Abstract
Among the phospholipases A2 (PLA2s) are the Group VI Ca2+-independent PLA2s (iPLA2s) and expression of multiple transcripts of iPLA2 in skeletal muscle has been reported. In the present study, phospholipase activity and sequential ATP and calmodulin affinity column chromatography analyses reveal that skeletal muscle iPLA2 exhibits properties characteristic of the iPLA2β isoform. The phospholipase activity of iPLA2β has been demonstrated to participate in signal transduction, cell proliferation, and apoptosis. We also report here that skeletal muscle from iPLA2β-null mice, relative to wild type muscle, exhibits a reduced capacity to oxidize palmitate but not palmitoyl-CoA or acetyl-CoA in the absence of changes in fatty acid transporters CD36 and CPT1 or β-hydroxyacyl-CoA dehydrogenase activity. Recently, purified iPLA2β was demonstrated to manifest a thioesterase activity which catalyzes hydrolysis of fatty acyl-CoAs. The liberated CoA-SH facilitates fatty acid transport into the mitochondria. In this regard, we find that fractions eluted from the ATP column and containing iPLA2β phospholipase activity also contained acyl-CoA thioesterase activity that was inhibited by the bromoenol lactone (BEL) suicide inhibitor of iPLA2β. We further find that acyl-CoA thioesterase activity in skeletal muscle preparations from iPLA2β-null mice is significantly reduced, relative to WT activity. These findings suggest that the absence of acyl-CoA thioesterase activity of iPLA2β can lead to reduced fatty acyl-CoA generation and impair fatty acid oxidation in iPLA2β-null mice. Our findings therefore reveal a novel function of iPLA2β, related not to its phospholipase activity but to its thioesterase activity, which contributes to optimal fatty acid oxidation in skeletal muscle.
Keywords: Affinity chromatography, iPLA2β-null, fatty acid oxidation, acyl-CoA thioesterase activity, skeletal muscle
Phospholipases A2 (PLA2) are a diverse group of enzymes that catalyze hydrolysis of the sn-2 substituent from glycerophospholipid substrates to yield a free fatty acid and a 2-lyso-phospholipid (1). At present, the recognized PLA2s are classified into 15 different groups that include the low MW secretory PLA2s and the higher MW Ca2+-dependent and Ca2+-independent PLA2s (2). Among the PLA2s is an 84 kDa cytosolic PLA2 that does not require Ca2+ for catalysis, is activated by ATP and inhibited by BEL, and is classified as Group VIA PLA2, designated iPLA2β (3–5). Inhibition of iPLA2β has been reported to suppress incorporation of arachidonic acid into membrane phospholipids of macrophage-like P388D1 cells (6, 7) leading to the suggestion that iPLA2β participates in phospholipid remodeling (8, 9). Findings in other cells, however, suggest that iPLA2β is involved in signal transduction (10–16).
The group VIA Ca2+-independent phospholipase A2 (iPLA2β) has been cloned from several sources and is encoded by mRNA species that yield proteins (expected molecular masses of 80–88 kDa) that contain a phospholipase motif preceded by eight ankyrin repeats in the N-terminal half of the molecule (17–19). An 88 kDa iPLA2β isoform is a product of a mRNA species that arises from an exon-skipping mechanism of alternate splicing and contains a 54 amino acid insert that interrupts the eighth ankyrin repeat (20, 21). Proteolytic processing of iPLA2β also yields truncated protein products that are constitutively active. These products arise from caspase-3-catalyzed cleavage of iPLA2β in human promonocytic U937 cells (22) and β-cells (23), and calmodulin-dependent cleavage of iPLA2β under in vitro conditions (24). Further, pancreatic islets predominantly express a catalytically active 70 kDa iPLA2β variant that is not a product of alternate splicing (25).
Multiple reports suggest that muscle tissue expresses more than one iPLA2 transcript. In one of the first such survey studies, Tang et al. (19) observed an iPLA2 transcript at 3 kb in Northern analyses of a mouse and rat multiple tissue blot. Subsequently, human skeletal muscle was shown to express four iPLA2 transcripts of 1.8, 2.0, 3.2, and 4.2 kb (20). The membrane-associated iPLA2γ in heart and skeletal muscle are thought to be products of an iPLA2 transcript of 3.4 kb (5, 26). Recently, we observed expression of two iPLA2 transcripts of 3.0 and 3.2 kb in several mouse tissues including skeletal muscle and pancreatic islets (27). We have previously demonstrated that iPLA2β is expressed in the β-cell but not in the non-β-cell population of islets (28). To determine if the iPLA2 expressed in skeletal muscle was analogous to that expressed in β-cells we examined the chromatographic profile and catalytic properties of skeletal muscle iPLA2. Here, we report that skeletal muscle expresses the iPLA2β isoform that manifests phospholipase and acyl-CoA thioesterase activity and participates in fatty acid oxidation.
Experimental Procedures
Materials
Materials used in these studies were obtained from the following (sources): [14C]-Palmitoyl-CoA (50 mCi/mmol, American Radiolabeled Chemicals, Inc., St. Louis, MO); (16:0/[14C]-18:2)-GPC (PLPC, 55 mCi/mmol), rainbow molecular mass standards, and enhanced chemiluminescence (ECL) reagent (Amersham, Arlington Heights, IL); SYBR Green PCR Kit (Applied Biosystems, Foster City, CA); Coomasie reagent, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) supplies, and Triton X-100 (BioRad, Hercules, CA); iPLA2 antibody (Cayman, Ann Arbor, MI); normal goat serum, Cy3-conjugated affinipure goat anti-rabbit IgG (H+L) (Jackson Immuno Research Laboratories, West Grove, PA); pentex fraction V fatty acid-free bovine serum albumin (Miles Laboratories, Eckert, IN); RIPA buffer (Pierce, Rockford, IL); peroxidase-conjugated goat anti-rabbit IgG, T-14 iPLA2, CPT1 and CD36 antibodies (Santa Cruz Biotech. Inc., Santa Cruz, CA); protease inhibitor cocktail, common reagents, and salts (Sigma Chemical Co., St. Louis, MO); and Immobolin-P PVDF membrane and Catch and Release V 2.0 immunoprecipitation kit (Upstate, Charlottesville, VA).
Animals and INS-1 cells
Male Sprague-Dawley rats (250 g) were obtained from Harlan Breeders (Indianapolis, IN). INS-1 β-cells were generously provided by Dr. C. Newgard (Duke University Medical Center, Durham, NC). A retroviral system was used to stably transfect INS-1 cells with a vector construct containing iPLA2β cDNA (OE INS-1 cells), as described (4) to generate iPLA2β overexpressing (OE) INS-1 cells. Wild type (WT) and iPLA2β-null mice were generously provided by Dr. John Turk (Washington University School of Medicine, St, Louis, MO).
Northern analyses of skeletal muscle iPLA2
Total RNA from skeletal muscle was isolated after solubilization in guanidinium thiocyanate by phenol/chloroform/isoamyl alcohol extraction and isopropyl alcohol precipitation and Northern analyses were performed using the Northern Max kit (29, 30). Tissue total RNA analyzed by electrophoresis was transferred to nylon membranes that were hybridized with iPLA2 cDNA probes labeled by random priming. The iPLA2 cDNA probe was amplified using reverse transcription-PCR (sense primer, 5'-TGTGACGTGGACAGCACTAGC; antisense primer, 5'-CCCCAGAGAAACGACTATGGA), which hybridizes to both short and long isoforms of iPLA2 (31). This region of cDNA represents the sequence that encodes amino acid residues 307–552 of the short isoform of iPLA2. A final stringency wash was followed by autoradiography, and the filters were then stripped and hybridized with cDNA probes to rat glyceraldehyde-3-phosphate dehydrogenase to mark RNA load (17).
