Abstract
Monoclonal antibodies (MAbs) directed against two phenotypically distinct ovine lentivirus (OvLV) strains were generated by fusion of BALB/c SP2/0-Ag 14 myeloma cells with spleen cells from mice immunized with purified OvLV. Hybridomas were selected by indirect enzyme-linked immunosorbent assay (ELISA) and analysis of reactivity on immunoblots. The majority (17 of 21) of the MAbs recognized the gag-encoded capsid protein, CA p27, of both strains. Four other MAbs recognized a smaller structural protein, presumably a matrix protein, MA p17. Three distinct epitopes on CA p27 and one on MA p17 were distinguished by the MAbs with competition ELISA. MAbs from each epitope group were able to recognize 17 North American field isolates of OvLV and the closely related caprine arthritis-encephalitis virus (CAEV). Analysis of the data indicated that these epitopes were highly conserved among naturally occurring isolates. A representative MAb from each epitope group of anti-CA p27 MAbs reacted with four field strains of OvLV and CAEV on immunoblots. An anti-MA p17 MAb recognized the same OvLV strains on immunoblots but failed to recognize CAEV. MAbs which recognize conserved epitopes of gag-encoded lentivirus proteins (CA p27 and MA p17) are valuable tools. These MAbs can be used to develop sensitive diagnostic assays and to study the pathogenesis of lentivirus infections in sheep and goats.
Full text
PDF


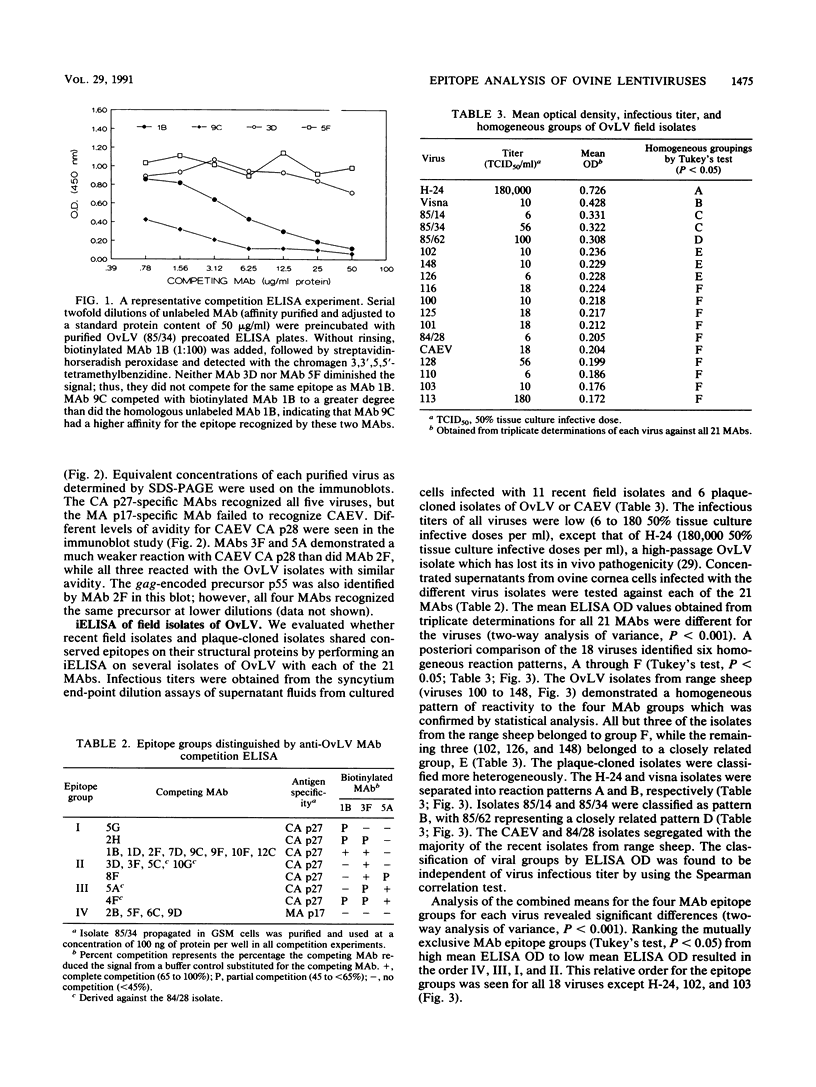
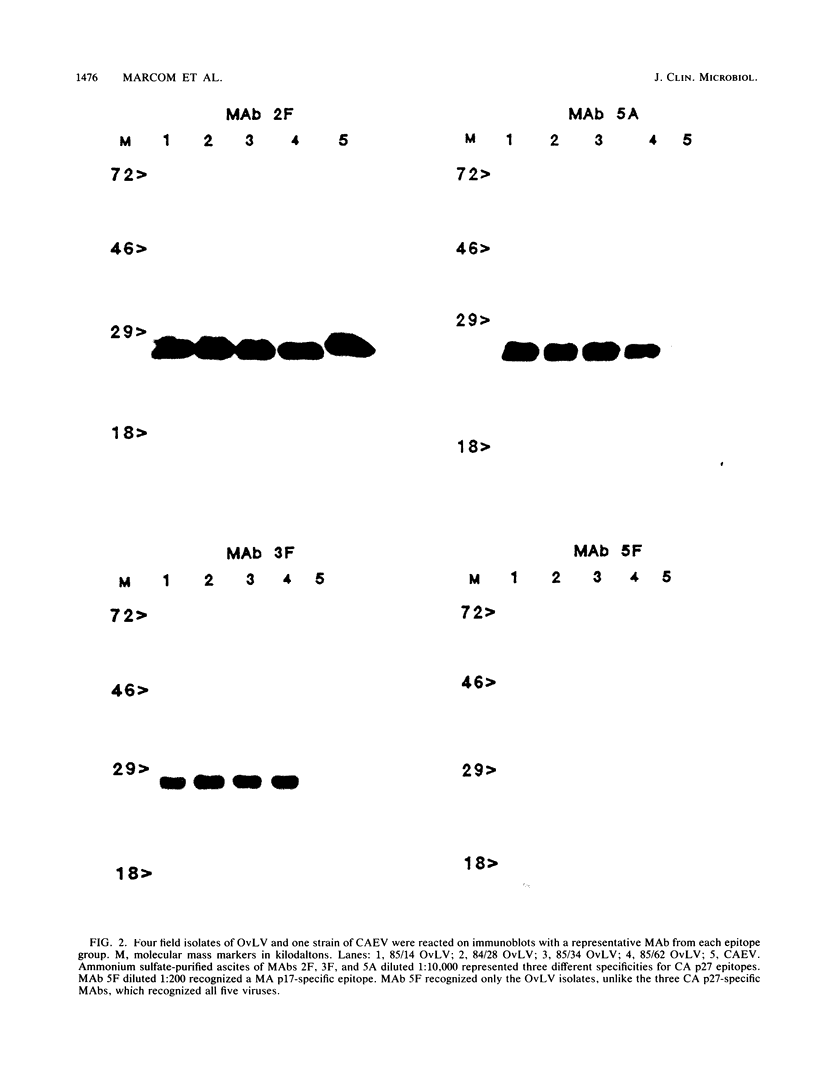
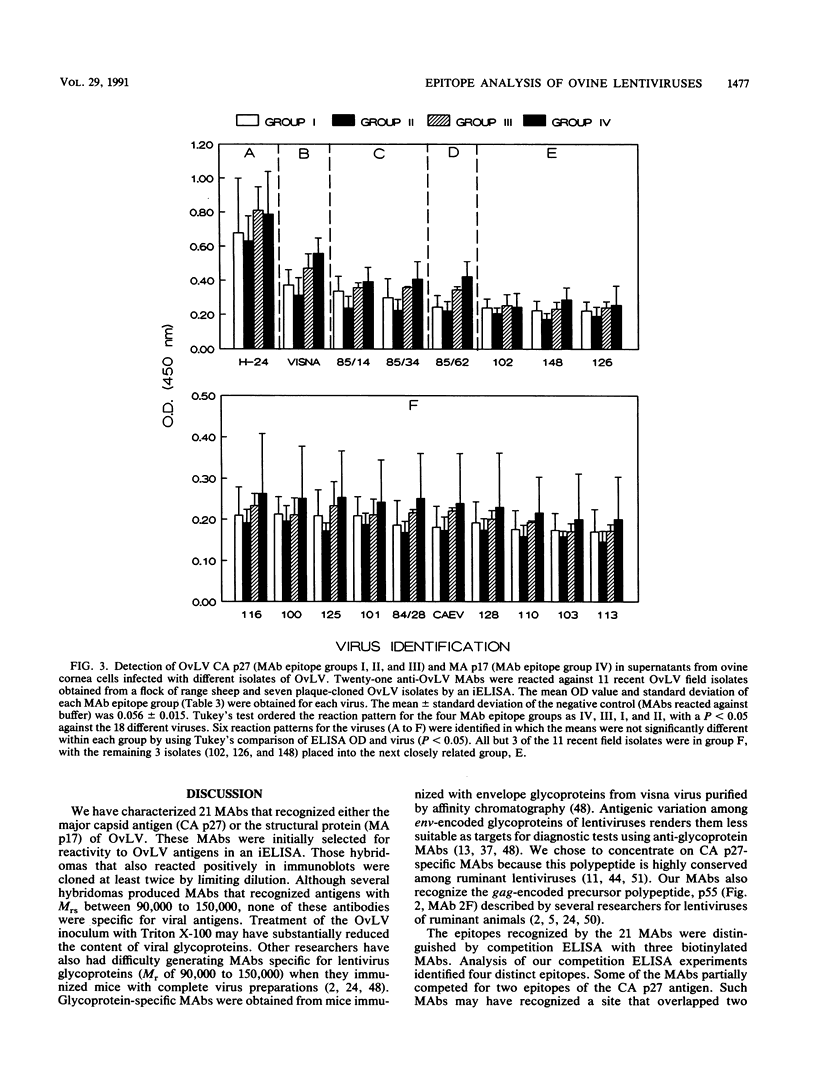


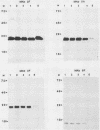
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archambault D., East N., Perk K., Dahlberg J. E. Development of an enzyme-linked immunosorbent assay for caprine arthritis-encephalitis virus. J Clin Microbiol. 1988 May;26(5):971–975. doi: 10.1128/jcm.26.5.971-975.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov L., Whalley J. M. Virus-specific polypeptides of caprine arthritis-encephalitis virus recognized by monoclonal antibodies to virion proteins p24 and p14. J Gen Virol. 1988 May;69(Pt 5):1097–1103. doi: 10.1099/0022-1317-69-5-1097. [DOI] [PubMed] [Google Scholar]
