Abstract
Sigma (σ) receptors, initially described as a subtype of opioid receptors, are now considered unique receptors. Pharmacological studies have distinguished two types of σ receptors, termed σ1 and σ2. Of these two subtypes, the σ1 receptor has been cloned in humans and rodents, and its amino acid sequence shows no homology with other mammalian proteins. Several psychoactive drugs show high to moderate affinity for σ1 receptors, including the antipsychotic haloperidol, the antidepressant drugs fluvoxamine and sertraline, and the psychostimulants cocaine and methamphetamine; in addition, the anticonvulsant drug phenytoin allosterically modulates σ1 receptors. Certain neurosteroids are known to interact with σ1 receptors, and have been proposed to be their endogenous ligands. These receptors are located in the plasma membrane and in subcellular membranes, particularly in the endoplasmic reticulum, where they play a modulatory role in intracellular Ca2+ signaling. Sigma1 receptors also play a modulatory role in the activity of some ion channels and in several neurotransmitter systems, mainly in glutamatergic neurotransmission. In accordance with their widespread modulatory role, σ1 receptor ligands have been proposed to be useful in several therapeutic fields such as amnesic and cognitive deficits, depression and anxiety, schizophrenia, analgesia, and against some effects of drugs of abuse (such as cocaine and methamphetamine). In this review we provide an overview of the present knowledge of σ1 receptors, focussing on σ1 ligand neuropharmacology and the role of σ1 receptors in behavioral animal studies, which have contributed greatly to the potential therapeutic applications of σ1 ligands.
Key Words: Sigma-1 receptors, learning and memory, depression and anxiety, schizophrenia, analgesia, pain, drugs of abuse, cocaine.
1. HISTORICAL OVERVIEW: DISCOVERY OF SIGMA RECEPTORS AND SIGMA RECEPTOR SUBTYPES
Sigma (σ) receptors were first described as a subclass of opioid receptors [102] to account for the psychotomimetic actions of (±)-SKF-10,047 (N-allylnormetazocine) and other racemic benzomorphans. This early confusion was due to the complex pharmacology of this racemic compound; later studies showed that (–)-SKF-10,047 binds to µ and κ opioids, whereas the (+)-isomer lacks affinity for opioid receptors but binds to PCP (phencyclidine) binding sites with low affinity, and to a different site with high affinity, which currently retains the designation of σ [reviewed in 112 and 124] amongst others.
Two different σ sites were distinguished based on their different drug selectivity pattern and molecular mass; these two σ sites are now known as σ1 and σ2 receptors [64]. It was reported that σ1 binding sites display stereospecificity towards dextrorotatory isomers of benzomorphans, whereas σ2 binding sites display reverse selectivity, i.e., levorotatory isomers display higher affinity than dextrorotatory isomers of σ ligands [e.g. 64, 165]. The molecular weight was found to differ between the two σ receptors subtypes: the σ1 receptor is a 29-kDa single polypeptide first cloned in 1996 [55], whereas σ2 receptors have not yet been cloned and have an apparent molecular weight of 18-21.5 kDa according to photolabeling studies [65, 159]. In spite of intensive efforts in research on the σ2 subtype in recent years [partially reviewed in 14; 142, 156, 114], the σ1 subtype is much better characterized, and is the focus of this review.
Sigma1 receptors have been thoroughly studied in an attempt to elucidate their possible neuropharmacological applications, mainly in learning and memory processes, depression and anxiety, schizophrenia, analgesia and some effects of certain drugs of abuse. In this review we describe some aspects of the general biology of σ1 receptors, but focus on σ1 ligand neuropharmacology and the role of σ1 receptors in behavioral animal studies, which have contributed greatly to the understanding of the possible neuropharmacological properties of σ1 receptors. Non-neuropharmacological effects of σ1 ligands such as cardiovascular effects or their effects on cancer and immunity, and their antitussive effects, are not covered in this review. Therefore this review will not go into detail on some aspects of σ1 receptor knowledge, and not all references will be cited.
2. MOLECULAR CHARACTERISTICS, DISTRIBUTION AND PHARMACOLOGICAL PROFILE OF SIGMA1 RECEPTORS
2.1. Cloning and Structure of σ1 Receptors
Significant progress in our knowledge of σ receptors was made when the σ1 receptor was cloned. The σ1 receptor is a 29-kDa single polypeptide which was first cloned in guinea-pig liver [55], and later in mouse kidney, a JAR human choriocarcinoma cell line, and in the rat and mouse brain [reviewed in 54]. The protein is composed by 223 amino acids and shows the typical σ1 binding profile [55, 84, 180]. The amino acid sequence of the σ1 receptor cloned from the human cell line is highly homologous to the σ1 receptor cloned from the other species [181], and shows no homology with other mammalian proteins, but shares approximately 30% identity with the yeast gene that encodes the C7–C8 sterol isomerase [141], and contains an endoplasmic reticulum retention signal at the NH2 terminus [55, 179]. Cloning of the σ1 receptor has contributed greatly to research in this field, making it possible to design specific antisense oligodeoxynucleotides to study σ1 receptor function (as will be described below) and develop σ1-receptor knockout mice [91].
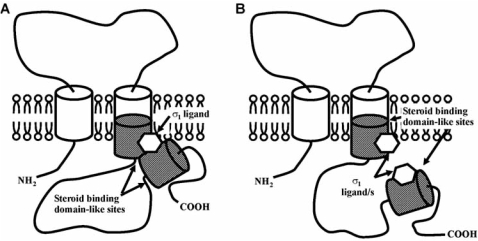
Several structures have been proposed for σ1 receptors. Initial studies proposed a single transmembrane domain structure [43, 55]. More recently, Aydar and coworkers presented evidence that the σ1 receptor in the plasma membrane has two transmembrane segments (when expressed in Xenopus laevis oocytes) with the NH2 and COOH termini on the cytoplasmic side of the membrane [3]. Recent studies proposed that in addition to the hydrophobic regions that constitute the putative transmembrane domains, there are two additional hydrophobic segments (one of them partially overlapping the second transmembrane domain), which were proposed to be steroid binding domain-like sites [27], and suggesting the existence of two different domains for ligand binding in the σ1 receptor [159], as previously proposed in earlier experiments [12]. This proposed model is illustrated in Fig. (1). The pharmacological characterization of these putative domains merits further study.
Fig (1).
Putative model for σ1 receptors proposed by Pal and coworkers [159]. Open cylinders represent the two putative transmembrane domains. Closed cylinders represent the steroid binding domain-like sites and the open hexagon represents a putative σ1 ligand. A, Possible spatial arrangement of the ligand binding site involving both steroid binding domain-like sites. B, Alternative model for ligand interaction with the σ1 receptor.
2.2. Anatomical and Subcellular Distribution of σ1 Receptors
2.2.1. Anatomical Distribution of σ1 Receptors
At the anatomical level σ1 receptors are widely distributed in peripheral organs [e.g. 192] and different areas of the central nervous system, where they have been thoroughly studied. They are widely distributed in the brain, but concentrated in specific areas involved in memory, emotion and sensory and motor functions [reviewed in 9, 54 and 146]. In these studies high to moderate levels of σ1 receptors were associated with the hippocampus, especially in the dentate gyrus, hypothalamus, olfactory bulb, several cortical layers, pons, the septum, the central gray, locus ceruleus, dorsal raphe, the substantia nigra pars compacta, the red nucleus and various motor cranial nerve nuclei. The cerebellum is not particularly enriched in σ1 receptors, although some of its areas, such as the Purkinje cell layer, have been reported to show considerable densities of σ1 receptors. In addition to the brain, σ1 receptors are also numerous in the spinal cord, mainly in the superficial layers of the dorsal horn [2].
2.2.2. Subcellular Distribution of σ1 Receptors
The subcellular distribution of σ1 receptors was firstly studied with radioligand binding in subcellular fractions, and more recently with immunochemical methods. Binding experiments with the σ1 radioligands [3H](+)-SKF-10,047, [3H](+)-3-PPP and [3H](+)-pentazocine showed that σ1 receptors are located in several types of mouse, rat and guinea pig brain membrane. These binding sites are more abundant in microsomal membranes, which is consistent with the endoplasmic reticulum retention signal of the cloned σ1 receptor [55, 179], but they are also present in nuclear, mitochondrial and synaptic membranes [17, 34, 38, 74]. Immunohistochemical studies further confirmed the existence of σ1 receptors in the endoplasmic reticulum not only in neurons [2], but also in many other cell types such as oligodendrocytes [160], lymphocytes [43], retinal cells [76] and certain cancer cells [62]. Detailed studies by Hayashi and Su in NG108 cells showed that σ1 receptors are located as highly clustered globular structures enriched in cholesterol and neutral lipids in the nuclear envelope and endoplasmic reticulum [reviewed in 62]. In neurons from the rat hypothalamus and hippocampus, electron microscopy studies showed that σ1 receptor immunostaining was mostly associated with neuronal perikarya, the membrane of mitochondria, some cisternae of the endoplasmic reticulum and dendrites, where it was localized in the limiting plasma membrane including the postsynaptic thickening [2].
2.3. Pharmacological Profile of σ1 Receptors: Xenobiotics and Endogenous Ligands
2.3.1. Exogenous Ligands for σ1 Receptors
As described in the introduction, one characteristic that distinguishes σ1 binding sites from σ2 receptors is that the σ1 receptor displays stereospecificity towards dextrorotatory isomers of benzomorphans (such as SKF-10,047 or pentazocine) [64, 165]. An interesting aspect of σ1 receptor pharmacology is that these receptors can bind, with high to moderate affinity, a wide spectrum of known compounds of very different structural classes and with different therapeutic and pharmacological applications, such as neuroleptics (e.g. haloperidol, nemopramide), antidepressants (e.g. fluvoxamine, clorgyline), antitussives (carbetapentane, dextromethorphan, dimemorfan), drugs for the treatment of neurodegenerative disorders such as Parkinson’s disease (amantadine) or Alzheimer’s disease (memantine, donepezil), and drugs of abuse (cocaine, methamphetamine) (Table 1). As for many other receptors, some allosteric modulators have been described for σ1 receptors, including the anticonvulsant drugs phenytoin (DPH) and ropizine (Table 1). The modulation of σ1 radioligand binding by DPH has been conventionally assumed to be a characteristic difference between σ1 and σ2 binding sites [see 121 and 165 for reviews]. However, we recently reported that DPH also discriminates between different σ1 ligands depending on their activities on σ1 receptors [32, 33].
Table 1.
Pharmacology of some Usual σ1 Receptor Ligands
| Compound | Subtype Selectivity | Affinity for σ1 Site* | Function on σ1 Site | Other Activities |
|---|---|---|---|---|
| Benzomorphans | ||||
| (+)-Pentazocine | σ1[61] | +++ [61] | Agonist [61] | - |
| (–)-Pentazocine | σ1/σ2[214] | ++ [214] | Agonist [31] | κ1 agonist, µ1, µ2, ligand, low affinity δ, and κ3 opioid ligand [31] |
| (+)-SKF-10,047 | σ1 [61] | +++ [61] | Agonist [61] | NMDA receptor ligand [61] |
| Antipsychotics | ||||
| Chlorpromazine | σ1/σ2 [108] | ++ [61] | ? [61] | Dopamine D2 antagonist [61] |
| Haloperidol | σ1/σ2 [61] | +++ [61] | Antagonist [61] | Dopamine D2 and D3 antagonist [75]; σ2 agonist [121] |
| Nemonapride | σ1/σ2? [61] | +++ [61] | ? [61] | Dopamine D2 antagonist [61] |
| Antidepressants | ||||
| Clorgyline | σ1 [74] | +++ [74] | Agonist? [9] | Irreversible monoamine oxidase A inhibitor [74] |
| Fluoxetine | σ1 [149] | + [149] | Agonist [61] | Selective 5-HT reuptake inhibitor [149, 61] |
| Fluvoxamine | σ1 [149] | +++ [149] | Agonist [61] | Selective 5-HT reuptake inhibitor[149, 61] |
| Imipramine | σ1 [149] | ++ [149] | Agonist [61] | Monoamine reuptake inhibitor [61] |
| Sertraline | σ1 [149] | ++ [149] | Agonist [9] | Selective 5-HT reuptake inhibitor [149] |
| Antitussives | ||||
| Carbetapentane | σ1/σ2 [19] | +++ [19] | Agonist [121] | Muscarinic antagonist [19] |
| Dextromethorphan | σ1 [182] | ++ [182] | Agonist [121] | NMDA receptor allosteric antagonist [93] |
| Dimemorfan | σ1/σ2 [182] | ++ [182] | Agonist [182, 217] | ? |
| Parkinson’s and/or Alzheimer’s disease | ||||
| Amantadine | ? | + [162] | Agonist? [162] | NMDA antagonist, antiviral properties [25] |
| Donepezil | σ1/σ2? [82] | +++? [82] | Agonist [126, 136,137] | Cholinesterase inhibitor [82] |
| Memantine | ? | + [162] | Agonist? [162] | NMDA antagonist, antiviral properties [25] |
| Drugs of abuse | ||||
| Cocaine | σ1/σ2 [111] | + [61, 111] | Agonist [61] | Monoamine transporters inhibitor, amongst other actions [175] |
| MDMA | σ1/σ2[15] | + [15] | ? | Preferential SERT inhibitor, among other actions [51] |
| Metamphetamine | σ1/σ2 [151] | + [151] | ? | Preferential DAT inhibitor, amongst other actions [45] |
| Putative endogenous ligands (neurosteroids) | ||||
| DHEAS | σ1 [61] | + [61] | Agonist [61] | GABAA negative modulator [121] |
| Pregnenolone sulfate | σ1 [61] | + [61] | Agonist [61] | NMDA positive/GABAA negative modulator [121] |
| Progesterone | σ1 [61] | + [32, 33, 70] | Antagonist [61] | NMDA negative/GABAA positive modulator [121] |
| Anticonvulsants | ||||
| Phenytoin (DPH) | σ1 [214] | Not applicable | Allosteric Modulator [32, 33, 214] | Delayed rectifier K+ channel blocker [152]; T-type Ca2+ current inhibitor [202]; Na+ current inhibitor [177] |
| Ropizine | σ1 [214] | Not applicable | Allosteric modulator [214] | ? |
| Other σ drugs | ||||
| BD 737 | σ1/σ2 [65] | +++ [54] | Agonist [54] | - |
| BD 1008 | σ1/σ2 [61] | +++ [61] | Antagonist [61] | σ2 agonist? [120] |
| BD 1047 | σ1 [107] | +++ [107] | Antagonist[107] | α adrenoceptor ligand [107] |
| BD 1063 | σ1 [107] | +++ [107] | Antagonist [107] | - |
| BMY 14802 | σ1/σ2 [108] | ++ [108] | Antagonist [54] | 5-HT1A agonist [106] |
| DTG | σ1/σ2 [61] | +++ [61] | ? [61] | σ2 agonist [121] |
| Dup 734 | σ1 [61] | +++ [61] | Antagonist [54] | 5-HT2 antagonist [200] |
| Eliprodil (SL-82.0715) | σ1/σ2 [56] | ++ [56] | ? [61] | NMDA antagonist,α1 adrenoceptor ligand [56] |
| E-5842 | σ1 [53] | +++ [53] | Antagonist [54] | Low to moderate affinity for dopamine, 5-HT and glutamate receptors [53] |
| Haloperidol Metabolite I | σ1 [108] | ++ [34, 108] | Antagonist [22] | - |
| Haloperidol Metabolite II | σ1/σ2 [108] | +++ [34, 108] | Irreversible antagonist [34] | Dopamine D2 and D3 ligand [75] |
| 4-IBP | σ1/σ2[77] | +++ [77] | Agonist [9] | Dopamine D2 ligand [77] |
| JO-1784 (Igmesine) | σ1 [61] | +++ [61] | Agonist [61] | - |
| Metaphit | σ1/σ2 [11] | ++ [34] | Irreversible antagonist [11] | Acylator of PCP and σ2 binding sites [11] |
| (+)-MR 200 | σ1/σ2 [173] | +++ [173] | Antagonist [100] | - |
| MS-377 | σ1 [61] | +++ [61] | Antagonist [61] | - |
| NE‑100 | σ1 [61] | +++ [61] | Antagonist [61] | - |
| OPC-14523 | σ1/σ2 [61] | +++ [61] | Agonist [54] | Agonist of pre- and post-synaptic 5-HT1A receptors [10]; SERT inhibitor [203] |
| Panamesine (EMD 57445) | σ1/σ2? [61] | +++? [61] | Antagonist [54] | One of its metabolites is a dopaminergic antagonist [61] |
| (+)-3-PPP | σ1/σ2[64] | ++ [32, 33] | Agonist [61] | σ2 agonist [121]; NMDA receptor ligand [68]; dopaminergic agonist [61] |
| PRE 084 | σ1 [61] | +++ [61] | Agonist [61] | - |
| Rimcazole (BW-234U) | σ1/σ2[110] | + [61] | Antagonist [61] | DAT inhibitor [110] |
| SA4503 | σ1 [61] | +++ [61] | Agonist [61] | - |
| SR 31742A | ? | +++ [61] | ? | High affinity for C8-C7 sterol isomerase [61] |
Ki or KD values:
< 50 nM;
< 500 nM;
< 10 µM.
