Abstract
The solution structures of four enolates derived from β-amino esters are investigated using 6Li NMR spectroscopy in conjunction with the method of continuous variation (method of Job). Ensembles of homo- and heteroaggregated enolates are generated by mixing enantiomers of a single enolate (R/S mixtures), opposite antipodes of two different enolates (R/S’ mixtures), and the same antipodes of two different enolates (R/R’ mixtures). The numbers of observable aggregates and their dependence on the mole fraction of the two enolates confirm the hexamer assignments. Inherent symmetries observable in the 6Li NMR spectra show the stereochemistry of chelation about the hexagonal drum.
Introduction
Very few reactive intermediates have captivated the attention of organic chemists more than lithium enolates.1 It is not surprising, therefore, that the underlying physical organic chemistry of enolates has garnered attention as well.2,3 Understanding structure-reactivity relationships of lithium enolates, however, requires knowing the structures of the aggregates.4,5,6 From the early structural studies of Jackman7 up to the more recent studies of Streitwieser,8 and others,9,10,11 progress has been made, but it is slow and, at times, contentious.11 The challenge stems in large part from a combination of high aggregate symmetries (cf., 1-4) and Li-O linkages that are opaque to NMR spectroscopy.12
 |
As part of a collaboration with Sanofi-Aventis to develop a plant-scale synthesis of Otamixaban,13 we were charged with the task of studying the alkylation of β-amino esters (eq 1).14 Although the mechanistic insights were interesting, it was the characterization of enolate 5 that proved acutely challenging. We solved the problem by generating an ensemble of aggregates from mixtures of antipodes (R)-5 and (S)-5 (eq 2). 6Li NMR spectroscopy showed that the number and relative integrations of the aggregates were highly characteristic of hexamers and inconsistent with monomers, dimers, and tetramers. The protocol formally falls under the rubric of the method of continuous variation15 (also called the method of Job16), which has found widespread application in chemistry and biochemistry.17 This strategy has now been generalized to a range of enolates representing the aggregates (2-4) anticipated from crystallographic studies.18, 19
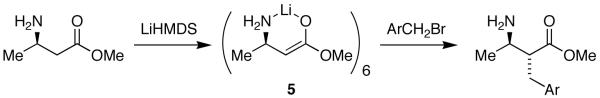 |
(1) |
| (2) |
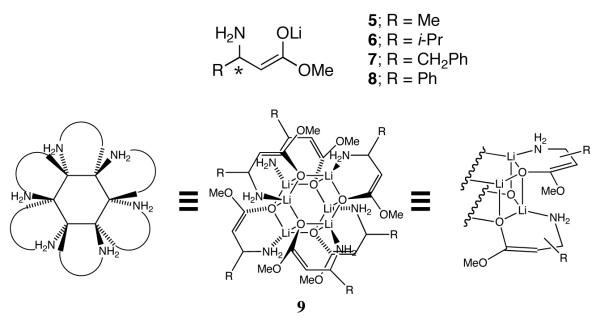
The study described herein focuses on the structures of β-amino ester enolates 6-8. By analogy to 5, enolates 6-8 were expected to form hexameric drums of general structure 9, and, indeed, they do. We focused on hexamers because they offer unique opportunities to examine new strategies and tactics.
 |
Background
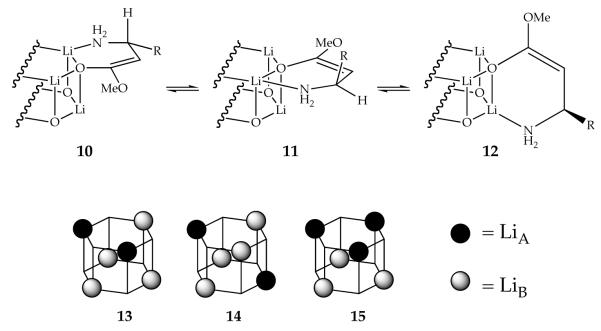
Applying the method of continuous variation to large ensembles in general and enolates 5-8 in particular presents acute challenges. Merely resolving a large number of species spectroscopically can be problematic given the relatively narrow (≈3 ppm) spectral range of 6Li, constituting a major focus throughout this manuscript. In addition, enolates 5-8 present considerable complexities stemming from stereoisomerism within the chelates (cf., 10-12) and positional isomerism (cf., 13 and 14) characteristic of the hexagonal drum.20 Indeed, 6Li NMR spectra recorded at the lowest attainable NMR probe temperatures (< -100 °C) are often intractably complex. Warming the probe, however, elicits rapid chelate-chelate,21 conformational exchange,22 and intraaggregate lithium-lithium exchanges,23 which cause the spectra to simplify.24,25 Rapid intraaggregate exchange in conjunction with slow interaggregate exchange causes each aggregate to appear as only a single 6Li resonance and affords separate resonances only for aggregates differing by subunit composition (cf., 4:2 versus 3:3 mixed aggregates; 14 and 15) or by virtue of their aggregation numbers (tetramers versus hexamers). Monitoring the relative 6Li resonance integrations as a function of mole fraction of the enolate mixtures and subsequent parametric fits3,14 afford multicomponent Job plots that prove highly diagnostic of aggregation state. Representative Job plots and new strategies for exploiting ensembles of β-amino ester enolates are described below.
 |
Results
We examined three strategies for characterizing enolates 6-8. Table 1 summarizes the predicted number of spectroscopically distinct structural forms observed for hexamers.26 In the first section, we describe mixtures of R and S enantiomers (so-called R/S mixtures) derived from a single β-amino ester enolate. This approach used to characterize enolate 514 is extended to enolates 6-8. A stereochemical degeneracy simplifies the 6Li NMR spectra. In the subsequent two sections, ensembles were generated from two structurally distinct enolates derived from opposite stereochemical series (R/S’ mixtures) and from the same stereochemical series (R/R’ mixtures). R/S’ and R/R’ mixtures lift the stereochemical degeneracy (Table 1). The three strategies present different technical challenges and offer complementary views of the hexameric aggregation state.27
Table 1.
Spectroscopically distinguishable aggregates in binary mixtures of enolates.a
| R/S mixtures | R/S’ mixtures | R/R’ mixtures |
|---|---|---|
| R6/S6 | R6 | R6 |
| R5S1/R1S5 | R5S’1 | R5R’1 |
| R4S2/R2S4 | R4S’2 | R4R’2 |
| R3S3 | R3S’3 | R3R’3 |
| R2S’4 | R2R’4 | |
| R1S’5 | R1R’5 | |
| S’6 | R’6 |
The R and S notations refer to enantiomers of a single enolate.27 The prime refers to subunits from a structurally distinct enolate. RmSn/RnSm refers to as.pectroscopically indistinguishable pair of enantiomers.
R/S Mixtures
We first examined the structures of enolates 6-8 using R/S mixtures derived from a single enolate following the protocol established previously.14 Enantiomeric pairs of aggregates--R6/S6, R5S1/R1S5 and R4S2/R2S4--are degenerate, resulting in ensembles comprising four rather than seven observable aggregates (Table 1). (Note: RmSn/RnSm refers to spectroscopically indistinguishable pairs of enantiomerically related aggregates.) Characterization of the isopropyl derivative 6 is illustrated emblematically as follows. 6Li NMR spectra recorded on R/S mixtures of enolate 6 under conditions optimized for fast intraaggregate exchange and slow interaggregate exchange (9.0 M THF in toluene, -20 °C) reveal four distinct resonances consistent with an ensemble of hexamers and inconsistent with monomers, dimers, and tetramers (Table 1).3 Serial dilution at fixed R/S proportions showed that the integrations are concentration independent, confirming a common aggregation number.
