, spirited person, a real leader in the area of stroke prevention, and active in the mission of the AHA. He loved life and unfortunately his life was cut short too quickly. I am extremely honored to be able to give this Bill Feinberg Lecture having known Bill.
I am also very thankful to have had great career mentors. Mentoring is very important for anybody involved in any aspect of research. My mentoring began back in medical school when I worked under an American Heart Association Student's Scholarship with Phil Wolf. He first introduced me to stroke epidemiology through the Framingham Study. To this day, Phil is still a very important mentor to me. I have also had the privilege of working with J.P. Mohr when I arrived in New York at the Neurological Institute. J.P. has been an incredible inspiration, constantly telling me to question everything, take nothing for granted, and he has been supportive of my independent career development. I am very appreciative to both of them, because I would not be here today without all of their support and mentoring.
Global Vascular Risk Concepts
The topic of my lecture is global vascular risk estimation. Today, we are often talking about global issues such as the global economy, global warming, global health, and, last year, Vladimir Hachinski told us about global solutions in his Willis Lecture.2 All of us recognize that the global stroke burden is on the rise. Stroke is a major public health burden, and in developing countries, it is becoming more of an issue as epidemiologic transitions occur.3 As more countries become developed and Westernized, deaths from infectious disease decline, certain vascular risk factors become more prevalent, and transitions to a greater preponderance of chronic disease, such as stroke, become more apparent. We are facing a worldwide problem in terms of the future predicted global stroke burden.
Although at this international stroke meeting we are hearing about the latest research on stroke pathophysiology, epidemiology, diagnosis, treatment, and prevention, we need to recognize that stroke is just one of the many silos of a larger vascular problem. We need to be interacting and thinking more globally. The other vascular silos include myocardial infarction, vascular death, peripheral arterial disease, angina, TIA, and vascular cognitive impairment. These conditions have a variety of common links, including sharing certain risk factors, and can be considered part of “global vascular risk.” “Global” has really a double implication because it implies a worldwide problem and encompasses multiple vascular outcomes. When we use the term “global vascular risk,” we need to recognize the worldwide impact of vascular disease, as well as think more broadly beyond just stroke.
One way of conceptualizing global vascular risk is through a pyramid diagram. (Figure 1). The top of the pyramid illustrates those who have clinically significant vascular disease, while the layers of vascular risk progress from the base upwards. At the base of the pyramid are those who are “at risk.” The wider base of the pyramid illustrates the greater global prevalence of those at risk, and as we progress upwards the risk of vascular disease increases. At the top, stroke, MI, peripheral arterial disease, and vascular death, which in 2002 accounted for 16.7 million deaths, are projected to account for 18.1 million deaths in 2010.4 At the base of the pyramid, we need to recognize the high global prevalence of vascular risk-inducing behaviors such as smoking, which is projected at 1.3 billion, and physical inactivity at 60% to 85% of the population. As we move up a pyramid stage, hypertension is estimated to occur in 600 million, obesity in 300 million, and diabetes close to 150 million. Subclinical diseases, which represent the transition states before clinical disease becomes apparent, are asymptomatic conditions usually undetected by patients and physicians in the clinic. Conditions such as asymptomatic carotid stenosis, increased carotid intima-media thickness (IMT), silent cerebral infarcts, and white matter hyperintensities are more difficult to estimate on the global prevalence scale.
Figure 1.

Determinants of global vascular risk.
When you look at this pyramid, you will also notice there is a color variation from blue to yellow, to orange, to red. You may wonder why I chose these colors. Well, as it turns out, these are the terrorism alert Homeland Security colors, with red being the most severe and blue being guarded. They are also the colors that have been used for pollen alert levels on The Weather Channel. Regardless, they truly conceptualize the state of global alarm that we need to consider these vascular conditions.