- Braun M. J., Clements J. E., Gonda M. A. The visna virus genome: evidence for a hypervariable site in the env gene and sequence homology among lentivirus envelope proteins. J Virol. 1987 Dec;61(12):4046–4054. doi: 10.1128/jvi.61.12.4046-4054.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., McGuire T. C. The lentiviruses: maedi/visna, caprine arthritis-encephalitis, and equine infectious anemia. Adv Virus Res. 1988;34:189–215. doi: 10.1016/s0065-3527(08)60518-7. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Stem T. A., Knowles D. P., McGuire T. C. Precursor polypeptides of caprine arthritis-encephalitis lentivirus structural proteins. J Gen Virol. 1988 Mar;69(Pt 3):675–681. doi: 10.1099/0022-1317-69-3-675. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Butcher A. C., Riegel C. A., McGrane V., Blair C. D., Teramoto Y. A., Winston S. Neutralizing determinants defined by monoclonal antibodies on polypeptides specified by bovine herpesvirus 1. J Virol. 1984 Nov;52(2):403–409. doi: 10.1128/jvi.52.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford T. B., Adams D. S., Cheevers W. P., Cork L. C. Chronic arthritis in goats caused by a retrovirus. Science. 1980 Feb 29;207(4434):997–999. doi: 10.1126/science.6153243. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Jackson T. A., Laird G. A. Prevalence of ovine progressive pneumonia in a sampling of cull sheep from western and midwestern United States. Am J Vet Res. 1977 Dec;38(12):2091–2093. [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Brogden K. A., Schmerr M. J. Failure of experimental vaccines to protect against infection with ovine progressive pneumonia (maedi-visna) virus. Vet Microbiol. 1987 Mar;13(3):201–204. doi: 10.1016/0378-1135(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Lehmkuhl H. D., Schmerr M. J., Brogden K. A. Ovine progressive pneumonia (maedi-visna) in sheep. Vet Microbiol. 1988 Jul;17(3):237–250. doi: 10.1016/0378-1135(88)90068-5. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Gaskin J. M., Perk K. Morphological and immunological comparison of caprine arthritis encephalitis and ovine progressive pneumonia viruses. J Virol. 1981 Sep;39(3):914–919. doi: 10.1128/jvi.39.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., Cutlip R. C., Lehmkuhl H. D., Brogden K. A. Ultrastructure and frequency of mastitis caused by ovine progressive pneumonia virus infection in sheep. Vet Pathol. 1986 Mar;23(2):184–189. doi: 10.1177/030098588602300212. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Wilcox G. E., Robinson W. F. Antigenic variation of caprine arthritis-encephalitis virus during persistent infection of goats. J Gen Virol. 1987 Dec;68(Pt 12):3145–3152. doi: 10.1099/0022-1317-68-12-3145. [DOI] [PubMed] [Google Scholar]