The integrations of the resonances corresponding to the RmSn aggregates of 6 were monitored as a function of mole fraction of the R and S antipodes. The equilibria are described according to eqs 3-5. A parametric fit affords the Job plot shown in Figure 1. The fit to the hexamer model is excellent. The R3S3 form is inordinately stable. Figure 2 displays the curves predicted from a purely statistical model.
Figure 1.
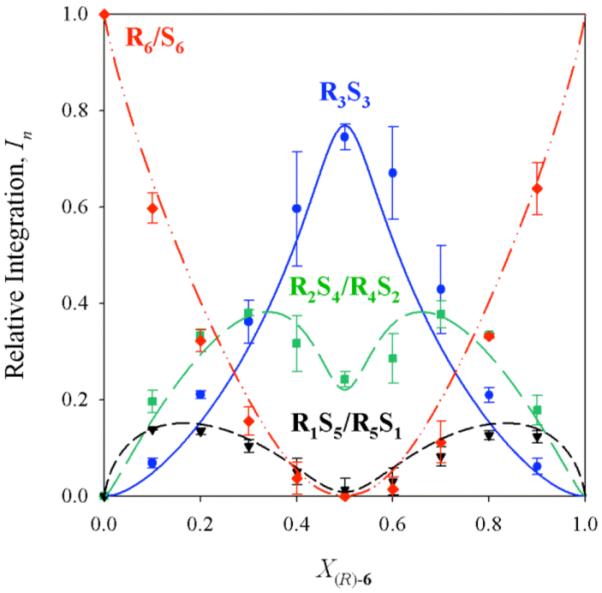
Job plot showing relative integrations (In)28 of RnS6-n hexamers as a function of the mole fraction of the R enantiomer (X(R)-6) for enolate 6. Samples were 0.10 M total enolate in 9.0 M THF/toluene, and spectra were recorded at -20 °C.
Figure 2.
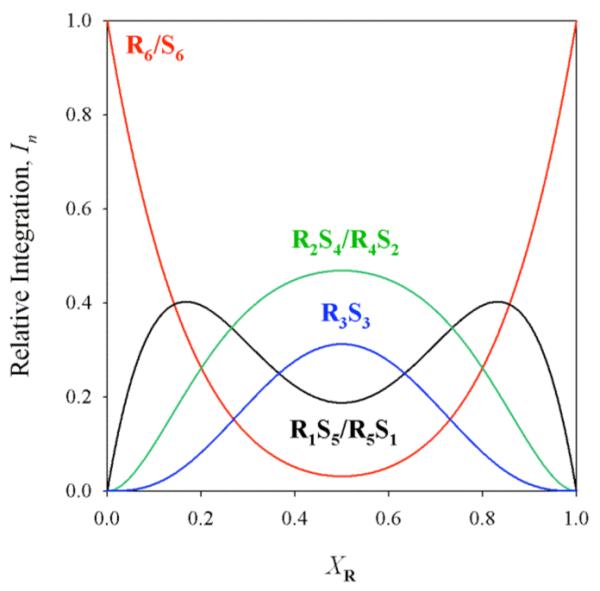
Statistical distribution of R/S mixtures of enolates.
| (3) |
| (4) |
| (5) |
The corresponding analysis of benzyl-substituted enolate 7 in 9.0 M THF at -25 °C revealed only three resonances, consistent with either an ensemble of tetramers (R4/S4, R3S1/R1S3, and R2S2) or an ensemble of hexamers in which two resonances are unresolved. Failure of the tetramer-based model was suggested by a poor parametric fit (Figure 3). By contrast, the fit to a hexamer model assuming the two resonances corresponding to the R3/S3 and R4S2/R2S4 hexamers are superimposed afforded a distinct improvement (Figure 4). The latter fit is further justified by the sporadic appearance of the fourth resonance as a shoulder. A similar problem surfaced during the analysis of R/S mixtures of phenyl-substituted enolate 8 in which resonances corresponding to R6 and R5S1/R1S5 do not resolve in 9.0 M THF. In the case of 8, however, all four 6Li resonances fully resolve in 3.0 M THF/toluene, affording a Job plot of comparable quality to that in Figure 1 consistent with hexamers (Supporting Information).
Figure 3.
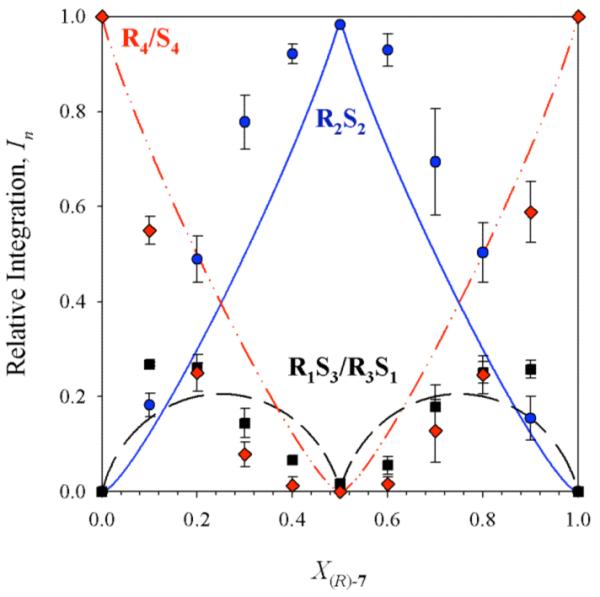
Job plot showing the relative integrations (In)28 of RnS4-n tetramers as a function of the mole fraction of the R enantiomer (X(R)-7) for enolate 7. The curves correspond to the parametric fit as described in the text. Samples were 0.10 M total enolate in 9.0 M THF/toluene, and spectra were recorded at -25 °C.
Figure 4.
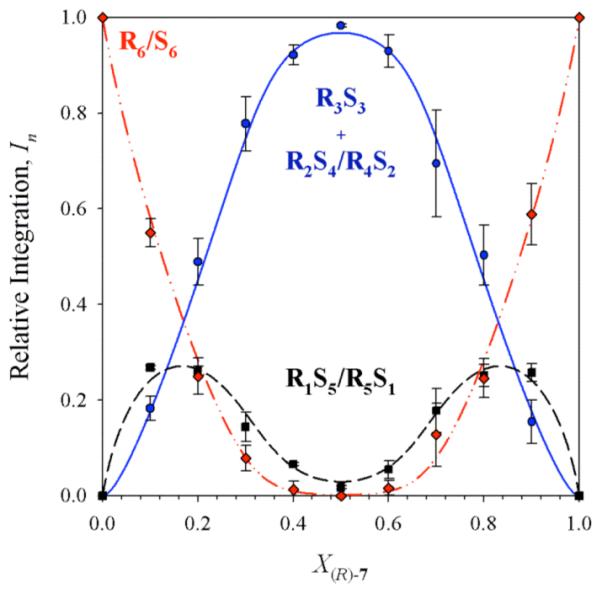
Job plot showing the relative integrations (In)28 of RnS6-n hexamers as a function of the mole fraction of the R enantiomer (X(R)-7) for enolate 7. The curves correspond to the parametric fit as described in the text. Samples were 0.10 M total enolate in 9.0 M THF/toluene, and spectra were recorded at -25 °C.