When we think about any chronic disease and the opportunities for prevention, we need to consider the progression of disease over time (Figure 2). Any disease curve can be modeled as progressing through various epochs starting with the disease-free when we are truly focusing on primordial prevention. Next, we begin to move into the preclinical disease period where we focus on primary prevention. Primordial prevention refers to reducing the risk of developing a vascular risk factor, whereas traditionally primary prevention deals with modification of risk factors to reduce the risk of clinical disease. Once some pathology has been detected, disease may be present, so late primary prevention will be focused on altering the progression of the pathology to reduce the risk of developing clinically symptomatic disease. As clinicians, we often get involved much later on the curve when symptoms have already developed and someone crosses the symptom threshold. Here, we more often deal with secondary and tertiary prevention, and we need to recognize that we are intervening later on the curve of disease progression when it may be too late. Secondary prevention is usually focused on measures to reduce the risk of a recurrent event among those at high-risk or with disease, whereas tertiary prevention typically refers to rehabilitation and improving outcomes among those with disease. Over time, there is progression from a healthy stage, with or without a genetic predisposition, to the development of vascular risk factors often induced by certain behaviors. Subclinical disease then develops and progresses until an individual becomes symptomatic crossing the threshold into clinical vascular disease with the onset of conditions such as MI, peripheral arterial disease, TIA, stroke, vascular cognitive impairment, and eventually leading to vascular death. At all stages, we need to be working on preventing the evolution or progression of disease, and shifting, as much as possible, our chronic disease curve to avoid the development of these outcomes. Our prevention programs need to be more broadly focused on the multiple different global vascular outcomes.
Figure 2.
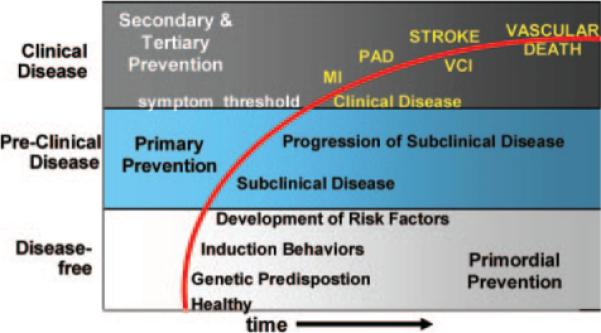
Primordial, primary, secondary, and tertiary prevention stages for global vascular risk.
The mission and strategic plan of the American Heart Association and American Stroke Association recognizes the importance of the global vascular burden. The mission statement calls for reducing disability and death from cardiovascular diseases and stroke. The 2010 impact goal is to reduce coronary heart disease, stroke, and risk by 25%. This statement includes “risk” as part of the goal. The AHA has worked toward these goals by issuing numerous guidelines and evidence-based recommendations, including those for primary and secondary stroke prevention.5,6 Such statements are aimed at changing practice and guiding healthcare providers to “get with the guidelines.” In these guidelines we sometimes struggle with the clinical trial evidence focusing on stroke as the main outcome, but, should we be talking about just preventing a stroke, or should we more broadly consider the prevention of stroke, MI, or vascular death?
Science Fiction: The Global Vascular Risk Tricorder
Science fiction has sometimes foretold the future. William Shatner, the actor who played Captain Kirk on the original Star Trek series, stated in a Discovery Channel broadcast, “With Star Trek, the medical community had been given a tantalizing glimpse into the future.” Back in 1966, one of the devices used by the crew was the “tricorder.” Now, I hope most know what a tricorder was, but if not, this was the diagnostic device used on the USS enterprise. It was a handheld tool used to get a complete readout of the health of a person in the field and provided a real-time complete picture of one's medical state from heartbeat to genetics.
Although such a device seems like a science fiction fantasy, data from multiple epidemiological studies are helping to develop many of the key inputs to build our future global vascular risk tricorder. Data that have greatly helped in this endeavor have come from studies such as the Framing-ham Study, Atherosclerosis Risk in Communities, the Cardiovascular Health Study, and from the multiple studies specifically evaluating stroke such as projects in Rochester, Minnesota, Greater Cincinnati Northern Kentucky, Brain Attack Surveillance in Corpus Christi, Baltimore–Washington, nationwide from the Reasons for Geographic and Racial Differences in Stroke, and in Northern Manhattan. Pooled analyses from multiple European cohorts have also generated global risk scores such as the SCORE project. To illustrate the components of our new global vascular risk tricorder, I will mainly cite data from our Northern Manhattan Cohort study, only because it is more familiar to me, but by no means wish to underestimate all of the terrific data that have resulted from the many other well-developed studies addressing these issues. All of these other studies are greatly helping in the development of more accurate global vascular risk estimation.
