Abstract
Purpose
A role for γδ T cells in immunoregulation has been shown in a number of studies, but in the absence of infection or induced disease, mice lacking γδ T cells generally appear to be healthy. However, we report here that certain mice lacking γδ T cells often spontaneously develop keratitis, characterized by a progressive and destructive inflammation of the cornea.
Methods
The keratitis developing in these mice was characterized in terms of prevalence in males vs. females, age of onset, and histological features. Attempts were made to understand the underlying causes of the disease by removing αβ T cells, altering sex hormones, and reconstituting γδ T cells.
Results
The development of keratitis in these mice depends upon the C57BL/10 genetic background, and is much more common among females than males. The incidence of the disease increases with age, exceeding 80% in females greater than 18 weeks old. We present evidence that the keratitis in these mice is at least partly autoimmune in nature, and that despite its prevalence in females, male hormones do not protect against the disease.
Conclusions
These findings indicate an important role for γδ T cells in maintaining immune balance in the eye. The mice described in this study represent a potential new small animal model of keratitis.
Introduction
γδ T cell function is not well-understood, and several hypotheses have been put forth to explain the role of these cells [reviewed in1]. Of these roles, two have enjoyed fairly wide acceptance. The first of these, that γδ T cells “bridge the gap” between innate and adaptive immunity, fits in well with the non-random distribution of these cells in epithelial sites, at the junction between the exterior and physiologic interior. The second hypothesis is that γδ T cells play an immunoregulatory role. There is a considerable body of evidence for this, and a number of reports indicate that distinct TCR-defined γδ T cells play particular immunoregulatory roles. Because αβ T cells comprise several functional types, it is not difficult to envision that for γδ T cells, both hypotheses are in fact correct. Reports showing that mice lacking γδ T cells (TCRδ−/− mice) are more susceptible to certain pathogens support the first hypohesis2–7, whereas other reports showing exaggerated inflammatory responses in the absence of γδ T cells support the second2, 8–14. However, one might expect that if γδ T cells are important in regulating immune responses, spontaneous autoimmunity might sometimes arise in TCRδ−/− mice. Mice on the FVB background have indeed been previously reported to develop a spontaneous dermatitis12, although this appears to be the only published example so far of unelicited autoimmunity in TCRδ−/− mice. Here, we report a second example: TCRδ−/− mice having the C57BL/10 background (B10.TCRδ−/−) frequently develop a spontaneous inflammation in the cornea of the eye (keratitis). This disease appears to arise at least partially from autoimmune mechanisms, and is substantially more prevalent in females than in males, affecting about 80% of females by 18 weeks of age. A low frequency of spontaneous keratitis was also noted in wildtype C57BL/10 (B10) females. We hypothesize that immune balance in the cornea of the eye is partially maintained by regulatory T cells of the γδ type, and that their absence can increase the susceptibility of the eye to autoimmune attack.
Materials and Methods
Mice
C57Bl/10J (B10) mice, C57BL/6J (B6) mice, B6 background mice with an inactivating mutation introduced into the TCR-Cδ gene [B6.TCRδ−/− mice8, 15], and B6 background mice with an inactivating mutation introduced into TCR-Cβ gene [B6.TCRβ−/− mice16] were originally obtained as breeding stock from the Jackson Laboratories (Bar Harbor, ME), and maintained in our facility under SPF conditions. The B10.TCRδ−/− strain was then established by crossing a B6.TCRδ−/− mouse with a B10 mouse, followed by 10 backcrosses onto the B10 background. Individuals to breed for the next generation were identified as those bearing the defective TCR-Cδ gene, as determined by Southern blotting of DNA from peripheral blood leukocytes, following digestion of the DNA with Hind III, and detecting the mutant gene with a probe for the neomycin resistance gene15. After the tenth backcross, mice homozygous for the mutant Cδ gene were established by intercrossing individuals heterozygous for the mutant allele, and offspring unable to produce any γδ T cells were identified by flow cytometry of blood T cells. A new line was established from these TCR-Cδ−/− homozygous individuals, and has been maintained in our facility for about 7 years by brother/sister mating. The B10.TCRβ−/− mice were established in a similar fashion, screening for the mutant Cβ gene by Southern blotting of DNA from peripheral blood leukocytes, which had been digested with Hind III, detecting the mutant Cβ using a probe for the neomycin resistance gene17. This strain was also established as a homozygous line following the tenth backcross, and has been maintained for about 6 years. B10.TCRβ−/−δ−/− mice were generated by crossing the B10.TCRδ−/− strain with B10.TCRβ−/− to generate F1 offspring, then intercrossing the F1 mice to create F2 offspring. F2 mice which produced neither αβ nor γδ T cells were identified by flow cytometry of purified peripheral blood T cells, and used to establish the new strain, which has been maintained in our facility by brother/sister mating for about 3 years. The studies described in this paper were reviewed and approved by the National Jewish Institutional Animal Care and Use Committee, and we have adhered to guidelines in the AVRO Animal Statement.