: not studied or unclear at the moment.
: no other pharmacological target has been described.
Haloperidol deserves special consideration among the σ ligands, because it is the most widely used σ1 antagonist in research on σ1 receptors, and its affinity is high enough to bind σ1 receptors in humans after a single oral dose [192]. In fact, haloperidol binds with similarly high affinity to dopamine D2 receptors and σ receptors, but its metabolites display preferential activity at σ receptors compared to dopamine D2 receptors [13]. Particularly interesting is the reduced metabolite of haloperidol (haloperidol metabolite II), which has high affinity for σ1 and σ2 receptors but shows much lower affinity for D2 receptors than the original compound [13, 108]. This compound was recently shown to be an irreversible σ1 ligand [34].
Some selective and high affinity σ1 drugs have been developed and are considered prototypical σ1 ligands. Examples are the σ1 agonists (+)-pentazocine, PRE 084, JO-1784 and SA4503, and the σ1 antagonists BD 1063 and NE-100. Table 1 summarizes the pharmacological activities on σ1 receptors, σ subtype selectivity and other known pharmacological activities of some σ ligands used in research (and also in therapeutics). Currently the number of σ ligands is increasing rapidly with the development of new compounds [35, 98, 109, 172 among others].
2.3.2. Putative Endogenous σ1 Ligands: Neurosteroids
Although the endogenous ligands for σ1 receptors have not yet been defined unequivocally, currently the neurosteroids are considered the most probable endogenous σ1 ligands. This term, first used by Baulieu, identifies steroids that are synthesized in the central and peripheral nervous systems, and includes pregnenolone, dehydroepiandrosterone (DHEA), their sulfate esters, progesterone, and allopregnenolone [reviewed in 5]. The physiological actions of neurosteroids include genomic actions and nongenomic neuromodulatory actions, the latter of which are presumably related with σ1 receptors [see 146 for a detailed review]. The interaction between neurosteroids and σ1 receptors was first suggested in 1988 [193] from in vitro experiments in guinea pig brain and spleen. Of the steroids tested, progesterone was the most potent inhibitor of σ1-specific radioligand binding; however, whether neurosteroids are the endogenous ligands of the σ1 receptor remains controversial because the affinity of progesterone for σ1 receptors does not appear to be high enough for an endogenous ligand [178]. In addition, other steroids such as DHEAS (DHEA sulfate), pregnenolone sulfate, testosterone and deoxycorticosterone exhibited even lower affinities for σ1 receptors than progesterone [61]. However, some reports support that neurosteroids are the σ1 receptor endogenous ligands. In many experimental paradigms, progesterone behaved like other known σ1 antagonists, and DHEA and pregnenolone sulfate act as other known σ1 agonists [see 127 and 146 for an extensive review]. The exogenous administration of neurosteroids led to a dose-dependent inhibition of in vivo σ1 radioligand binding [117, 219], and modifications in endogenous levels of neurosteroids (e.g., after adrenalectomy, castration, ovariectomy or during pregnancy) affected σ1 responses [6, 7, 208]. The cloned σ1 protein presents homologies with the steroid binding site of several steroidogenic enzymes, which supports the specific interaction of σ1 receptors with neurosteroids [27, 127, 159]. We have therefore included them in Table 1 as putative σ1 endogenous ligands.
3. MODULATION OF CELLULAR EFFECTS BY SIGMA1 RECEPTORS
One of the earliest questions about the cellular effects of σ1 receptors concerned their possible coupling to G-proteins. This issue has been studied with different experimental approaches, and the results reported to date are as profuse as they are contradictory [reviewed in 9 and 54]. Even some selective σ1 agonists seemed to act through G-proteins (JO-1784), whereas others ((+)-pentazocine) did not under the same experimental conditions [143]. Now that the σ1 receptor has been cloned [55], it seems clear that the cloned receptor does not have the typical structure of a G-protein-coupled receptor with seven transmembrane domains; however, the existence of a metabotropic σ1 receptor subtype different of the cloned type cannot be ruled out yet [e.g. 104]. Although the coupling of σ1 receptors to G-proteins remains controversial, the modulatory role of σ1 receptors in the activity of some ion channels, different kinds of neurotransmission (mainly glutamatergic) and in second messenger systems, particularly the phospholipase C/protein kinase C/inositol 1,4,5-trisphosphate (PLC/PKC/InsP3) system, has been extensively reported.
3.1. Modulation of Plasmalemmal Ion Channels
3.1.1. Potassium Channels
Potassium channels have been shown to constitute an important target for σ drugs. It has been shown that σ ligands inhibited K+ currents in several experimental preparations [97, 103, 188, 189, 220, 224]. In some of these studies,known σ1 agonists and antagonists produced the same effects [220, 224]. These results might reflect the participation of σ2 activity, since it was recently reported that σ2 ligands can also modulate K+ currents [142]. However, other recent studies showed that the selective σ1 agonists (+)-pentazocine and JO-1784 reduced several K+ currents in frog melanotropic cells [188, 189], and prevented the activation of small-conductance calcium-activated K+ channels (SK channels) in rat hippocampal slices [103]. These effects were reversed by known σ1 antagonists (NE-100 or haloperidol) [103, 188]. Regarding the molecular mechanism of the effects of σ1 receptors in K+ currents, it was proposed that σ1 receptors and K+ channels must be in close proximity for any functional interaction to occur [97, 128], and in fact the heterologous expression of σ1 receptors with voltage-gated K+ channels Kv 1.4 and 1.5, in Xenopus oocytes, resulted in modulation of the channel function in the absence of any σ ligand, and greater modulation in the presence of SKF-10,047 [3]. Moreover, Kv 1.4 channel not only colocalized [128] but also co-immunoprecipitated with σ1 receptor proteins, indicating that σ1 receptors are directly associated with these K+ channels [3]. In addition, σ1 photolabeling with radioiodinated probes identified high-molecular-mass protein complexes, suggesting that σ1 receptors may exist as oligomers or interact with protein partners either constitutively or through ligand binding [159]. The formation of these complexes might help explain the wide variety of actions produced by σ1 ligands in the central nervous system.
3.1.2. Calcium Channels
Sigma1 ligands have also been reported to modulate plasmalemmal voltage-dependent calcium channels. Interaction between σ receptors and Ca2+ channels was suggested from studies in which the increase in intracellular Ca2+ concentration ([Ca2+]i) mediated by depolarization was diminished by σ ligands in neuronal cultures or forebrain synaptosomes [reviewed in 9, 54, 145]. However, in some of these experiments σ1 agonists and σ1 antagonists produced the same effects, which might be also due to the participation of σ2 receptors [reviewed in 145]. In addition, the selective σ1 agonists (+)-pentazocine and PRE 084 induced opposite effects on the increase in [Ca2+]i induced by depolarization with KCl in NG108 cells, but both effects were reverted by σ1 receptor antisense oligodeoxynucleotide [59], suggesting that they were both mediated by the cloned σ1 receptor. It therefore seems clear that more studies are necessary to clarify the role of these receptors in plasmalemmal voltage-dependent calcium channels.
3.2. Neurotransmitter Systems and σ1 Receptors: Modulation of N-methyl-D-aspartate (NMDA) Neurotransmission
Many studies have shown that σ1 receptors are able to modulate several neurotransmitter systems. It has been reported that σ1 receptors can potentiate glutamatergic neurotransmission [partially reviewed in 9 and 146], enhance cholinergic neurotransmission [partially reviewed in 118; 71], enhance serotonergic neurotransmission [reviewed in 9], negatively modulate the GABAergic system [47, 148], diminish noradrenaline release [20], and modulate dopaminergic neurotransmission [reviewed in 124]. The direction of modulation of the dopaminergic system has been especially controversial because the contradictory results reported thus far make it difficult to reach solid conclusions. The conflicting results probably reflect the use of drugs with different degrees of selectivity for σ1 receptors, and different routes of administration [reviewed extensively in 124].
Among the modulatory effects on different neurotransmitter systems by σ1 receptors, the modulation of glutamatergic neurotransmission has been described in greater detail than others. It has been reported that σ1 receptors can enhance spontaneous glutamate release in the hippocampus [42, 138], potentiate glutamate release induced by brain-derived neurotrophic factor [222], potentiate the increase in [Ca2+]i induced by glutamate in pyramidal neurons [144], and facilitate long-term potentiation in the rat hippocampus [26, 94, 103]. Of the three subtypes of glutamate-gated ion channels (NMDA, kainate and AMPA-kainate receptors), the connection between σ1 and NMDA receptors has been widely explored, mainly in studies of the NMDA-induced firing activity in the dorsal hippocampus. In this model σ1 agonists such as the selective agonists JO-1784 and (+)-pentazocine, the putative agonist DHEA, and the antidepressants clorgyline and sertraline (but not paroxetine or tranylcypromine, which showed much lower affinities for σ1 receptors) were able to modulate NMDA-induced firing. The effect of these ligands was reversed by known σ1 antagonists such as haloperidol, NE-100 or BMY-14802, and also by the putative endogenous ligand progesterone [reviewed in 9 and 146]. Interestingly, in these studies σ1 agonists showed a bell-shaped dose-response curve characterized by low-dose stimulation and high-dose inhibition. This type of dose-response curve indicates hormesis [18], and is well documented for σ1 receptor activation not only in the modulation of NMDA-induced firing, but also in many other experimental approaches, as will be described below.
Particularly interesting are the studies that related steroidal tonus under physiological conditions with the σ-mediated potentiation of glutamatergic neurotransmission in the hippocampus. This effect was strongly affected by increased levels of progesterone in pregnancy [7], and by decreased levels of this neurosteroid after ovariectomy [6]. A molecular mechanism was recently proposed by which σ1 receptor activation increases the NMDA receptor response. Ca2+ entering the cells through the NMDA receptors activates a Ca2+-activated K+ current, underlain by SK channels, which in turn shunts the NMDA receptor responses. Selective σ1 agonists prevented SK channel opening, and consequently increased the NMDA receptor response, emphasizing the importance of the σ1 receptor as a postsynaptic regulator of synaptic transmission [103]. Importantly, the modulation of several neurotransmitter systems mentioned above may be a consequence, at least partially, of the modulation of NMDA receptors. It has been reported in this connection that σ1 ligands can modulate dopaminergic [reviewed in 124], cholinergic [reviewed in 146], serotonergic [197] or noradrenergic systems [reviewed in 9] through NMDA receptors.
In summary, σ1 receptors modulate several neurotransmitter systems, and it seems that the modulation of NMDA responses by σ1 receptors plays a pivotal role in the modulation of neurotransmission by σ1 ligands.
3.3. Sigma1 Receptors as Modulators of Intracellular Messenger Systems
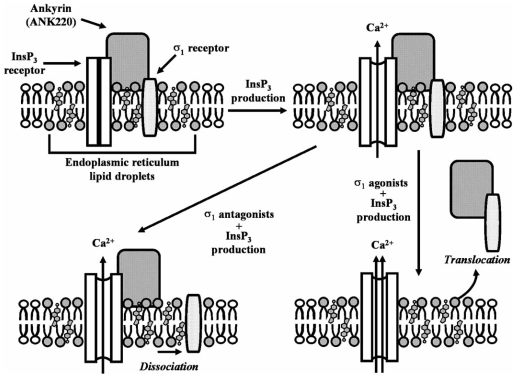
The modulation of metabotropic responses by σ1 receptors, particularly the increase in [Ca2+]i after stimulation of InsP3 receptors at the endoplasmic reticulum, has been described in detail. The mechanism of modulation of the PLC/PKC/InsP3 system by σ1 receptors appears to be a complex one involving the translocation of σ1 receptors to the plasma membrane and the nucleus; this was proposed as a mechanism by which an intracellular receptor modulates metabotropic responses [147, 59]. Sigma1 receptors are localized in highly clustered globular structures associated with the endoplasmic reticulum, which contain moderate amounts of free cholesterol and neutral lipids, forming lipid droplets [62], in which σ1 receptor, ankyrin (specifically the ANK220 isomer) and the InsP3 receptors form a complex [60]. Sigma1 receptor activation by agonists induces the dissociation of the σ1 receptor-ANK220 complex from the InsP3 receptors [60], potentiating the calcium efflux induced by receptors that activates the PLC system (such as receptors for bradykinin and brain-derived neurotrophic factor) [59, 60, 70, 162, 222]. The enhancement of calcium efflux, which followed a bell-shaped curve [59], has been reported not only with known selective σ1 agonists such as PRE 084 or (+)-pentazocine [59, 60, 70], but also with other compounds such as the neurosteroids pregnenolone, pregnenolone sulfate and DHEA [59, 70], amantadine and memantine [162]. In the presence of a σ1 antagonist, σ1 receptors are dissociated from ankyrin and InsP3 receptors, which remain on the endoplasmic reticulum [60] where they impede the potentiation by σ1 agonists of bradykinin-induced Ca2+ efflux [59, 70, 162]. This latter effect was also prevented by the putative σ1 antagonist progesterone [59, 70], and by specific σ1 receptor antisense oligodeoxynucleotides [59]. Under basal conditions σ1 ligands did not affect [Ca2+]i [59, 69], and the cells needed to be stimulated to make appropriate levels of InsP3 available for the modulation of [Ca2+]i by σ1 receptor agonists. Additionally, the silencing of InsP3 receptors resulted in a decrease in σ1 receptor mARN levels [154], underscoring the relationship between this second messenger system and σ1 receptors. This proposed model of modulation by σ1 receptors of InsP3-mediated calcium efflux is illustrated in Fig. (2).
Fig (2).
