Abstract
Background
CB1 cannabinoid receptors in the brain are known to participate in the regulation of reward-based behaviors, however, the contribution of each of the endocannabinoid transmitters, anandamide and 2-arachidonoylglycerol (2-AG), to these behaviors remains undefined. To address this question, we assessed the effects of URB597, a selective anandamide deactivation inhibitor, as a reinforcer of drug-seeking and drug-taking behavior in squirrel monkeys.
Methods
We investigated the reinforcing effects of the fatty acid amide hydrolase (FAAH) inhibitor URB597 in monkeys trained to intravenously self-administer Δ9-tetrahydrocannabinol (THC), anandamide or cocaine, and quantified brain endocannabinoid levels using liquid chromatography/mass spectrometry. We measured brain FAAH activity using an ex vivo enzyme assay.
Results
URB597 (0.3 mg/kg, intravenous) blocked FAAH activity and increased anandamide levels throughout the monkey brain. This effect was accompanied by a marked compensatory decrease in 2-AG levels. Monkeys did not self-administer URB597 and the drug did not promote reinstatement of extinguished drug-seeking behavior previously maintained by THC, anandamide, or cocaine. Pretreatment with URB597 did not modify self-administration of THC or cocaine even though, as expected, it significantly potentiated anandamide self-administration.
Conclusions
In the monkey brain, the FAAH inhibitor URB597 increases anandamide levels while causing a compensatory down-regulation in 2-AG levels. These effects are accompanied by a striking lack of reinforcing properties, which distinguishes URB597 from direct-acting cannabinoid agonists such as THC. Our results reveal an unexpected functional heterogeneity within the endocannabinoid signaling system, and suggest that FAAH inhibitors might be used therapeutically without risk of abuse or triggering of relapse to drug abuse.
Keywords: Anandamide, FAAH, self-administration, reinstatement, URB597, 2-arachidonoylglycerol
INTRODUCTION
Drugs that activate cannabinoid receptors, the molecular target of Δ9-tetrahydrocannabinol (THC) in marijuana, reduce nausea and emesis produced by chemotherapy (1), alleviate pain symptoms associated with central and peripheral neuropathies (2,3), decrease pain and spasticity in multiple sclerosis (4,5), and improve psychomotor deficits in Tourette’s syndrome (6). Despite such broad therapeutic potential, the clinical usefulness of these agents is limited by their psychotropic and reinforcing effects, which account for the remarkable prevalence of marijuana as an abused drug (7,8). The rewarding properties of plant-derived or synthetic cannabinoid drugs are reasonably well understood. Mechanistically, they have been linked to the ability of these substances to activate CB1-type cannabinoid receptors in the central nervous system, enhance activity of midbrain dopaminergic neurons, and elicit dopamine release in the reward-controlling shell region of the nucleus accumbens (7,8). By contrast, the contribution of endocannabinoid signals to the regulation of normative reward-based behaviors is still unclear, despite indications that pharmacological or genetic interruption of CB1 receptor activity strongly affects such behaviors (9).
One important set of questions that remains unanswered relates to the chemical neuroanatomy of endocannabinoid-mediated reward and, in particular, to the functions served by individual endocannabinoid substances in reward modulation. Two such substances have been characterized: anandamide and 2-arachidonoylglycerol (2-AG) (10).Both compounds are produced in and released from neuronal and glial cells upon demand, and are eliminated by uptake into cells followed by intracellular hydrolysis. In the brain, anandamide is primarily hydrolyzed by the postsynaptic membrane-associated amidase, fatty acid amide hydrolase (FAAH), which also cleaves other non-cannabinoid fatty-acid ethanolamides such as oleoylethanolamide (OEA) (11,12). On the other hand, 2-AG is predominantly hydrolyzed by the presynaptic cytosolic lipase, monoacylglycerol lipase (MGL) and, to a lesser extent, by two additional membrane-associated lipases, ABHD6 and ABHD12 (13,14).
The existence of distinct biochemical pathways mediating the deactivation of anandamide and 2-AG suggests that selective pharmacological interruption of each of these pathways might help define the contribution of individual endocannabinoid signals to the modulation of reward. The compound URB597 is a potent and selective inhibitor of intracellular FAAH activity (15,16,17). In rodents, URB597 increases brain anandamide levels without changing the levels of 2-AG (17,18). Moreover, the drug elicits antinociceptive, anxiolytic-like, and antidepressant-like effects (17,18,19,20,21), which are likely mediated by enhanced anandamide activity at CB1 receptors, because they are attenuated by the CB1 antagonists rimonabant and AM251 (17,18,19). Importantly, URB597 does not cause place preference or substitute for THC in rat drug-discrimination tests, an indication that it may lack hedonic properties (18).
In the present study, we investigated the rewarding properties of URB597 in squirrel monkeys, a primate species that has been extensively used to model human reward-based behavior and has provided precious insights into the reinforcing effects of cannabinoids (22,23,24,25,26). We first determined the effects of URB597 on endocannabinoid levels in areas of the brain associated with reward, memory and emotional responses to stress. Next, we tested whether URB597 would either be self-administered by monkeys or would alter their self-administration of THC and cocaine. Finally, to assess the potential of URB597 to precipitate relapse to abuse in abstinent individuals, we examined its ability to reinstate extinguished drug-seeking behavior.
METHODS AND MATERIALS
Subjects
Twenty three adult male squirrel monkeys (Saimiri sciureus) weighing 0.9 to 1.1 kg were housed in individual cages in a temperature- and humidity-controlled room with unrestricted access to water. Monkeys were fed (two hours after the session) a daily ration consisting of five biscuits of high protein monkey diet (Lab Diet 5045, PMI Nutrition International, Richmond, Indiana) and two pieces of Banana Softies (Bio-Serv, Frenchtown, NJ) that maintained their body weights at a constant level throughout the study. Fresh fruits, vegetables and environmental enrichment were provided daily.