Histology
Mice were sacrificed by CO2 inhalation, and the eyes excised and fixed in 10% neutral-buffered formalin, then paraffin-embedded. Sections (5 µm) were made of each paraffin block at a variety of levels, and were stained with hematoxylin/eosin. Some sections were also stained with Gomori’s silver stain or with Gram’s stain in an attempt to reveal any infectious fungi, or Gram-positive or -negative bacteria, respectively, that might be present (not shown).
Immunofluorescence
Corneas were frozen in OCT compound, as previously described for lung tissue18, and acetone-dehydrated 10 µm sections stained with a pan-reactive anti-TCRβ monoclonal antibody (H57-59719) plus an anti-hamster immunoglobulin biotin-conjugated antibody (Jackson ImmunoResearch, cat. no. 127-065-160) and Cy3-conjugated streptavidin (Jackson ImmunoResearch, cat. no. 016-160-084), together with either anti-CD4 (clone GK1.520) or anti-CD8α (clone 53.6.721) monoclonal antibodies plus an anti-rat immunoglobulin Cy5-conjugated F(ab’)2 antibody (Jackson ImmunoResearch, cat. no. 712-176-153).
Flow cytometry
Two-color flow cytometry was carried on nylon-wool purified spleen or peripheral blood cells as previously described22. Before staining, cells were pre-blocked with 5–10 µg/ml of a monoclonal antibody against Fc receptors to reduce non-specific staining [2.4G223]. Monoclonal antibodies grown, purified, labeled in our own laboratory and used for flow cytometry include anti-Cδ FITC [GL324], anti-Cβ FITC [H57-59719], and anti-CD3-Biotin [KT325]. Streptavidin-PE (Biosource, Camarillo, CA) was used as a secondary reagent to detect the biotinylated antibody. Samples were analyzed on either a FACSCAN or FACSCalibur flow cytometer and the data processed using FlowJo software.
αβ TCR depletion
B10.TCRδ−/− female mice at 6–7 weeks of age were treated with 100 µg of anti-αβ TCR monoclonal antibody [H57-59719], diluted in Hanks BSS, by i.v. injection in 0.2cc. This antibody was grown and purified in our laboratory using standard methods. The antibody was re-injected every 6–8 days until the mice reached 18 weeks of age. Control mice were treated in the same way using an equivalent amount of purified Syrian hamster gamma globulin (Jackson ImmunoResearch, West Grove, PA; Cat. No. 007-000-002). The mice were monitored by gross observation every 2 weeks for keratitis, and scored as described in Results.
Cyclosporin A treatment
Cyclosporin A (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO at a concentration of 7.5 mg/ml, and 0.1 cc injected intraperitoneally every 3–4 days, in B10.TCRδ−/− females, starting at 6 weeks of age. Sham-treated controls were similarly injected at the same time but with DMSO only. The treatment was continued until the mice reached 16 weeks of age.
Adoptive T cell transfer
Splenic T cells were purified from adult (6 weeks old or greater) female B10 mice. Briefly, cells were dispersed by passage through steel mesh screens, and washed once in Iscove's Complete Tumor Medium26 containing 5% FBS. The erythrocytes were then lysed with Gey’s solution, and the cells washed again, and passed over a nylon wool column27 to enrich for T cells. To eliminate αβ T cells, most NK cells, and B cells, the cells were next treated with a cocktail of biotinylated monoclonal antibodies specific for the αβ-TCR [H57-597 (16)], NK1.1 (PK136, BD Pharmingen), and CD19 (1D3, BD Pharmingen), washed, and then incubated with streptavidin-conjugated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), in accordance with the manufacturer’s recommendations. After washing, the cells were passed over an LD column (Miltenyi Biotec, Bergisch Gladbach, Germany) and the nonadherent population (enriched for γβ T cells) collected. A small aliquot of the purified γβ T cells was then stained with anti-CD3 [KT3-biotin25] and anti-Cδ [GL3-FITC28] monoclonal antibodies plus PE-streptavidin to determine purity by flow cytometry (generally 70–80%), and the remainder resuspended in Hank’s BSS plus 5% heat-inactivated FBS and injected intravenously into 6–8 week old female B10.TCRδ−/− mice, using 0.5–1.0 × 105 cells/mouse. Sham-treated matched controls were injected with Hank’s BSS plus 5% heat-inactivated FBS only. Adoptively-transferred mice were scored for keratitis on alternate weeks until 18–19 weeks old.