Immunoblotting analyses
iPLA2 was immunoprecipitated from skeletal muscle using the Catch and Release V 2.0 kit, according to manufacturer’s instructions (Upstate, Charlottesville, VA). Briefly, skeletal muscle was homogenized in RIPA buffer (100 mg tissue/ml buffer) containing protease inhibitor cocktail (50 µl/ml) and iPLA2 was immunoprecipitated (O/N, 4 °C) using primary antibody (from Cayman) directed against iPLA2 (12 µg/ml lysates). Bound fraction was subsequently recovered, boiled in sample buffer, analyzed by SDS-PAGE (7.5%), and transferred onto Immobolin-P PVDF membranes. T-14 iPLA2 primary antibody (0.0015 µg/µl) and peroxidase-conjugated goat anti-rabbit IgG (1:40,000) secondary antibody were then used for iPLA2 immunoblotting analyses. Skeletal muscle homogenates prepared for substrate oxidation studies (described below) were used for immunoblotting analyses of CPT1 and CD36. Protein was analyzed by SDS-PAGE (8%), transferred onto PVDF membranes, and probed for CPT1 and CD36 (primary antibodies 1:200). Peroxidase-conjugated goat anti-rabbit IgG was used as the secondary antibody (CPT1 1:10,000; CD36 1:5,000). GAPDH was used as loading control. Immunoreactive protein bands were visualized by ECL.
Elution of iPLA2 by sequential chromatography
Rat skeletal muscle (12g) was sonicated (Sonics & Materials Vibra Cell, 12%, 10 sec) in homogenization buffer (10 mM HEPES, 1 mM EDTA, 340 mM sucrose, 1 mM DTT, pH 7.5) and the pooled sonicate was centrifuged (1000 × g, 10 min, 4 °C). Protein in the supernatant was precipitated overnight with the addition of ammonium sulfate (50%). The mixture was then centrifuged (20,000 × g, 30 min), and the protein pellet was resuspended in buffer A (10 mM HEPES, 1 mM EDTA, 340 mM sucrose, 1 mM DTT, 1 mM triton X-100, pH 7.5) and loaded onto an ATP-agarose affinity column (1.5 ml). Buffer A containing ATP (1 mM) was then added to the column to elute iPLA2 and Ca2+-independent phospholipase activity and protein in each fraction were determined. Fractions containing activity were then combined and the concentration of calcium in the pooled sample was increased to 5 mM prior to loading onto calmodulin-Sepharose column. The void was collected and re-loaded and the column was washed with buffer A containing 0.5 mM Ca2+. iPLA2 was eluted with 8 mM EGTA-containing buffer A and each fraction was assayed for Ca2+-independent phospholipase activity and protein content.
Skeletal muscle iPLA2β phospholipase activity
Gastrocnemius muscle was excised from rats and 1 ml of homogenization buffer (250 mM sucrose, 40 mM Tris-HCl, 1 mM EGTA, pH 7.1 at 4 °C) containing protease inhibitor cocktail (1:100) was added to 100 mg tissue. The muscle was homogenized by sonication (2 × 15 sec, 12% pulse) and the cytosol obtained, as described (23, 28). Cytosolic protein concentration was determined using Coomassie reagent. Ca2+-independent PLA2 enzymatic activity was then determined in 30 µg protein aliquot of cytosol in the absence and presence of ATP (10 mM) or BEL (10 µM) by ethanolic injection (5 µl) of the substrate 1-palmitoyl-2-[14C]-linoleoyl-sn-glycero-3-phosphocholine (5 µM) in assay buffer (40 mM Tris, pH 7.5, 5 mM EGTA) and quantitated, as described (23, 25). To test the effects of BEL on activity, the sample protein was first pre-incubated (2 min, RT) with BEL before addition of substrate.
Skeletal muscle homogenate preparation for substrate oxidation
Homogenization procedure was performed as previously described (32). Briefly, ~75 mg of gastrocnemius muscle was thoroughly minced with scissors in 200 µl of homogenization buffer containing: 250 mM sucrose, 1 mM EDTA, 10 mM Tris·HCl, and 2 mM ATP, pH 7.4. The homogenization buffer was then brought up to yield a 20 fold (w/v) dilution. This was then transferred to a 3 ml Teflon on glass homogenization vessel. The muscle was then homogenized twice on ice at 12 passes over a 30 s time period at ~1,500 rpm. Homogenates were kept on ice for no more than 1h until oxidation experiments could be performed.
Substrate oxidation
14CO2 production and release from [1-14C]-glucose, [1-14C]-palmitate, [1-14C]-acetyl CoA, and [1-14C]-palmitoyl-CoA were used to assess oxidation as described (33), with some variation. Briefly, palmitate and palmitoyl-CoA (100 µM) were bound to 0.5% BSA (final concentration) and acetyl-CoA and glucose were added directly to the reaction buffer. Each substrate was brought up in reaction buffer containing: 100 mM sucrose, 10 mM Tris·HCl, 10 mM KPO4, 100 mM KCl, 1 mM MgCl2·6H2O, 1 mM l-carnitine, 0.1 mM malate, 2 mM ATP, 0.05 mM coenzyme A, and 1 mM dithiothreitol, pH 7.4. Aliquots (80 µl) of the diluted muscle homogenates were plated in triplicate in a 100 well trapping device. Reaction buffer (325 µl) was placed in the bottom of each well. In addition, 1N NaOH (400 µl) was placed in a 500 µl microcentrifuge tube without the cap, which was also placed in each well to allow CO2 trapping. The trapping device was then sealed with parafilm, then a siliconized rubber gasket, and finally the lid of the trapping device and allowed to incubate in a shaking incubator at 37 °C for 1h. Reactions were terminated by the addition of 100 µl of 70% perchloric acid via injection ports in the trapping device lid. The trapping device was placed back into the shaking incubator and released 14CO2 was trapped for 1h at room temperature. CO2 released during substrate oxidation and trapped in NaOH was quantitated by liquid scintillation spectrometry and expressed as CO2 produced/mg protein.
β-HAD activity
Total β-hydroxyacyl-CoA dehydrogenase activity was measured as previously described (34) with some variation. Briefly, muscle homogenate prepared as described above was freeze-fractured 3 times in liquid nitrogen and allowed to thaw. The reactions were started by the addition of 100 µM acetoacetyl-CoA and the absorbance was measured at 340 nm over a 5 min period.
Acyl-CoA thioesterase activity assay
To identify iPLA2β-associated thioesterase activity, hydrolysis of [1-14C]-palmitoyl-CoA by iPLA2β eluted from the ATP affinity column or in skeletal muscle cytosol prepared from WT and iPLA2β-null mice was determined. Fractions eluted from the ATP affinity column containing phospholipase activity were pooled and an aliquot (containing 1 µg protein) was pre-incubated without or with BEL (10 µM, 2 min, room temperature) and acyl-CoA thioesterase activity was assayed in the presence of 0.5–5 µM substrate. Activity in skeletal muscle cytosol (containing 30 µg protein) was assayed in the presence of 0.5–10 µM substrate. The reaction (in a total volume of 200 µl) was started by ethanolic injection of the substrate [1-14C]-palmitoyl-CoA in assay buffer (40 mM Tris, pH 7.5, 5 mM EGTA). Following an incubation period of 2 min, the reaction was stopped by addition of butanol (100 µl). The mixture was then centrifuged and hydrolysed [14C]-fatty acid contained in an aliquot of the butanol layer was separated by TLC and quantitated by liquid scintillation spectrometry.