One group of five monkeys was used for experiments with the anandamide self-administration baseline: all monkeys had a history of anandamide self-administration (6754, 67F4, 42A, 70F4, 1568). Another group of four monkeys was used for experiments with the THC self-administration baseline: three monkeys had a history of THC self-administration (434, 37B, 453) and one monkey had history of anandamide self-administration (66B2). Another group of four monkeys was used for experiments with the cocaine self-administration baseline; all monkeys had a history of cocaine self-administration (70F7, 01714B, 5045, 39B). A group of ten monkeys with no prior exposure to cannabinoids was used for neurochemical analyses (26–90, 421F, 2598, 2557, 3533, 1701-05, 3668-04, 33B-03, S457–04, 4–85).
Adult male Wistar rats (270–300 g), n = 6–12 per group, were used for evaluation of brain lipid levels in rats. The animals were housed at constant room temperature and humidity under a 12-h light/dark cycle. Food and water were available ad libitum.
Monkeys and rats were maintained in facilities fully accredited by AALAC and experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the Intramural Research Program, NIDA, NIH, and followed the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2004).
Apparatus and self-administration procedure
Experimental chambers and other apparatus used in this study were the same as previously described (22). Monkeys were surgically prepared with chronic indwelling venous catheters (polyvinyl chloride) (27). The catheters were connected to polyethylene tubing, which passed out of the isolation chamber where it was attached to a motor-driven syringe pump. Before the start of each session, monkeys were placed into Plexiglas chairs and restrained in the seated position by waist locks. Before each session, catheters were flushed with 1 ml of saline and one priming injection was delivered (calculated to fill the dead space of the catheter).
At the start of the session, the white house light was turned off and green stimulus lights were turned on. In the presence of the green lights, monkeys were required to make 10 responses on a lever (10-response fixed-ratio schedule of reinforcement; FR10) to produce an injection of anandamide, THC or cocaine. Completion of 10 responses on the lever turned off the green lights and produced an intravenous (i.v.) injection of 40 µg/kg of anandamide, 4 µg/kg of THC or 30 µg/kg of cocaine paired with a 2-s illumination of amber stimulus lights (brief stimulus). Duration of each injection was 0.2 s and injection volume was 0.2 ml. Each injection was followed by a 60-s timeout period, during which the chamber was dark and lever presses had no programmed consequences. One-hour sessions were conducted five days per week (typically Monday to Friday). All monkeys had learned to respond under the FR10 schedule for the particular training drug prior to beginning this study.
Dose-effect curve determinations
When responding for the training dose of drug (40 µg/kg anandamide, 4 µg/kg THC or 30 µg/kg cocaine) was stable for at least five consecutive sessions (less than 15% variability), self-administration behavior was extinguished by replacing drug with its vehicle for five consecutive sessions, followed by replacement of vehicle with a different dose of a drug for five sessions. This order of testing was repeated until the dose-effect curve for each drug was constructed (anandamide doses: 3, 10, 30, 56, 100 µg/kg/injection; THC doses: 1, 2, 4, 8 µg/kg/injection; cocaine doses: 1, 3, 30, 100 µg/kg/injection). This was followed by determination of a dose-effect curve for URB597 (doses ranged from 1, 3, 10, 30, 100 µg/kg/injection) in each group of monkeys. Each URB597 dose was studied for four to five consecutive sessions and each dose-condition was separated by four to five consecutive sessions of vehicle substitution.
Pretreatment tests
Afterwards, we studied the effects of three-day pre-session treatment with 0.3 mg/kg URB597 or its vehicle (given i.v., 30 min before session) on self-administration of the 3 and 56 µg/kg doses of anandamide, 1 and 4 µg/kg doses of THC or 1 and 30 µg/kg doses of cocaine. URB597 was administered only after at least three consecutive sessions of stable responding for each drug-dose with vehicle pretreatment was reached (less than 15% variability). Monkeys were injected with vehicle or URB597 in their home cage.
Drug-induced reinstatement
After completing the previous experiments, monkeys self-administered the training dose of each drug (THC, anandamide or cocaine) for at least five sessions until responding was stable (less than 15% variability over three sessions). Self-administration behavior was then extinguished by substituting vehicle for THC, anandamide, or cocaine, but maintaining the presentation of the brief-stimulus associated with each injection. Then we tested reinstatement of extinguished drug-taking behavior by priming injections of THC (40 µg/kg, i.v., given immediately before the session) or URB597 (0.3 mg/kg, i.v., given 30 minutes before the session) in all three groups of monkeys. Reinstatement effects of each pretreatment were studied for three consecutive sessions starting after at least three days of stable vehicle extinction. Monkeys received injections of vehicle or URB597 in their home cage. THC was injected in the chair immediately before the session.
Brain removal procedure
Food was withheld 12 h prior to this procedure. Anaesthesia was induced and maintained with isoflurane (1.0-2%). Monkeys were weighed, prepared with a venous line (lateral saphenous vein), placed on a surgery table and kept warm by heat lamps. Body temperature and ECG were monitored throughout anaesthesia. After animals were stabilized (usually after 5–15 minutes of anaesthesia), URB597 (0.3 mg/kg) or its vehicle was intravenously injected 1 h prior to euthanasia and the venous line was flushed with 0.5 ml of saline. Body temperature, SPO2, pulse and rate of respiration were recorded every 10 min. After 1 h, monkeys were euthanized with Euthasol (0.1 ml/kg i.v.). Death was confirmed by the absence of respiration and heart beat on ECG. Brains were quickly removed, the cerebella were separated and forebrains were dissected. Each hemisphere was cut into 3 parts by two coronal sections made at approximately AP +12.5 and −5 (28). Brain fragments were snap-frozen in isopentane (−50°C), wrapped in aluminium foil, and placed in dry ice. Samples were stored in the freezer (−80°C) for 2 days and then shipped on dry ice to the University of California Irvine for analyses.