Orchiectomy
B10.TCRδ−/− male mice were orchiectomized at 3–4 weeks of age, before weaning and prior to the descent of the testes. Mice were anesthetized by i.p. injection of 1.25% tribromoethanol (Avertin, Sigma-Aldrich Co., St. Louis, MO) in PBS, using about 25 µl per gram of body weight, to keep the mouse anesthetized for about 30 minutes. Abdominal hair was shaved, and the shaved area swabbed with 70% ethanol. A lateral incision about 0.5 cm wide was then made through the skin of the lower abdomen, followed by a slightly smaller incision through the peritoneal membrane. Each testis/epididymis was then located with forceps and exposed. Both testes were tied off together with a resorbable suture (Dexon II 4-0), and excised by cutting above the suture knot with scissors. The peritoneum was next closed with 1–2 sutures, and the abdominal skin with 2–3 sutures. Finally, antibiotic ointment (Neosporin) was applied with a sterile swab to the sutured skin. While the mice were still unconscious, a time-release pellet containing either 17-β estradiol (Innovative Research of America, Sarasota, FL; Cat. No. NE-121, 1.7 mg 90-day release pellets) or a placebo pellet (Cat. No. NC-111, containing the same biodegradable carrier-binder) was also implanted under the skin at the dorsal neck area of each mouse, using a 10-gauge precision trochar. Following pellet implantation, mice were wrapped in gauze and placed under a warming lamp until they regained consciousness. During the recovery period (approximately 15 minutes), 0.2–0.4 cc of sterile saline was injected i.p. to prevent dehydration. After regaining consciousness, the mice were placed in a clean cage and restored to their mothers, and thereafter were weighed daily to verify that they were healing properly; a weight gain was expected by the second day post-surgery. Any mice that failed to show a weight gain by day 3 were euthanized. Antibiotic ointment was applied daily to the surgical site at the time of weighing for 5–7 days, after which time the mice were weaned from their mothers and no further treatment was provided. Starting at 6–7 weeks of age, the mice were monitored by gross observation every 2 weeks for keratitis, and scored as described in Results.
Microsatellite mapping
B10.TCRδ−/− × B6.TCRδ−/− F2 females were generated, and tail clips obtained from each when the mice were weaned. DNA was purified from the tail clips as follows: ~1 cm-long tail clips were placed in 0.5 ml of digestion buffer (10 mM Tris pH 7.5, 400 mM NaCl, 100 mM EDTA, 0.6% SDS, 800 µg/ml Proteinase K) in a 1.5 ml microcentrifuge tube and incubated overnight at 56°C. 150 µl of 6M NaCl (saturated) was then added to each sample and the tubes shaken vigorously by hand for approximately 30 seconds. The samples were then centrifuged at room temperature at top speed in a microcentrifuge, and 0.5 ml of the supernatant transferred to a new tube containing 750 µl of 95% ethanol. The tubes were then inverted gently by hand and the precipitated DNA lumps transferred to a new tube containing 200 µl of TE (10 mM Tris ph 7.5 + 1 mM EDTA), using a small pipetteman tip. The samples were placed at 37°C for several hours, and shaken by hand a few times to rehydrate the DNA. The concentration of DNA in each sample was then determined by optical density at 260 nm with a spectrophotometer. The DNA samples from each mouse were tested by PCR to amplify a chromosome 4 or 13 microsatellite marker whose size in B10 differs from that in B6 (D4Mit157 and D13Mit115; ResGen, InVitrogen Corp., Frederick, MD), using ~6 ng of DNA in each 20 µl reaction, plus 0.6 µl of each microsatellite primer (6.6µM), and PCR conditions as recommended by InVitrogen. All results were verified by repeating each PCR at least once. The F2 mice that provided each DNA sample were scored by gross observation on alternate weeks for keratitis until they reached 18–19 weeks of age.
Statistics
Fisher’s Exact Test for analysis of a 2 × 2 contingency table (one-tailed) was used to calculate p-values, using GraphPad Prism software. As indicated in Fig. 3, the significance of experimental values obtained was in some cases determined by comparison with historical controls.