- Gates N. L., Everson D. O., Hulet C. V. Effects of thin ewe syndrome on reproductive efficiency. J Am Vet Med Assoc. 1977 Dec 15;171(12):1266–1267. [PubMed] [Google Scholar]
- Gates N. L., Winward L. D., Gorham J. R., Shen D. T. Serologic survey of prevalence of ovine progressive pneumonia in Idaho range sheep. J Am Vet Med Assoc. 1978 Dec 15;173(12):1575–1577. [PubMed] [Google Scholar]
- Gogolewski R. P., Adams D. S., McGuire T. C., Banks K. L., Cheevers W. P. Antigenic cross-reactivity between caprine arthritis-encephalitis, visna and progressive pneumonia viruses involves all virion-associated proteins and glycoproteins. J Gen Virol. 1985 Jun;66(Pt 6):1233–1240. doi: 10.1099/0022-1317-66-6-1233. [DOI] [PubMed] [Google Scholar]
- Goyal S. M., Khan M. A., McPherson S. W., Robinson R. A., Boylan W. J. Prevalence of antibodies to seven viruses in a flock of ewes in Minnesota. Am J Vet Res. 1988 Apr;49(4):464–467. [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., König C. D., Bakker J., de Boer M. J., Pekelder J. J., Sol J., Vellema P., de Vries G. Maedi-visna control in sheep. III: Results and evaluation of a voluntary control program in The Netherlands over a period of four years. Vet Q. 1987 Nov;9 (Suppl 1):29S–36S. [PubMed] [Google Scholar]
- Houwers D. J., König C. D., de Boer G. F., Schaake J., Jr Maedi-visna control in sheep. I. Artificial rearing of colostrum-deprived lambs. Vet Microbiol. 1983 Apr;8(2):179–185. doi: 10.1016/0378-1135(83)90064-0. [DOI] [PubMed] [Google Scholar]
- Houwers D. J., König C. D., de Boer G. F., Schaake J., Jr Maedi-visna control in sheep. I. Artificial rearing of colostrum-deprived lambs. Vet Microbiol. 1983 Apr;8(2):179–185. doi: 10.1016/0378-1135(83)90064-0. [DOI] [PubMed] [Google Scholar]
- Huffman E. M., Kirk J. H., Winward L., Gorham J. R. Serologic prevalence of ovine progressive pneumonia in a western range flock of sheep. J Am Vet Med Assoc. 1981 Apr 1;178(7):708–710. [PubMed] [Google Scholar]
- Hussain K. A., Issel C. J., Rwambo P. M., Arnizaut A. B., Ball J. M., Schnorr K. L., Montelaro R. C. Identification of gag precursor of equine infectious anaemia virus with monoclonal antibodies to the major viral core protein, p26. J Gen Virol. 1988 Jul;69(Pt 7):1719–1724. doi: 10.1099/0022-1317-69-7-1719. [DOI] [PubMed] [Google Scholar]