In one set of experiments, rats were sacrificed by rapid decapitation under light anaesthesia 2 h after injection of URB597 or vehicle. In other experiments, rats were food deprived for 12 h and sacrificed by decapitation 1 h after injection of URB597 or vehicle, while maintained under isoflurane anaesthesia for the duration of the experiment. In either case, brains were rapidly removed, and the hippocampus and prefrontal cortex was dissected from the fresh tissue over ice. Brain regions were frozen in dry ice, and stored at −80°C until lipid and enzymatic analyses.
Lipid analyses
Select regions of the brain left hemisphere were dissected over dry ice, using a stereotaxic atlas as a guide (28). Lipids were extracted with methanol-chloroform and fractionated by silica gel chromatography. Anandamide, 2-AG and OEA were quantified by liquid chromatography/mass spectrometry (LC/MS), as described (29). Lipid extractions on all brain regions were done at the same time. All analytical standards were prepared in the laboratory (30), except for 2-AG which was purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Enzyme assays
Please see supplemental material.
Drugs
Please see supplemental material.
Statistical analyses
Cumulative-response records were obtained during all sessions to assess within-session patterns of responding. Rates of responding during self-administration sessions are expressed as responses per second averaged over the one-hour session, with responding during time-outs not included in calculations. Injections per session represent total number of injections delivered per session. Data for dose-effect curves are expressed as mean response rates and numbers of injections per session ± SEM over the last three sessions. In addition, total intake of anandamide, THC or cocaine for each session was calculated. Reinstatement data and effects of pretreatment with URB597 on drug self-administration are expressed as mean ± SEM of total numbers of injections per session over three sessions. Statistical analysis (SigmaStat program; Jandel Scientific, USA) was done using single-factor repeated measures ANOVA to assess differences between vehicle and test-drug pretreatment conditions or between different doses of anandamide, THC, cocaine or URB597 and vehicle. Significant main effects were analyzed further by subsequent paired comparisons to control values using Dunnett’s test (Figure 5). Bonferroni t-test was used (Figures 6 and 7) when the number of observations did not allow for the use of the Dunnett’s test. Differences between effects of vehicle and URB597 pretreatment on lipid levels and FAAH activity were analyzed using single-factor ANOVA. Differences were considered statistically significant when p < 0.05.
Figure 5.
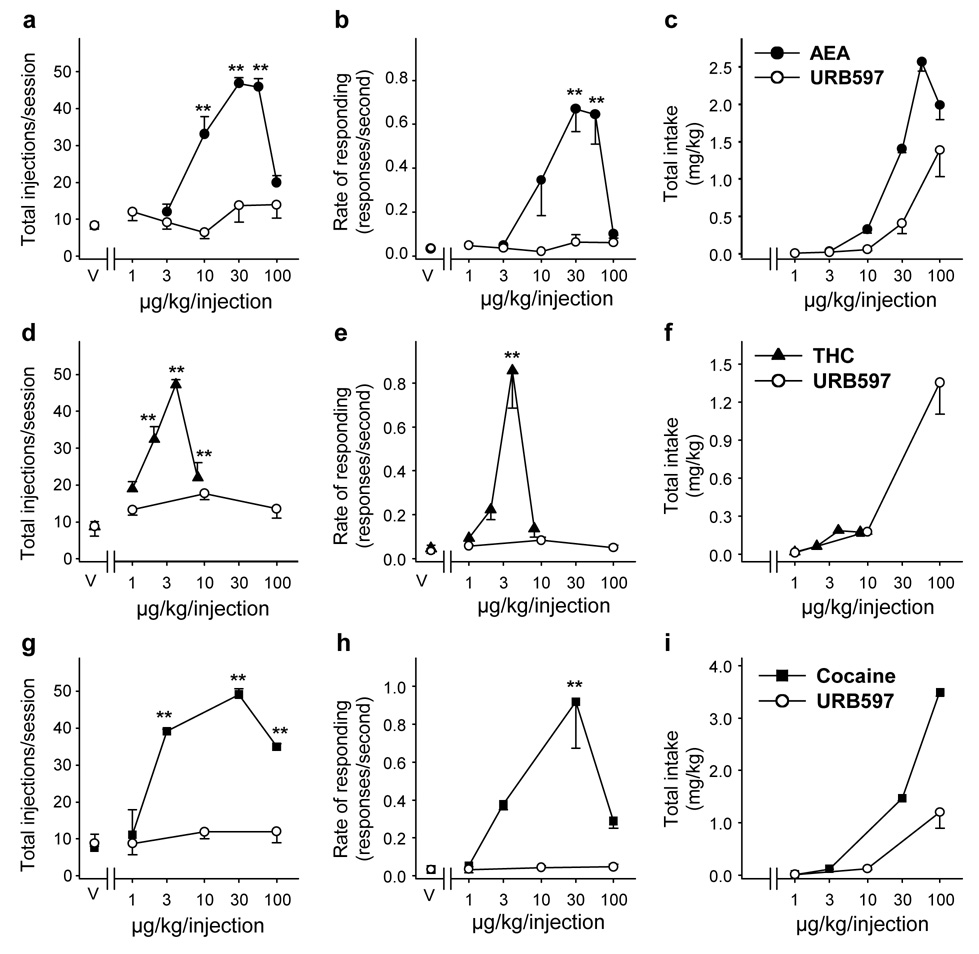
Dose-response curves for self-administration of URB597 in squirrel monkeys that had previously self-administered anandamide (AEA, a–c), THC (d–f) or cocaine (g–i). Number of injections per session (a, d, g), overall rates of responding (b, e, h) in the presence of the green light signaling drug availability, and total drug intake per session (c, f, i). Each point represents the mean ± SEM of the last three sessions under each URB597 (empty circles), anandamide (solid circles, n = 4), THC (solid triangles, n = 3) or cocaine (solid squares, n = 3) unit dose condition, and under a vehicle (V) condition. Single-factor ANOVA for repeated measures, post-hoc comparisons (Dunnett’s test) vs. vehicle conditions: *p < 0.05, **p < 0.01.