Model of modulation by σ1 receptors of InsP3-mediated calcium efflux, proposed by Hayashi and Su [60, 63]. InsP3 receptors, ANK220 and σ1 receptor form a complex in lipid droplets on the endoplasmic reticulum, which contain moderate amounts of free cholesterol and neutral lipids. In the presence of a σ1 agonist, the σ1 receptor-ANK220 complex is dissociated from InsP3 receptors and translocated. As a result InsP3 binding to its receptor increases and Ca2+ efflux is enhanced. In the presence of a σ1 antagonist, ANK220 remains coupled to InsP3 receptor, but σ1 receptor is dissociated from the complex, impeding the potentiation of calcium efflux by σ1 agonists.
An additional mechanism by which σ1 receptors can modulate other receptors located in the plasma membrane was recently proposed. It was reported that σ1 receptors can affect the levels of plasma membrane lipid rafts by changing the lipid components therein [199]. This membrane reconstitution could in turn affect the function of the proteins it contains, such as neurotransmitter receptors or tropic factor receptors. In fact, σ1 receptors play an important role in neurite sprouting [see 62 for a more complete review].
In summary, σ1 receptors translocate from lipid droplets on the endoplasmic reticulum when stimulated by agonists, modulating intracellular Ca2+ mobilizations at the endoplasmic reticulum after activation of the PLC/PKC/InsP3 system, and enhancing the cellular effects of different receptors.
4. THERAPEUTIC POTENTIAL OF SIGMA1 RECEPTORS
Given the widespread distribution of σ1 receptors in the central nervous system and their modulatory role at cellular, biochemical and neurotransmission levels (see above), σ1 ligands appear to be useful in different therapeutic fields such as depression and anxiety, amnesic and cognitive deficits, psychosis, analgesia and treatment for drugs of abuse. These potential therapeutic applications are reviewed briefly below.
4.1. Role of σ1 Receptors in Learning and Memory
The central cholinergic and glutamatergic neurotransmission systems play a crucial role in learning and memory functions. Cholinergic function is disturbed in some memory pathologies such as Alzheimer’s disease and pathological ageing, in which deficits in cortical cholinergic activity have been observed [4]. In addition, NMDA receptors are involved in the induction of different forms of synaptic plasticity (such as long-term potentiation) which are thought to be the synaptic substrate for learning and memory processes [166]. As described in the section ‘Neurotransmitter systems and σ1 receptors: modulation of N-methyl-D-aspartate (NMDA) neurotransmission’, σ1 agonists facilitate long-term potentiation in the rat hippocampus. However, the administration of large doses of σ1 agonists or antagonists (+)-SKF-10,047, (+)-pentazocine, PRE 084, JO-1784, SA4503, DTG, BMY 14802, haloperidol, BD 1047 or NE-100, or even the downregulation of σ1 receptor expression by antisense oligodeoxynucleotides, failed to affect learning in control animals. This finding suggests that σ1 receptors are not involved in normal memory functions [see 118, 120, 121, 127, and 146 for reviews]. Bearing in mind the typical modulatory role of σ1 receptors, it is not surprising that they have been found to modulate memory and learning processes when a state of pharmacological or pathological imbalance is induced.
4.1.1. Role of σ1 Receptors in Memory and Learning Impairment Induced by Drugs, Chemicals or Brain Lesions Affecting Cholinergic or Glutamatergic Neurotransmission
The learning impairment induced by the cholinergic muscarinic antagonist scopolamine, the nicotinic antagonist mecamylamine, or by cortical cholinergic dysfunction induced by ibotenic acid injection in the basal forebrain were attenuated or reversed by several σ1 agonists, including the selective σ1 agonists (+)-pentazocine, JO-1874 and SA4503 [reviewed in 118, 121 and 146]. In addition, the memory impairments induced by the serotonin (5-HT) depleter p-chloroamphetamine (PCA), which also involves cholinergic dysfunction [115], were attenuated in a bell-shaped manner by the administration of (±)-pentazocine, (+)-3-PPP, DTG, and (+)-SKF-10,047 [115, 116]. The effects of σ1 agonists in scopolamine-induced amnesia were reversed by known σ1 antagonists including haloperidol and NE-100, and by the downregulation of σ1 receptor expression by specific antisense oligodeoxynucleotides (reviewed in 118, 121 and 146). Interestingly, the putative σ1 agonists pregnenolone sulfate and DHEAS were also effective in scopolamine-induced amnesia model, and their effects were reversed by NE-100 and progesterone [120, 121 and 146].
As noted above, NMDA receptors also play an important role in learning and memory processes. The σ ligands (+)-SKF-10,047, (+)-pentazocine, JO-1784, DTG, PRE 084 and SA4503, and also the putative endogenous σ1 agonists DHEAS and pregnenolone sulfate attenuated the learning deficits induced by dizocilpine (MK-801), a noncompetitive NMDA receptor antagonist, in rats and mice presented with different mnesic tasks. The anti-amnesic effect of σ1 agonists was reverted by several known σ1 antagonists such as haloperidol, BMY 14802, NE-100 and BD 1047, by the putative endogenous σ1 antagonists progesterone [partially reviewed in 120, 121 and 146; 127], and by the administration of antisense oligodeoxynucleotides against σ1 receptors [122, 123, 126]. Cholinesterase inhibitors such as rivastigmine, tacrine and donepezil also attenuated dizocilpine-induced learning impairments [126]; however, only the effect of donepezil (which is also a potent σ1 ligand, see Table 1) was blocked by BD 1047 or antisense treatment [126].
Repeated exposure to carbon monoxide (CO) gas induced long-lasting but delayed amnesia which was measurable about one week after exposure. Like models of ischemia, this model involves the neurotoxicity of excitatory amino acids, and the hippocampal cholinergic system appears markedly affected by hypoxic toxicity [reviewed in 118]. Sigma1 ligands have been shown to have neuroprotective properties in models of ischemia [partially reviewed in 118, 16, 81]. Consistent with this neuroprotective action is the observation that the σ ligands (+)-SKF-10,047, PRE 084, JO-1784 and DTG reversed CO-induced amnesia, and their effects were prevented by NE-100, BMY 14802 and BD 1047 [partially reviewed in 120, 135]. Donepezil and some other cholinesterase inhibitors have also been tested in this behavioral model of amnesia, and it was found that all drugs showed anti-amnesic properties, but the pre-administration of BD 1047 blocked only the effect of donepezil [135]. Interestingly, in this model of amnesia the σ1 antagonists BD 1008 and haloperidol also showed anti-amnesic effects that were not reversed by NE-100, so it was suggested that these drugs might produce their effects through their σ2 agonistic activity [120]. The role of σ1 receptors in these experimental models is summarized in Table 2.
Table 2.
Summary of the Effects of σ1 Receptors in Experimental Models of Learning and Memory (see References and Text for Detailed Information)
| Involvement of σ1 Receptors in Learning and Memory | ||||
|---|---|---|---|---|
| Behavioral Assays | ||||
| Effect of σ1 Agonists | Effect of σ1 Antagonism | |||
| Cognitive impairment induced by | Drugs, chemicals or brain lessions | Scopolamine [121, 146] | Improvement | Reversion of the effects of σ1 agonists |
| Mecamylamine [121] | Improvement | Not tested | ||
| Basal forebrain lesion [121, 146] | ||||
| PCA [115, 116] | ||||
| Dizocilpine [120, 121, 122, 123, 126, 127, 146] | Improvement | Reversion of the effects of σ1 agonists | ||
| CO [16, 81, 118, 120, 135] | ||||
| Ageing-related diseases | Aged animals [146] | Improvement | Not tested | |
| Senescence-accelerated mice [118, 146] | Improvement | Reversion of the effects of σ1 agonists | ||
| β25-35-amyloid-related peptide (Alzheimer disease-type amnesia) [119, 137, 217] | ||||
| Alterations during pregnancy | Stress [134] | Improvement | Reversion of the effects of σ1 agonists | |
| Cocaine administration [133] | ||||
| Cognitive amelioration induced by | Low doses of cocaine [171] | Enhancement | Inhibition | |
| Effects on mechanisms involved in memory and learning impairment or potentiation | ||||
| Impairment | Neuronal injury induced by ischemia [16, 81, 118] or β25-35-amyloid-related peptide [99] | Neuroprotective effects | Reversion of the effects of σ1 agonistsa | |
| Potentiation | Long-term potentiation [26, 94, 103] | Enhancement | Reversion of the effects of σ1 agonists | |
Some nonselective σ1 antagonists exert neuroprotective effects [reviewed in 118], which may be due to a non-σ1-mediated mechanism.
4.1.2. Role of σ1 Receptors in Cognitive Impairments in Ageing: Alzheimer Disease
In models related with the memory deficits of ageing, σ1 agonists were also effective in attenuating the learning deficits in aged mice, aged rats and in senescence-accelerated mice, [reviewed in 118, 146]. Moreover, in the model of Alzheimer’s disease-type amnesia induced by β25-35-amyloid related peptide, which involves both cholinergic and glutamatergic neurotransmission through NMDA receptors [121], the σ1 receptor agonists (+)-pentazocine, PRE 084, SA4503, (+)-SKF-10,047, the antitussive drug dimemorfan and the putative σ1 agonists DHEAS and pregnenolone sulfate attenuated amnesia in a bell-shaped manner. The effects of σ1 agonists were reverted by haloperidol, BD 1047 and the putative σ1 antagonist progesterone [119, 137, 217]. Donepezil and other cholinesterase inhibitors were also tested in this behavioral model, but only the effects of donepezil were partially reversed by BD 1047, suggesting that the anti-amnesic effects of this drug involve both its cholinergic and σ1 agonistic properties [137]. These findings are consistent with the neuroprotective action of the σ1 agonist PRE 084, which attenuated cell death in cultured cortical neurons co-incubated with β25-35-amyloid related peptide, and this effect was reversed by the selective σ1 antagonist NE-100 [99]. The effects of σ1 ligands in ageing-related cognitive impairment are summarized in Table 2.
4.1.3. Other Ameliorative Effects of σ1 Agonists on Learning and Memory
Stress during pregnancy directly affects the neurophysiological development of the fetus with deleterious consequences observable throughout the individual’s lifetime [89], and can result in impairments in learning and memory processes [134]. The σ1 agonist JO-1784 reversed the learning deficits induced by prenatal stress, in a BD 1063-sensitive manner [134]. In addition, it is known that repeated cocaine treatment in utero can induce learning and memory impairment in the offspring. It was recently found that this process can be reverted by the σ1 agonist JO-1784 or DHEA, in a BD 1063-sensitive manner [133]. On the other hand, cocaine administered at very low doses (much lower doses than those which induce learning and memory impairments) can enhance memory storage in mice [72]. The ameliorating effects of cocaine on memory can be enhanced by the σ1 agonist JO-1784 and also by the putative σ1 agonist DHEA, and masked by the σ1 antagonist BD 1047 and also by the putative σ1 antagonist progesterone [171]. The hyperlocomotion, toxic effects, and reward properties induced by this psychostimulant are observed at much higher doses, and the effects of σ1 ligands on these effects will be described later in section 4.5.1 on cocaine and σ1 receptors. These effects of σ1 agonists on cognitive impairment due to alterations during pregnancy, as well as their role in the ameliorative effects of low doses of cocaine, are summarized in Table 2.
In summary, σ1 agonists appear to be promising pharmacological tools against memory and learning disorders resulting from pharmacological or pathological alterations (see Table 2). Among the memory and learning disorders, Alzheimer’s disease (the most common form of late-life dementia) is characterized by a cognitive decline, and effective treatment remains elusive. Sigma1 agonists could thus provide an alternative treatment against the cognitive deficits of this disease.
4.2. Role of σ1 Receptors in Depression and Anxiety
Several neurotransmitter systems are important in the pathophysiology of depression and anxiety. Depression likely involves dysfunction in brain areas that are modulated by monoaminergic systems such as the frontal cortex and the hippocampus [reviewed in 39]. Given that σ1 ligands play a modulatory role in several neurotransmitter systems, and that they can bind several known antidepressants (Table 1), they have been studied as possible pharmacological tools against these mood disorders.
4.2.1. Depression and σ1 Receptors
The effects of σ1 ligands were tested in behavioral studies used to predict the antidepressant activity of drugs. The selective σ1 agonists SA4503 and (+)-pentazocine decreased immobility time in the tail suspension test, and this effect was antagonized by NE-100 [207]. Many σ1 agonists have been tested in the forced swimming test, for example SA4503, (+)-pentazocine, JO-1784, DHEAS, pregnenolone sulfate, donepezil, and some novel σ selective compounds such as UMB23, among others. The decrease in immobility in the forced swimming test induced by the σ1 agonists was blocked by known σ1 antagonists [partially reviewed in 121; 126, 185, 208, 210, 218]. Interestingly, the extracts of the flowering plant Hypericum perforatum (St. John’s wort), which are used as antidepressants, appear to exert their therapeutic actions through σ1 receptors [reviewed in 132]. Additional experiments have related endogenous neurosteroidal levels with σ1 receptor function. In adrenalectomized and castrated mice, the effect of JO-1784 and PRE 084 in the forced swimming test was enhanced compared to control animals, and these effects were blocked by the selective σ1 antagonist BD 1047 [208]. In addition, the antidepressant efficacy of the selective agonist JO-1784 was enhanced in 12-month-old senescence-accelerated (SAM) mice, which showed decreased levels of progesterone [163]. Moreover, in animals acutely treated with β25-35-amyloid related peptide, which does not modify their immobility time, the effects of the selective σ1 agonists JO-1784 and PRE 084 were facilitated, presumably because of a decrease in progesterone levels in the hippocampus [209].
An important consideration is that σ1 agonists were able to potentiate the firing of serotonergic neurons of the dorsal raphe nucleus, as early as after 2 days of treatment, whereas SSRI- and monoamine oxidase inhibitor-induced changes took several weeks to emerge. The rapid effect of σ1 agonists has been proposed to predict a more rapid onset of antidepressant efficacy compared to existing medications [8]. Because of the typically modulatory role of σ1 receptors, OPC-14523, a compound with high affinity for σ1 receptors, 5-HT1A receptors, and serotonin transporter (SERT) (Table 1) was developed, and was found to produce a marked antidepressant-like effect in the forced swimming test after a single oral administration. This effect was reversed by both σ1 and 5-HT1A antagonists [203]. Moreover, and also in keeping with the modulatory role of σ1 receptors, the combined administration of the selective σ1 receptor agonist (+)-pentazocine and venlafaxine [41], or the co-administration of pramipexole and sertraline [167], at subeffective doses, showed a synergistic antidepressant-like effect, as did the co-administration of SA4503 and memantine or amantadine [186]. Importantly, the antidepressant-like effects of these drugs were reversed by known selective σ1 antagonists, and also by progesterone [41, 167, 186].
It is known that NMDA receptor subunit 1 is decreased in the prefrontal cortex or hippocampus of depressive patients [92, 155]. In the olfactory bulbectomized rat model of depression, animals show a decrease in NMDA receptor subunit 1 in these areas, and exhibit behavioral deficits which resemble the psychomotor agitation, loss of interest, and cognitive dysfunction of depression. Repeated treatment with SA4503 ameliorated the behavioral deficits, and also reversed the decrease in the protein expression of NMDA receptor subunit 1. These effects of SA4503 were blocked by the co-administration of NE-100 and by acute treatment with the NMDA receptor antagonist dizocilpine [216]. These findings document the strong relationship between depression, NMDA receptors and σ1 receptors.