R/S’ Mixtures
We examined mixtures of structurally distinct enolates derived from opposite absolute configuration (R/S’ mixtures).27 The R/S’ mixtures should be similar to the R/S mixtures except that the enantiomeric degeneracy has been removed: An ensemble of RmS’n hexamers would appear as seven discrete aggregates (Table 1). Resolution of seven 6Li resonances was, at times, more difficult because intraaggregate exchange rates varied slightly among aggregates of different stoichiometries. Parametric fits3,14 to equilibria in eqs 6-10 were carried out in each case. The studies were arbitrarily restricted to mixtures of enolates 6-8 with enolate 5. (No such restriction would exist in the event that an enolate resists characterization.)
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
Analysis of R/S’ mixtures of enolates 5 and 6 was reasonably successful. All seven resonances were readily observed. At nearly equimolar concentrations (X(R)-5 = 0.4-0.6), the resonances corresponding to R4S’2 and R2S’4 were readily observed but difficult to accurately integrate separately. Accordingly, the two resonances were summed, affording the Job plot illustrated in Figure 5. The increased stability of R3S’3 was anticipated from results for the R/S mixtures (vide supra).
Figure 5.
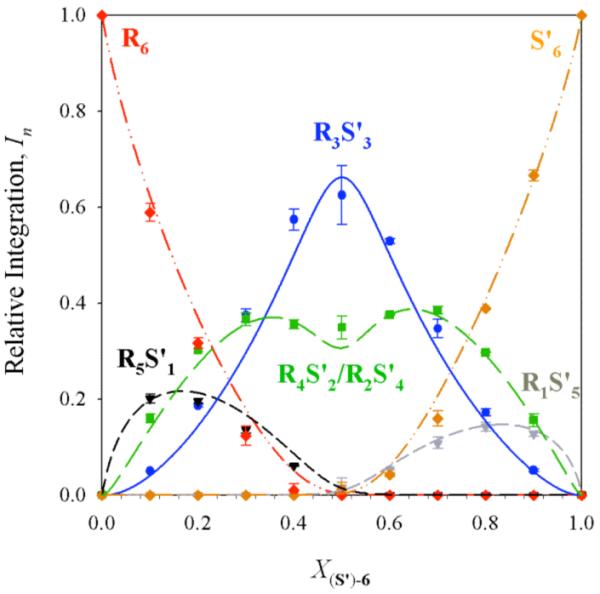
Job plot showing relative integrations (In)28 of RnS6-n hexamers as a function of the mole fraction of the S enantiomer (X(S’)-6) for enolate 6. The curves correspond to the parametric fit as described in the text. Samples were 0.10 M total enolate in 3.0 M THF/toluene, and spectra were recorded at -30 °C.
R/S’ mixtures derived from enolates 5 and 7 also successfully afforded a Job plot, albeit with the requisite summation of the resonances corresponding to R4S’2 and R2S’4 (Supporting Information). By contrast, R/S’ mixtures of 5 and 8 afford ensembles of aggregates that are qualitatively familiar, but poor resolution precludes a detailed analysis.
R/R’ Mixtures
Mixtures of structurally distinct enolates derived from the same stereochemical series (R/R’ mixtures) afford an ensemble of seven spectroscopically distinct hexamers. Each of the RmR’n hexamers should mimic the optically pure (R6) forms. Success depended critically on the choice of enolates.
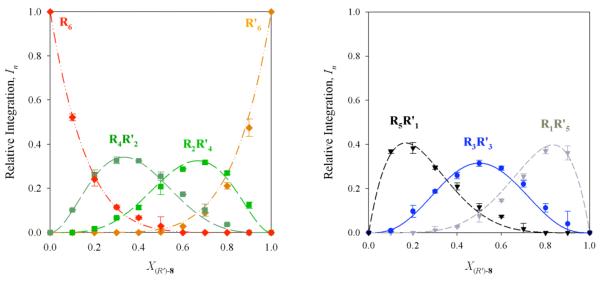
6Li spectra of R/R’ mixtures derived from enolates 5 and 8 in the limit of rapid intraaggregate exchange (-10 °C) display seven well-resolved resonances bracketed by the resonances of homoaggregates of 5 and 8 (Figure 6). The resulting Job plot (Figure 7) is both instructive and aesthetically pleasing. (Figure 7 represents a single parametric fit displayed in two parts for visual clarity.) Superposition of the curves from the parametric fit in Figure 7 with the analogous curves anticipated from a purely statistical distribution shows a striking similarity (Figure 8).
Figure 6.
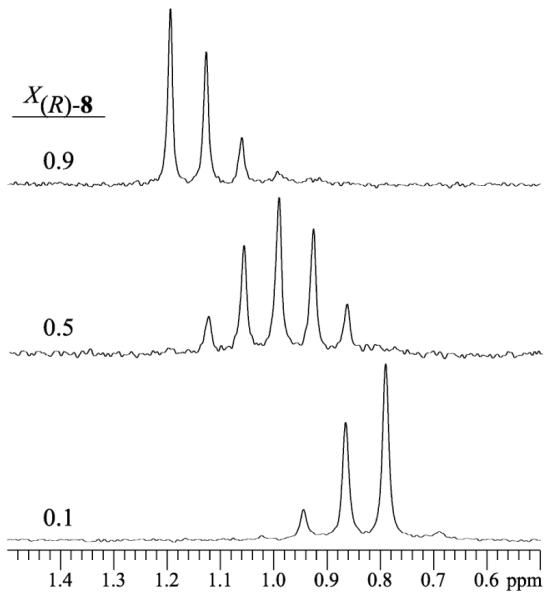
6Li NMR spectra recorded on R/R’ mixtures (the same stereochemical series) derived from enolates 5 and 8. Samples were 0.10 M total enolate in 3.0 M THF/toluene, and spectra were recorded at -40 °C.
Figure 7.
Job plot showing the relative integrations (In)28 of RnR’6-n hexamers of enolates 5 and 8 as a function of the mole fraction of the R enantiomer of enolate analogous curves anticipated from a purely statistical distribution shows a striking similarity (Figure 8). 8 (X(R)-8). The curves correspond to parametric fits as described. Samples were 0.10 M total enolate in 3.0 M THF/toluene, and spectra were recorded at -40 °C.
Figure 8.