Figure 6.
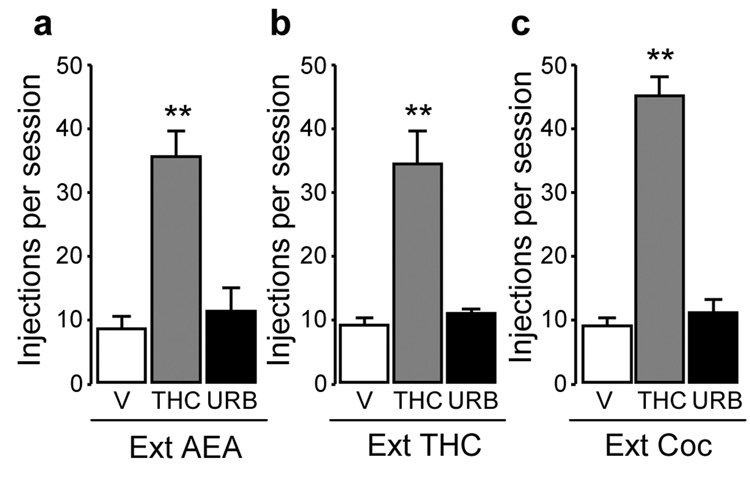
Reinstatement of extinguished (Ext) anandamide (AEA, a), Δ9-tetrahydrocannabinol (THC) (b), or cocaine (c) seeking behavior by priming injections of THC (40 µg/kg, i.v.) (gray bars), but not by vehicle (V, empty bars) or URB597 (0.3 mg/kg, i.v.) (URB, solid bars). Data are mean ± SEM from 3–5 monkeys over three sessions under each condition. Single-factor ANOVA for repeated measures, post-hoc comparisons (Bonferroni t-test) vs. vehicle pretreatment: **p < 0.01.
Figure 7.
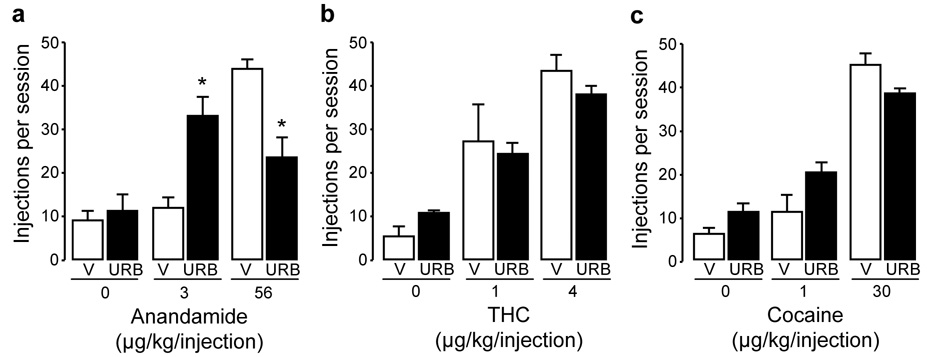
Effects of 30 min pretreatment with URB597 (0.3 mg/kg, i.v.) (URB, solid bars) or vehicle (V, empty bars) on responding maintained by different doses of anandamide (a), Δ9-tetrahydrocannabinol (THC) (b), or cocaine (c) injections. Each bar represents the mean ± SEM from 3–5 monkeys over three sessions under each condition. Single-factor ANOVA for repeated measures, post-hoc comparisons (Bonferroni t-test) vs. vehicle pretreatment: *p < 0.05.