In addition to the modulatory role of σ1 receptors in NMDA- and 5-HT-mediated responses related with depression, a complementary mechanism of action of σ1 ligands in this disorder has been reported to involve their effects in neuroplasticity processes. The mechanism of action of some antidepressants may involve neurotrophic actions [150], and it was reported that treatment with (+)-pentazocine or the antidepressants imipramine and fluvoxamine (which exhibit affinity for σ1 receptors, see Table 1) enhanced growth factor-induced neurite sprouting in PC12 cells, and also upregulated σ1 receptors [198]. The enhancement of growth factor-induced neurite sprouting by these drugs was mimicked by the overexpression of σ1 receptors [199].
In humans σ1 receptors can bind fluvoxamine at therapeutic doses [73], suggesting that this receptor might mediate some of the effects of this antidepressant; it has also been reported that JO-1784, at doses of 20 mg/day, exhibited a stronger antidepressant effect than the known antidepressant fluoxetine at the same dose in clinical trials. However, at 100 mg/day JO-1784 was no different from the placebo [9], which is in keeping with the bell-shaped dose-response curves induced by σ1 agonists in several behavioral, biochemical, and electrophysiological paradigms.
In summary, σ1 agonists showed good antidepressant effects in several behavioral models, probably because of their enhancement of serotonergic and glutamatergic neuronal functions as well as their neurotrophic actions (see Table 3). Due to the typically modulatory role of σ1 receptors, the design of drugs with mixed affinity for σ1 and other receptors related with depression, and the combined treatment of σ1 agonists with known antidepressant drugs, may offer good prospects in terms of efficacy.
Table 3.
Summary of the Involvement of σ1 Receptors in Depression (see References and Text for Additional Information)
| Involvement of σ1 Receptors in Depression | |||
|---|---|---|---|
| Effect of σ1 Agonists | Effect of σ1 Antagonists | ||
| Behavioral experimental models | Tail suspension test [207] | Improvement | Reversion of the effects of σ1 agonists |
| Forced swimming test [121, 126, 185, 208, 210, 218] | |||
| Olfactory bulbectomy [216] | |||
| Mechanisms associated with antidepressant activity | Firing of serotonergic neurons [8, 203] | Potentiation | Reversion of the effects of σ1 agonists |
| Neurotrophic actions [198] | Potentiation of growth factor-induced neurite sprouting | ||
| Mechanisms associated with depression | Decrease of NMDA receptor subunit 1 [216] | Reversion | Reversion of the effects of σ1 agonists |
4.2.2. Anxiety and σ1 Receptors
Evidence of anxiolytic activity of σ1 ligands was reported in the conditioned fear stress model, in which (+)-SKF-10,047, JO-1784, the neurosteroids pregnenolone sulfate and DHEAS, and also the antitussive dextromethorphan attenuated the motor suppression induced by previous electric footshock [79, 80, 153, 211], in a bell-shaped manner [211]. In addition, the effects of σ1 agonists on motor suppression were reversed by the known σ1 antagonist NE-100 and progesterone [153, 211]. In contrast, (+)-pentazocine lacked any effect in this model [79, 80]. Interestingly, the concentration of σ1 active steroids was altered in the plasma and brain of stressed mice, and it was therefore hypothesized that endogenous levels of neurosteroids might be involved in the expression of conditioned fear stress responses via σ1 receptors [153, 211]. In agreement with these results, animals treated chronically with β1-40-amyloid related peptide, in which progesterone levels in the hippocampus and cortex were decreased, exhibited facilitation of the effect of the σ1 agonists JO-1784, (+)-SKF-10,047 and DHEAS [211].
The effects of σ1 ligands have also been assayed in other behavioral tests such as sexual dysfunction induced by stress, marble-burying behavior and colonic motor disturbances induced by fear. It was reported recently that DHEA attenuated stress-induced sexual dysfunction in rats in a NE-100 dependent manner [140]. In the marble-burying behavior test, considered a potential model of obsessive–compulsive disorder on the basis of behavioral similarity, the effect of fluvoxamine was antagonized by BD 1063 and BD 1047, but not by the σ2 antagonist SM-21, suggesting again that the interaction of fluvoxamine with σ1 receptors contributes to its antidepressant effects. In addition, the σ1 agonists (+)-SKF-10,047 and PRE 084 slightly inhibited marble-burying behavior [44]. Gue and coworkers [52] showed that JO-1784 suppressed stress-induced colonic motor disturbances induced by fear stress in rats, in a model that mimicked the gastrointestinal tract disorders frequently present in anxiety, and this effect was reversed by BMY 14802 [52]. Subsequently, JO-1784 showed good results in clinical trials in a phase-1-model of functional diarrhea [213]. The results described above (summarized in Table 4) suggest that σ1 receptors play an important role in the modulation of anxiety.
Table 4.
Summary of the Involvement of σ1 Receptors in Anxiety (see References Cited in the Text for Detailed Information)
| Involvement of σ1 Receptors on Anxiety | |||
|---|---|---|---|
| Effect of σ1 Agonists | Effect of σ1 Antagonists | ||
| Behavioral experimental models | Conditioned fear stress [79, 80, 153, 211] | Improvement | Reversion of the effects of σ1 agonists |
| Sexual dysfunction induced by stress [140] | |||
| Marble-burying behavior test [44] | |||
| Colonic motor disturbances induced by fear [52] | |||
| Clinical trials (phase-1) | Functional diarrhea [213] | Improvement | Not tested |
4.3. Schizophrenia and σ1 Receptors
The dopamine hypothesis of schizophrenia, which involves enhanced mesolimbic dopamine function, remains the dominant hypothesis for the pathophysiology of this disorder, particularly regarding the appearance of positive symptoms [40]. In addition, it is important to consider the glutamatergic system. In fact, the blockade of NMDA receptors by PCP induces schizophrenia-like psychosis in humans [24, 212]. Because several antipsychotics possess high to moderate affinities for σ1 receptors (Table 1), researchers were inspired to test σ1 receptor ligands in several animal models of schizophrenia.
4.3.1. Role of σ1 Receptors in Behavioral Models of Schizophrenia in which Dopaminergic Function is Prominently Enhanced
In behavioral animal models in which the dopaminergic function is affected, such as apomorphine-induced climbing, amphetamine-induced locomotor activity, and behavioral sensitization by the repeated administration of psychostimulants, promising results have been reported using σ1 antagonists (summarized in Table 5). The nonselective σ1 antagonist BMY 14802, panamesine, E-5842 and MS-377 inhibit apomorphine-induced climbing [53, 187, 195, 201]. In addition, DTG, SR 31742A, panamesine, rimcazole and E-5842 inhibit amphetamine-induced locomotor activity [53, 164, 176, 187]. However, rimcazole and BD 1047 had little effect on apomorphine-induced climbing, and in addition, this latter compound had little effect on acute amphetamine-induced hyperlocomotion [187]. In models of behavioral sensitization with the repeated administration of psychostimulants—a pharmacological model of schizophrenia [21]— σ1 antagonism inhibited sensitization to methamphetamine [1, 196, 205] and cocaine [206, 221]. It was therefore suggested that σ1 antagonists may be suitable for maintenance therapy in persons with stable schizophrenia rather than for the treatment of acute psychotic features.
Table 5.
Summary of the Involvement of σ1 Receptors in Schizophrenia (see References and Text for Detailed Information)
| Involvement of σ1 Receptors on Schizophrenia | ||||
|---|---|---|---|---|
| Effect of σ1 Agonists | Effect of σ1 Antagonists | |||
| Behavioral experimental models | Dopaminergic function prominently enhanced | Apomorphine-induced climbing [53, 187, 195, 201] | Not tested | Inhibition |
| Amphetamine-induced locomotor activity [53, 164, 176, 187] | ||||
| Behavioral sensitization induced by repeated administration of psychostimulants [1, 196, 205, 206, 221] | ||||
| Glutamergic function prominently disturbed | PCP-induced stereotyped behaviors [63, 187, 195] | Not tested | Inhibition | |
| Dizocilpine-induced hyperlocomotion in monoamine depleted mice [157] | Enhancement | Reversion of the effects of σ1 agonists | ||
| PCP-induced cognitive deficits [58] | Improvement | Reversion of the effects of σ1 agonists | ||
| Clinical trials | Only with BMY 14802, eliprodil and panamesine [61] | Not tested | Inconclusive results | |
4.3.2. Role of σ1 Receptors in Behavioral Models of Schizophrenia in which Glutamatergic Function is Prominently Disturbed
As said before, in addition to dopaminergic dysfunction, alterations in glutamatergic neurotransmission are also involved in schizophrenia. Sigma1 ligands modified animal behavior in some glutamatergic models of this disease (summarized in Table 5). PCP-induced head weaving, which is insensitive to selective D2 antagonists, was attenuated by NE-100, haloperidol, BMY 14802, Dup 734 and MS-377 [63, 195]. Recent reports also showed that BD 1047, rimcazole and panamesine attenuated PCP-induced head twitching [187]. In addition, selective σ1 receptor agonists such as (+)-pentazocine, and also 3-(+)-PPP and (+)-SKF-10,047, enhanced the psychotomimetic effect (hyperlocomotion) of dizocilpine in monoamine-depleted mice, and this enhancement was blocked by NE-100 [157], suggesting that σ1 receptor blockade may be effective for negative symptoms of schizophrenia, which are hypothesized to be mediated, at least in part, by glutamatergic neurotransmission. Among the negative symptoms of schizophrenia, cognitive deficits are core features of the illness and predict vocational and social disabilities for patients [90]. It has been extensively reported that σ1 agonists play an important role in memory processes (as described in the section 4.1., ‘Role of σ1 receptors in learning and memory’). In fact, SA4503, DHEAS, and fluvoxamine (a SSRI with high affinity for σ1 receptors), but not paroxetine (an SSRI without affinity for σ1 receptors) improved the PCP-induced cognitive deficits in the novel object recognition test, and these effects were antagonized by the co-administration of NE-100 [58]. In addition, the antipsychotic (and also σ1 antagonist) drug haloperidol was ineffective in this behavioral model [57]. These results suggest that σ1 agonists are potentially useful for the cognitive deficits of schizophrenia.
4.3.3. Sigma1 Receptors and Extrapyramidal Side Effects
The extrapyramidal effects of neuroleptics are considered one of the most problematic side effects of these drugs. It has been suggested that σ receptors mediate the undesirable motor side effects of antipsychotic drugs [reviewed in 54 and 214], an effect classically attributed to the σ2 subtype [e.g., 215]. Although it was found that the affinities of several neuroleptics for σ receptors (both σ1 and σ2) correlated well with their risk of producing acute dystonic reactions [108], it is known that the blockade of σ1 receptors with other more selective antagonists such as NE-100 [158], MS-377 [195], E-5842 [53] or BMY 14802 [50] (at effective doses for the test used) does not induce motor side effect. These findings suggest that the blockade of σ1 receptors is not enough in itself to induce extrapyramidal side effects, so additional mechanisms are probably be involved.
4.3.4. Clinical Trials with σ1 Ligands in Schizophrenia
Some clinical trials have been done with rimcazole, BMY 14802, eliprodil (SL-82.0715) and panamesine. The trials with rimcazole and BMY 14802 yielded inconclusive results [reviewed in 61]; however, eliprodil reduced scores for negative but not positive symptoms, whereas panamesine reduced both positive and negative symptoms. However, a metabolite of panamesine has potent antidopaminergic properties which might explain its effect against the positive symptoms, so further research is needed to determine whether these effects are wholly or partly mediated by σ1 receptors [reviewed in 61].
In summary, due to the complex pathogenesis of schizophrenia and the differential effects of σ1 antagonists (which improve the behavior of animals in models based on the motor effects of dopaminergic stimulants or NMDA antagonists) and σ1 agonists (which improve the cognitive deficits induced by PCP) (summarized in Table 5), treatment based exclusively on σ1 ligands would probably be complex.
4.4. Sigma1 Receptors and Analgesia
Sigma1 receptors are distributed in the central nervous system in areas of great importance in pain control, such as the superficial layers of the spinal cord dorsal horn, the periaqueductal gray matter, the locus ceruleus and rostroventral medulla [2, 88]. As will be described below, they may be involved in the modulation of opioid analgesia, and may also play an important role in nociception in the absence of opioid drugs.
4.4.1. Modulation of Opioid Analgesia by σ1 Receptors
Chien and Pasternak were the first to report the involvement of σ1 receptors in analgesia [28]: they clearly demonstrated that σ1 receptors play an important role in the modulation of opioid analgesia in the tail-flick test. The systemic administration of σ1 agonists, including the selective σ1 agonist (+)-pentazocine, antagonized the antinociception induced by morphine in the tail-flick test [28-30, 130]. Further experiments with other opioids confirmed the role of σ1 receptors in opioid analgesia. (+)-Pentazocine also diminished δ-, κ1, and κ3 opioid antinociception [29, 130, 173]. In addition, σ1 antagonists such as haloperidol and (+)-MR 200 not only reversed the effects of agonists, but also increased opioid-induced antinociception, indicating the presence of a tonically active anti-opioid σ1 system [28-30, 100, 173].
The anatomical location of the modulation of opioid analgesia by σ1 receptors has been determined with different routes of administration of opioids, σ1 receptor ligands and antisense oligodeoxynucleotides. The intrathecal (i.t.) administration of (+)-pentazocine did not reverse the spinal (i.t.) analgesic effect of morphine in the tail-flick test, suggesting that the modulation of opioid analgesia by σ1 receptors in this test does not occur at the spinal level [130]. Interestingly, the supraspinal (intracerebroventricular, i.c.v.) administration of (+)-pentazocine decreased the analgesic effect of agonists for the κ and μ opioid receptors nalorphine and nalbuphine [130]; in addition, the analgesia induced by the supraspinal (i.c.v.) administration of the selective µ-opioid agonist DAMGO was enhanced by the σ1 antagonist (+)-MR 200 administered subcutaneously [100]. Further experiments based on the selective blockade of σ1 receptor synthesis by the i.c.v. administration of specific antisense oligodeoxynucleotides confirmed the supraspinal location of the modulation of opioid analgesia [87, 130, 161]. Finally, a more detailed approach was tested recently by Mei and Pasternak [131], who used microinjections of morphine in conjunction with (+)-pentazocine, haloperidol, or both in three brainstem nuclei: the periaqueductal gray, rostroventral medulla and locus ceruleus. The activity of σ1 receptors was found to differ depending on the area. Whereas both the locus ceruleus and rostroventral medulla were sensitive to (+)-pentazocine, the periaqueductal gray was not. The rostroventral medulla was particularly interesting, because it was the only region with evidence for tonic σ1 activity (enhanced by haloperidol), and it was also able to modulate the analgesia from morphine administered to the periaqueductal grey.
In contradistinction to results in the tail-flick test, it was found that the systemic administration of (+)-SKF-10,047 or NE-100 was unable to modulate κ1 opioid analgesia in the acetic acid-induced writhing test [66]. Although the doses used in this study might have been too low to prevent the participation of σ1 receptors in the modulation of κ1 opioid analgesia in the acetic acid-induced writhing, i.c.v. treatment with σ1 antisense oligodeoxynucleotides also failed to affect this response [67], suggesting that the supraspinal inhibition of σ1 receptors does not affect κ opioid analgesia in this behavioral test. This findings may indicate that the supraspinal σ system modulates only some opioid analgesic effects, probably depending on the type of pain evaluated (i.e., depending on the behavioral model used). Further research with different models is needed to characterize the role of the supraspinal σ system in opioid analgesia. The role of σ1 receptors on opioid analgesia in behavioral experimental models is summarized in Table 6.