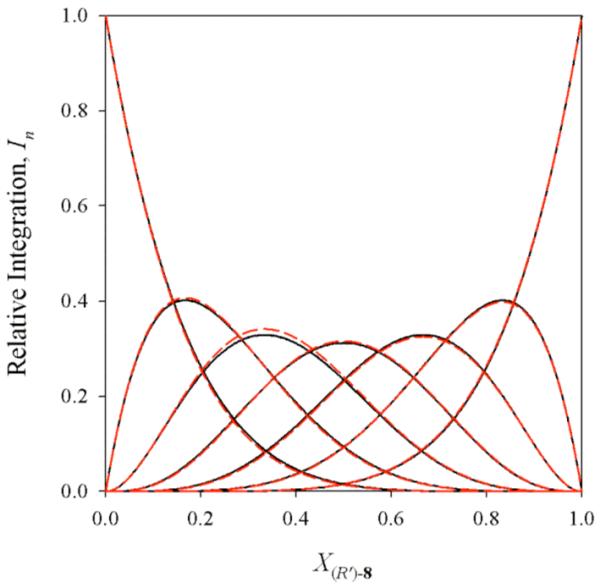
Superposition of the parametric fit from Figure 7 (red) with the results anticipated from a statistical distribution (black).
R/R’ mixtures derived from methyl- and isopropyl-substituted enolates 5 and 6 (respectively) presented minor resolution problems. The 6Li signals of R6 and R5R’1 (R = 5; R’ = 6) were observable as discrete resonances but were poorly resolved, requiring summation of their integrations when fitting the data (Supporting Information). Mixtures of the methyl- and benzyl-substituted enolates 5 and 7 afforded a poorly-resolved ensemble of aggregates due to a small chemical shift dispersion.
Stereochemistry of Chelation
With the hexameric aggregation state established, it is instructive to examine the 6Li NMR spectra recorded in the low temperature limit in which all intraaggregate exchanges are slow. We discuss the results in the context of the possible structural forms shown in Chart 1. Spectra are archived in Supporting Information.
Chart 1.
Li and O have been omitted for clarity and N = NH2.
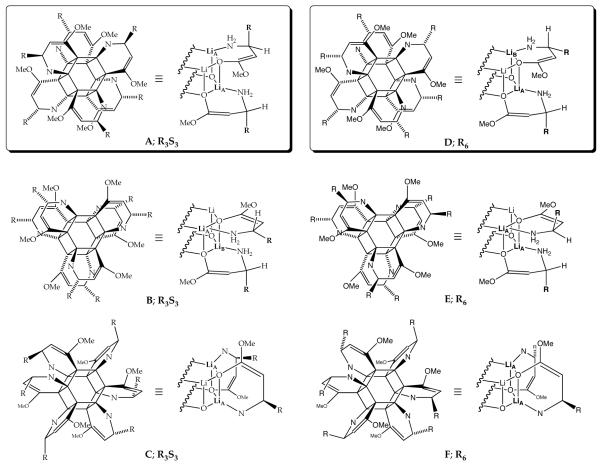
Let us first consider the structures of the R3S3 hexamer available as the predominant species in equimolar R/S mixtures (racemates). R3S3 hexamers derived from racemates of 5-8 display an inordinate stability relative to less symmetric counterparts of unequal stoichiometry. Of the three high-symmetry structural types represented by A, B, and C, isomers A and C should each display a single 6Li resonance, whereas B should display a pair of resonances in a 1:1 ratio. Isomer B appears to be excluded. In light of the crystal structure of 5 showing a hexamer of type A,14 we deem A as the probable form in solution. The orientation in the alkyl substituents away from the perimeter of A also logically accounts for the unusual stability.
The R3S’3 hexamers formed from the R/S’ mixtures (pseudo racemates) display the same unusual stability as the R3S3 aggregates. The reduced symmetry of R3S’3 hexamers causes the R antipodes to reside on one hexagonal face and the S’ antipodes on the other, resulting in two 6Li resonances (1:1). Warming causes the two resonances to coalesce, which we attribute to intraaggregate exchange of the lithium nuclei. Although this experiment does not distinguish A and C, the consistency with A is satisfying.
The homochiral (R6 and S6) aggregates appear to be hexagonal drum D. The R6 and S6 homoaggregates of 6-8 each showed two resonances (1:1) at ≤-90 °C, consistent with D and inconsistent with E and F. Wrapping the six chelates of E in the opposite direction affords a second isomer in which both alkyl groups are in the equatorial positions around the perimeter, but it seems unlikely that the two stereoisomers would be of equal stability. The homoaggregates of 5, however, initially presented a minor conundrum. Previously reported14 spectra recorded on solutions of optically pure 5 revealed a single 6Li resonance, suggesting that the R6 and S6 forms of 5 are not type D. Results from enolates 6-8 suggested that the rates of chelate exchange may be high for the relatively small methyl-substituted enolate. Indeed, reinvestigation of optically pure enolate 5 showed that the R6 and S6 forms display two resonances albeit at <-120 °C and low THF concentration. Therefore, enolates 5-8 are all assigned as hexamer type D.
In the case of the R/S and R/S’ mixtures, the R3S3 and R3S’3 are inordinately stable and show distinct preferences for a single positional isomer, resulting in relatively clean spectra with minor mounds in the baseline in equimolar mixtures. By contrast, the R/R’ mixtures should afford the statistically distributed RmR’n aggregates and nearly isoenergetic positional isomers (see 13 and 14). Each positional isomer could display multiple inequivalent lithium sites (as many as six per isomer). Indeed, the R/R’ mixtures afforded only broad mounds regardless of stoichiometry.
Discussion
We are currently developing methods for characterizing a wide range of lithium enolates, all of which are based on the method of continuous variation.3,14 Recent studies, for example, show that standard enolates unadorned with further functionality are either dimers or tetramers, depending on the choice coordinating solvent.14 A primary goal of the study described herein was to exploit enolates 5-8 derived from β-amino esters to examine methods and strategies for characterizing highly aggregated enolates. We open the discussion by summarizing the structural assignments. This is followed by a more detailed analysis of how 6Li NMR spectroscopy in conjunction with the method of continuous variation (Job plots) afforded the critical aggregation numbers.
Enolate structures
Job plots salted throughout the results section and discussed below show that enolates 5-8 are hexameric. We believe that all of the homo- and heteroaggregated hexamers are of general structure 9 bearing chelates wrapped around the hexagonal drum in the same direction. Direct support for this assertion is based on 6Li NMR spectroscopic studies of a smaller subset that includes: (1) R6 and S6 homochiral hexamers derived from optically pure samples (denoted by R and S), (2) R3S3 heterochiral hexamers prevalent in racemic samples, (3) R3S’3 hexamers prevalent in equimolar mixtures of structurally distinct enolates of opposite absolute configuration, and (4) all RmR’n (and SmS’n) hexamers prepared from mixtures of structurally distinct enolates of the same absolute configuration. The structures of the RmSn and RmS’n hexamers in which m ≠ n ≠ 0 (e.g., 5:1 and 4:2) are assigned as general structure 9 only by analogy.
One consequence of the hexagonal drum 9 is that subunits of opposite absolute configuration exemplified by R3S3 and R3S’3 mixed hexamers allow optimal disposition of the alkyl side chains as shown in hexamer A (Chart 1). Type A hexamers are reflected in the x-ray structure of rac-514 and display inordinate stability in solution (vide infra). Conversely, the homochiral aggregates R6 and S6 as well as the RmR’n hexamers derived from pairs of enolates within the same enantiomeric series appear to be type D hexagonal drums in which the β-alkyl substituents point away from the perimeter on one hexagonal face and toward the perimeter on the other.