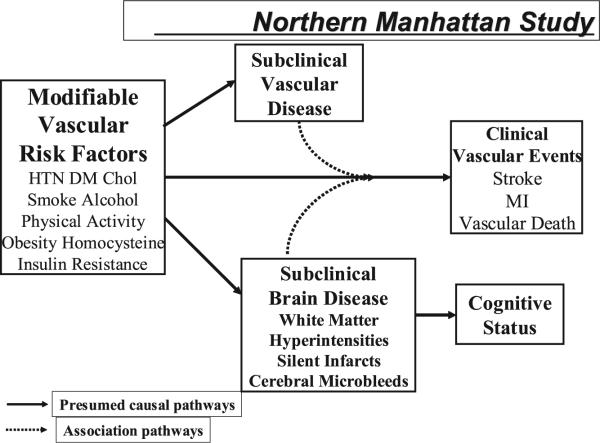
The Northern Manhattan Study (NOMAS) includes observations on a tri-ethnic, population-based, prospective cohort of 3298 stroke-free subjects (688 whites, 801 blacks, 1732 Hispanics, 77 other) who have been followed annually for multiple outcomes including stroke, MI, and death.7,8 Baseline enrollment was initiated in 1993. With a median of 6.5 years of follow-up, 220 strokes, 205 myocardial infarctions, and 304 vascular deaths have been documented. We are now able to evaluate the relationship of some of our baseline variables as components predicting global vascular risk with a particular focus on modifiable factors (Figure 3).
Figure 3.
Study design of the Northern Manhattan Study.
Inputs for Our Global Vascular Risk Estimator
Sociodemographics and Prior Disease
As we think about the key inputs for our global vascular risk tricorder, it is clear that age, gender, race–ethnicity, and social cultural factors, that sometimes are difficult for us to measure, have important inputs into our model. Our original NOMAS data have demonstrated race–ethnic differences in stroke incidence.9,10 Blacks had a 2.4-fold increased annual stroke incidence, and Hispanics a 2-fold increased annual stroke incidence compared with whites living in the same community. Moreover, socioeconomic disparities, which may explain some race–ethnic disparities, exist in our NOMAS community cohort, with 82% of our white group having completed high school, 63% of our blacks, and only 22% of our Hispanics. In NOMAS, we have included age, gender, race–ethnicity, and education in our global vascular risk estimation model. We also recognize that the greatest vascular risks occur among subjects at the top of the pyramid who already have had a stroke or TIA, cardiac disease, or peripheral arterial disease. In our global vascular risk estimation model, we have found that baseline cardiac diseases, as well as peripheral arterial disease, are both independent predictors of stroke, MI, and vascular death.
Vascular Risk-Inducing Behaviors
Multiple studies have provided essential data on the importance of vascular risk-inducing behaviors. These behaviors are at the base of our global vascular risk pyramid because they are quite prevalent and can lead to other vascular risk factors. In NOMAS, we have been able to learn about some of these vascular risk-inducing behaviors, particularly among lower socioeconomic groups. Smoking, physical inactivity, alcohol, and dietary factors are some of the vascular risk-inducing behaviors that we and others have considered. In our global vascular risk model, cigarette smoking is an independent predictor of stroke, MI, or vascular death. Prior smoking increases the risk of stroke, MI, or vascular death by a factor of ≈1.3. Current smoking increases it even further, with a 2.1-fold increased hazard rate indicating a modifiable dose-response relationship. Physical activity is protective, with light physical activity providing some reduction in the hazard (HR=0.9), but moderate to heavy physical activity reduces the hazards of stroke, MI, and vascular death even further (HR=0.6). Alcohol has a complicated relationship with global vascular risk that is likely dependent on dose. Light to moderate alcohol consumption of no >2 drinks per day actually reduces the hazard of stroke, MI, and vascular death.8 Heavy drinking of >5 drinks per day increases vascular risk.11 Studies of the effects of diet on vascular risk are difficult and require reliable measurements in large samples to detect associations. In NOMAS, we have preliminary reports on the deleterious effects of dietary fat and sodium, but the key dietary components of our global vascular risk estimator are still elusive.12,13
Vascular Risk Modulators
As we move up the vascular risk pyramid, we enter the stage I have classified as “vascular risk modulators.” I have used the term “modulator” to help express the continuous relationship that many of these components have with global vascular risk prediction. Recording blood pressure, rather than hyper-tension, fasting glucose, not just diabetes, lipid levels, not just hyperlipidemia, and waist circumference, not just obesity, may provide more predictive value. These modulators are all continuous, rather than categorical, variables. It is important for us to recognize that as clinicians, we sometimes categorize, often because it is just easier to illustrate relationships and guide treatment recommendations. It is sometimes more convenient for us to think about classifying someone as hypertensive versus not, but we may lose information and precision because many of these relationships are continuous. They have linear or nonlinear associations with the outcomes of interest, and there may be interactions across various subgroups by age, gender, and race–ethnicity. Moreover, we are now becoming more familiar with addressing constellations of risk modulators that could have more complex quantitative relationships as we combine these continuous variables together.