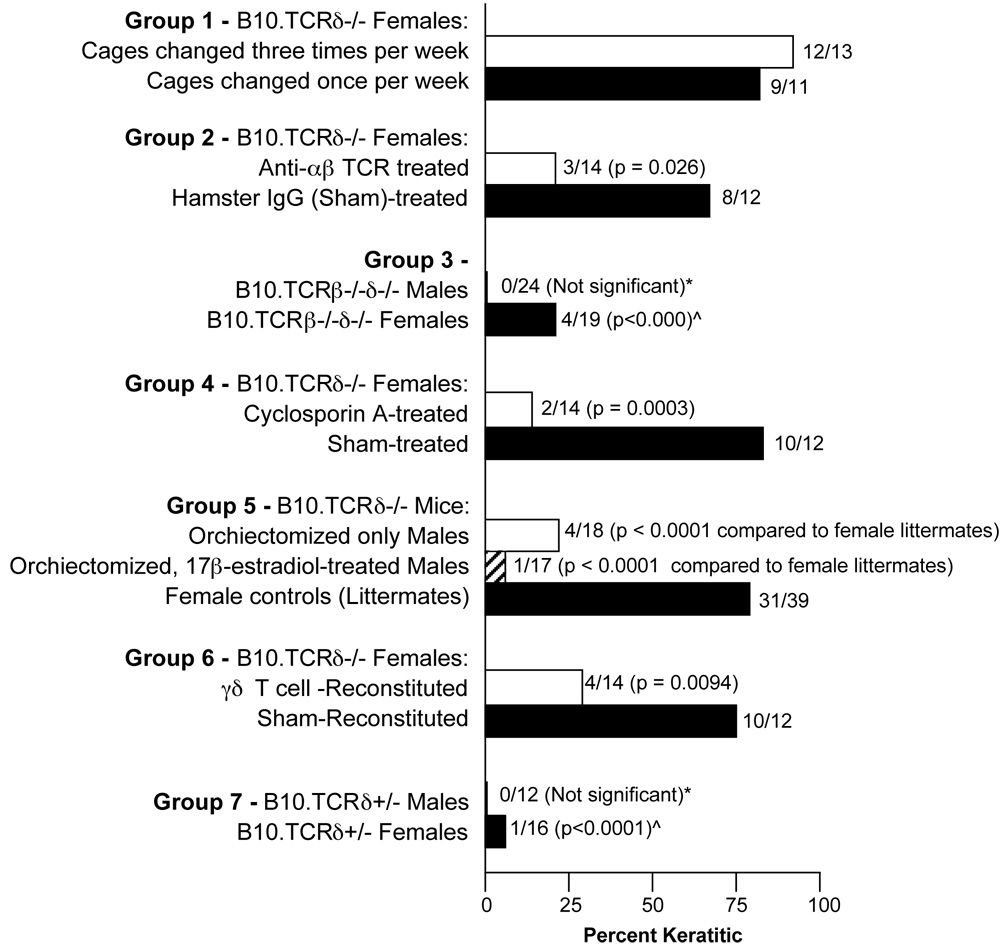
Fig. 3.
Keratitis frequency in B10-background mice. Bar graphs indicate percentage of mice in each category that had developed keratitis by 18–19 weeks of age, except for Group 4, in which mice were instead assessed for keratitis slightly earlier, at 15–16 weeks of age, due to the death of several mice. The actual number of mice with keratitis is indicated as a fraction of the total number examined for each category, shown next to each bar. (Note: in Group 4, one cyclosporin A-treated mouse developed keratitis early on but was clear of discernible disease after several weeks of Cyclosporin A treatment. This mouse was still counted as keratitic.) *Compared to B10.TCRδ−/− male historical controls, of which 5/35 were keratitic. ^Compared to B10.TCRδ−/− female historical controls, of which 29/36 were keratitic.
Results
Mice lacking γδ T cells develop spontaneous keratitis
A new mouse strain incapable of producing γδ T cells, due to insertion of the neomycin resistance gene into a constant region gene for TCR-δ15, was generated by backcrossing C57BL/6-background mice carrying this mutation (B6.TCRδ−/− mice) 10 times onto the C57BL/10J (B10) background. Though this new line (B10.TCRδ−/−) appeared to be generally healthy, a high frequency of opaque eyes was noted among adult females (Fig. 1A). Surprisingly, neither female nor male B6.TCRδ−/− mice raised in the same facility under the equivalent conditions showed the same tendency (however, mice having eyes with a similar appearance also were observed to be frequent among older female B10.TCRβ−/− mice; these mice are being examined as part of a separate study, currently in progress). Mice with opaque or cloudy eyes were more rarely noted among wildtype B10 females, and occasionally among B10.TCRδ−/− males (Fig. 1B), but were virtually absent among B10 males and either male or female B6 mice (not shown). A histological examination of the opaque eyes of these mice revealed a mild to severe inflammation in the cornea (keratitis). Infiltrates in the corneal stroma, particularly the portion proximal to the corneal epithelium (Fig. 1C), were evident, along with the presence of blood vessels in this normally avascular tissue, and in some cases, a hypertrophy of the epithelial layer (Fig. 1C5), often grossly visible as a white thickening in the center of the eye. The infiltrating inflammatory cells included a high frequency of granulocytes in the inflamed corneal stroma (Fig. 1D). Many CD4+ and some CD8+ αβ T cells were also evident by immunofluorescent staining of frozen sections from keratitic corneas (Fig. 1E, 1F).