Method of Job
The most probable forms of lithium enolates--monomers, dimers, tetramers, and hexamers (1-4)--are indistinguishable by standard NMR spectroscopic methods due to the high symmetry and opaque Li-O linkages. The aggregate structures emerge when mixtures of structurally related enolates afford ensembles of mixed aggregates. The number of possible aggregates in the ensemble increases with aggregate size and is highly diagnostic (Table 1).29 Thus the total number of aggregates observed is revealing. The quantitative dependence of these ensembles on the mole fraction of the enolate subunits completes a compelling picture.
Chemical shifts and resolution
Success of the strategy for determining the aggregation state depends critically on the resolution of the discrete aggregates by 6Li NMR spectroscopy. Although some problems presented by enolates 5-8 may be specific to the β-amino ester enolates, the lessons are proving useful as the method is extended to other classes of enolates.
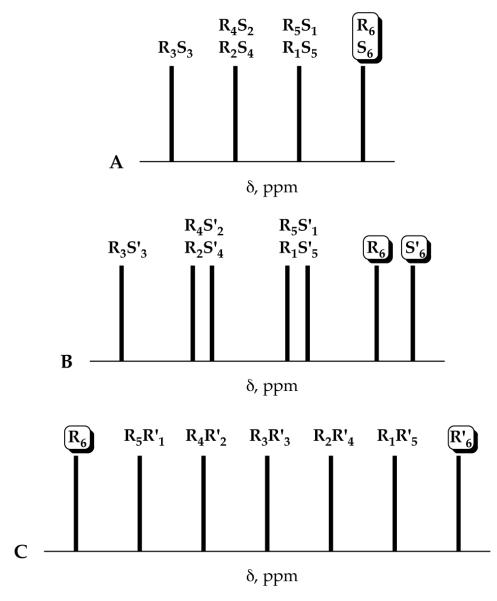
Let us first consider the chemical shift dispersion resulting from pairs of enantiomers in R/S mixtures (Figure 9A). A relatively small number of resonances (four) arise because there are three degenerate pairs. The 6Li resonances are bracketed upfield by the homochiral R6/S6 aggregates and downfield by the heterochiral R3S3 aggregate. The chemical shifts of the aggregates reflect the degree of heterochirality. The nature of the hydrocarbon does not significantly influence the resolution,30 but the proportion of THF in THF/hydrocarbon mixtures is important. Although one has limited control over the resolution, the resolution was adequate in most cases.
Figure 9.
Chemical shift dispersion of enolate aggregates derived from (A) R/S mixtures, (B) R/S’ mixtures, and (C) R/R’ mixtures.
R/S’ mixtures present a different scenario (Figure 9B). Because the R and S’ enantiomers come from structurally different enolates, the degeneracy caused by enantiomerism is eliminated: All seven RmS’n hexamers are spectroscopically distinct (Table 1). The resonances are bracketed by the homochiral R6 and S’6 forms upfield and the R3S’3 heterochiral form downfield. Clustering of resonances within the R4S’2/R2S’4 and R5S’1/R1S’5 pairs causes some resolution problems that are often alleviated by adjusting the THF concentration. The chemical shift dispersion can be controlled by judicious pairing of enolates based on the chemical shifts of the R6 and S’6 homoaggregates. At the outset, we had the simple notion that maximally resolved R6 and S’6 pairs would afford maximally resolved ensembles of mixed aggregates. This was wrong. Widely separated resonances for the R6 and S’6 cause the resonances of the mixed aggregates to merge unpredictably. The best resolution is observed by pairing R6 and S’6 aggregates with minimum chemical shift separation (as shown in Figure 9B), conditions causing the R/S’ mixtures to mimic the R/S mixtures.
The R/R’ mixtures afford seven 6Li resonances bracketed by the resonances of the two homoaggregates (Figure 9C). The chemical shifts of the RmR’n mixed aggregates correlate predictably with stoichiometry. Resolution is maximized by pairing R6 and R’6 homoaggregates with the largest chemical shift separation. Tactically, this allows one homoaggregate with an unusual chemical shift--the phenyl substituted enolate 8--to be paired with the others. Faced with a particularly difficult resolution problem one might explicitly design a derivative with a β substituent (such as a naphthyl or phenanthryl group) to impart an especially wide chemical shift dispersion. Additional importance of the R/R’ mixtures is underscored by the parametric fits.
Parametric Fits
The Job plots and affiliated parametric fits3,14 are fully consistent with hexamers. Although in several instances, limited resolution demands that resonances corresponding to two aggregates are summed (see Figures 4 and 5), the underlying math is flexible, and the conclusions appear robust. We remind the reader, however, that the primary goal is to exploit an ensemble of heteroaggregates to provide insights into the structures of the homoaggregates. To that end, control experiments showing that the proportion of all aggregates are independent of the concentration provide important support to the assignments. The parametric fits resulting from the three protocols offer complementary perspectives of the aggregates.
Using R/S mixtures of a single enolate is the simplest of the three protocols in that only four curves must be fit (Table 1). Enolates 6 and 8 afforded excellent results (Figure 1). Unusual stabilities of the R3S3 aggregates afford distributions that deviate markedly from statistical (Figure 2). Poor resolution for R/S mixtures of enolate 7 afforded a resonance count consistent with tetramers rather than hexamers. Fortunately, the parametric fit to a tetramer model (Figure 3) was decidedly worse than the fit to the hexamer model (Figure 4), and subsequent prodding revealed the missing resonance. Both the number and mole-fraction dependencies and absolute-concentration independencies of the aggregates are consistent with hexamers and argue strongly that the Rn and Sn homoaggregates are necessarily R6 and S6.
The R/S’ mixtures afford ensembles containing seven potentially distinguishable aggregates (no degenerate pairs). Although we were forced to sum two resonances in some cases, the fits are excellent. One might ask what would happen if an R6 hexamer and S’4 tetramer were mixed. It seems most probable that an intractable mess would result, but that begs the question: Would an ensemble containing six hexamers and a tetramer be distinguishable from an ensemble of seven hexamers? Probably not from the parametric fit alone. The independence of the ensemble on the absolute enolate concentration provides support.
Job plots based on R/R’ mixtures offer the most convincing view. The seven observable aggregates are nearly statistically distributed type D hexamers differing only in the β-alkyl group (Figure 8). This is highly consistent with a type D hexamer in which the various alkyl substituents are interchangeable isoenergetically.
Conclusion
The studies described above can be distilled to a single concept: Formation of mixed aggregates from mixtures of closely related organolithium species provides insight into the spectroscopically opaque homoaggregates.29 The method is an unusually complex application of the method of continuous variation.15,16,17 The strategy relies on high resolution NMR spectroscopy (6Li in this instance) and powerful curve fitting algorithms. We focused this portion of a more broadly-based study on β-aminoester enolates 5-8 because previous studies strongly suggested that they would form hexagonal drums. Such hexamers present challenges that are unique in both detail and severity when compared with dimer- and tetramer-based ensembles.3 Nonetheless, the underlying issues are likely to surface again during studies of other highly functionalized enolates, and strategies developed herein should prove instructive.