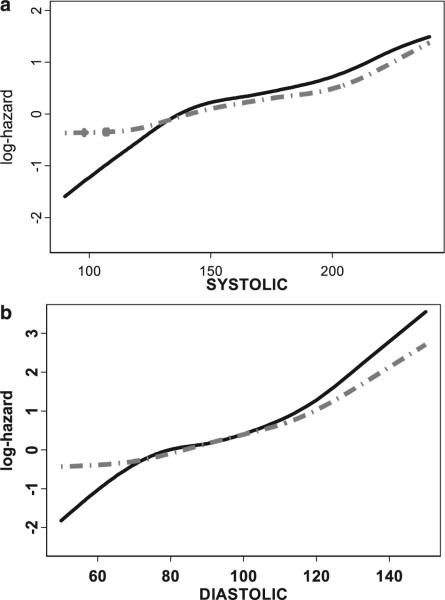
Data from NOMAS illustrate some of these continuous relationships with specific vascular risk modulators. The relationship between blood pressure and the hazards for specific vascular outcomes are shown in the log-hazard curves in Figure 4. The range of systolic blood pressure is enumerated on the x-axis from 100 mm Hg to ≈250 mm Hg. The log-hazards for stroke and stroke, MI, or vascular death are enumerated on the y-axis. A linear increase in hazard is illustrated for stroke, as well as stroke, MI, or vascular death as outcomes. A similar relationship is also found for diastolic blood pressure. Blood pressure is a great example of a continuous vascular risk modulator and an important component in our model for predicting global vascular risk. In NOMAS, as well as in the Framingham Stroke model, we have also found an interaction between diastolic blood pressure and taking an anti-hypertension medication, with a greater risk among those on medicines, which probably reflects a greater severity of hypertension.
Figure 4.
Relationship between (a) systolic blood pressure (mm Hg) and (b) diastolic blood pressure (mm Hg) and the log-hazard for stroke (solid line), and stroke, myocardial infarction, and vascular death (dotted line) from the Northern Manhattan Study.
Waist circumference, a potential measure of obesity and abdominal adipose tissue, may be another important continuous variable predictive of global vascular risk. In NOMAS, we have found an increasing hazard of stroke, MI, or vascular death as waist circumference increases in both women and men. A significant interaction was detected with a different relationship for men and women. A smaller waist circumference had an increased hazard among women compared with the same waist circumference in that of men. When we use categorical classifications of waist circumference, there are differential cut points by gender to define an increased risk. This illustrates the need to think about gender-specific relationships when we evaluate vascular risk factors.
Fasting blood glucose, not just diabetes, may be another continuous vascular risk modulator. We recognize that borderline elevations of fasting blood glucose may indicate insulin resistance that pre-dates definite diabetes. In NOMAS, we have observed an increasing hazard of stroke, MI, and vascular death with increasing fasting blood glucose levels and an interaction among those with and without any baseline cardiac disease. Fasting blood sugar increased the risk of stroke, MI, and vascular death, which increased even greater among those who had pre-existing cardiac disease.