- Janvier B., Archinard P., Mandrand B., Goudeau A., Barin F. Linear B-cell epitopes of the major core protein of human immunodeficiency virus types 1 and 2. J Virol. 1990 Sep;64(9):4258–4263. doi: 10.1128/jvi.64.9.4258-4263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa O., Lairmore M. D., DeMartini J. C. Analysis of antibody responses to phenotypically distinct lentiviruses. J Clin Microbiol. 1990 Apr;28(4):764–770. doi: 10.1128/jcm.28.4.764-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Eklund C. M., Lopez C., Hadlow W. J. Isolation of a virus from the lungs of Montana sheep affected with progressive pneumonia. Virology. 1968 Jul;35(3):483–484. doi: 10.1016/0042-6822(68)90228-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lairmore M. D., Poulson J. M., Adducci T. A., DeMartini J. C. Lentivirus-induced lymphoproliferative disease. Comparative pathogenicity of phenotypically distinct ovine lentivirus strains. Am J Pathol. 1988 Jan;130(1):80–90. [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Rosadio R. H., DeMartini J. C. Ovine lentivirus lymphoid interstitial pneumonia. Rapid induction in neonatal lambs. Am J Pathol. 1986 Oct;125(1):173–181. [PMC free article] [PubMed] [Google Scholar]
- Leis J., Baltimore D., Bishop J. M., Coffin J., Fleissner E., Goff S. P., Oroszlan S., Robinson H., Skalka A. M., Temin H. M. Standardized and simplified nomenclature for proteins common to all retroviruses. J Virol. 1988 May;62(5):1808–1809. doi: 10.1128/jvi.62.5.1808-1809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light M. R., Schipper I. A., Molitor T. W., Tilton J. E., Slanger W. D. Progressive pneumonia in sheep: incidence of natural infection and establishment of clean flocks. J Anim Sci. 1979 Nov;49(5):1157–1160. doi: 10.2527/jas1979.4951157x. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Adams D. S., Johnson G. C., Klevjer-Anderson P., Barbee D. D., Gorham J. R. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am J Vet Res. 1986 Mar;47(3):537–540. [PubMed] [Google Scholar]
- McGuire T. C., Brassfield A. L., Davis W. C., Cheevers W. P. Antigenic and structural variation of the p28 core polypeptide of goat and sheep retroviruses. J Gen Virol. 1987 Aug;68(Pt 8):2259–2263. doi: 10.1099/0022-1317-68-8-2259. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Norton L. K., O'Rourke K. I., Cheevers W. P. Antigenic variation of neutralization-sensitive epitopes of caprine arthritis-encephalitis lentivirus during persistent arthritis. J Virol. 