Experimental Section
Reagents and Solvents
Substrates were prepared using literature procedures described in Supporting Information. THF was distilled from solutions containing sodium benzophenone ketyl. Hydrocarbon solvents were distilled from blue solutions containing sodium benzophenone ketyl with approximately 1% tetraglyme to dissolve the ketyl. The diphenylacetic acid used to check n-BuLi solution titers was recrystallized from methanol and sublimed at 120 °C under full vacuum.31 [6Li]LiHMDS used to prepare the enolates was recrystallized.32 Air- and moisture-sensitive materials were manipulated under argon using standard glove box, vacuum line, and syringe techniques.
Preparation of compounds
The precursors to 5-7 are literature compounds prepared using an Arndt-Eistert homologation of the corresponding α-amino acids.33 The precursor to 8 was prepared according to a literature procedure.34 Detailed procedures can be found in supporting information.
Enolization
The enolates were prepared in situ for NMR spectroscopy. After flame drying the NMR tube, the appropriate stock solutions were added, and the tube was sealed. Samples were warmed to 0 °C for 15-30 minutes and were thoroughly mixed prior to collecting the spectrum. Integrations were preformed using the deconvolution macro included with VNMR software.
Parametric Fit
Data was fit parametrically to mathematical models described previously.3
Supplementary Material
Acknowledgment
We thank the National Science Foundation for direct support of this work as well as Dupont Pharmaceuticals, Merck Research Laboratories, Pfizer, Sanofi-Aventis, R. W. Johnson, Boehringer-Ingelheim, and Schering-Plough for indirect support.
Footnotes
Supporting Information: Experimental procedures, NMR spectra, and Job plots. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Footnotes
- 1(a).Green JR. Science of Synthesis. 8a. Georg Thieme Verlag; New York: 2005. pp. 427–486. [Google Scholar]; (b) Schetter B, Mahrwald R. Angew. Chem. Int. Ed. 2006;45:7506. doi: 10.1002/anie.200602780. [DOI] [PubMed] [Google Scholar]; (c) Arya P, Qin H. Tetrahedron. 2000;56:917. [Google Scholar]; (d) Caine D. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 1. Pergamon; New York: 1989. p. 1. [Google Scholar]; Martin SF. Vol. 1. p. 475. Ibid. [Google Scholar]; (e) Plaquevent J-C, Cahard D, Guillen F, Green JR. Science of Synthesis. Vol. 26. Georg Thieme Verlag; New York: 2005. pp. 463–511. [Google Scholar]; (f) Katritzky Alan R., Taylor Richard J. K., editors. Comprehensive Organic Functional Group Transformations II. Elsevier; Oxford, UK: 1995. pp. 834–835. [Google Scholar]; (g) Cativiela C, Diaz-de-Villegas MD. Tetrahedron: Asymmetry. 2007;18:569. [Google Scholar]; (h) Dugger RW, Ragan JA, Ripin DHB. Org. Process Res. Dev. 2005;9:253. [Google Scholar]; (i) Farina V, Reeves JT, Senanayake CH, Song JJ. Chem. Rev. 2006;106:2734. doi: 10.1021/cr040700c. [DOI] [PubMed] [Google Scholar]; (j) Wu G, Huang M. Chem. Rev. 2006;106:2596. doi: 10.1021/cr040694k. [DOI] [PubMed] [Google Scholar]
- 2.For a discussion of prior efforts to elucidate structure-reactivity relationships of enolates and other O-Li species see ref 3.
- 3.Liou LR, McNeil AJ, Ramirez A, Toombes GES, Gruver JM, Collum DB. J. Am. Chem. Soc. 2008;130 doi: 10.1021/ja7100642. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collum DB, McNeil AJ, Ramirez A. Angew. Chem. Int. Ed. 2007;49:3002. doi: 10.1002/anie.200603038. [DOI] [PubMed] [Google Scholar]
- 5(a).Hsieh HL, Quirk RP. Anionic Polymerization: Principles and Practical Applications. Marcel Dekker; New York: 1996. [Google Scholar]; (b) Szwarc M, editor. Ions and Ion Pairs in Organic Reactions. 1 and 2. Wiley; New York: 1972. [Google Scholar]; (c) Wardell JL. Chapter 2. In: Wilkinson G, Stone FGA, Abels FW, editors. Comprehensive Organometallic Chemistry. Vol. 1. Pergamon; New York: 1982. [Google Scholar]; (d) Wakefield BJ. The Chemistry of Organolithium Compounds. Pergamon Press; New York: 1974. [Google Scholar]; (e) Brown TL. Pure Appl. Chem. 1970;23:447. [Google Scholar]
- 6.The rate law provides the stoichiometry of the transition structure relative to that of the reactants: Edwards JO, Greene EF, Ross J. J. Chem. Educ. 1968;45:381.
- 7(a).Jackman LM, Bortiatynski J. Adv. Carbanion Chem. 1992;1:45.Jackman LM, Lange BC. Tetrahedron. 1977;33:2737.Jackman LM, Chen X. J. Am. Chem. Soc. 1997;119:8681.Jackman LM, Petrei MM, Smith BD. J. Am. Chem. Soc. 1991;113:3451.Jackman LM, Scarmoutzos LM, DeBrosse CW. J. Am. Chem. Soc. 1987;109:5355.Jackman LM, Haddon RC. J. Am. Chem. Soc. 1973;95:3687.Jackman LM, DeBrosse CW. J. Am. Chem. Soc. 1983;105:4177.Also, see: Quan W, Grutzner JB.J. Org. Chem 1986514220Jackman LM, Rakiewicz EF. J. Am. Chem. Soc. 1991;113:1202.Jackman LM, Smith BD. J. Am. Chem. Soc. 1988;110:3829. doi: 10.1021/ja00226a021.
- 8(a).Streitwieser A. J. Mol. Model. 2006;12:673. doi: 10.1007/s00894-005-0045-3. [DOI] [PubMed] [Google Scholar]; (b) Streitwieser A, Wang DZ. J. Am. Chem. Soc. 1999;121:6213. [Google Scholar]; (c) Leung SS-W, Streitwieser A. J. Org. Chem. 1999;64:3390. doi: 10.1021/jo990075p. [DOI] [PubMed] [Google Scholar]; (d) Wang DZ, Kim Y-J, Streitwieser A. J. Am. Chem. Soc. 2000;122:10754. [Google Scholar]; (e) Kim Y-J, Streitwieser A. Org. Lett. 2002;4:573. doi: 10.1021/ol017175d. [DOI] [PubMed] [Google Scholar]; (f) Kim Y-J, Wang DZ. Org. Lett. 2001;3:2599. doi: 10.1021/ol0162872. [DOI] [PubMed] [Google Scholar]; (g) Streitwieser A, Leung SS-W, Kim Y-J. Org. Lett. 1999;1:145. doi: 10.1021/ol990604b. [DOI] [PubMed] [Google Scholar]; (h) Abbotto A, Leung SS-W, Streitwieser A, Kilway KV. J. Am. Chem. Soc. 1998;120:10807. [Google Scholar]; (i) Leung SS-W, Streitwieser A. J. Am. Chem. Soc. 1998;120:10557. [Google Scholar]; (j) Abu-Hasanayn F, Streitwieser A. J. Org. Chem. 1998;63:2954. [Google Scholar]; (k) Abu-Hasanayn F, Streitwieser A. J. Org. Chem. 1996;118:8136. [Google Scholar]; (l) Gareyev R, Ciula JC, Streitwieser A. J. Org. Chem. 1996;61:4589. doi: 10.1021/jo960013o. [DOI] [PubMed] [Google Scholar]; (m) Abu-Hasanayn F, Stratakis M, Streitwieser A. J. Org. Chem. 1995;60:4688. [Google Scholar]; (n) Dixon RE, Williams PG, Saljoughian M, Long MA, Streitwieser A. Magn. Reson. Chem. 1991;29:509. [Google Scholar]
- 9(a).Suzuki M, Koyama H, Noyori R. Bull. Chem. Soc. Jpn. 2004;77:259. [Google Scholar]; (b) Suzuki M, Koyama H, Noyori R. Tetrahedron. 2004;60:1571. [Google Scholar]; (c) Pospisil PJ, Wilson SR, Jacobsen EN. J. Am. Chem. Soc. 1992;114:7585. [Google Scholar]
- 10.Biddle MM, Reich HJ. J. Org. Chem. 2006;71:4031. doi: 10.1021/jo0522409. [DOI] [PubMed] [Google Scholar]
- 11.The structure and reactivity of lithium enolates during methacrylate polymerizations have garnered considerable attention and has been reviewed: Zune C, Jerome R. Prog. Polymer Sci. 1999;24:631. Also, see: Baskaran D. Prog. Polym. Sci. 2003;28:521.