There are also attempts to try to bring different vascular risk modulators together as constellations of risk factors, such as in the definition of the metabolic syndrome. Metabolic syndrome, as currently defined, however, is still considered a categorical variable defined by having at least 3 other categorical risk conditions: an elevated triglyceride, an elevated blood pressure, an elevated fasting blood sugar, an elevated waist circumference, or low HDL. Each factor is defined by categorical cut points. In NOMAS, we have demonstrated an increased risk of stroke and stroke, MI, or vascular death among those with metabolic syndrome with an adjusted hazard ratio of 1.6 for ischemic stroke, and a hazard of 1.4 for stroke, MI, and vascular death.14 The concept of combining vascular risk factors may also increase the prevalence of those classified as abnormal who may have been missed using classic definitions. A more complex approach may require classifying a metabolic index on a continuous scale through some quantitative summation of all of these continuous vascular risk modulators. Further definitions and evolutions of this concept are likely to occur, particularly as we move to thinking about the metabolic syndrome on a continuous scale.
It is also important to recognize that there are worldwide shifts occurring in the global impact of vascular risk factors, particularly as countries develop. The prevalence of vascular risk factors, such as blood pressure, tobacco use, and cholesterol, increases as countries evolve from high-mortality developing countries to lower-mortality developing countries, to developed countries. The epidemiologic transition that occurs as countries develop and people survive longer will mean an even greater burden across the world of stroke, MI, and vascular death.
Subclinical Vascular Diseases
As risk factors continue uncontrolled over time, subclinical diseases develop that may increase the risk of stroke, MI, and vascular death. Potential inputs to our global vascular risk-estimating tricorder include subclinical diseases characterized by newer imaging techniques. Subclinical vascular diseases include measurements of carotid plaque, carotid intima-media thickness, carotid distensibility, brachial endothelial reactivity, and aortic arch atheroma. These conditions may be subclinical markers that are related to underlying vascular risk factors, as well as predictors of the clinical outcomes of stroke, MI, and vascular death. In NOMAS, we have been able to show that imaging a small amount of carotid plaque (75th percentile >1.8 mm), not enough to get a surgeon or interventionalist too excited, was associated with an increased risk of stroke, MI, and vascular death. The risk attenuated with adjustment of other vascular risk factors, but remained a significant predictor of global vascular risk. We have also demonstrated that irregular carotid plaque is a more potent risk factor for stroke than flat plaque.15
Subclinical Brain Diseases
With the increasing use of more sensitive quantitative brain MRI techniques, the detection of subclinical brain disease is no longer a topic for science fiction. Conditions such as white matter hyperintensities, silent cerebral infarcts, brain atrophy, and cerebral microbleeds may be related to the individual underlying risk factors, as well as predictors of stroke, MI, and vascular death. Data from the Cardiovascular Health study and the Rotterdam study have demonstrated relationships between these conditions and the risk of vascular outcomes, as well as potential predictors of cognitive status.16–18 In NOMAS we have found associations between cognitive impairment and quantitative white matter hyperintensity volume, particularly impairments in motor dexterity and executive function.19,20 Subclinical brain disease, therefore, is another new frontier in terms of trying to measure and quantitate these factors as they relate to risk factors, and perhaps use in our global vascular risk tricorder.
Biomarkers
Results of various blood tests often labeled as serum biomarkers may also be helpful in global vascular risk prediction. New tests are being developed to detect those who may have an increased risk of stroke, MI, or vascular death. Such tests may be helpful for risk stratification, particularly among those at intermediate risk, as well as for monitoring the effects of modification. In NOMAS, we have found that elevations of white blood cell counts are related to the risk of stroke, MI, and vascular death, and that levels of other inflammatory biomarkers, including TNF receptor and IL-2, are related to subclinical carotid disease.21–23 Biomarkers may also provide an important opportunity to add blood test results to our global vascular risk models, but we need to establish whether they are independently predictive after adjusting for conventional risk factors, add incremental value to risk prediction, and are cost-effective.24
Subclinical markers of disease may also help us in the design and conduct of randomized clinical trials by serving as surrogate end points in proof of concept phase 2 trials. Monitoring for a vascular response to therapeutic interventions has been accomplished in multiple randomized trials using statins and carotid IMT as the outcome. Subclinical markers also help us with screening and risk stratification particularly for persons with an intermediate risk, as well as help in clarifying pathogenesis and in detecting new genes.