RESULTS
Effects of URB597 on FAAH activity and brain endocannabinoid levels
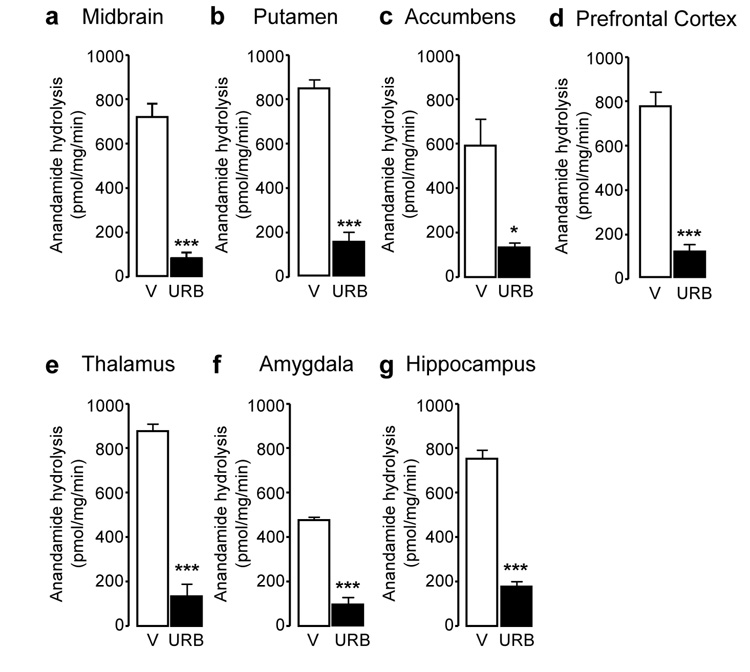
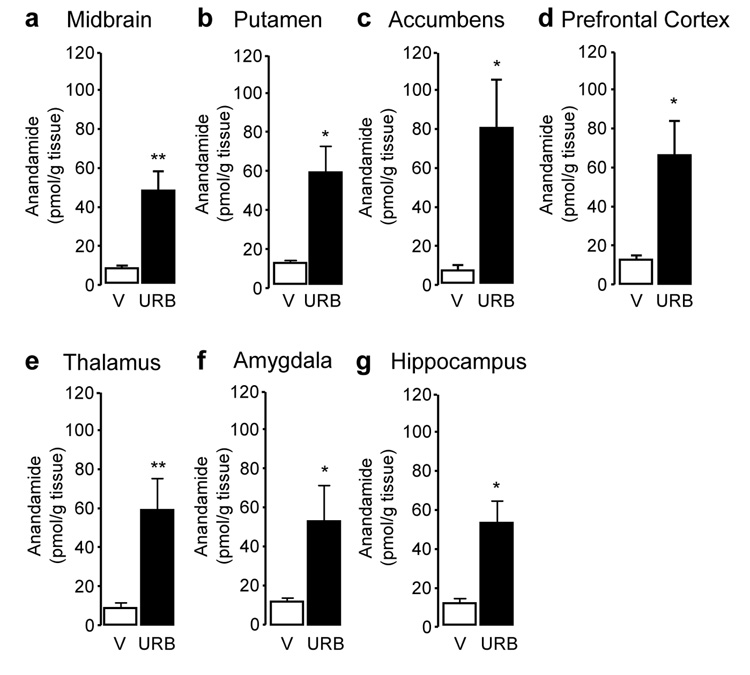
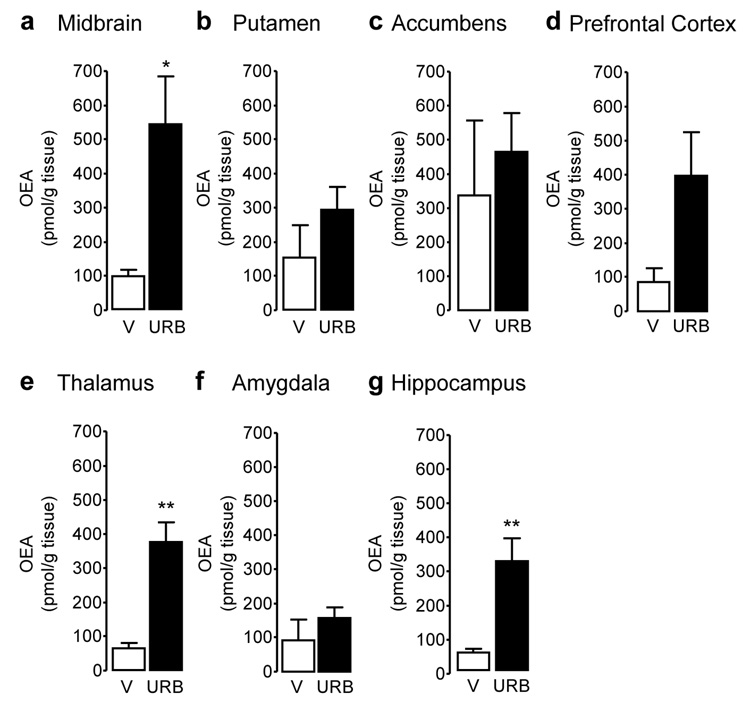
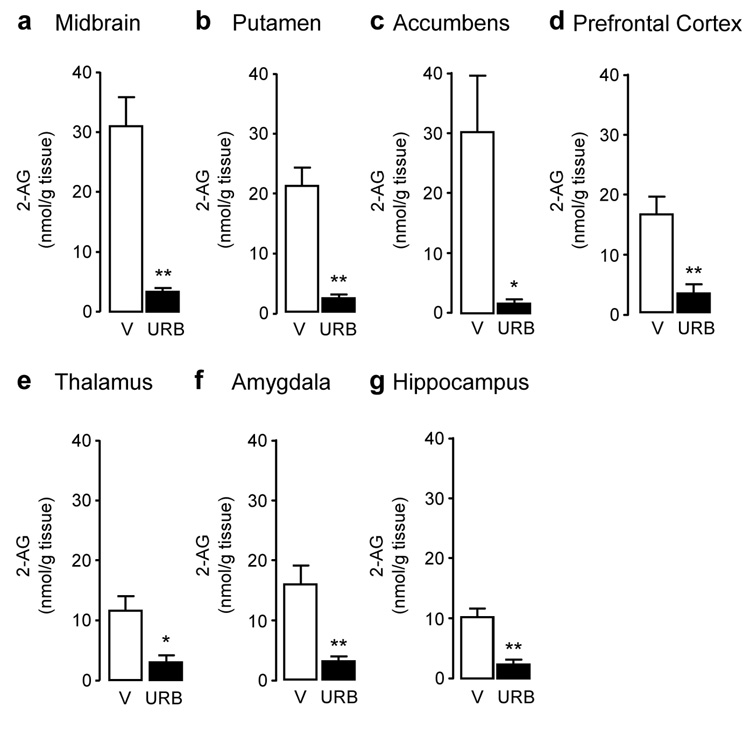
Systemic administration of URB597 (0.3 mg/kg, intravenous, i.v.) resulted in a marked inhibition of FAAH activity in all brain areas examined (Figure 1) (midbrain: F(1,6) = 56.88, p < 0.001; putamen: F(1,6) = 126.93, p < 0.001; nucleus accumbens: F(1,6) = 8.52, p = 0.027; prefrontal cortex: F(1,6) = 53.04, p < 0.001; thalamus: F(1,6) = 153.84, p < 0.001; amygdala: F(1,5) = 162.93, p < 0.001; hippocampus: F(1,6) = 107.27, p < 0.001). As previously observed in rodents (17,29), FAAH inhibition was accompanied by an increase in the levels of anandamide (Figure 2) and OEA (Figure 3), a non-endocannabinoid FAAH substrate (31). Surprisingly, because in contrast with prior results obtained in rats (32), URB597 treatment did not significantly increase OEA levels in putamen, nucleus accumbens and amygdala, and only marginally increased them in prefrontal cortex, suggesting that enzyme activities other than FAAH catalyze OEA hydrolysis in these regions of the monkey brain. Moreover, and again in contrast with previous rodent data (17,29), URB597 administration significantly decreased 2-AG levels in monkey brain (Figure 4). Parallel experiments confirmed that URB597 (1 mg/kg, intraperitoneal) does not affect 2-AG levels in rat hippocampus, even when experimental conditions closely matched those used in monkeys (12 h food deprivation, ongoing isoflurane anaesthesia) (Table 1). Similar results were obtained in the prefrontal cortex (data not shown). The ability of URB597 to reduce 2-AG levels in the monkey brain was not attributable to a direct effect of the drug on 2-AG-metabolizing enzymes, as incubation with URB597 (30 min) only altered DGL or MGL activities in monkey brain homogenates at concentrations significantly higher than those required to inhibit FAAH (residual DGL activity in putamen, as percent of control: 1 µM URB597, 106.6 ± 0.4; 10 µM URB597, 114.8 ± 6.2; residual MGL activity in putamen: 1 µM URB597, 78.0 ± 0.9, 10 µM URB597, 50.8 ± 0.3; mean ± SEM; n = 3) (17).
Figure 1.
Fatty-acid amide hydrolase (FAAH) activity in squirrel monkey brain regions 1 hour after injection of URB597 (0.3 mg/kg, i.v.). (a) Midbrain, (b) putamen, (c) nucleus accumbens, (d) prefrontal cortex, (e) thalamus, (f) amygdala, and (g) hippocampus. Activity was measured as ex vivo [3H] anandamide hydrolysis (pmol/mg protein/min). Data are mean ± S.E.M (n = 3–5); *p<0.05, ***p<0.001 (single-factor ANOVA); empty bars, vehicle (V); solid bars, URB597 (URB).
Figure 2.
Anandamide tissue levels in squirrel monkey brain regions 1 hour after injection of URB597 (0.3 mg/kg, i.v.). (a) Midbrain, (b) putamen, (c) nucleus accumbens, (d) prefrontal cortex, (e) thalamus, (f) amygdala, and (g) hippocampus. Data are mean ± S.E.M. (n = 4–5); *p<0.05, **p<0.01 (single-factor ANOVA); empty bars, vehicle (V); solid bars, URB597 (URB).