- 12(a).The rapid relaxation of the highly quadrupolar 17O nucleus would preclude observing 6Li-17O coupling. For leading references to 6Li-15N and 6Li-13C see: Günther HJ. Brazil. Chem. 1999;10:241.Collum DB. Acc. Chem. Res. 1993;26:227.
- 13(a).Guertin KR, et al. Bioorg. Med. Chem. Lett. 2002;12:1671. doi: 10.1016/s0960-894x(02)00213-5. [DOI] [PubMed] [Google Scholar]; (b) Czekaj M, et al. Bioorg. Med. Chem. Lett. 2002;12:1667. doi: 10.1016/s0960-894x(02)00212-3. [DOI] [PubMed] [Google Scholar]; (c) Chandramouli SV, O’Brien MK, Powner TH. WO Patent 0040547. 2000; (d) Nagula G, Huber VJ, Lum C, Goodman BA. Org. Lett. 2000;2:3527. doi: 10.1021/ol006614q. [DOI] [PubMed]
- 14(a).McNeil AJ, Toombes GES, Gruner SM, Lobkovsky E, Collum DB, Chandramouli SV, Vanasse BJ, Ayers TA. J. Am. Chem. Soc. 2004;126:16559. doi: 10.1021/ja045144i. [DOI] [PubMed] [Google Scholar]; (b) McNeil AJ, Toombes GES, Chandramouli SV, Vanasse BJ, Ayers TA, O’Brien MK, Lobkovsky E, Gruner SM, Marohn JA, Collum DB. J. Am. Chem. Soc. 2004;126:5938. doi: 10.1021/ja049245s. [DOI] [PubMed] [Google Scholar]; (c) McNeil AJ, Collum DB. J. Am. Chem. Soc. 2005:127, 5655. doi: 10.1021/ja043470s. [DOI] [PubMed] [Google Scholar]
- 15.Gil VMS, Oliveira NC. J. Chem. Educ. 1990;67:473. [Google Scholar]
- 16.Job P. Ann. Chim. 1928;9:113. [Google Scholar]
- 17(a).Huang CY. Method Enzymol. 1982;87:509. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]; (b) Hirose K. J. Incl. Phenom. 2001;39:193. [Google Scholar]; (c) Likussar W, Boltz DF. Anal. Chem. 1971;43:1265. [Google Scholar]
- 18(a).Seebach D. Angew. Chem., Int. Ed. Engl. 1988;27:1624. [Google Scholar]; (b) Setzer WN, Schleyer P. v. R. Adv. Organomet. Chem. 1985;24:353. [Google Scholar]; (c) Williard PG. Chapter 1.1. In: Trost BM, Fleming I, editors. Comprehensive Organic Synthesis. Vol. 1. Pergamon; New York: 1991. [Google Scholar]; (d) Pauer F, Power PP. In: Lithium Chemistry: A Theoretical and Experimental Overview. Sapse A-M, Schleyer P. v. R., editors. Wiley & Sons; New York: 1995. pp. 295–392. [Google Scholar]
- 19(a).For crystal structures of hexameric O-lithiated species, see: Simple enolates: Nichols MA, Leposa CM, Hunter AD, Zeller MJ. Chem. Crystallogr. 2007;37:825.Rodriques-Delgado A, Chen EY-X. J. Am. Chem. Soc. 2005;127:961. doi: 10.1021/ja044671z.Willard PG, Carpenter GB. J. Am. Chem. Soc. 1986;108:462. doi: 10.1021/ja00263a016.Chelating enolates: Chivers T, Downard A, Yap GPA.Inorg. Chem 1998375708Jastrzebski JTBH, van Koten G, van de Mieroop WF. Inorg. Chim. Acta. 1988;142:169.Maetzke T, Hidber CP, Seebach D. J. Am. Chem. Soc. 1990;112:8248.Waldmueller D, Mayer B, Braun M, Hanuschik A, Krueger C, Guenot P. Chem. Ber. 1992;125:2779.Chelating amino alkoxides: Tian X, Woski M, Lustig C, Pape T, Froehlich R, Le Van D, Bergander K, Mitzel NW.Organometallics 20052482Strohmann C, Seibel T, Schildbach D. J. Am. Chem. Soc. 2004;126:9876. doi: 10.1021/ja048216e.Armstrong DR, Davies RP, Raithby PR, Snaith R, Wheatley AEH. New J. Chem. 1999;23:499.Armstrong DR, Clegg W, Hodgson SM, Snaith R, Wheatley AEH. J. Organomet. Chem. 1998;550:233.Herberich GE, Spaniol TP, Fischer A. Chem. Ber. 1994;127:1619.Phenolates: Rajeswaran M, Begley WJ, Olson LP, Huo S.Polyhedron 2007263653Jackman LM, Cizmeciyan D, Williard PG, Nichols MA. J. Am. Chem. Soc. 1993;115:6262.Alkoxides: Nekola H, Olbrich F, Behrens UZ.Anorg. Allg. Chem 20026282067Chisholm MH, Drake SR, Naiini AA, Streib WE. Polyhedron. 1991;10:805.Higher aggregates: Andrews PC, MacLellan JG, Mulvey RE, Nichols PJ.J. Chem. Soc. Dalton Trans 200281651Maetzke T, Seebach D. Organometallics. 1990;9:3032.Allan JF, Nassar R, Specht E, Beatty A, Calin N, Henderson KW. J. Am. Chem. Soc. 2004;126:484. doi: 10.1021/ja038420m.Vilardo JS, Fanwick PE, Rothwell IP. Polyhedron. 1998;17:769.
- 20.Positional isomerism does not exist in either cyclic dimers or cubic tetramers.
- 21(a).For examples of chelate exchange see Aubrecht KB, Lucht BL, Collum DB. Organometallics. 1999;18:2981.Reich HJ, Goldenberg WS, Sanders AW, Jantzi KL, Tzschucke CC.J. Am. Chem. Soc 20031253509 and references cited therein.Fraenkel G, Winchester WR. J. Am. Chem. Soc. 1989;111:3794.