Genetics and Risk Detection
On the Star Trek Enterprise, a mutation scanner was available to detect for genetic anomalies. Although we may not be quite there yet, we are learning more about the genetic determinants of global vascular risk. A genetic predisposition is at the base of our vascular risk pyramid, but we currently know very little about which genetic markers are predictive of risk. At this International Stroke Meeting, there are multiple abstracts being presented regarding new polymorphisms related to vascular risk factors or stroke itself. Therefore, genetic predisposition could become an important input to our vascular risk estimator.
In Northern Manhattan, we have been trying to look at the importance of genetics in a variety of ways. Although we know that environmental determinants are clearly related to stroke, vascular risk factors, as well as subclinical vascular diseases, we are less knowledgeable about the genetic determinants of these conditions. We also know that stroke is a complex disorder with multiple phenotypes, multiple patho-genetic mechanisms, and likely multiple genes involved. Studies using quantitative subclinical traits such as carotid IMT, distensibility, and left ventricular mass, which are less complex disorders, may help elucidate the genetic factors predisposing to stroke.25 In the Northern Manhattan Family Study, we have enrolled nearly 1500 family members from 108 high-risk Hispanic probands from NOMAS. Many of our Hispanic families are large. The mean family size was nearly 11 with our largest family having 56 members enrolled, and 52% of our families have >10 members enrolled.
Carotid IMT is one of the key quantitative traits measured among our families. It is a continuous measure and has a normal distribution in our family cohort. Every family member has some measure of IMT, so every person contributes to the estimate of heritability and to the detection of quantitative trait loci through genetic linkage. Our initial publications have focused on estimating the heritability of some of these traits before initiating a whole genome wide scan. Carotid IMT had a heritability of 38%, distensibility 17%, LV mass 47%, homocysteine 38%, and waist circumference 47%.26–28 Metabolic syndrome defined by the current definition as a categorical variable had a heritability of 19%.29 Overall, the detection of genetic factors are still in their infancy, but will likely be important in the design of our future global vascular risk tricorder.
Global Vascular Risk Estimation
Despite the recent advances in imaging, biomarkers, and genetics, we are probably not yet ready to incorporate all of these factors into a cost-effective global vascular risk estimator. We are not yet certain about how much incremental value these new measures provide over the conventional vascular risk factors. Our current global vascular risk tricorder includes the following variables: sociodemographics (age, gender, race–ethnicity, and education), vascular risk-inducing behaviors (smoking, alcohol, physical activity), vascular risk modulators (systolic blood pressure, diastolic blood pressure, and a diastolic blood pressure hypertensive medication interaction, waist circumference, and a waist circumference gender interaction, fasting blood sugar, fasting blood sugar and a cardiac disease interaction, and HDL), and the existence of clinical vascular disease (any cardiac disease, a cardiac disease medication interaction, peripheral vascular disease). Many of these factors were part of the Framingham Risk profile, whereas others such as physical activity, alcohol, HDL, waist circumference, and fasting blood sugar were not. Future editions of the global vascular risk tricorder may include a Doppler probe to measure subclinical carotid vascular disease, and perhaps a serum and DNA analyzer, when we clarify all the additional predictive inputs to our models. More complex global vascular risk models, however, will need to address the incremental predictive value of adding more inputs to the model, cost considerations when results of imaging or blood tests are used, and statistical issues regarding the handling of missing data.
Refined Prediction Leads to Better Prevention
Whereas estimating global vascular risk may be important for prediction, the real key to reducing the number of deaths due to vascular disease is to improve our ability to modify global risk.30 An improved ability to predict risk should lead to better prevention efforts. The goal of mass approaches would be to shift the entire population's global vascular risk score through policy changes, behavioral shifts, and economic disincentives and incentives (Figure 5a). These require concerted efforts working through the AHA and other organizations to lobby congress to make broad policy changes to help shift the whole population to a lower global vascular risk score. Collaborative partnerships combining the strengths of the AHA, American Cancer Society, and American Diabetes Association have already been initiated, and need to be enhanced to address cardiovascular disease and diabetes, as well as cancer.31 Economic disincentive programs such as increased taxes on cigarettes and alcohol, as well as economic incentive programs such as tax credits for physical activity or joining a gym, are possible approaches. Government-sponsored programs to ban trans-fats in restaurants, restrict smoking in public areas, increase physical activity programs in public schools, modify school lunch menus, and educate the public about the benefits of a healthy lifestyle are other potential mass approaches for global vascular risk prevention. As health care professionals, in contrast, we usually get involved through the high-risk approach (Figure 5b). Our focus is on detecting those at the highest levels on the distribution of global vascular risk, and trying to reduce risk through behavior modifications and medications.