Statistical analyses
Data were converted to mean ± SEM values and the Students’ t-test was applied to determine significant differences between two samples (p < 0.05). Statistical difference (p < 0.05) between WT and iPLA2β-null thioesterase activity was determined by determining area under curve (AUC).
RESULTS
Skeletal muscle expresses iPLA2 mRNA and protein
Skeletal expression of iPLA2 was identified by Northern, immunoblotting, and enzymatic activity analyses. As shown in Figure 1, skeletal muscle expressed a transcript of ~3kb, an iPLA2-immunoreactive band with an apparent molecular mass of 85 kDa, and Ca2+-independent phospholipase activity that was stimulated by ATP and inhibited by BEL. These properties are characteristic of iPLA2β expressed in insulinoma cells, pancreatic islets, and heart (28, 35–38).
FIGURE 1. Expression of iPLA2β message, protein, and phospholipase catalytic activity in rat skeletal muscle.
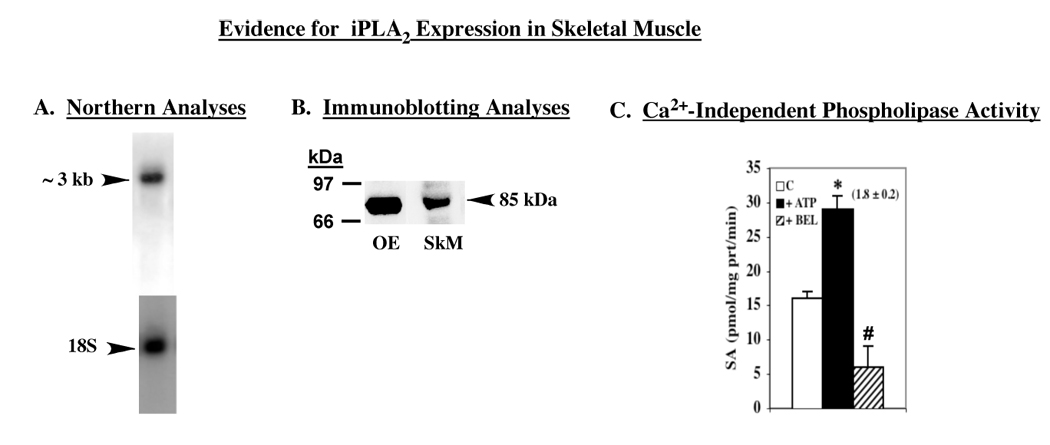
A. Northern analyses. B. Immunoblotting analyses. iPLA2 was immunoprecipitated from skeletal muscle lysates and the immunoprecipitated proteins were analysed by SDS-PAGE and transferred onto Immobolin-P PVDF membrane. The electroblot was then probed with antibodies against iPLA2β (T-14) and immunoreactive protein was visualized by enhanced chemiluminescence (ECL). C. Phospholipase catalytic activity. An aliquot (30 µg protein) of cytosol prepared from rat gastrocnemius muscle was used to determine Ca2+-independent phospholipase activity of iPLA2β in the absence and presence of ATP (1 mM) or BEL (10 µM) and the mean specific activities ± SEMs are presented (n = 6). Value presented in parenthesis over the ATP bar represents fold-stimulation of activity, relative to control. (*,#Significantly different from control activity, p < 0.005 and p < 0.0001, respectively).
Elution of rat skeletal muscle iPLA2 by sequential ATP and calmodulin affinity chromatography
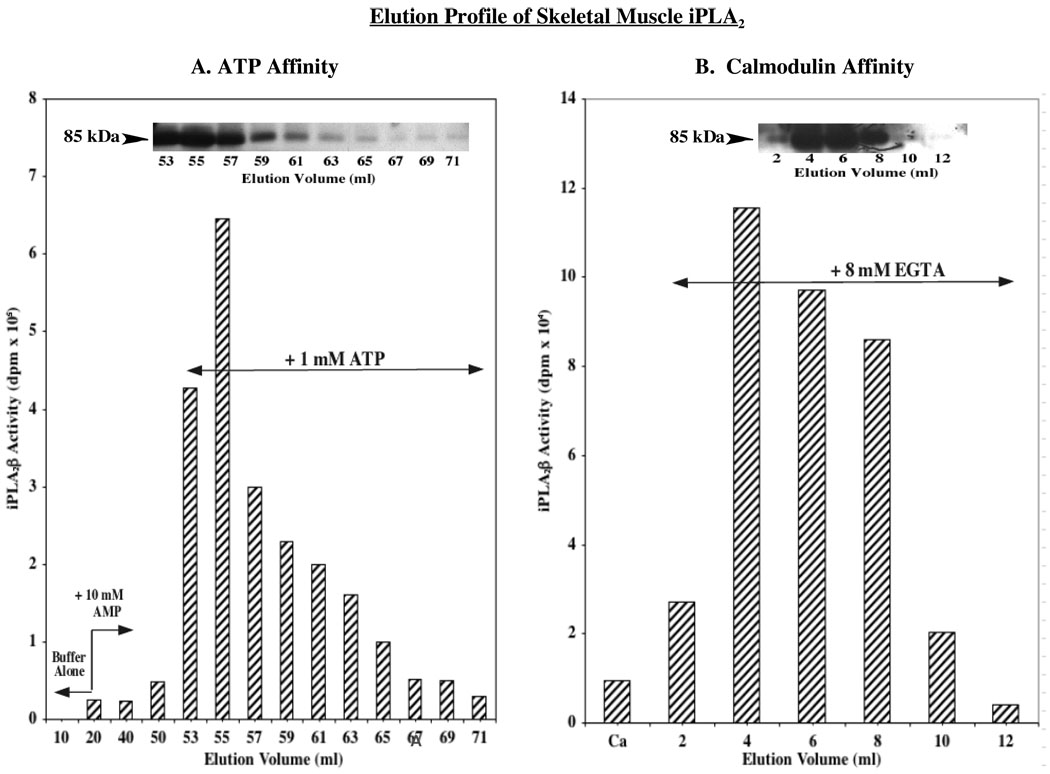
To further establish the identity of skeletal muscle iPLA2 isoform, sequential ATP and calmodulin affinity column chromatography, a scheme used previously in the purification of iPLA2β (39), was used to elute skeletal muscle iPLA2. As illustrated in Figure 2A, fractions eluted from the ATP column with the addition of 1 mM ATP contained Ca2+-independent phospholipase activity that was associated with an immunoreactive protein band that migrated with apparent mass of 85 kDa on SDS-PAGE analyses (Figure 2A inset). The phospholipase activity-containing fractions were then pooled, and the [Ca2+] in the pool was increased to 5 mM to facilitate binding to calmodulin-Sepharose. Subsequent addition of EGTA promoted recovery of Ca2+-independent phospholipase activity (Figure 2B) that was associated with an immunoreactive band that migrated with an apparent mass of 85 kDa (Fig. 2B inset).
FIGURE 2. ATP and calmodulin affinity column chromatography profiles of iPLA2β elution from skeletal muscle.