- 22(a).For discussions of conformational equilibrations, see Clegg W, Liddle ST, Snaith R, Wheatley AEH. New J. Chem. 1998;22:1323.Boche G, Fraenkel G, Cabral J, Harms K, van Eikema Hommes NJR, Lohrenz J, Marsch M, Schleyer P. v. R. J. Am. Chem. Soc. 1992;114:1562.Remenar JF, Lucht BL, Kruglyak D, Romesberg FE, Gilchirst JH, Collum DB. J. Org. Chem. 1997;62:5748.
- 23(a).Time averaging of lithium nuclei within aggregates (intraaggregate exchanges) is well precedented: Arvidsson PI, Ahlberg P, Hilmersson G. Chem. Eur. J. 1999;5:1348.Bauer W. J. Am. Chem. Soc. 1996;118:5450.Bauer W, Griesinger C. J. Am. Chem. Soc. 1993;115:10871.DeLong GT, Pannell DK, Clarke MT, Thomas RD. J. Am. Chem. Soc. 1993;115:7013.Thomas RD, Clarke MT, Jensen RM, Young TC. Organometallics. 1986;5:1851.Bates TF, Clarke MT, Thomas RD. J. Am. Chem. Soc. 1988;110:5109.Fraenkel G, Hsu H, Su BM. In: Lithium: Current Applications in Science, Medicine, and Technology. Bach RO, editor. Wiley; New York: 1985. pp. 273–289.Heinzer J, Oth JFM, Seebach D. Helv. Chim. Acta. 1985;68:1848.Fraenkel G, Henrichs M, Hewitt JM, Su BM, Geckle MJ. J. Am. Chem. Soc. 1980;102:3345.Lucht BL, Collum DB. J. Am. Chem. Soc. 1996;118:3529.
- 24.For examples of solvent exchanges, see: Lucht BL, Collum DB.Acc. Chem. Res 1999321035 and references cited therein.
- 25(a).Arvidsson PI, Ahlberg P, Hilmersson G. Chem. Eur. J. 1999;5:1348. [Google Scholar]; (b) Bauer W. J. Am. Chem. Soc. 1996;118:5450. [Google Scholar]; (c) Bauer W, Griesinger C. J. Am. Chem. Soc. 1993;115:10871. [Google Scholar]; (d) DeLong GT, Pannell DK, Clarke MT, Thomas RD. J. Am. Chem. Soc. 1993;115:7013. [Google Scholar]; (e) Thomas RD, Clarke MT, Jensen RM, Young TC. Organometallics. 1986;5:1851. [Google Scholar]; (f) Bates TF, Clarke MT, Thomas RD. J. Am. Chem. Soc. 1988;110:5109. [Google Scholar]; (g) Fraenkel G, Hsu H, Su BM. In: Lithium: Current Applications in Science, Medicine, and Technology. Bach RO, editor. Wiley; New York: 1985. pp. 273–289. [Google Scholar]; (h) Heinzer J, Oth JFM, Seebach D. Helv. Chim. Acta. 1985;68:1848. [Google Scholar]; (i) Fraenkel G, Henrichs M, Hewitt JM, Su BM, Geckle MJ. J. Am. Chem. Soc. 1980;102:3345. [Google Scholar]; (j) Lucht BL, Collum DB. J. Am. Chem. Soc. 1996;118:3529. [Google Scholar]
- 26.Ensembles of dimers and tetramers would afford three and five aggregates, respectively, which would afford two and three spectroscopically distinct resonances, respectively. Dimeric and tetrameric ensembles have been discussed in detail previously.ref 3
-
27.We refer to the enolates as R or S according to the structures as follows:
In the case of a phenyl moiety (R = Ph), however, the Cahn-Ingold-Prelog (CIP) rules would formally reverse the stereochemical designation. We have taken the liberty ignoring the reversal to minimize confusion within the text.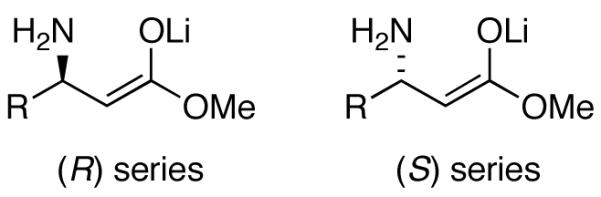
- 28.The relative integration (In) was previously referred as aggregate mole fraction (Xn’, ref 14). We have changed it to avoid using two, distinctly different mole fraction terms and to include provisions for mixtures of aggregates which are not of the same aggregation number.
- 29(a).For instances in which ensembles were used to investigate homoaggregation, see: Kissling RM, Gagne MR. J. Org. Chem. 2001;66:9005. doi: 10.1021/jo016105h.Galiano-Roth AS, Michaelides EM, Collum DB. J. Am. Chem. Soc. 1988;110:2658.Reich HJ, Goldenberg WS, Gudmundsson BÖ, Sanders AW, Kulicke KJ, Simon K, Guzei IA. J. Am. Chem. Soc. 2001;123:8067. doi: 10.1021/ja010489b.Gilchrist JH, Harrison AT, Fuller DJ, Collum DB. J. Am. Chem. Soc. 1990;112:4069.Hoffmann D, Collum DB. J. Am. Chem. Soc. 1998;120:5810.Fraenkel G, Henrichs M, Hewitt M, Su BM. J. Am. Chem. Soc. 1984;106:255.Novak DP, Brown TL. J. Am. Chem. Soc. 1972;94:3793.Desjardins S, Flinois K, Oulyadi H, Davoust D, Giessner-Prettre C, Parisel O, Maddaluno J. Organometallics. 2003;22:4090.Günther H. In: Advanced Applications of NMR to Organometallic Chemistry. Gielen M, Willem R, Wrackmeyer B, editors. Wiley & Sons; New York: 1996. pp. 247–290.Weingarten H, Van Wazer JR. J. Am. Chem. Soc. 1965;87:724.Góralski P, Legoff D, Chabanel M. J. Organomet. Chem. 1993;456:1.
- 30.Hydrocarbon effects on chemical shift, aggregation state, and reactivity of organolithiums can be pronounced. For examples and leading references, see Godenschwager PF, Collum DB. J. Am. Chem. Soc. 2007;129:12023. doi: 10.1021/ja074018m.
- 31.Kofron WG, Baclawski LM. J. Org. Chem. 1976;41:1879. [Google Scholar]
- 32.Romesberg FE, Bernstein MP, Gilchrist JH, Harrison AT, Fuller DJ, Collum DB. J. Am. Chem. Soc. 1993;115:3475. [Google Scholar]
- 33.Podlech J, Seebach D. Liebigs Ann. 1995:1217. [Google Scholar]
- 34(a).Davis FA, Reddy RE, Szewczyk JM. J. Org. Chem. 1995;60:7037. [Google Scholar]; (b) Davis FA, Reddy RE, Szewczyk JM, Reddy GV, Portonovo PS, Zhang H, Fanelli D, Reddy RT, Zhou P, Carroll PJ. J. Org. Chem. 1997;62:2555. doi: 10.1021/jo970077e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.