Table 6.
Summary of the Involvement of σ1 Receptors in Analgesia (see Text and References for Detailed Information, as Administration Routes of Drugs)
| Involvement of σ1 Receptors on Analgesia | |||
|---|---|---|---|
| Behavioral Experimental Models | Effect of σ1 Agonists | Effect of σ1 Antagonism | |
| Modulation of opioid analgesia | Tail-flick test [28-30, 87, 100, 130, 161, 173] | Inhibition | Enhancement |
| Acetic acid-induced writhings [66, 67] | Inactive (very low doses tested)a | Inactive (very low doses tested)a | |
| Pain modulation in the absence of opioid drugs | Tail-flick test [22, 28-30, 100, 130, 161] | Inactive | Inactive |
| Acetic acid-induced writhings [66, 67] | Inactive(very low doses tested)a | Inactive(very low doses tested)a | |
| Nociceptive flexor response test [204] | Nociception | Reversion of the effects of σ1 agonists | |
| Formalin-induced pain [22, 23, 86] | Reversion of the effects of σ1 antagonists | Antinociception | |
| Plantar test [85] | Thermal hyperalgesiab | Reversion of the effects of σ1 agonists | |
| von Frey test [85] | Mechanical allodyniab | Reversion of the effects of σ1 agonists | |
Additional experiments using higher doses of σ1 ligands should be performed.
Selective σ1 agonists should be tested.
In addition, some recent reports showed that haloperidol and chlorpromazine, two neuroleptics that bind to σ sites (Table 1), inhibit the antianalgesia induced by nalbuphine in men [48]. Although the authors did not attribute this inhibition to σ receptors, this possibility cannot be fully ruled out, and would suggest that interaction between the σ and opioid systems is important in clinical terms. However, this issue also needs to be addressed in further clinical studies.
4.4.2. Analgesic Effect of σ1 Receptor Ligands
The role of σ1 ligands in the absence of opioid drug has also been investigated. Several σ1 ligands or antisense treatments have been proved to be inactive in the tail-flick test [22, 28-30, 100, 130, 161], as well as in the acetic acid-induced writhing test [66, 67] (although higher doses of σ1 ligands should be tested to ensure their lack of involvement in acetic acid-induced writhing). However, other reports showed that σ1 receptors are able to modulate nociception in other behavioral tests in the absence of an opioid drug. Ueda and coworkers [204] showed that the σ1 agonists (+)-pentazocine and SA4503, (+)-3-PPP, and also the putative σ1 agonists DHEAS and pregnenolone sulfate (administered intraplantarly) can induce nociception even when used alone in the nociceptive flexor response test. The effect of the σ1 agonists was reverted by the known σ1 antagonists NE-100, BD 1047 or the putative σ1 endogenous antagonist progesterone [204]. Other studies in our laboratory with the formalin test showed that formalin-induced nociception was attenuated not only by the systemic administration of haloperidol, haloperidol metabolite II and haloperidol metabolite I (with an order of potency which correlated with their affinity for σ1 receptors) [22], but also in σ1 receptor knockout mice [23]. Recent experiments with the same behavioral test found that in contradistinction to the supraspinal action of σ1 antagonists on the modulation of opioid analgesia, the i.t. administration of the σ1 receptor antagonists BD 1047 and BMY 14802 dose-dependently reduced formalin-induced pain behaviors in the second phase but not in the first phase of the formalin test [86]. This underscored the importance of spinal σ1 receptors in the second phase of formalin-induced pain. These results were consistent with previous findings which showed that haloperidol, haloperidol metabolite I and haloperidol metabolite II were more effective in the second than in the first phase of formalin-induced pain [22]. In agreement with these behavioral studies, it was also reported that antagonism of spinal σ1 receptors suppressed phosphorylation of the NR1 subunit of spinal NMDA receptors [86], which are important for maintaining spinal sensitization associated with the second phase of the formalin test [190]. From these results it was proposed that σ1 receptors may be important in models in which spinal sensitization occurs (without ruling out other analgesic effects in other models), and in fact, the putative σ1 agonist DHEA induced mechanical allodynia and thermal hyperalgesia when administered i.t., and the effects were reversed by BD 1047 [85]. This hypothesis deserves further investigation in other models of pain, especially in models of tonic pain in which central sensitization occurs. The results obtained in the behavioral models described above (summarized in Table 6) suggest that σ1 receptors play an important role in nociception in the absence of opioid drugs.
In summary, σ1 receptors are not only able to modulate opioid antinociception, at least in the tail-flick test, but may also play an active role in nociception in the absence of opioid drugs in some behavioral models (see Table 6).
4.5. Sigma1 Receptors and Drugs of Abuse
As shown before (Table 1), σ1 receptors can bind several drugs of abuse. It is therefore not surprising that σ1 ligands can modulate some of the effects of these drugs. Among the drugs of abuse studied to date, the involvement of σ1 receptors in the actions of cocaine has been extensively investigated, but σ1 receptors also appear to underlie the effects of other substances such as methamphetamine, MDMA (3,4-methylenedioxymethamphetamine) and ethanol, as will be described below.
4.5.1. Cocaine and σ1 Receptors
Cocaine is generally thought to act as a dopamine reuptake inhibitor to produce its reinforcing effects, although other mechanisms might also be important [105]. Cocaine binds preferentially to σ1 receptors rather than to σ2 [111], and the affinity of cocaine for σ1 receptors is in the micromolar range (Table 1), as is its affinity for its main pharmacological target, the dopamine transporter (DAT) [175]. Cocaine levels in the post-mortem brain of addicts were estimated to be between 0.1 and 4 µM [78], which is close to the Ki value of cocaine for σ1 receptors. In recent years several excellent and promising studies have been performed with σ1 ligands against the effects of cocaine, as described below.
4.5.1.1. Modulation by σ1 Receptors of the Acute Effects of Cocaine
The ability of compounds to attenuate the acute locomotor effects of cocaine is often used as an initial screening tool to identify agents able to block the psychostimulant activity of this drug of abuse. Convulsions and lethality, on the other hand, represent a measure of cocaine toxicity, and can result from exposure to acute large doses. Many σ1 antagonists have been reported to significantly prevent the acute locomotor stimulatory effects, convulsions or lethality induced by cocaine in rodents, including haloperidol, BD 1008 (and some of its analogs such as the selective σ1 antagonists BD 1047 and BD 1063), BMY 14802, panamesine and rimcazole (and some of its analogs), among others [partially reviewed in 112 and 124; 37, 95, 113]. Furthermore, the administration of antisense oligodeoxynucleotides that knock down brain σ1 receptors mimicked the effects of pharmacological σ1 antagonism on the locomotor stimulatory effects or convulsions induced by cocaine [109, 111]. Particularly interesting are the studies in which post-treatment of mice with the novel σ receptor antagonists LR132 and YZ-011, after cocaine administration, also attenuated cocaine-induced lethality after an overdose. However, BD 1063 was unable to prevent death under these conditions, and the authors hypothesized that this result was due to differences in pharmacokinetics [109, 111]. The ability of σ receptor antagonists to prevent death after an overdose of cocaine in animals suggests a clinical application potentially worth further study. In contradistinction to the positive effects of σ1 antagonists, the administration of DTG, the novel σ1 agonists BD1031 and BD1052, or the selective σ1 agonist SA4503 exacerbated locomotor stimulatory actions and the toxic effects (measured as convulsions and lethality rate) of the acute administration of cocaine [109, 111, 129, 184]. The results obtained in these behavioral models (summarized in Table 7) suggest that σ1 receptors play an important role in the acute effects of cocaine. In addition to σ1 receptors, it has been proposed that the σ2 subtype might also be a good pharmacological target against cocaine-induced actions [partially reviewed by 113; 114, 156].
Table 7.
Summary of the Involvement of σ1 Receptors in the Behavioral Effects Induced by Cocaine (see References and Text for Detailed Information)
| Involvement of σ1 Receptors in Cocaine-Induced Behavioral Effects | |||||
|---|---|---|---|---|---|
| Behavioral Experimental Model | Effect of σ1 Agonists | Effect of σ1 Antagonism | |||
| Acute effects of cocaine | Psychostimulant effects | Locomotor activity [37, 95, 111, 109, 112, 124] | Potentiation | Inhibition | |
| Toxicity | Convulsions [37, 109, 111, 112, 113, 124] | ||||
| Lethality [37, 112, 113, 124] | |||||
| Repeated administration of cocaine | Self-reported effects of cocaine | Drug discrimination test [83, 95, 221] | Not tested | Slight or no effect | |
| Nervous system plasticity | Locomotor sensitization [206, 221] | Not tested | Inhibition | ||
| Rewarding properties | During addictive behavior | Conditioned place preference [168, 169] | Not tested | Inhibition | |
| Self-administration [101] | Not tested | No effect | |||
| After extinction of addictive behavior | Conditioned place preference after priming injection of drugs [170] | Reactivation | Inhibition | ||
| Discriminative stimulus associated with cocaine availability for self-administration [101] | Not tested | Inhibition | |||
4.5.1.2. Modulation by σ1 Receptors of the Effects of Repeated Cocaine Administration
Several σ1 antagonists have also been tested in behavioral models that used repeated doses of this drug of abuse. The σ1 antagonists rimcazole and some of its analogs, and other putative σ antagonists did not alter or only slightly altered the discriminative stimulus of cocaine [83, 95, 221], indicating that the interaction between cocaine and σ receptor ligands might be more complex than an exclusively competitive antagonism. Other studies that involved the repeated administration of cocaine found that σ receptor antagonists significantly prevented the development of cocaine-induced locomotor sensitization [206, 221], which is considered a measurable index of nervous system plasticity resulting from repeated exposure to cocaine [112]. The effects of σ1 antagonism on the rewarding properties of this drug of abuse have been explored with promising results. In the conditioned place preference test, the selective σ1 receptor antagonists BD 1047 and NE-100 attenuated the acquisition [168, 169] and also the expression of cocaine-induced conditioned place preference [169]. In addition, treatment with σ1 antisense oligodeoxynucleotide was effective against the acquisition of conditioned place preference, indicating the specificity of these effects [168]. However, in cocaine self-administration experiments, Martin-Fardon and coworkers found that BD 1047 was inactive against the acute reinforcing effects of cocaine, supposedly because both the reinforcing quality and relevant neuroadaptive changes are likely to differ in rats subjected to involuntary administration (as in conditioned place preference) vs. self-administration of cocaine [101]. After extinction, cocaine addictive behavior can be reactivated by a discriminative stimulus associated with cocaine administration, or by a priming injection of cocaine (in self-administration or conditioned place preference experiments, respectively). These processes were both blocked by BD 1047 [101, 170], and the latter one was also blocked by σ1 antisense oligodeoxynucleotides [170]. Interestingly, the σ1 agonists PRE 084 and JO-1784 were unable to induce conditioned place preference [169], but the administration of the latter σ1 agonist, or even DHEA, was enough to reactivate conditioned place preference after extinction, in a BD 1047-sensitive manner [170]. The results in these behavioral models (summarized in Table 7) suggest that σ1 receptors play an important role in neuronal plasticity after repeated cocaine administration, and that σ1 antagonists could be useful to prevent craving and relapse of cocaine addiction.
It has been reported that σ1 receptor density changes after repeated treatment with cocaine [96, 169, 170, 183, 223]. Particularly interesting is the σ1 receptor upregulation in the caudate putamen (an important area in the drug reward mechanism), which was not produced in dopamine D1 receptor knockout mice [223]. Consistent with this finding was that cocaine treatment in the neuroblastoma cell line B- 104 (lacking in dopamine transporter or receptors), was also unable to induce σ1 receptor upregulation [36], suggesting a close relationship between dopamine and σ1 receptors. In fact, it has been proposed that both D1 receptors and σ1 receptors are involved in cocaine-induced life-long alterations in neurons [194].
4.5.1.3. Effects of σ1 Ligands on Cocaine-Induced Immune System Depression
Different experiments have been designed to investigate the effects of cocaine other than its acute toxicity or rewarding properties, specifically, modulation of the immune system by cocaine. It was recently reported that cocaine can enhance alveolar cell carcinoma growth in mice, and that this effect was mimicked by PRE 084 and reversed by BD 1047. Increased tumor growth induced by cocaine or PRE 084 was accompanied by an increase in IL-10 and a decrease in IFN-γ production [46]. In addition, the selective σ1 antagonist BD 1047 blocked enhancement of the replication of HIV-1 in mice with severe combined immunodeficiency implanted with HIV-1-infected human peripheral blood mononuclear cells [174], and also in human microglial cell cultures [49]. These reports suggest that σ1 receptors are involved in the cocaine-induced depression of the immune system.
In summary, σ1 antagonists appear to be potentially useful not only against acute cocaine toxicity or addiction, but also against the noxious modulation of the immune system in cocaine consumers. In addition, σ1 agonists, as described in section 4.1. ‘Role of σ1 receptors in learning and memory,’ may be useful against some behavioral alterations induced by repeated cocaine exposure in utero. It therefore seems clear that cocaine produces its behavioral and biochemical effects, at least in part, through its interaction with σ1 receptors, and that σ1 ligands should be considered for the development of potential therapies to treat different aspects of cocaine abuse.
4.5.2. Other Drugs of Abuse and σ1 Receptors
Methamphetamine, like cocaine, also binds to σ1 receptors in the micromolar range (Table 1), and with a 20-fold higher affinity than for σ2 receptors [151], so it is not unexpected that σ1 ligands modulate some effects of this psychostimulant. Early studies found that the σ1 antagonists NE 100, BMY 10802 and MS-377 modulated the acute motor effects of methamphetamine only weakly, if at all [158, 196, 205]. However, more recent studies showed that the selective σ1 antagonists BD 1063 and BD 1047, as well as σ1 antisense oligodeoxynucleotide, inhibited methamphetamine-induced locomotor activity [151]. It was also recently reported that like cocaine or methamphetamine, the compound MDMA (‘ecstasy’), which is structurally related to methamphetamine, showed preferential affinity for σ1 receptors rather than for the σ2 subtype, and that BD 1063 also attenuated the locomotor activity induced by this compound [15]. Furthermore, BMY 14802 and MS-377, two known σ1 antagonists, inhibited the behavioral sensitization induced by the repeated administration of methamphetamine [1, 196, 205]. As in studies with repeated cocaine administration, it was thought that σ1 receptors might play a role in neuronal plasticity after the repeated administration of methamphetamine. Sigma1 receptor levels were recently found to be unaltered in rats passively treated with this psychostimulant; however, in rats self-administered with methamphetamine, σ1 receptors were upregulated in the rat midbrain, an area involved in learning and reward processes, but not in the cerebellum, frontal cortex, striatum and hippocampus. These observations underscored the role of σ1 receptors in neuronal plasticity after the consumption of psychostimulants [191].