Skeletal muscle protein was prepared as described in Methods and loaded onto an ATP-agarose column. The column was washed with buffer alone and then with buffer containing AMP (10 mM) prior to elution of iPLA2β with buffer containing ATP (1 mM). Fractions containing Ca2+-independent phospholipase activity were pooled, supplemented with CaCl2, and loaded onto a calmodulin-Sepharose column. The column was washed with buffer and Ca2+-independent phospholipase activity was subsequently eluted with buffer containing EGTA (8 mM). A. ATP-Agarose affinity chromatography. B. Calmodulin-Sepharose affinity chromatography. (Insets, iPLA2β immunoblotting analyses). (Ca, 0.5 mM Ca2+).
Two additional bands at ca. 100 and 60 kDa (not shown) were evident in fractions collected from both the ATP and calmodulin columns. However, mass spectrometry analyses revealed that neither of the bands contained iPLA2β and that the higher band corresponded to glycogen phosphorylase and the lower band to precursor of albumin. Collectively, the findings presented in Figure 1 and Figure 2 indicate that skeletal muscle express an iPLA2 that is similar to the β-isoform of iPLA2β expressed in insulinoma cells, pancreatic islets, and heart (28, 35–38).
Substrate oxidation in skeletal muscle
In view of the low phospholipase A2 activity in skeletal muscle, other potential catalytic activities of iPLA2β in skeletal muscle were considered. Recently, it was suggested that iPLA2β manifests an acyl-CoA thioesterase activity under in vitro conditions and that saturated acyl-CoA substrates (14–16C) are its preferred substrates (40). Because fatty acyl-CoAs are important substrates of mitochondrial β-oxidation, we examined whether skeletal muscle fatty acid oxidation differed in WT and iPLA2β-null mice. The mice were generated and genotyped by Southern analyses, as described earlier (29). Subsequently, Northern analyses confirmed the presence of iPLA2β message in skeletal muscle from WT mice and its absence in skeletal muscle from the iPLA2β-null mice (29). To verify that the WT mouse skeletal muscle expressed analogous protein and catalytic activity, analyses were performed in gastrocnemius muscle. As shown in Figure 3, an immunoprecipitation protocol used to identify rat skeletal muscle iPLA2β yielded an iPLA2-immunoreactive band in WT muscle with an apparent molecular mass of 85 kDa. Cytosolic preparations from the WT skeletal muscle were found to express a Ca2+-independent phospholipase activity that was stimulated by ATP and inhibited by BEL. In contrast, muscle from iPLA2β-null mice expressed background phospholipase activity that was not stimulatable by ATP. The analyses in mice therefore, collectively, indicated that iPLA2β is expressed in skeletal muscle of WT but not iPLA2β-null mice.
FIGURE 3. Expression of iPLA2β protein in WT mouse and phospholipase catalytic activity in WT and iPLA2β-null mice skeletal muscle.
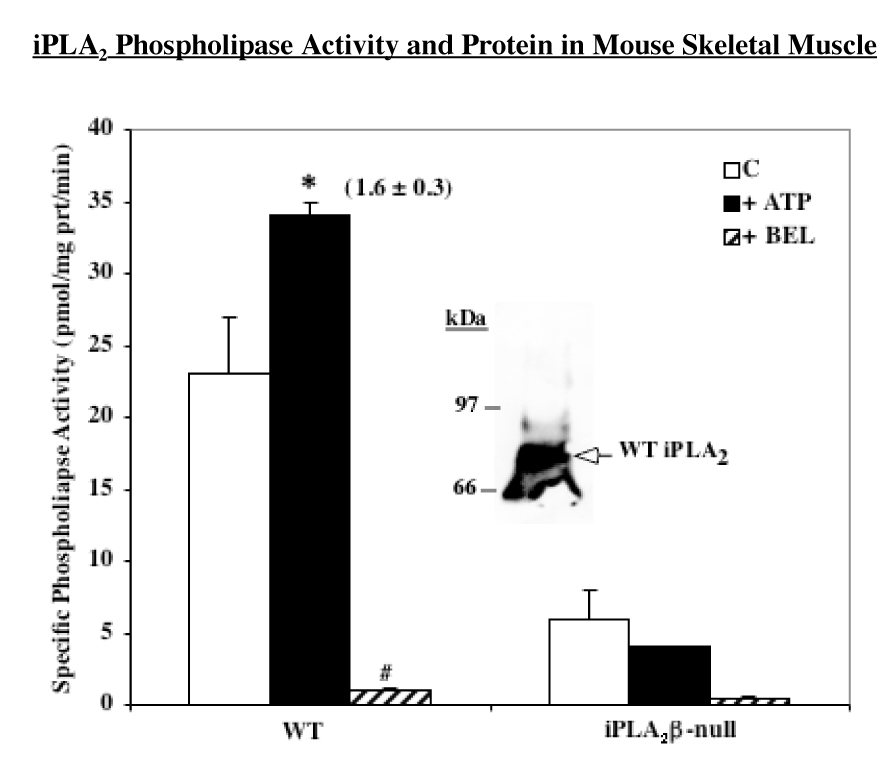
iPLA2 was immunoprecipitated from mouse skeletal muscle and processed as in Figure 1. An aliquot (30 µg protein) of cytosol prepared from mouse gastrocnemius muscle was used to determine Ca2+-independent phospholipase activity of iPLA2β in the absence (Control) and presence of ATP (1 mM) or BEL (10 µM) and the mean specific activities ± SEMs are presented (n = 6). Value presented in parenthesis over the ATP bar represents fold-stimulation of activity, relative to control. (*#Significantly different from control activity, p < 0.05 and p < 0.0001, respectively).
To examine whether iPLA2β contributes to substrate oxidation, homogenates were prepared from gastrocnemicus muscle isolated from WT and iPLA2β-null mice and 14CO2 production and release from [1-14C]-glucose, [1-14C]-palmitate, [1-14C]-acetyl-CoA, and [1-14C]-palmitoyl-CoA were determined. As shown in Figure 4A, glucose oxidation was not affected by the absence of iPLA2β. Unexpectedly, palmitate oxidation was reduced by nearly 40% in the iPLA2β-null muscle (Figure 4B), relative to wild type muscle, while oxidation of acetyl-CoA and palmitoyl-CoA were similar in the WT and iPLA2β-null groups. These findings suggest that the absence of iPLA2β does not interfere with the β-oxidation pathway or the Kreb’s cycle but may impair the processing of palmitate prior to its entry into the β-oxidation pathway.
FIGURE 4. Substrate oxidation in skeletal muscle of WT and iPLA2β-null mice.