Figure 5.
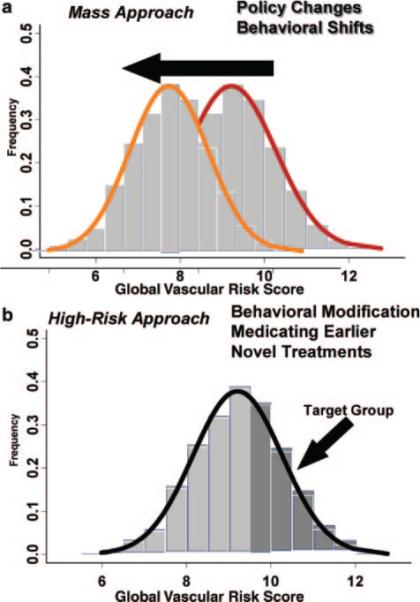
Shifting the global vascular risk score through the (a) mass approach and (b) high-risk approach. The histogram depicts the distribution of the global vascular risk score in our NOMAS cohort.
Earlier and more refined global vascular risk estimation, now and in the future, should lead to advances in our understanding of ways to modify risk. Novel approaches are needed to reduce the risk of global vascular risk. We need to develop more innovative methods to modify behavior and testing new cost-effective medications that will need to be started very early before any clinical disease has been detected.32 We need to shift our paradigm and continue to think more globally, in terms of the risk of global vascular outcomes, and in terms of the impact of vascular disease on global public health. The goal is to make our current predictions for the mortality from vascular diseases in 2010 wrong. In the words of Louis Pasteur, “When thinking about a disease, I never dream of a remedy for it, but instead a means of preventing it.”33
Acknowledgments
I express my thanks to all of the subjects who have enrolled in our Northern Manhattan Study and Northern Manhattan Family Study and the many research assistants who have helped collect, enter, and analyze data for the studies. I also specifically acknowledge the support of Bernadette Boden-Abala, Tanja Rundek, Mitchell Elkind, Myunghee Paik, Clinton Wright, Edison Sabala, and Janet Derosa.
Sources of Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS 29993 and 40807).
Footnotes
Disclosures
Dr Sacco serves as a consultant to Boehringer Ingelheim, BMSSanofi Partnership, Merck, and Glaxo Smith Kline.
References
- 1.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, Manolio TA, Whisnant JP, Wolf PA. Risk Factors Panel—American Heart Association Prevention Conference IV. Stroke. 1997;28:1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 2.Hachinski V. The 2005 Thomas Willis Lecture. Stroke and vascular cognitive impairment: a transdisciplinary, translational and transactional approach. Stroke. 2007;38:1396. doi: 10.1161/01.STR.0000260101.08944.e9. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 4.Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. World Health Organization in collaboration with the Centers for Disease Control and Prevention. World Health Organization; 2004. Available at: http://www.who.int/cardiovascular_diseases/resources/atlas/en/ [Google Scholar]
- 5.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly Hayes M, Nixon JV, Sacco RL, American Heart Association/American Stroke Association Stroke Council. Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group. Cardiovascular Nursing Council. Clinical Cardiology Council. Nutrition, Physical Activity, and Metabolism Council. Quality of Care and Outcomes Research Interdisciplinary Working Group. American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group. Cardiovascular Nursing Council. Clinical Cardiology Council. Nutrition, Physical Activity, and Metabolism Council. the Quality of Care and Outcomes Research Interdisciplinary Working Group The American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort. The Northern Manhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 8.Elkind MSV, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 10.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Elkind M, Boden-Albala B, Lin I-F, Kargman DE, Hauser WA, Shea S, Paik M. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999;281:53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 12.White H, Szumski A, Boden-Albala B, Williams A, Paik MC, Sacco RL. Dietary intake of total fat as a possible risk factor for ischemic stroke: the Northern Manhattan Study. Stroke. 2005;36:427. [Google Scholar]
- 13.Williams A, White H, Szumski A, Boden-Albala B, Paik MC, Sacco RL. Dietary intake of salt as a risk factor for ischemic stroke: the Northern Manhattan Stroke (NOMAS). Stroke. 2005;36:460. [Google Scholar]