Figure 3.
Oleoylethanolamide (OEA) tissue levels in squirrel monkey brain regions 1 hour after injection of URB597 (0.3 mg/kg, i.v.). (a) Midbrain, (b) putamen, (c) nucleus accumbens, (d) prefrontal cortex, (e) thalamus, (f) amygdala, and (g) hippocampus. Data are mean ± S.E.M. (n = 4–5); *p<0.05, **p<0.01 (single-factor ANOVA); empty bars, vehicle (V); solid bars, URB597 (URB).
Figure 4.
2-Arachidonoylglycerol (2-AG) tissue levels in squirrel monkey brain regions 1 hour after injection of URB597 (0.3 mg/kg, i.v.). (a) Midbrain, (b) putamen, (c) nucleus accumbens, (d) prefrontal cortex, (e) thalamus, (f) amygdala, and (g) hippocampus. Data are mean ± S.E.M. (n = 4–5); *p<0.05, **p<0.01 (single-factor ANOVA); empty bars, vehicle (V); solid bars, URB597 (URB).
Table 1.
Endocannabinoid levels in rat hippocampus after URB597 administration.
| Endocannabinoid | Vehiclea | URB597a | Vehicleb | URB597b |
|---|---|---|---|---|
| Anandamide (pmol/g) | 6.62 ± 0.86 | 17.71 ± 2.80** | 4.29 ± 0.60 | 7.60 ± 1.09* |
| 2-AG (nmol/g) | 5.83 ± 0.34 | 6.14 ± 0.41 | 6.06 ± 0.42 | 5.47 ± 0.36 |
Mean ± SEM (n = 12a or 6b)
p < 0.05
p = 0.001 vs. vehicle; single-factor ANOVA
Endocannabinoid levels measured 2 h after URB597 (1 mg-kg−1, intraperitoneal, i.p.).
Endocannabinoid levels measured 1 h after URB597 (1 mg-kg−1, i.p.) in food-deprived rats (12 h) maintained on isoflurane anesthesia for the duration of the experiment.
Lack of URB597 self-administration in monkeys that previously self-administered anandamide, THC or cocaine
Before the experiments, three groups of squirrel monkeys had learned to self-administer anandamide, THC or cocaine under a ten-response, fixed-ratio schedule of i.v. drug injection (every 10th lever-press response produced a drug injection followed by a one min timeout; FR10, TO 1-min). Varying the injection dose of anandamide, THC or cocaine resulted in inverted, U-shaped, dose-effect curves. Anandamide (Figure 5b; F(5,15) = 11.91, p < 0.001), THC (Figure 5e; F(4,8) = 16.83, p < 0.001) or cocaine (Figure 5h; F(4,8) = 9.41, p < 0.001) maintained significantly higher rates of responding and higher numbers of injections per session (Figure 5a, anandamide: F(5,15) = 47.05, p < 0.001; Figure 5d, THC: F(4,8) = 43.59, p < 0.001; Figure 5g, cocaine: F(4,8) = 22.28, p < 0.001) than vehicle. Maximal responding was maintained by doses of 30 and 56 µg/kg anandamide per injection (Figure 5b, 0.67 ± 0.10 and 0.65 ± 0.14 response per second, respectively), 4 µg/kg THC per injection (Figure 5e, 0.86 ± 0.17 response per second) and 30 µg/kg cocaine per injection (Figure 5h, 0.92 ± 0.25 response per second). When vehicle injections were substituted for any of these drugs, rates of responding and number of injections self-administered per session rapidly decreased to very low levels (Figure 5).