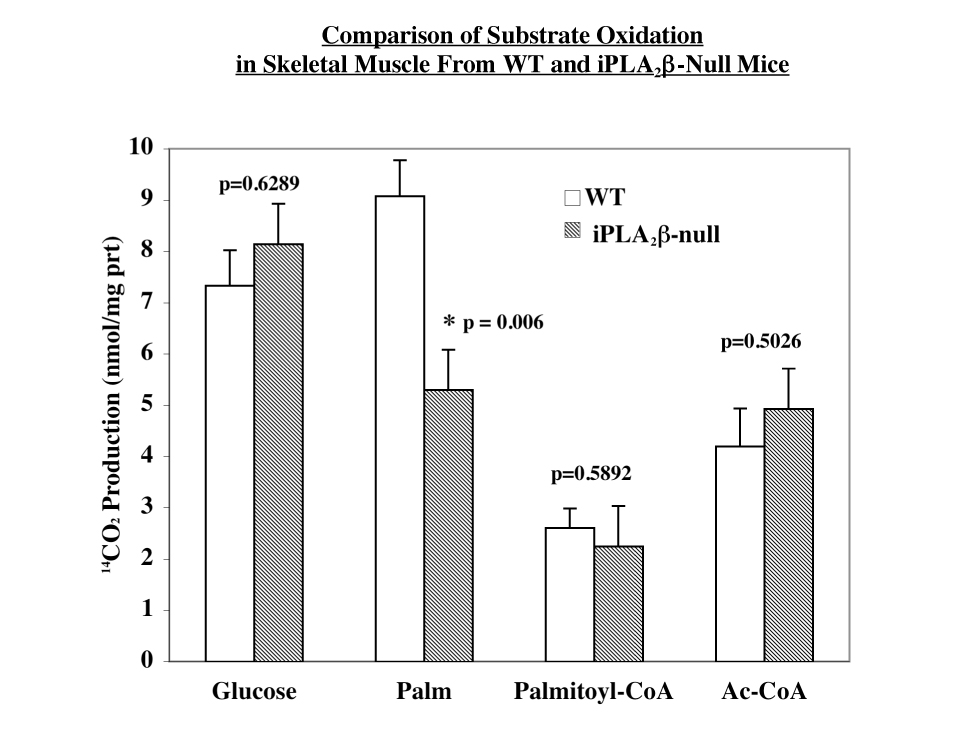
A. Glucose oxidation. B. Palmitate, palmitoyl-CoA, and acetyl CoA oxidation. Oxidation was measured as 14CO2 production from [14C]-labeled substrates in skeletal muscle homogenates prepared from gastrocnemius muscle. Results are mean ± SEM (n = 6-18 in each group). (*iPLA2β-null group significantly different from WT group.)
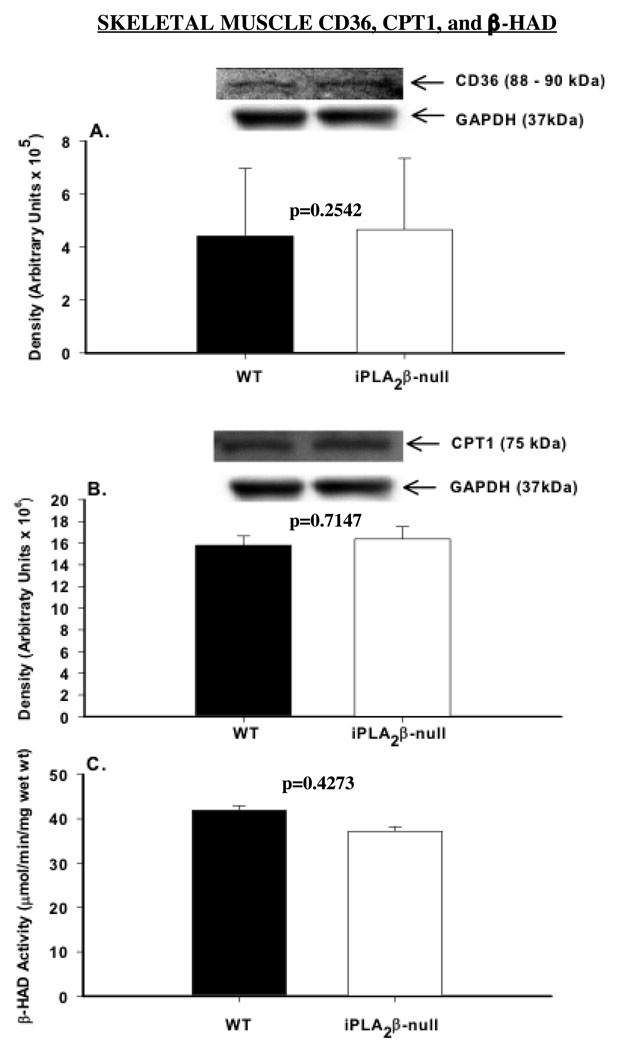
Fatty acid transport and β-hydroxyacyl-CoA dehydrogenase (β-HAD)
Fatty acid transport across the plasma membrane and the inner mitochondrial membrane occurs via facilitated diffusion requiring CD36 and carnitine palmitoyltransferase 1 (CPTI). We therefore examined whether the abundance of CD36 or CPT1 is altered in iPLA2β-null mice. As shown in Figures 5A and B, expression levels of CD36 and CPT1 were similar in WT and iPLA2β-null skeletal muscle. Next, we measured the activity of β-HAD which catalyzes dehydrogenation of l-β-hydroxyacyl-CoA to β-ketoacyl-CoA in the β-oxidation pathway. This step yields an NADH, which donates its electrons to the respiratory chain to generate ATP. β-HAD activity was also found to be similar in the WT and iPLA2β-null skeletal muscle (Figure 5C). Consistent with the oxidation results, these findings indicate that the expression and/or activity of key components of the fatty acid oxidation pathway required to deliver an acetyl-CoA residue to the Kreb’s cycle are normal in the iPLA2β-null skeletal muscle. However, these findings support the possibility that the defect in muscle palmitate oxidation in the absence of iPLA2β is most likely due to reduced availability of the appropriate substrate to these components.
FIGURE 5. CPT-1 and CD36 protein expression and β-HAD activity in skeletal muscle of WT and iPLA2β-null mice.
Skeletal muscle homogenates were prepared from gastrocnemius and processed for immunoblotting analyses of (A) CPT-1 and (B) CD36. Each panel shows quantified data and each bar represents the mean ± SEM (n = 4 in each group) of the respective protein. Inserts are immunoblots for each protein and corresponding GAPDH control. (C) β-HAD activity. β-hydroxyacyl-CoA dehydrogenase activity prepared from gastrocnemius skeletal muscle homogenates. Data presented are means ± SEMs of β-HAD activity (n = 4 in each group).