1988 Sep;62(9):3488–3492. doi: 10.1128/jvi.62.9.3488-3492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedrig M., Hinkula J., Weigelt W., L'age-Stehr J., Pauli G., Rosen J., Wahren B. Epitope mapping of monoclonal antibodies against human immunodeficiency virus type 1 structural proteins by using peptides. J Virol. 1989 Aug;63(8):3525–3528. doi: 10.1128/jvi.63.8.3525-3528.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Poss M. L., Demartini J. C. Animal lentivirus vaccines: problems and prospects. Vet Immunol Immunopathol. 1989 Feb;20(3):183–212. doi: 10.1016/0165-2427(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Gonda M. A., Narayan O. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986 May;58(2):665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Molineaux S. M., Narayan O. Genetic variation among lentiviruses: homology between visna virus and caprine arthritis-encephalitis virus is confined to the 5' gag-pol region and a small portion of the env gene. J Virol. 1984 Sep;51(3):713–721. doi: 10.1128/jvi.51.3.713-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quérat G., Barban V., Sauze N., Filippi P., Vigne R., Russo P., Vitu C. Highly lytic and persistent lentiviruses naturally present in sheep with progressive pneumonia are genetically distinct. J Virol. 1984 Nov;52(2):672–679. doi: 10.1128/jvi.52.2.672-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P. A. Visna of sheep; a slow, demyelinating infection. Br J Exp Pathol. 1958 Oct;39(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Bhaduri L. M., Narayan O., Clements J. E. Topographical rearrangements of visna virus envelope glycoprotein during antigenic drift. J Virol. 1987 Apr;61(4):1019–1028. doi: 10.1128/jvi.61.4.1019-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowring L., Haase A. T., Charman H. P. Serological definition of the lentivirus group of retroviruses. J Virol. 1979 Feb;29(2):523–528. doi: 10.1128/jvi.29.2.523-528.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Filippi P., Quérat G., Sauze N., Vitu C., Russo P., Delori P. Precursor polypeptides to structural proteins of visna virus. J Virol. 1982 Jun;42(3):1046–1056. doi: 10.1128/jvi.42.3.1046-1056.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Zeelon E. P., Sweet R. W., Harter D. H., Spiegelman S. Immunological cross-reactions of the major internal protein component from "slow" viruses of sheep. Virology. 1977 Feb;76(2):851–854. doi: 10.1016/0042-6822(77)90264-1. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]