After establishing dose-response curves for the three groups of monkeys, vehicle was substituted for THC, anandamide or cocaine for 5 to 10 sessions and URB597 was then substituted for vehicle. Each URB597 dose was studied for 4−5 consecutive sessions and each dose-condition was separated by 4–5 consecutive sessions of vehicle extinction. Varying the injection dose of URB597 (1, 3, 30, 100 µg/kg/injection) did not result in inverted U-shaped dose-effect curves in any of the groups of monkeys (Figure 5). Self-administration behavior was never significantly above vehicle levels at any dose of URB597 or any of the subjects tested. Neither number of injections per session (Figure 5a, anandamide group: F(6,17) = 1.00, p = 0.46; Figure 5d, THC group: F(3,6) = 3.91, p = 0.08; Figure 5g, cocaine group: F(3,6) = 1.20, p = 0.39) nor rates of responding (Figure 5b, anandamide group: F(6,17) = 0.810, p = 0.58; Figure 5e, THC group: F(3,6) = 4.36, p = 0.06; Figure 5h, cocaine group: F(3,6) = 1.00, p = 0.46) differed significantly from vehicle. When URB597 was available at an injection dose of 100 µg/kg, intake over the 1-hour session reached an average of 1.31 ± 0.17 mg/kg (Figure 5c), more than four times greater than the 0.3 mg/kg dose of URB597 that produced a 10-fold increase in brain anandamide levels (Figure 2).
Reinstatement of extinguished THC-, cocaine- or anandamide-seeking behavior by a priming injection of THC, but not URB597
Next, we examined the effects of a priming injection of THC (40 µg/kg, i.v.) in monkeys experienced with anandamide, THC, or cocaine and then tested the impact of a priming injection of URB597 at a dose that suppresses FAAH activity (0.3 mg/kg, i.v.; Figure 1). At this dose, URB597 did not affect responding for food under an FR10, TO 1-min schedule (F(1,8) = 0.12, p = 0.74; data not shown), when given for 5 consecutive sessions 30 min before session inception. Testing of priming injections always started after vehicle extinction had been stable for at least three days and reinstatement tests with priming injections were conducted for three consecutive sessions at each dose. THC produced a significant reinstatement of drug-taking behavior in the three groups of monkeys, while URB597 did not reinstate drug-taking in any of the animals (Figure 6a, anandamide group: F(2,7) = 22.87, p <0.001; Figure 6b, THC group: F(2,6) = 23.19, p = 0.002; Figure 6c, cocaine group: F(2,4) = 131.85, p < 0.001).
Effects of URB597 on self-administration of THC, cocaine, or anandamide
Finally, we investigated whether AAH inhibition influences self-administration of low and peak doses of anandamide, THC, or cocaine. An i.v. dose of 0.3 mg/kg of URB597, which strongly inhibited FAAH activity (Figure 1), decreased the number of self-administered injections of 56 µg/kg anandamide (Figure 7a, F(1,4) = 19.56, p = 0.011), and increased the number of self-administered injections of 3 µg/kg anandamide (Figure 7a, F(1,4) = 14.25, p = 0.02). This leftward shift in the anandamide dose-response curve confirmed that URB597 was pharmacologically active under our experimental conditions. By contrast, URB597 did not produce significant changes in self-administration behavior for injections of 1 and 4 µg/kg THC (Figure 7b, THC 1: F(1,3) = 0.153, p = 0.72; THC 4: F(1,4) = 3.63, p = 0.15) or 1 and 30 µg/kg cocaine (Figure 7c, cocaine 1: F(1,2) = 7.75, p = 0.11; cocaine 30: F(1,3) = 6.06, p = 0.09)).
DISCUSSION
We found that the selective FAAH inhibitor URB597 suppresses FAAH activity and increases anandamide levels in regions of the squirrel monkey brain that participate in motivational, cognitive and emotional functions. This effect is accompanied by a marked decrease in the levels of 2-AG, a major endocannabinoid substance in the brain, even though URB597 does not affect activities of 2-AG-metabolizing enzymes such as DGL and MGL. We further observed that URB597 does not display overt reinforcing property in monkeys over a broad range of experimental conditions. Indeed, the drug did not reinforce self-administration behavior even when its cumulative intake exceeded by several folds a fully effective dose for FAAH inhibition. Furthermore, neither previous cocaine nor THC exposure predisposed monkeys to self-administer URB597: even monkeys that had previously self-administered anandamide at very high rates failed to respond to the FAAH inhibitor. Lastly, URB597 did not affect the reinforcing effects of THC or cocaine, and did not reinstate extinguished drug-seeking behavior in monkeys that had previously self-administered THC or cocaine. We interpret these results to indicate (i) that URB597, by enhancing anandamide signaling, causes a compensatory down-regulation in 2-AG mobilization; and (ii) the potentiation of anandamide-mediated transmission produced by URB597 is insufficient per se to produce reinforcing effects. Our findings further imply that FAAH inhibitors such as URB597 – which have demonstrated analgesic, anxiolytic, antidepressant and antihypertensive properties in rodents (17,18,21,32,33) – may be used in humans without anticipated risk of inducing abuse or provoking relapse to drug use in abstinent individuals.