The role of σ1 receptors in the behavior of other drugs of abuse has also been explored, and it was found that the σ1 antagonist BD 1047 was effective against ethanol-induced locomotor stimulation, conditioned place preference, taste aversion and some symptoms of the abstinence syndrome after chronic ethanol consumption [125, 135]. Interestingly, σ1 receptor expression was increased in the hippocampus of mice after chronic ethanol consumption. However, both the σ1 agonist JO-1784 and the antagonist BD 1047 shared some ameliorating properties against the abstinence syndrome after chronic ethanol consumption [135]. These observations suggest a new pharmacological target for alleviating ethanol addiction and abstinence syndrome after withdrawal, although more behavioral tests should be performed. Interestingly, an association has been suggested between polymorphisms in the σ1 receptor gene and alcoholism [139], supporting the role of σ1 receptors in chronic ethanol consumption.
5. CONCLUSIONS AND PERSPECTIVES
At the present time it seems logical to attribute the neuropharmacological properties of σ1 ligands to the neuromodulatory role of σ1 receptors. They act as intracellular amplifiers for signal transductions involving InsP3 receptors, are clearly able to modulate neurotransmitter systems (mainly through NMDA receptors) and ion channels (such as K+ channels), and may play an important role in neuroplasticity processes. Because of this typically modulatory nature of σ1 receptors, σ1 ligands are usually devoid of effect per se under control conditions in many experimental situations. In fact, and in agreement with the modulatory role of σ1 receptors, σ1 knockout mice do not display any overt phenotype. However, data showed that σ1 ligands are highly active when a pharmacological or pathological imbalanced state arises. In addition, and also due to the modulatory role of σ1 receptors, the combined administration of σ1 receptor ligands and medications with a known therapeutic effect has been shown to improve the effects of the latter (at least in behavioral models of depression and in opioid-mediated analgesia), resulting in the need for lower doses to reach therapeutic concentrations. This synergistic action of σ1 ligands and low doses of other known drugs merits further study in additional behavioral models. Of particular interest is the bell-shaped dose-response curve of σ1 agonists in in vitro experiments, in behavioral tests in which σ1 agonists are active (i.e., learning and memory processes, depression and anxiety), and even in some clinical trials. These data strongly suggest that researchers should take hormesis into account in order to design informative experiments or clinical trials with σ1 agonists.
In the light of our current knowledge, it seems clear that σ1 agonists are promising pharmacological tools against memory and learning disorders, and also against depression and anxiety. Although some previous findings suggest that σ1 antagonists might be potentially useful tools against some symptoms of schizophrenia, currently the most promising therapeutic targets for σ1 antagonism are nociception and some deleterious effects of certain drugs of abuse (such as cocaine, methamphetamine and ethanol). Importantly, many drugs used routinely in therapeutics show affinity for σ1 receptors (see Table 1), and exert the same effects as other more selective σ1 ligands in many behavioral tests and in vitro assays. Therefore, the therapeutical properties of these drugs might be due, at least in part, to their interaction with σ1 receptors. Interestingly, several drugs have been proved to be effective against diseases (in behavioral animal models) different from those for which they are prescribed in clinical practice, through their interaction with σ1 receptors. These findings raise the possibility of new therapeutic applications with drugs routinely used in therapeutics.
ACKNOWLEDGMENTS
We thank Professor J.M. Baeyens for his expert advice and K. Shashok for improving the use of English in the manuscript. The authors were partially supported by grant CTS 109 from the Junta de Andalucía, grant SAF 2006-06122 from the Spanish Ministerio de Educación y Ciencia (MEC), FEDER funds, a grant from Laboratorios Dr. Esteve, S.A., and a grant from the Centro para el Desarrollo Tecnológico Industrial (Genius Pharma project). F.R. Nieto and J.M. Entrena were supported by Formación de Profesorado Universitario grants, and C.M. Cendán by a Postdoctoral grant (all from the MEC).
REFERENCES
- 1.Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr. Res. 1994;12:251–257. doi: 10.1016/0920-9964(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 3.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 4.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 5.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J. Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron R, de Montigny C, Debonnel G. Pregnancy reduces brain sigma receptor function. Br. J. Pharmacol. 1999;127:1769–1776. doi: 10.1038/sj.bjp.0702724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermack JE, Debonnel G. Modulation of serotonergic neurotransmission by short- and long-term treatments with sigma ligands. Br. J. Pharmacol. 2001;134:691–699. doi: 10.1038/sj.bjp.0704294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bermack JE, Debonnel G. The role of sigma receptors in depression. J. Pharmacol. Sci. 2005;97:317–336. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- 10.Bermack JE, Debonnel G. Effects of OPC-14523, a combined sigma and 5-HT1a ligand, on pre- and post-synaptic 5-HT1a receptors. J. Psychopharmacol. 2007;21:85–92. doi: 10.1177/0269881106063996. [DOI] [PubMed] [Google Scholar]
- 11.Bluth LS, Rice KC, Jacobson AE, Bowen WD. Acylation of σ receptors by Metaphit, an isothiocyanate derivative of phencyclidine. Eur. J. Pharmacol. 1989;161:273–277. doi: 10.1016/0014-2999(89)90859-5. [DOI] [PubMed] [Google Scholar]
- 12.Bowen WD, Hellewell SB, McGarry KA. Evidence for a multi-site model of the rat brain σ receptor. Eur. J. Pharmacol. 1989;163:309–318. doi: 10.1016/0014-2999(89)90200-8. [DOI] [PubMed] [Google Scholar]
- 13.Bowen WD, Moses EL, Tolentino PJ, Walker JM. Metabolites of haloperidol display preferential activity at σ receptors compared to dopamine D-2 receptors. Eur. J. Pharmacol. 1990;177:111–118. doi: 10.1016/0014-2999(90)90260-d. [DOI] [PubMed] [Google Scholar]
- 14.Bowen WD. Sigma receptors: recent advances and new clinical potentials. Pharm. Acta Helv. 2000;74:211–218. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 15.Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine and σ1 receptors. Eur. J. Pharmacol. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucolo C, Marrazzo A, Ronsisvalle S, Ronsisvalle G, Cuzzocrea S, Mazzon E, Caputi A, Drago F. A novel adamantane derivative attenuates retinal ischemia-reperfusion damage in the rat retina through σ1 receptors. Eur. J. Pharmacol. 2006;536:200–203. doi: 10.1016/j.ejphar.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Cagnotto A, Bastone A, Mennini T. [3H](+)-pentazocine binding to rat brain sigma1 receptors. Eur. J. Pharmacol. 1994;266:131–138. doi: 10.1016/0922-4106(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- 19.Calderon SN, Izenwasser S, Heller B, Gutkind JS, Mattson MV, Su TP, Newman AH. Novel 1-phenylcycloalkane-carboxylic acid derivatives are potent and selective σ1 ligands. J. Med. Chem. 1994;37:2285–2291. doi: 10.1021/jm00041a006. [DOI] [PubMed] [Google Scholar]
- 20.Campana G, Bucolo C, Murari G, Spampinato S. Ocular hypotensive action of topical flunarizine in the rabbit: role of σ1 recognition sites. J. Pharmacol. Exp. Ther. 2002;303:1086–1094. doi: 10.1124/jpet.102.040584. [DOI] [PubMed] [Google Scholar]
- 21.Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- 22.Cendan CM, Pujalte JM, Portillo-Salido E, Baeyens JM. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology (Berl) 2005;182:485–493. doi: 10.1007/s00213-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 23.Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in σ1 receptor knockout mice. Eur. J. Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Chavez-Noriega LE, Marino MJ, Schaffhauser H, Campbell HUC, Conn PJ. Novel potential therapeutics for schizophrenia: focus on the modulation of metabotropic glutamate receptor function. Curr. Neuropharmacol. 2005;3:9–34. [Google Scholar]
- 25.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Dai XN, Sokabe M. Chronic administration of dehydroepiandrosterone sulfate (DHEAS) primes for facilitated induction of long-term potentiation via sigma 1 (σ1) receptor: optical imaging study in rat hippocampal slices. Neuropharmacology. 2006;50:380–392. doi: 10.1016/j.neuropharm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Hajipour AR, Sievert MK, Arbabian M, Ruoho AE. Characterization of the cocaine binding site on the sigma-1 receptor. Biochemistry. 2007;46:3532–3542. doi: 10.1021/bi061727o. [DOI] [PubMed] [Google Scholar]
- 28.Chien CC, Pasternak GW. Functional antagonism of morphine analgesia by (+)-pentazocine: evidence for an anti-opioid σ1 system. Eur. J. Pharmacol. 1993;250:R7–R8. doi: 10.1016/0014-2999(93)90650-7. [DOI] [PubMed] [Google Scholar]
- 29.Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J. Pharmacol. Exp. Ther. 1994;271:1583–1590. [PubMed] [Google Scholar]
- 30.Chien CC, Pasternak GW. Sigma antagonists potentiate opioid analgesia in rats. Neurosci. Lett. 1995;190:137–139. doi: 10.1016/0304-3940(95)11504-p. [DOI] [PubMed] [Google Scholar]
- 31.Chien CC, Pasternak GW. (-)-Pentazocine analgesia in mice: interactions with a σ receptor system. Eur. J. Pharmacol. 1995;294:303–308. doi: 10.1016/0014-2999(95)00552-8. [DOI] [PubMed] [Google Scholar]
- 32.Cobos EJ, Baeyens JM, Del Pozo E. Phenytoin differentially modulates the affinity of agonist and antagonist ligands for σ1 receptors of guinea pig brain. Synapse. 2005;55:192–195. doi: 10.1002/syn.20103. [DOI] [PubMed] [Google Scholar]
- 33.Cobos EJ, Lucena G, Baeyens JM, Del Pozo E. Differences in the allosteric modulation by phenytoin of the binding properties of the σ1ligands [3H](+)-pentazocine and [3H]NE-100. Synapse. 2006;59:152–161. doi: 10.1002/syn.20230. [DOI] [PubMed] [Google Scholar]
- 34.Cobos EJ, del Pozo E, Baeyens JM. Irreversible blockade of sigma-1 receptors by haloperidol and its metabolites in guinea pig brain and SH-SY5Y human neuroblastoma cells. J. Neurochem. 2007;102:812–825. doi: 10.1111/j.1471-4159.2007.04533.x. [DOI] [PubMed] [Google Scholar]
- 35.Collina S, Loddo G, Urbano M, Linati L, Callegari A, Ortuso F, Alcaro S, Laggner C, Langer T, Prezzavento O, Ronsisvalle G, Azzolina O. Design, synthesis, and SAR analysis of novel selective σ1 ligands. Bioorg. Med. Chem. 2007;15:771–783. doi: 10.1016/j.bmc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Cormaci G, Mori T, Hayashi T, Su TP. Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates σ-1 receptors in B-104 cells: Implication for neuroplasticity. J. Pharmacol. Exp. Ther. 2007;320:202–210. doi: 10.1124/jpet.106.108415. [DOI] [PubMed] [Google Scholar]
- 37.Daniels A, Ayala E, Chen W, Coop A, Matsumoto RR. N-[2-(m-methoxyphenyl)ethyl]-N-ethyl-2-(1-pyrrolidinyl) ethylamine (UMB 116) is a novel antagonist for cocaine-induced effects. Eur. J. Pharmacol. 2006;542:61–68. doi: 10.1016/j.ejphar.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 38.DeHaven-Hudkins DL, Lanyon LF, Ford-Rice FY, Ator MA. σ recognition sites in brain and peripheral tissues. Characterization and effects of cytochrome P450 inhibitors. Biochem. Pharmacol. 1994;47:1231–1239. doi: 10.1016/0006-2952(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 39.Delgado PL, Moreno FA. Role of norepinephrine in depression. J. Clin. Psychiatry. 2000;61(Suppl 1):5–12. [PubMed] [Google Scholar]
- 40.Depatie L, Lal S. Apomorphine and the dopamine hypothesis of schizophrenia: a dilemma? J. Psychiatry. Neurosci. 2001;26:203–220. [PMC free article] [PubMed] [Google Scholar]
- 41.Dhir A, Kulkarni SK. Involvement of sigma-1 receptor modulation in the antidepressant action of venlafaxine. Neurosci. Lett. 2007;420:204–208. doi: 10.1016/j.neulet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Dong LY, Cheng ZX, Fu YM, Wang ZM, Zhu YH, Sun JL, Dong Y, Zheng P. Neurosteroid dehydroepiandrosterone sulfate enhances spontaneous glutamate release in rat prelimbic cortex through activation of dopamine D1 and sigma-1 receptor. Neuropharmacology. 2007;52:966–974. doi: 10.1016/j.neuropharm.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Dussossoy D, Carayon P, Belugou S, Feraut D, Bord A, Goubet C, Roque C, Vidal H, Combes T, Loison G, Casellas P. Colocalization of sterol isomerase and sigma1 receptor at endoplasmic reticulum and nuclear envelope level. Eur. J. Biochem. 1999;263:377–386. doi: 10.1046/j.1432-1327.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- 44.Egashira N, Harada S, Okuno R, Matsushita M, Nishimura R, Mishima K, Iwasaki K, Orito K, Fujiwara M. Involvement of the sigma1 receptor in inhibiting activity of fluvoxamine on marble-burying behavior: comparison with paroxetine. Eur. J. Pharmacol. 2007;563:149–154. doi: 10.1016/j.ejphar.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 46.Gardner B, Zhu LX, Roth MD, Tashkin DP, Dubinett SM, Sharma S. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J. Neuroimmunol. 2004;147:95–98. doi: 10.1016/j.jneuroim.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Garrone B, Magnani M, Pinza M, Polenzani L. Effects of trazodone on neurotransmitter release from rat mossy fibre cerebellar synaptosomes. Eur. J. Pharmacol. 2000;400:35–41. doi: 10.1016/s0014-2999(00)00378-2. [DOI] [PubMed] [Google Scholar]
- 48.Gear RW, Lee JS, Miaskowski C, Gordon NC, Paul SM, Levine JD. Neuroleptics antagonize nalbuphine antianalgesia. J. Pain. 2006;7:187–191. doi: 10.1016/j.jpain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-β1. Int. Immunopharmacol. 2006;6:1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Gewirtz GR, Gorman JM, Volavka J, Macaluso J, Gribkoff G, Taylor DP, Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- 51.Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 52.Gue M, Junien JL, Del Rio C, Bueno L. Neuropeptide Y and sigma ligand (JO 1784) suppress stress-induced colonic motor disturbances in rats through sigma and cholecystokinin receptors. J. Pharmacol. Exp. Ther. 1992;261:850–855. [PubMed] [Google Scholar]
- 53.Guitart X, Codony X, Ballarín M, Dordal A, Farré AJ. E-5842: a new potent and preferential σ ligand: preclinical pharmacological profile. CNS Drug Rev. 1998;4:201–224. [Google Scholar]
- 54.Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl) 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 55.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashimoto K, London ED. Interactions of erythro-ifenprodil, threo-ifenprodil, erythro-iodoifenprodil, and eliprodil with subtypes of σ receptors. Eur. J. Pharmacol. 1995;273:307–310. doi: 10.1016/0014-2999(94)00763-w. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. Eur. J. Pharmacol. 2005;519:114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-Induced Cognitive Deficits in Mice are Improved by Subsequent Subchronic Administration of Fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2006;32:514–522. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi T, Maurice T, Su TP. Ca2+ signaling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J. Pharmacol. Exp. Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- 60.Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi T, Su TP. σ-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS. Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi T, Su TP. The Sigma Receptor: Evolution of the Concept in Neuropsychopharmacology. Curr. Neuropharmacol. 2005;3:1–15. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 65.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of σ1 and σ2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 66.Hiramatsu M, Hoshino T, Kameyama T, Nabeshima T. Involvement of κ-opioid and σ receptors in short-term memory in mice. Eur. J. Pharmacol. 2002;453:91–98. doi: 10.1016/s0014-2999(02)02388-9. [DOI] [PubMed] [Google Scholar]