Skeletal muscle iPLA2β expresses acyl-CoA thioesterase activity that is reduced in the iPLA2β-null mice
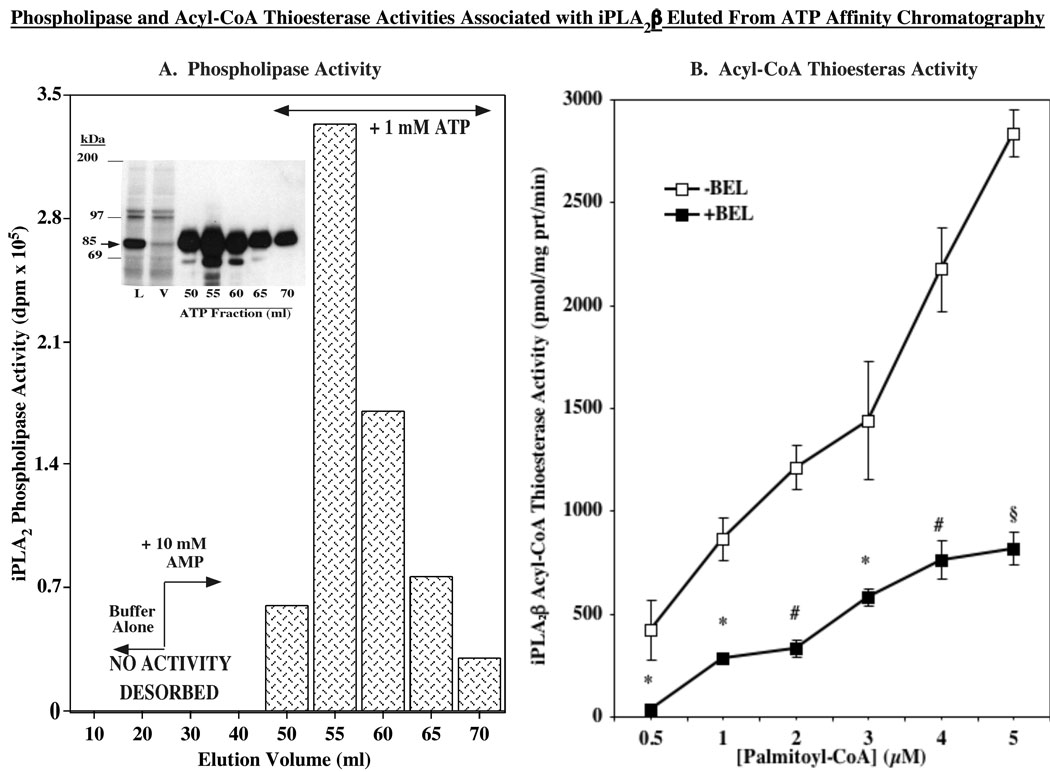
During periods of elevated fatty acid oxidation the majority of mitochondrial CoA-SH is bound to long-chain free fatty acids at the outer mitochondrial membrane (41). Mitochondrial thioesterase activity (also known as acyl-CoA thioesterase) catalyzes the hydrolysis of fatty acyl-CoAs to free fatty acid and CoA-SH and the liberated CoA-SH supports fatty acid transport and subsequent oxidation (42). In view of the recent demonstration that iPLA2β manifests an acyl-CoA thioesterase-like activity (40), we predicted that in its absence there is a decrease in the availability of CoA-SH to support optimal fatty acid oxidation. To examine the contribution of iPLA2β to the needed acyl-CoA thioesterase activity during fatty acid oxidation in skeletal muscle, generation of radiolabeled fatty acid from hydrolysis of [1-14C]-palmitoyl-CoA was determined by two approaches. Because the skeletal muscle iPLA2β was demonstrated to be similar to the one in pancreatic islets, β-cell iPLA2β overexpressed in INS-1 cells was eluted from the ATP affinity column (Figure 6A). As described previously (25), pancreatic islets and insulinoma cells express catalytically active 85 and 70 kDa iPLA2β isoforms and iPLA2β-immunoreacive bands at these apparent molecular masses are evident in the ATP column eluants (Figure 6A, inset). The fractions containing iPLA2β phospholipase activity were pooled and used to assess acyl-CoA thioesterase activity of iPLA2β in the absence and presence of BEL. As seen in Figure 6B, a [substrate]-dependent acyl-CoA thioesterase activity was evident in the pooled fraction in the absence of BEL. However, in the presence of BEL there was a dramatic reduction in thioesterase activity, raising the possibility that the skeletal muscle iPLA2β also manifests such activity. We therefore compared thioesterase activity in skeletal muscle prepared from WT and iPLA2β-null mice. We previously reported that skeletal muscle from WT, but not from iPLA2β-null mice, expresses iPLA2β message. As shown in Figure 7, WT expressed an acyl-CoA thioesterase activity that was nearly linear over the range of [1-14C]-palmitoyl-CoA concentrations tested. Muscle from iPLA2β-null mice, not unexpectedly, also expressed an acyl-CoA thioesterase activity. However, the acyl-CoA thioesterase activity in the absence of iPLA2β was significantly reduced, relative to WT activity, as the substrate concentration was increased from 0.5 to 10 µM (AUC: WT, 10547 ± 942 vs. KO, 7319 ± 482, p = 0.0133). Calculation of protein yields (µg protein/mg wet weight) revealed no significant difference between the two groups (WT, 88 ± 10 and KO, 90 ± 12, p = 0.8723, n=12 in each group), suggesting that general changes in skeletal muscle protein content do not contribute to the observed decrement in thioesterase activity in the iPLA2β-null muscle. Taken together, these two approaches indicate that an acyl-CoA thioesterase activity is manifested by skeletal muscle iPLA2β raising the possibility that such activity is necessary for optimal fatty acid oxidation.
FIGURE 6. iPLA2β-associated phospholipase and acyl-CoA thioesterase activity.
A. Phospholipase activity. Partially-purified iPLA2β was prepared from iPLA2β-overexpressing INS-1 cells (30 × T225 flasks), as described (25). Ca2+-independent phospholipase activity of iPLA2β was determined in eluants from the ATP affinity column using [14C]-PLPC as the substrate. (Inset, immunoblotting analyses of iPLA2β in ATP column eluants). B. Acyl-CoA thioesterase activity. Fractions containing phospholipase activity were pooled, protein concentration determined, and acyl-CoA thioesterase specific activity was measured in 1 µg protein aliquots using [14C]-palmitoyl-CoA as substrate in the absence or presence of BEL (10 µM). (*,#,§BEL-treated group significantly different from control group, p < 0.05, p < 0.01, and p < 0.005, respectively, n=3).
Figure 7. Acyl-CoA thioesterase activity manifested by skeletal muscle iPLA2β.
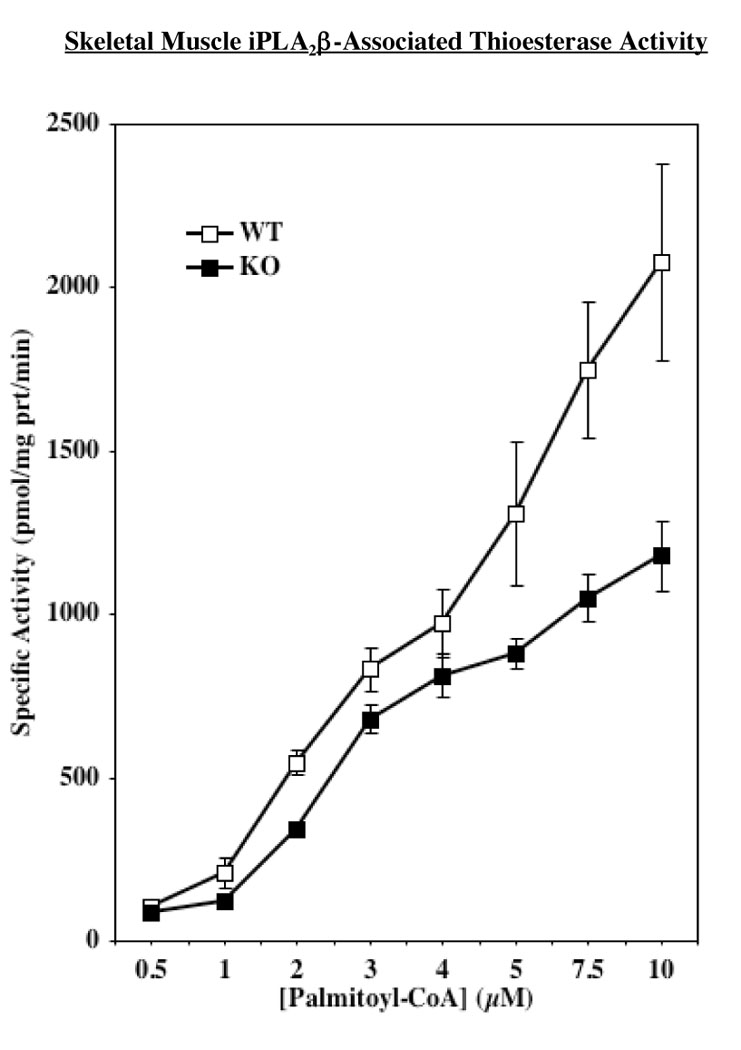
Cytosol was prepared from muscle isolated from WT and iPLA2β-null mice and acyl-CoA thioesterase activity was measured in 30 µg protein aliquots. The mean ± SEMs of WT and KO muscle acyl-CoA thioesterase specific activity in the presence of [14C]-palmitoyl-CoA (0.50–10 µM) substrate are presented (n = 8 in each group). iPLA2β-null activity significantly (p = 0.0133) reduced, relative to WT activity, as reflected by AUC analyses.