- 14.Boden-Albala B, Lee HS, Rundek T, Paik MC, Sacco RL. Race-ethnicity, stroke risk and the metabolic syndrome: findings from the Northern Manhattan Stroke Study. Stroke. 2004;35:243. [Google Scholar]
- 15.Prabhakaran S, Rundek T, Ramas R, Elkind MS, Paik MC, Boden-Albala B, Sacco RL. Carotid plaque surface irregularity predicts ischemic stroke: the northern Manhattan study. Stroke. 2006;37:2696–2701. doi: 10.1161/01.STR.0000244780.82190.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr, Cardiovascular Health Study Collaborative Research Group White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 17.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, Rotterdam Scan Study Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 18.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 19.Wright CB, Guzman J, Stern Y, Sacco RL, Decarli C. Delayed memory and motor function are associated with white matter hyperintensities. American Heart Association International Stroke Conference. Stroke. 2006;37:641. [Google Scholar]
- 20.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkind MS, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology. 2005;64:2121–2125. doi: 10.1212/01.WNL.0000165989.12122.49. [DOI] [PubMed] [Google Scholar]
- 22.Elkind MS, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke. 2002;33:31–38. doi: 10.1161/hs0102.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkind MS, Rundek T, Sciacca RR, Ramas R, Chen HJ, Boden-Albala B, Rabbani L, Sacco RL. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis. 2005;180:181–187. doi: 10.1016/j.atherosclerosis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 25.Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Hank Juo S-H, Sacco RL. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6 and hepatic lipase genes: The Northern Manhattan Prospective Cohort Study. Stroke. 2002;333:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juo SH, Lin HF, Rundek T, Sabala EA, Boden-Albala B, Park N, Lan MY, Sacco RL. Genetic and environmental contributions to carotid intima-media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke. 2004;35:2243–2247. doi: 10.1161/01.STR.0000142132.20442.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juo SH, Di Tullio MR, Lin HF, Rundek T, Boden-Albala B, Homma S, Sacco RL. Heritability of left ventricular mass and other morphologic variables in Caribbean Hispanic subjects: the Northern Manhattan Family Study. J Am Coll Cardiol. 2005;46:735–737. doi: 10.1016/j.jacc.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juo S-H, Rundek T, Lin H-F, Chen R, Lan M-Y, Huang JS, Boden-Albala B, Sacco RL. Heritability of carotid artery distensibility in Hispanics. Stroke. 2005;36:2357–2361. doi: 10.1161/01.STR.0000185926.05011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin H-F, Boden-Albala B, Juo S-H, Park N, Rundek T, Lan M-Y, Sacco RL. Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia. 2005;48:2006–2012. doi: 10.1007/s00125-005-1892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkind MS. Implications of stroke prevention trials: treatment of global risk. Neurology. 2005;65:17–21. doi: 10.1212/01.wnl.0000171745.13592.cb. [DOI] [PubMed] [Google Scholar]
- 31.Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K, Thun MJ, American Cancer Society. American Diabetes Association. American Heart Association Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35:1999–2010. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 32.Radziszewska B, Hart RG, Wolf PA, D'Agostino RB, Sr, Cutler JA. Clinical research in primary stroke prevention: needs, opportunities, and challenges. Neuroepidemiology. 2005;25:91–104. doi: 10.1159/000086342. [DOI] [PubMed] [Google Scholar]
- 33.Pasteur L. Faite a l'Association amicale des anciens eleves de l'Ecole Centrale des Arts et manufactures (groupe de Paris). Le Genie Civil. 1884;5:133–135. [Google Scholar]