- 67.Hiramatsu M, Hoshino T. Involvement of κ-opioid receptors and σ receptors in memory function demonstrated using an antisense strategy. Brain Res. 2004;1030:247–255. doi: 10.1016/j.brainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 68.Hofner G, Wanner KT. [3H]ifenprodil binding to NMDA receptors in porcine hippocampal brain membranes. Eur. J. Pharmacol. 2000;394:211–219. doi: 10.1016/s0014-2999(00)00084-4. [DOI] [PubMed] [Google Scholar]
- 69.Hong W, Werling LL. Binding of σ receptor ligands and their effects on muscarine-induced Ca2+ changes in SH-SY5Y cells. Eur. J. Pharmacol. 2002;436:35–45. doi: 10.1016/s0014-2999(01)01606-5. [DOI] [PubMed] [Google Scholar]
- 70.Hong W, Nuwayhid SJ, Werling LL. Modulation of bradykinin-induced calcium changes in SH-SY5Y cells by neurosteroids and sigma receptor ligands via a shared mechanism. Synapse. 2004;54:102–110. doi: 10.1002/syn.20069. [DOI] [PubMed] [Google Scholar]
- 71.Horan B, Gifford AN, Matsuno K, Mita S, Ashby CR Jr. Effect of SA4503 on the electrically evoked release of 3H-acetylcholine from striatal and hippocampal rat brain slices. Synapse. 2002;46:1–3. doi: 10.1002/syn.10107. [DOI] [PubMed] [Google Scholar]
- 72.Introini-Collison IB, McGaugh JL. Cocaine enhances memory storage in mice. Psychopharmacology (Berl) 1989;99:537–541. doi: 10.1007/BF00589905. [DOI] [PubMed] [Google Scholar]
- 73.Ishikawa M, Ishiwata K, Ishii K, Kimura Y, Sakata M, Naganawa M, Oda K, Miyatake R, Fujisaki M, Shimizu E, Shirayama Y, Iyo M, Hashimoto K. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: A positron emission tomography study using [11C]SA4503. Biol. Psychiatry. 2007;62:878–883. doi: 10.1016/j.biopsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Itzhak Y, Stein I, Zhang SH, Kassim CO, Cristante D. Binding of σ-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of σ-receptor subtypes. J. Pharmacol. Exp. Ther. 1991;257:141–148. [PubMed] [Google Scholar]
- 75.Jaen JC, Caprathe BW, Pugsley TA, Wise LD, Akunne H. Evaluation of the effects of the enantiomers of reduced haloperidol, azaperol, and related 4-amino-1-arylbutanols on dopamine and σ receptors. J. Med. Chem. 1993;36:3929–3936. doi: 10.1021/jm00076a022. [DOI] [PubMed] [Google Scholar]
- 76.Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest. Ophthalmol. Vis. Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.John CS, Vilner BJ, Bowen WD. Synthesis and characterization of [125I]-N-(N-benzylpiperidin-4-yl)-4- iodobenzamide, a new σ receptor radiopharmaceutical: high-affinity binding to MCF-7 breast tumor cells. J. Med. Chem. 1994;37:1737–1739. doi: 10.1021/jm00038a002. [DOI] [PubMed] [Google Scholar]
- 78.Kalasinsky KS, Bosy TZ, Schmunk GA, Ang L, Adams V, Gore SB, Smialek J, Furukawa Y, Guttman M, Kish SJ. Regional distribution of cocaine in postmortem brain of chronic human cocaine users. J. Forensic Sci. 2000;45:1041–1048. [PubMed] [Google Scholar]
- 79.Kamei H, Kameyama T, Nabeshima T. (+)-SKF-10,047 and dextromethorphan ameliorate conditioned fear stress through the activation of phenytoin-regulated σ1 sites. Eur. J. Pharmacol. 1996;299:21–28. doi: 10.1016/0014-2999(95)00830-6. [DOI] [PubMed] [Google Scholar]
- 80.Kamei H, Noda Y, Kameyama T, Nabeshima T. Role of (+)-SKF-10,047-sensitive sub-population of σ1 receptors in amelioration of conditioned fear stress in rats: association with mesolimbic dopaminergic systems. Eur. J. Pharmacol. 1997;319:165–172. doi: 10.1016/s0014-2999(96)00851-5. [DOI] [PubMed] [Google Scholar]
- 81.Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J. Pharmacol. Exp. Ther. 2006;319:1355–1365. doi: 10.1124/jpet.106.107557. [DOI] [PubMed] [Google Scholar]
- 82.Kato K, Hayako H, Ishihara Y, Marui S, Iwane M, Miyamoto M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci. Lett. 1999;260:5–8. doi: 10.1016/s0304-3940(98)00943-4. [DOI] [PubMed] [Google Scholar]
- 83.Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur. J. Pharmacol. 2003;468:109–119. doi: 10.1016/s0014-2999(03)01638-8. [DOI] [PubMed] [Google Scholar]
- 84.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem. Biophys. Res. Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 85.Kibaly C, Meyer L, Patte-Mensah C, Mensah-Nyagan AG. Biochemical and functional evidence for the control of pain mechanisms by dehydroepiandrosterone endogenously synthesized in the spinal cord. FASEB J. 2008;22:93–104. doi: 10.1096/fj.07-8930com. [DOI] [PubMed] [Google Scholar]
- 86.Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with σ1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br. J. Pharmacol. 2006;148:490–498. doi: 10.1038/sj.bjp.0706764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.King M, Pan YX, Mei J, Chang A, Xu J, Pasternak GW. Enhanced κ-opioid receptor-mediated analgesia by antisense targeting the σ1 receptor. Eur. J. Pharmacol. 1997;331:R5–R6. doi: 10.1016/s0014-2999(97)01064-9. [DOI] [PubMed] [Google Scholar]
- 88.Kitaichi K, Chabot JG, Moebius FF, Flandorfer A, Glossmann H, Quirion R. Expression of the purported sigma1 (σ1) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J. Chem. Neuroanat. 2000;20:375–387. doi: 10.1016/s0891-0618(00)00106-x. [DOI] [PubMed] [Google Scholar]
- 89.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci. Biobehav. Rev. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 90.Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr. Res. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (σ 1) knockout mice. Eur. J. Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 92.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 93.LePage KT, Ishmael JE, Low CM, Traynelis SF, Murray TF. Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology. 2005;49:1–16. doi: 10.1016/j.neuropharm.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, Zhou R, Cui S, Xie G, Cai W, Sokabe M, Chen L. Dehydroepiandrosterone sulfate prevents ischemia-induced impairment of long-term potentiation in rat hippocampal CA1 by up-regulating tyrosine phosphorylation of NMDA receptor. Neuropharmacology. 2006;51:958–966. doi: 10.1016/j.neuropharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Banister SD, Christie MJ, Banati R, Meikle S, Coster MJ, Kassiou M. Trishomocubanes: novel σ ligands modulate cocaine-induced behavioural effects. Eur. J. Pharmacol. 2007;555:37–42. doi: 10.1016/j.ejphar.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates Fra-2 and σ-1 receptor gene and protein expression in brain regions involved in addiction and reward. J. Pharmacol. Exp. Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- 97.Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J. Physiol. 2000;526(3):527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marrazzo A, Prezzavento O, Pappalardo MS, Bousquet E, Iadanza M, Pike VW, Ronsisvalle G. Synthesis of (+)- and (-)-cis-2-[(1-adamantylamino)-methyl]-1-phenylcyclopropane derivatives as high affinity probes for σ1 and σ2 binding sites. Farmaco. 2002;57:45–53. doi: 10.1016/s0014-827x(01)01170-3. [DOI] [PubMed] [Google Scholar]
- 99.Marrazzo A, Caraci F, Salinaro ET, Su TP, Copani A, Ronsisvalle G. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16:1223–1226. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- 100.Marrazzo A, Parenti C, Scavo V, Ronsisvalle S, Scoto GM, Ronsisvalle G. In vivo evaluation of (+)-MR200 as a new selective sigma ligand modulating MOP, DOP and KOP supraspinal analgesia. Life Sci. 2006;78:2449–2453. doi: 10.1016/j.lfs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of σ1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- 102.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 103.Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maruo J, Yoshida A, Shimohira I, Matsuno K, Mita S, Ueda H. Binding of [35S]GTPγS stimulated by (+)-pentazocine sigma receptor agonist, is abundant in the guinea pig spleen. Life Sci. 2000;67:599–603. doi: 10.1016/s0024-3205(00)00651-2. [DOI] [PubMed] [Google Scholar]
- 105.Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc. Natl. Acad. Sci. USA. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matos FF, Korpinen C, Yocca FD. 5-HT1A receptor agonist effects of BMY-14802 on serotonin release in dorsal raphe and hippocampus. Eur. J. Pharmacol. 1996;317:49–54. doi: 10.1016/s0014-2999(96)00699-1. [DOI] [PubMed] [Google Scholar]
- 107.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel σ receptor ligands: antidystonic effects in rats suggest σ receptor antagonism. Eur. J. Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 108.Matsumoto RR, Pouw B. Correlation between neuroleptic binding to σ1 and σ2 receptors and acute dystonic reactions. Eur. J. Pharmacol. 2000;401:155–160. doi: 10.1016/s0014-2999(00)00430-1. [DOI] [PubMed] [Google Scholar]
- 109.Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting σ1 receptors produce anti-cocaine effects in mice. Eur. J. Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- 110.Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, Hauck NA. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001;41:878–886. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 111.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 112.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. σ receptors: potential medications development target for anti-cocaine agents. Eur. J. Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 113.Matsumoto RR, Gilmore DL, Pouw B, Bowen WD, Williams W, Kausar A, Coop A. Novel analogs of the σ receptor ligand BD1008 attenuate cocaine-induced toxicity in mice. Eur. J. Pharmacol. 2004;492:21–26. doi: 10.1016/j.ejphar.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 114.Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Effects of UMB24 and (+/-)-SM 21, putative σ2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol. Biochem. Behav. 2007;86:86–91. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsuno K, Senda T, Matsunaga K, Mita S, Kaneto H. Similar ameliorating effects of benzomorphans and 5-HT2 antagonists on drug-induced impairment of passive avoidance response in mice: comparison with acetylcholinesterase inhibitors. Psychopharmacology (Berl) 1993;112:134–141. doi: 10.1007/BF02247374. [DOI] [PubMed] [Google Scholar]
- 116.Matsuno K, Senda T, Matsunaga K, Mita S. Ameliorating effects of σ receptor ligands on the impairment of passive avoidance tasks in mice: involvement in the central acetylcholinergic system. Eur. J. Pharmacol. 1994;261:43–51. doi: 10.1016/0014-2999(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 117.Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to σ1 receptors in the mouse forebrain. J. Neurosci. Res. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 118.Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (σ) receptor ligands. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 119.Maurice T, Su TP, Privat A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate B25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- 120.Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (σ1) receptor: pharmacological evidence and therapeutic opportunities. Jpn. J. Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- 121.Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the σ1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res. Brain Res. Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 122.Maurice T, Phan VL, Urani A, Guillemain I. Differential involvement of the sigma1 (σ1) receptor in the anti-amnesic effect of neuroactive steroids, as demonstrated using an in vivo antisense strategy in the mouse. Br. J. Pharmacol. 2001;134:1731–1741. doi: 10.1038/sj.bjp.0704355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maurice T, Phan VL, Privat A. The anti-amnesic effects of sigma1 (σ1) receptor agonists confirmed by in vivo antisense strategy in the mouse. Brain Res. 2001;898:113–121. doi: 10.1016/s0006-8993(01)02152-7. [DOI] [PubMed] [Google Scholar]
- 124.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma1 (σ1) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci. Biobehav. Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 125.Maurice T, Casalino M, Lacroix M, Romieu P. Involvement of the sigma1 receptor in the motivational effects of ethanol in mice. Pharmacol. Biochem. Behav. 2003;74:869–876. doi: 10.1016/s0091-3057(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 126.Maurice T, Meunier J, Feng B, Ieni J, Monaghan DT. Interaction with σ1 protein, but not N-methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J. Pharmacol. Exp. Ther. 2006;317:606–614. doi: 10.1124/jpet.105.097394. [DOI] [PubMed] [Google Scholar]
- 127.Maurice T, Gregoire C, Espallergues J. Neuro(active) steroids actions at the neuromodulatory sigma1 (σ1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol. Biochem. Behav. 2006;84:581–597. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 128.Mavlyutov TA, Ruoho AE. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J. Mol. Signal. 2007;2:8. doi: 10.1186/1750-2187-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCracken KA, Bowen WD, De Costa BR, Matsumoto RR. Two novel σ receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur. J. Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 130.Mei J, Pasternak GW. σ1 receptor modulation of opioid analgesia in the mouse. J. Pharmacol. Exp. Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- 131.Mei J, Pasternak GW. Modulation of brainstem opiate analgesia in the rat by σ1 receptors: a microinjection study. J. Pharmacol. Exp. Ther. 2007;322:1278–1285. doi: 10.1124/jpet.107.121137. [DOI] [PubMed] [Google Scholar]
- 132.Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75:1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 133.Meunier J, Maurice T. Beneficial effects of the sigma1 receptor agonists igmesine and dehydroepiandrosterone against learning impairments in rats prenatally exposed to cocaine. Neurotoxicol. Teratol. 2004;26:783–797. doi: 10.1016/j.ntt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 134.Meunier J, Gue M, Recasens M, Maurice T. Attenuation by a sigma1 (σ1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br. J. Pharmacol. 2004;142:689–700. doi: 10.1038/sj.bjp.0705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meunier J, Ieni J, Maurice T. Antiamnesic and neuroprotective effects of donepezil against learning impairments induced in mice by exposure to carbon monoxide gas. J. Pharmacol. Exp. Ther. 2006;317:1307–1319. doi: 10.1124/jpet.106.101527. [DOI] [PubMed] [Google Scholar]
- 136.Meunier J, Demeilliers B, Celerier A, Maurice T. Compensatory effect by sigma1 (σ1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav. Brain. Res. 2006;166:166–176. doi: 10.1016/j.bbr.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 137.Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid β25-35 peptide-induced toxicity in mice involve an interaction with the σ1 receptor. Br. J. Pharmacol. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons.Possible role of metabotropic σ1-like receptors. J. Biol. Chem. 2002;277:28725–28732. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- 139.Miyatake R, Furukawa A, Matsushita S, Higuchi S, Suwaki H. Functional polymorphisms in the sigma1 receptor gene associated with alcoholism. Biol. Psychiatry. 2004;55:85–90. doi: 10.1016/j.biopsych.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 140.Mizuno T, Yotsuyanagi S, Nagasaka Y, Namiki M. Dehydroepiandrosterone alleviates copulatory disorder induced by social stress in male rats. J. Sex. Med. 2006;3:612–618. doi: 10.1111/j.1743-6109.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- 141.Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8-C7 isomerase. Br. J. Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monassier L, Manoury B, Bellocq C, Weissenburger J, Greney H, Zimmermann D, Ehrhardt JD, Jaillon P, Baro I, Bousquet P. σ2-receptor ligand-mediated inhibition of inwardly rectifying K+ channels in the heart. J. Pharmacol. Exp. Ther. 2007;322:341–350. doi: 10.1124/jpet.107.122044. [DOI] [PubMed] [Google Scholar]