Discussion
In view of reports suggesting expression of multiple transcripts of muscle iPLA2 in humans and rodents, in the present study we examined the chromatographic profile and catalytic properties of skeletal muscle iPLA2. Our findings reveal that rodent skeletal muscle (a) contains an iPLA2 transcript of ~3 kb, (b) expresses iPLA2-immunoreactive protein with an apparent molecular mass of 85 kDa, (c) manifests iPLA2 phospholipase activity that is stimulated by ATP and inhibited by BEL, and (d) expresses iPLA2 that exhibits affinity for ATP and calmodulin during sequential chromatography. These properties are analogous to those of iPLA2β expressed in islets, β-cells, and cardiac muscle (28, 35–38) and suggest that skeletal muscle expresses an 85 kDa isoform of iPLA2β protein.
We previously reported that INS-1 insulinoma cells and pancreatic islets express an additional 70 kDa iPLA2β-immunoreactive protein that is catalytically active and not generated by a mechanism of alternate splicing of iPLA2 transcript (25). Sequential chromatography of the skeletal protein revealed three protein bands that migrated with apparent molecular masses of ca. 100 kDa, 85 kDa, and 60 kDa. However, the phospholipase activity of skeletal muscle iPLA2β appears to be manifested by the iPLA2β-immunoreactive 85 kDa isoform. This is supported by the findings that only the iPLA2β-immunoreactive band that eluted with an apparent molecular mass of 85 kDa correlated with phospholipase activity elution and mass spectrometry analyses revealing that the ~100 kDa and 60 kDa protein bands corresponded to glycogen phosphorylase and precursor of albumin, respectively.
Though the specific phospholipase activity of skeletal muscle iPLA2β appears to be low, it has been suggested that iPLA2 phospholipase activity can influence skeletal muscle contractile function by modulating cytosolic oxidant activity (43). In view of the absence of a prominent Ca2+-independent phospholipase A2 activity in the skeletal muscle, it is of interest to note that an acyl-CoA thioesterase activity was recently attributed to iPLA2β (40). That study demonstrated a preferential hydrolysis of 14–16C acyl-CoAs by iPLA2β. Site-directed mutagenesis of the phospholipase catalytic serine (GTS465TG) abolished the phospholipase as well as the acyl-CoA thioesterase activity of iPLA2β. Pretreatment with BEL also significantly inhibited both catalytic activities. These observations taken together with our findings that palmitate but not acetyl-CoA or palmitoyl-CoA oxidation is reduced in the absence of changes in fatty acid transport proteins CD36 and CPT1 or β-HAD activity in the skeletal muscle from iPLA2β-null mice, raise the possibility that acyl-CoA thioesterase activity of iPLA2β might be important in fatty acid oxidation in skeletal muscle.
Thioesterase activity is critical to support fatty acid oxidation because it results in the liberation of CoA-SH from fatty acyl-CoAs. CoA-SH is required to facilitate fatty acid transport into the mitochondria, the β-oxidation pathway step catalyzed by β-ketothiolase dehydrogenase, and the Kreb’scycle reaction catalyzed by α-ketoglutarate dehydrogenase. Hence, the possibility that acyl-CoA thioesterase activity of iPLA2β is required for optimal fatty acid oxidation was first examined using iPLA2β eluted from the ATP affinity column. Incubation of an aliquot of the eluant pool containing phospholipase activity with [1-14C]-palmitoyl-CoA resulted in hydrolysis of the substrate that was inhibited by the addition of BEL. This suggested that the measured acyl-CoA thioesterase activity is most likely manifested by iPLA2β. To overcome the possibility of non-specific effects of BEL or presence of non- iPLA2β proteins in the partially-purified prepartion from the ATP column, acyl-CoA thioesterase activity was next determined in skeletal muscle from WT and iPLA2β-null mice. Consistent with the earlier demonstration of iPLA2β message in skeletal muscle of WT but not from iPLA2β-null mice (29), catalytic activity characteristic of iPLA2β was not evident in skeletal muscle of the iPLA2β-null mice.
As expected, both WT and iPLA2β-null preparations expressed significant acyl-CoA thioesterase activity. However, AUC analyses revealed that the activity in the iPLA2β-null group, relative to the WT group, was significantly diminished as the concentration of the [1-14C]-palmitoyl-CoA substrate was increased from 0.5 to 10 µM. These findings therefore are the first demonstration of expression of acyl-CoA thioesterase activity by skeletal muscle iPLA2β. In summary, our findings indicate that skeletal muscle expresses an 85 kDa iPLA2β isoform that manifests both phospholipase and acyl-CoA thioesterase activities. Further, they suggest that the acyl-CoA thioesterase activity of iPLA2β could be an important contributor for optimal fatty acid oxidation by skeletal muscle.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Samuel Smith and Dr. Mary Wohltmann for expert technical assistance and Dr. Christopher M. Jenkins for helpful discussions concerning thioesterase activity measurements.
This work was supported by grants from the National Institutes of Health RO1-69455, R37-DK34388, P41-RR00954, P60-DK20579, P30-DK56341, and T32-DK007296-27 (to M.J.C.)
The abbreviations used are
- AUC
area under curve
- BEL
bromoenol lactone suicide inhibitor of iPLA2β
- β-HAD
β-hydroxyacyl-CoA dehydrogenase
- CPT1
carnitine palmitoyltransferase 1
- cPLA2
group IV cytosolic phospholipase A2
- iPLA2β
β-isoform of group VIA calcium-independent phospholipase A2
- OE
iPLA2β-overexpressing cells
- PLA2
phospholipase A2
- SEM
standard error of the mean.