The pharmacological profile of URB597 is strikingly different from that of THC and other direct-acting CB1 receptor agonists. Studies in rodents have shown that URB597 does not produce THC-like effects such as catalepsy, hypothermia or hyperphagia (17,34). Further, URB597 does not mimic the discriminative-stimulus (subjective) actions of THC (18,35). Even further, URB597 does not increase dopamine levels in the nucleus accumbens shell of rats, a defining neurochemical feature of reinforcing drugs (35,36). Finally, URB597 does not elicit conditioned place preferences indicative of rewarding properties in rats (18). However, experiments in rodents, such as those outlined above, are insufficient to model human reward-based behaviors and to predict the addictive potential of drugs. Thus the present results provide the first unequivocal demonstration that URB597 lacks THC-like reinforcing properties, and suggest that this FAAH inhibitor might be used in therapy without anticipated risk of abuse or triggering relapse to drug use.
Exogenous anandamide exerts potent reinforcing effects in monkeys (24). Thus, it may be surprising that the ability of URB597 to potentiate brain anandamide signaling does not translate into overt rewarding properties. However, there are two plausible reasons why URB597 does not support self-administration responding. First, exogenous and endogenous anandamide might each access distinct subpopulations of CB1 receptors in the brain. In particular, systemic administration could allow anandamide to reach a receptor pool that is normally engaged by 2-AG. In this context, the observation that treatment with URB597 decreases 2-AG levels in the monkey brain suggests the existence of a compensatory mechanism aimed at reducing 2-AG signaling in the face of enhanced anandamide signaling. Such a mechanism might account, at least in part, for the inability of URB597 to serve as a reinforcer. Consistent with this idea, a recent report suggests that pharmacological or genetic disruption of FAAH activity causes a down-regulation of 2-AG production in acutely dissected rodent striatal slices, which is reportedly due to vanilloid TRPV1 receptor activation (37). However, we were unable to replicate this observation in live animals even when using doses of URB597 that completely suppressed FAAH activity and significantly increased anandamide levels (17,18, present manuscript). Another possibility is that the kinetics of CB1 receptor activation may differ between anandamide and URB597 administration, as the former is likely to produce a more rapid recruitment of CB1 receptors than the latter. It is well established that effectiveness of drug reinforcement in monkeys depends on a rapid drug distribution throughout the brain (38,39,40,41,42). Irrespective of the mechanism involved, the impact of 2-AG down-regulation on the broad pharmacological properties of URB597 in primates remains to be determined.
In conclusion, our findings with URB597 unmask a previously unsuspected functional heterogeneity within the endocannabinoid signaling system in the brain, and suggest that FAAH inhibitors such as URB597 might be used therapeutically without risk of abuse or triggering relapse to drug abuse.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, by National Institute on Drug Abuse (NIDA) Grants, 012447, and 017538 (to DP); and by the Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD.
We thank Kathleen Stilwell, Dr. Ira Baum, Phil White and Dean Shumway for assistance during the brain removal procedure, and Nataliya Gross and Saurabh Sharma for assistance with biochemical experiments. We also thank Dr. Leigh Panlilio for his comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
DP is an inventor in a patent disclosing FAAH inhibitors, owned by the University of California, Irvine and licensed to Organon Biosciences, a unit of Schering-Plough. All other authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Davis MP. Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin Investig Drugs. 2008;17:85–95. doi: 10.1517/13543784.17.1.85. [DOI] [PubMed] [Google Scholar]
- 2.Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290:1757–1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- 3.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 4.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 5.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23:17–24. doi: 10.1185/030079906x158066. [DOI] [PubMed] [Google Scholar]
- 6.Muller-Vahl KR. Cannabinoids reduce symptoms of Tourette's syndrome. Expert Opin Pharmacother. 2003;4:1717–1725. doi: 10.1517/14656566.4.10.1717. [DOI] [PubMed] [Google Scholar]
- 7.Justinova Z, Goldberg SR, Heishman SJ, Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005;81:285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms-a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 9.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 11.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 12.Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 13.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mor M, Rivara S, Lodola A, Plazzi PV, Tarzia G, Duranti A, et al. Cyclohexylcarbamic acid 3'- or 4'-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies. J Med Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- 16.Tarzia G, Duranti A, Tontini A, Piersanti G, Mor M, Rivara S, et al. Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J Med Chem. 2003;46:2352–2360. doi: 10.1021/jm021119g. [DOI] [PubMed] [Google Scholar]
- 17.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 18.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo R, Loverme J, La RG, Compton TR, Parrott J, Duranti A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3'- carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- 22.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Delta(9)-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 23.Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- 24.Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, et al. Blockade of THC-Seeking Behavior and Relapse in Monkeys by the Cannabinoid CB(1)-Receptor Antagonist Rimonabant. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- 28.Emmers R, Akert K. A stereotaxic atlas of the brain of the squirrel monkey (Saimiri Sciureus) Madison: University of Wisconsin Press; 1963. [Google Scholar]
- 29.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 30.Astarita G, Ahmed F, Piomelli D. Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J Lipid Res. 2008;49:48–57. doi: 10.1194/jlr.M700354-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Fu J, Gaetani S, Oveisi F, LoVerme J, Serrano A, Rodriguez de Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 32.Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- 35.Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- 36.Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 37.Maccarrone M, Rossi S, Bari M, De CV, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- 38.Kato S, Wakasa Y, Yanagita T. Relationship between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol Biochem Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- 39.Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Wakasa Y, Takada K, Yanagita T. Reinforcing effect as a function of infusion speed in intravenous self-administration of nicotine in rhesus monkeys. Nihon Shinkei Seishin Yakurigaku Zasshi. 1995;15:53–59. [PubMed] [Google Scholar]
- 41.Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.