- 143.Monnet FP, Debonnel G, Bergeron R, Gronier B, de Montigny C. The effects of sigma ligands and of neuropeptide Y on N-methyl-D-aspartate-induced neuronal activation of CA3 dorsal hippocampus neurones are differentially affected by pertussin toxin. Br. J. Pharmacol. 1994;112:709–715. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by σ1 receptor agonists in rat hippocampal neurons. J. Pharmacol. Exp. Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- 145.Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol. Cell. 2005;97:873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- 146.Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: molecular, physiological, and behavioral aspects. J. Pharmacol. Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- 147.Morin-Surun MP, Collin T, Denavit-Saubie M, Baulieu EE, Monnet FP. Intracellular σ1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc. Natl. Acad. Sci. USA. 1999;96:8196–8199. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol. Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- 149.Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of σ receptors in rat brain. Eur. J. Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 150.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 152.Nobile M, Lagostena L. A discriminant block among K+ channel types by phenytoin in neuroblastoma cells. Br. J. Pharmacol. 1998;124:1698–1702. doi: 10.1038/sj.bjp.0702035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noda Y, Kamei H, Kamei Y, Nagai T, Nishida M, Nabeshima T. Neurosteroids ameliorate conditioned fear stress: an association with sigma1 receptors. Neuropsychopharmacology. 2000;23:276–284. doi: 10.1016/S0893-133X(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 154.Novakova M, Bruderova V, Sulova Z, Kopacek J, Lacinova L, Kvetnansky R, Vasku A, Kaplan P, Krizanova O, Jurkovicova D. Modulation of expression of the sigma receptors in the heart of rat and mouse in normal and pathological conditions. Gen. Physiol. Biophys. 2007;26:110–117. [PubMed] [Google Scholar]
- 155.Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci. Lett. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 156.Nuwayhid SJ, Werling LL. Sigma2 (σ2) receptors as a target for cocaine action in the rat striatum. Eur. J. Pharmacol. 2006;535:98–103. doi: 10.1016/j.ejphar.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 157.Okuyama S, Imagawa Y, Tomisawa K. Behavioral Evidence for Modulation by Sigma Ligands of (+)MK-801-induced Hyperlocomotion in Monoamine-depleted Mice. Neuropharmacology. 1996;35:467–474. doi: 10.1016/0028-3908(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 158.Okuyama S, Nakazato A. NE-100: a novel sigma receptor antagonist. CNS Drug Rev. 1996;2:226–237. [Google Scholar]
- 159.Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the σ-1 receptor ligand binding site using a novel photoprobe. Mol. Pharmacol. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- 160.Palacios G, Muro A, Vela JM, Molina-Holgado E, Guitart X, Ovalle S, Zamanillo D. Immunohistochemical localization of the σ1-receptor in oligodendrocytes in the rat central nervous system. Brain Res. 2003;961:92–99. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- 161.Pan YX, Mei J, Xu J, Wan BL, Zuckerman A, Pasternak GW. Cloning and characterization of a mouse σ1 receptor. J. Neurochem. 1998;70:2279–2285. doi: 10.1046/j.1471-4159.1998.70062279.x. [DOI] [PubMed] [Google Scholar]
- 162.Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E. Involvement of the sigma1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci. 2004;19:2212–2220. doi: 10.1111/j.0953-816X.2004.03297.x. [DOI] [PubMed] [Google Scholar]
- 163.Phan VL, Miyamoto Y, Nabeshima T, Maurice T. Age-related expression of σ1 receptors and antidepressant efficacy of a selective agonist in the senescence-accelerated (SAM) mouse. J. Neurosci. Res. 2005;79:561–572. doi: 10.1002/jnr.20390. [DOI] [PubMed] [Google Scholar]
- 164.Poncelet M, Santucci V, Paul R, Gueudet C, Lavastre S, Guitard J, Steinberg R, Terranova JP, Breliere JC, Soubrie P. Neuropharmacological profile of a novel and selective ligand of the sigma site: SR 31742A. Neuropharmacology. 1993;32:605–615. doi: 10.1016/0028-3908(93)90057-a. [DOI] [PubMed] [Google Scholar]
- 165.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of σ binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 166.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 167.Rogoz Z, Skuza G. Mechanism of synergistic action following co-treatment with pramipexole and fluoxetine or sertraline in the forced swimming test in rats. Pharmacol. Rep. 2006;58:493–500. [PubMed] [Google Scholar]
- 168.Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- 169.Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 170.Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. The sigma1 (σ1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology (Berl.) 2004;175:154–162. doi: 10.1007/s00213-004-1814-x. [DOI] [PubMed] [Google Scholar]
- 171.Romieu P, Lucas M, Maurice T. σ1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology. 2006;31:1431–1443. doi: 10.1038/sj.npp.1300885. [DOI] [PubMed] [Google Scholar]
- 172.Ronsisvalle G, Marrazzo A, Prezzavento O, Pasquinucci L, Falcucci B, Di Toro RD, Spampinato S. Substituted 1-phenyl-2-cyclopropylmethylamines with high affinity and selectivity for sigma sites. Bioorg. Med. Chem. 2000;8:1503–1513. doi: 10.1016/s0968-0896(00)00072-9. [DOI] [PubMed] [Google Scholar]
- 173.Ronsisvalle G, Marrazzo A, Prezzavento O, Cagnotto A, Mennini T, Parenti C, Scoto GM. Opioid and sigma receptor studies. New developments in the design of selective sigma ligands. Pure Appl. Chem. 2001;73:1499–1509. [Google Scholar]
- 174.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and σ-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J. Leukoc. Biol. 2005;78:1198–1203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 175.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 176.Rückert NG, Schmidt WJ. The σ receptor ligand 1,3-di-(2-tolyl)guanidine in animal models of schizophrenia. Eur. J. Pharmacol. 1993;233:261–267. doi: 10.1016/0014-2999(93)90059-q. [DOI] [PubMed] [Google Scholar]
- 177.Rush AM, Elliott JR. Phenytoin and carbamazepine: differential inhibition of sodium currents in small cells from adult rat dorsal root ganglia. Neurosci. Lett. 1997;226:95–98. doi: 10.1016/s0304-3940(97)00258-9. [DOI] [PubMed] [Google Scholar]
- 178.Schwarz S, Pohl P, Zhou GZ. Steroid binding at sigma-"opioid" receptors. Science. 1989;246:1635–1638. doi: 10.1126/science.2556797. [DOI] [PubMed] [Google Scholar]
- 179.Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997;241:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- 180.Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. Cloning and functional characterization of a σ receptor from rat brain. J. Neurochem. 1998;70:922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- 181.Seth P, Ganapathy ME, Conway SJ, Bridges CD, Smith SB, Casellas P, Ganapathy V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta. 2001;1540:59–67. doi: 10.1016/s0167-4889(01)00117-3. [DOI] [PubMed] [Google Scholar]
- 182.Shin EJ, Nah SY, Kim WK, Ko KH, Jhoo WK, Lim YK, Cha JY, Chen CF, Kim HC. The dextromethorphan analog dimemorfan attenuates kainate-induced seizures via σ1 receptor activation: comparison with the effects of dextromethorphan. Br. J. Pharmacol. 2005;144:908–918. doi: 10.1038/sj.bjp.0705998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol. Teratol. 2006;28:173–180. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 184.Skuza G. Effect of sigma ligands on the cocaine-induced convulsions in mice. Pol. J. Pharmacol. 1999;51:477–483. [PubMed] [Google Scholar]
- 185.Skuza G, Rogoz Z. A potential antidepressant activity of SA4503, a selective σ1 receptor agonist. Behav. Pharmacol. 2002;13:537–543. doi: 10.1097/00008877-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 186.Skuza G, Rogoz Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J. Physiol. Pharmacol. 2006;57:217–229. [PubMed] [Google Scholar]
- 187.Skuza G, Rogoz Z. Effect of BD 1047, a sigma1 receptor antagonist, in the animal models predictive of antipsychotic activity. Pharmacol. Rep. 2006;58:626–635. [PubMed] [Google Scholar]
- 188.Soriani O, Foll FL, Roman F, Monnet FP, Vaudry H, Cazin L. A-Current down-modulated by σ receptor in frog pituitary melanotrope cells through a G protein-dependent pathway. J. Pharmacol. Exp. Ther. 1999;289:321–328. [PubMed] [Google Scholar]
- 189.Soriani O, Le Foll F, Galas L, Roman F, Vaudry H, Cazin L. The σ-ligand (+)-pentazocine depresses M current and enhances calcium conductances in frog melanotrophs. Am. J. Physiol. 1999;277:E73–E80. doi: 10.1152/ajpendo.1999.277.1.E73. [DOI] [PubMed] [Google Scholar]
- 190.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J. Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl.) 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- 192.Stone JM, Arstad E, Erlandsson K, Waterhouse RN, Ell PJ, Pilowsky LS. [123I]TPCNE-a novel SPET tracer for the sigma-1 receptor: first human studies and in vivo haloperidol challenge. Synapse. 2006;60:109–117. doi: 10.1002/syn.20281. [DOI] [PubMed] [Google Scholar]
- 193.Su TP, London ED, Jaffe JH. Steroid binding at σ receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 194.Su TP, Hayashi T. Cocaine affects the dynamics of cytoskeletal proteins via sigma1 receptors. Trends Pharmacol. Sci. 2001;22:456–458. doi: 10.1016/s0165-6147(00)01740-5. [DOI] [PubMed] [Google Scholar]
- 195.Takahashi S, Sonehara K, Takagi K, Miwa T, Horikomi K, Mita N, Nagase H, Iizuka K, Sakai K. Pharmacological profile of MS-377, a novel antipsychotic agent with selective affinity for σ receptors. Psychopharmacology (Berl) 1999;145:295–302. doi: 10.1007/s002130051061. [DOI] [PubMed] [Google Scholar]
- 196.Takahashi S, Miwa T, Horikomi K. Involvement of σ1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci. Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- 197.Takahashi S, Horikomi K, Kato T. MS-377, a novel selective σ1 receptor ligand, reverses phencyclidine-induced release of dopamine and serotonin in rat brain. Eur. J. Pharmacol. 2001;427:211–219. doi: 10.1016/s0014-2999(01)01254-7. [DOI] [PubMed] [Google Scholar]
- 198.Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves σ-1 receptors implications for antidepressants. J. Pharmacol. Exp. Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- 199.Takebayashi M, Hayashi T, Su TP. σ-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse. 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- 200.Tam SW, Steinfels GF, Gilligan PJ, Schmidt WK, Cook L. DuP 734 [1-(cyclopropylmethyl)-4-(2'(4''-fluorophenyl)-2'-oxoethyl)- piperidine HBr] a sigma and 5-hydroxytryptamine2 receptor antagonist: receptor-binding, electrophysiological and neuropharmacological profiles. J. Pharmacol. Exp. Ther. 1992;263:1167–1174. [PubMed] [Google Scholar]
- 201.Taylor DP, Eison MS, Moon SL, Schlemmer RF Jr, Shukla UA, VanderMaelen CP, Yocca FD, Gallant DJ, Behling SH, Boissard CG. A role for σ binding in the antipsychotic profile of BMY 14802? NIDA. Res. Monogr. 1993;133:125–157. [PubMed] [Google Scholar]
- 202.Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J. Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- 203.Tottori K, Miwa T, Uwahodo Y, Yamada S, Nakai M, Oshiro Y, Kikuchi T, Altar CA. Antidepressant-like responses to the combined sigma and 5-HT1A receptor agonist OPC-14523. Neuropharmacology. 2001;41:976–988. doi: 10.1016/s0028-3908(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 204.Ueda H, Inoue M, Yoshida A, Mizuno K, Yamamoto H, Maruo J, Matsuno K, Mita S. Metabotropic neurosteroid/σ-receptor involved in stimulation of nociceptor endings of mice. J. Pharmacol. Exp. Ther. 2001;298:703–710. [PubMed] [Google Scholar]
- 205.Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (σ) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci. 1992;50:L129–L134. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- 206.Ujike H, Kuroda S, Otsuki S. σ Receptor antagonists block the development of sensitization to cocaine. Eur. J. Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- 207.Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of σ receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 208.Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by σ1-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J. Pharmacol. Exp. Ther. 2001;298:1269–1279. [PubMed] [Google Scholar]
- 209.Urani A, Romieu P, Roman FJ, Maurice T. Enhanced antidepressant effect of sigma1 (σ1) receptor agonists in β25-35--amyloid peptide-treated mice. Behav. Brain Res. 2002;134:239–247. doi: 10.1016/s0166-4328(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 210.Urani A, Romieu P, Portales-Casamar E, Roman FJ, Maurice T. The antidepressant-like effect induced by the sigma1 (σ1) receptor agonist igmesine involves modulation of intracellular calcium mobilization. Psychopharmacology (Berl.) 2002;163:26–35. doi: 10.1007/s00213-002-1150-y. [DOI] [PubMed] [Google Scholar]
- 211.Urani A, Romieu P, Roman FJ, Yamada K, Noda Y, Kamei H, Manh TH, Nagai T, Nabeshima T, Maurice T. Enhanced antidepressant efficacy of σ1 receptor agonists in rats after chronic intracerebroventricular infusion of β-amyloid-(1-40) protein. Eur. J. Pharmacol. 2004;486:151–161. doi: 10.1016/j.ejphar.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 212.van Berckel BNM. glutamate and schizophrenia. Curr. Neuropharmacol. 2003;1:351–370. [Google Scholar]
- 213.Volz HP, Stoll KD. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S214–S220. doi: 10.1055/s-2004-832680. [DOI] [PubMed] [Google Scholar]
- 214.Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol. Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 215.Walker JM, Bowen WD, Patrick SL, Williams WE, Mascarella SW, Bai X, Carroll FI. A comparison of (-)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of σ2 receptors in motor function. Eur. J. Pharmacol. 1993;231:61–68. doi: 10.1016/0014-2999(93)90684-a. [DOI] [PubMed] [Google Scholar]
- 216.Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nitta A, Nabeshima T. Role of N-methyl-D-aspartate Receptors in Antidepressant-Like Effects of σ1 Receptor Agonist 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine Dihydrochloride (SA-4503) in Olfactory Bulbectomized Rats. J. Pharmacol. Exp. Ther. 2007;322:1305–1314. doi: 10.1124/jpet.107.124685. [DOI] [PubMed] [Google Scholar]
- 217.Wang HH, Chien JW, Chou YC, Liao JF, Chen CF. Anti-amnesic effect of dimemorfan in mice. Br. J. Pharmacol. 2003;138:941–949. doi: 10.1038/sj.bjp.0705117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (σ) receptor agonists produce antidepressant-like effects in mice. Eur. Neuropsychopharmacol. 2007;17:708–716. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Waterhouse RN, Chang RC, Atuehene N, Collier TL. In vitro and in vivo binding of neuroactive steroids to the sigma-1 receptor as measured with the positron emission tomography radioligand [18F]FPS. Synapse. 2007;61:540–546. doi: 10.1002/syn.20369. [DOI] [PubMed] [Google Scholar]
- 220.Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, Jackson MB. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J. Physiol. 1999;517(2):391–406. doi: 10.1111/j.1469-7793.1999.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J. Pharmacol. Exp. Ther. 1993;266:473–482. [PubMed] [Google Scholar]
- 222.Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- 223.Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- 224.Zhang H, Cuevas J. σ Receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J. Pharmacol. Exp. Ther. 2005;313:1387–1396. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]