REFERENCES
- 1.Gijon MA, Leslie CC. Phospholipases A2. Semin. Cell Dev. Biol. 1997;8:297–303. doi: 10.1006/scdb.1997.0151. [DOI] [PubMed] [Google Scholar]
- 2.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso DJ, Jenkins CM, Sims HF, Cohen JM, Yang J, Gross RW. Complex transcriptional and translational regulation of iPLAγ resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur. J. Biochem. 2004;271:4709–4724. doi: 10.1111/j.1432-1033.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2β) indicate a signaling rather than a housekeeping role for iPLA2β. J. Biol. Chem. 2001;276:13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Takeya R, Sumimoto H. A novel intracellular membrane-bound calcium-independent phospholipase A2. Biochem. Biophys. Res. Comm. 2000;272:320–326. doi: 10.1006/bbrc.2000.2776. [DOI] [PubMed] [Google Scholar]
- 6.Balsinde J, Balboa MA, Dennis EA. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- 7.Balsinde J, Bianco ID, Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chilton FH, Fonteh AN, Surette ME, Triggiani M, Winkler JD. Control of arachidonate levels within inflammatory cells. Biochim. Biophys. Acta. 1996;1299:1–15. doi: 10.1016/0005-2760(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 9.Dennis EA. The biosynthesis of phospholipids. Methods Enzymol. 1992;209:1–4. doi: 10.1016/0076-6879(92)09003-l. [DOI] [PubMed] [Google Scholar]
- 10.Boilard E, Surette ME. Anti-CD3 and concanavalin a-induced human t cell proliferation is associated with an increased rate of arachidonate-phospholipid remodeling. Lack of involvement of group IV and group VI phospholipase A2 in remodeling and increased susceptibility of proliferating T cells to CoA-independent transacylase inhibitor-induced apoptosis. J. Biol. Chem. 2001;276:17568–17575. doi: 10.1074/jbc.M006152200. [DOI] [PubMed] [Google Scholar]
- 11.Derrickson BH, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through Gq/G11 and the calcium-independent phospholipase A2. Am. J. Physiol. Renal Physiol. 1997;272:F781–F788. doi: 10.1152/ajprenal.1997.272.6.F781. [DOI] [PubMed] [Google Scholar]
- 12.Isenovic E, LaPointe MC. Role of Ca2+-independent phospholipase A2 in the regulation of inducible nitric oxide synthase in cardiac myocytes. Hypertension. 2000;35:249–254. doi: 10.1161/01.hyp.35.1.249. [DOI] [PubMed] [Google Scholar]
- 13.Maggi LB, Jr, Moran JM, Scarim AL, Ford DA, Yoon J-W, McHowat J, Buller RML, Corbett JA. Novel role for calcium-independent phospholipase A2 in the macrophage antiviral response of inducible nitric-oxide synthase expression. J. Biol. Chem. 2002;277:38449–38455. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 14.Tithof PK, Olivero J, Ruehle K, Ganey PE. Activation of neutrophil calcium-dependent and -independent phospholipases A2 by organochlorine compounds. Toxicol. Sci. 2000;53:40–47. doi: 10.1093/toxsci/53.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Tithof PK, Peters-Golden M, Ganey PE. Distinct phospholipases A2 regulate the release of arachidonic acid for eicosanoid production and superoxide anion generation in neutrophils. J. Immunol. 1998;160:953–960. [PubMed] [Google Scholar]
- 16.Williams SD, Ford DA. Calcium-independent phospholipase A2 mediates CREB phosphorylation and c-fos expression during ischemia. Am. J. Physiol. Heart. Circ. Physiol. 2001;281:H168–H176. doi: 10.1152/ajpheart.2001.281.1.H168. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J. Biol. Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 18.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J. Biol. Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A Novel Cytosolic Calcium-independent phospholipase A2 contains eight ankyrin motifs. J. Biol. Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 20.Larsson Forsell PKA, Kennedy BP, Claesson H-E. The human calcium-independent phospholipase A2 gene: Multiple enzymes with distinct properties from a single gene. Eur. J. Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 21.Larsson PKA, Claesson HE, Kennedy BP. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J. Biol. Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 22.Atsumi G-I, Murakami M, Kojima K, Hadano A, Tajima M, Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IV cytosolic phospholipase A2α inhibits stimulus-induced arachidonate release, whereas that of type VI CA2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 23.Ramanadham S, Hsu FF, Zhang S, Jin C, Bohrer A, Song H, Bao S, Ma Z, Turk J. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2β) and suppressed by inhibition of iPLA2β. Biochemistry. 2004;43:918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2β. Implications for structure and function. J. Biol. Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 25.Ramanadham S, Song H, Hsu FF, Zhang S, Crankshaw M, Grant GA, Newgard CB, Bao S, Ma Z, Turk J. Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2 (iPLA2β) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2 beta transcript. Biochemistry. 2003;42:13929–13940. doi: 10.1021/bi034843p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J. Biol. Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- 27.Bao S, Song H, Wohltmann M, Ramanadham S, Jin W, Bohrer A, Turk J. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express group via phospholipase A2 and effects of metabolic stress on glucose homeostasis. J. Biol. Chem. 2006;281:20958–20973. doi: 10.1074/jbc.M600075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross RW, Ramanadham S, Kruszka KK, Han X, Turk J. Rat and human pancreatic islet cells contain a calcium ion independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by adenosine triphosphate and is specifically localized to islet beta-cells. Biochemistry. 1993;32:327–336. doi: 10.1021/bi00052a041. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramanadham S, Zhang S, Ma Z, Wohltmann M, Bohrer A, Hsu FF, Turk J. Delta6-, Stearoyl CoA-, and Delta5-desaturase enzymes are expressed in beta-cells and are altered by increases in exogenous PUFA concentrations. Biochim. Biophys. Acta. 2002;1580:40–56. doi: 10.1016/s1388-1981(01)00189-5. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z, Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog. Nucleic Acid Res. Mol. Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim J-Y, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 33.Cortright RN, Sandhoff KM, Basilio JL, Berggren JR, Hickner RC, Hulver MW, Dohm GL, Houmard JA. Skeletal Muscle Fat Oxidation Is Increased in African-American and White Women after 10 days of Endurance Exercise Training. Obesity. 2006;14:1201–1210. doi: 10.1038/oby.2006.137. [DOI] [PubMed] [Google Scholar]
- 34.Holloway GP, Lally J, Nickerson JG, Alkhateeb H, Snook LA, Heigenhauser GJF, Calles-Escandon J, Glatz JFC, Luiken JJFP, Spriet LL, Bonen A. Fatty acid binding protein facilitates sarcolemmal fatty acid transport but not mitochondrial oxidation in rat and human skeletal muscle. J Physiol. 2007;582:393–405. doi: 10.1113/jphysiol.2007.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanadham S, Wolf MJ, Li B, Bohrer A, Turk J. Glucose-responsitivity and expression of an ATP-stimulatable, Ca2+-independent phospholipase A2 enzyme in clonal insulinoma cell lines. Biochim. Biophys. Acta. 1997;1344:153–164. doi: 10.1016/s0005-2760(96)00139-7. [DOI] [PubMed] [Google Scholar]
- 36.Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2. Implications for cardiac cycle-dependent alterations in phospholipolysis. J. Biol. Chem. 1996;271:20989–20992. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- 37.Ramanadham S, Hsu F-F, Bohrer A, Ma Z, Turk J. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J. Biol. Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- 38.Hazen SL, Gross RW. Human myocardial cytosolic Ca2+-independent phospholipase A2 is modulated by ATP. Concordant ATP-induced alterations in enzyme kinetics and mechanism-based inhibition. Biochem. J. 1991;280:581–587. doi: 10.1042/bj2800581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf MJ, Gross RW. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J. Biol. Chem. 1996;271:30879–30885. doi: 10.1074/jbc.271.48.30879. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2β: Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J. Biol. Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 41.Hunt MC, Nousiainen SEB, Huttunen MK, Orii KE, Svensson LT, Alexson SEH. Peroxisome proliferator-induced long chain acyl-CoA thioesterases comprise a highly conserved novel multi-gene family involved in lipid metabolism. J. Biol. Chem. 1999;274:34317–34326. doi: 10.1074/jbc.274.48.34317. [DOI] [PubMed] [Google Scholar]
- 42.MacLellan JD, Gerrits MF, Gowing A, Smith PJS, Wheeler MB, Harper M-E. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54:2343–2350. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- 43.Gong MC, Arbogast S, Guo Z, Mathenia J, Su W, Reid MB. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J. Appl. Physiol. 2006;100:399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]