Fig. 1.
A, B10.TCRδ−/− female mice frequently develop corneal opacities. Examples of B10.TCRδ−/− females with varying degrees of corneal opacity are shown, along with an unaffected eye for comparison (upper left panel). B, Overall percentage of mice with keratitis among different strains, determined by gross examination. Mice of mixed ages, all 7 weeks of age or older, were included. Total numbers of males (M) and females (F) that were examined are indicated for each strain. C, Hematoxylin/eosin stained sections of corneas from mice with opaque eyes. C1, Low magnification to show the overall structure of a normal B10 mouse eye. C2, Higher magnification of the normal cornea shown in C1. C3, Cornea of a B10.TCRδ−/− mouse showing slight opacity and a reddish cast to the eye. C4, Cornea of a B10.TCRδ−/− mouse with slightly greater corneal opacity and a reddish eye. C5, Cornea of a B10.TCRδ−/− mouse with severe opacity and central thickening, due to a hypertrophied corneal epithelium. C6, From a severely keratitic eye; the outer epithelium appears to have been sloughed (note: C2–C6 are at the same magnification). D, High magnification of the cornea from a severely keratitic eye. E, Immunofluorescent staining of frozen section of keratitic B10.TCRδ−/− cornea stained with anti-TCRβ (red) and anti-CD4 (blue); double positive CD4 αβ T cells are pink. F, Immunofluorescent staining of frozen section of keratitic B10.TCRδ−/− cornea stained with anti-TCRβ (red) and anti-CD8α (blue); double positive CD8 αβ T cells appear pink. In both E and F, the autofluorescent background (green) has been deliberately enhanced to reveal the tissue structure.
Differences in the eye disease of B10 background mice
When mice were examined periodically to look for the onset of disease, differences between B10.TCRδ−/− and wildtype B10 females were noted. As can be seen (Fig. 2a), in B10.TCRδ−/− mice, the disease develops at various timepoints in individual females, such that about half of the mice have obvious keratitis by 9–10 weeks of age. The incidence increases with age, reaching ~80% by 18 weeks. In contrast, B10 wildtype females appear to most commonly develop the disease early on, by 7 weeks of age, and show no apparent increase over time, such that keratitis remains rare among B10 females even as they grow older. A scoring system was established to follow the disease (Fig. 2B), based on a system previously used to score herpes simplex virus-induced keratitis29; each eye is scored between 0 and 5, such that the maximum total score possible is 10. Total scores over time for three different cohorts of B10.TCRδ−/− age-matched females individuals are shown in Fig. 2C, each raised at a different time in our facility. Small differences observed between groups examined at distinct times may indicate that there is an environmental influence on the likelihood of disease onset as well. We tested whether this might be the result of differences in the frequency of cage changing, but this did not appear to influence either the disease incidence (Fig. 3, Group 1) or severity (not shown). In mice that received a score of 5 for a single eye, the inflammation was sometimes found to have spread to areas beyond the cornea. In some such mice, the eye appeared to be phthisical, with globe atrophy, and in a few cases was virtually absent.
Fig. 2.
A, Incidence of keratitis as a function of age in B10.TCRδ−/− vs. wildtype B10 females. Mice from 4–7 different litters were examined at the indicated ages over the course of one year. B, Severity scoring system. C, Cohorts of female B10.TCRδ−/− littermates were scored for severity of keratitis every 2 weeks from 6 to 18 weeks of age; each group represents a cohort raised at one particular time. Each symbol represents an individual mouse. The total number of individuals in each cohort group is indicated in parentheses. The maximum possible score for an individual mouse is 10.
Keratitis in B10.TCRδ−/− females appears to be due to an autoimmune process
Several features of the keratitis in B10TCRδ−/− mice suggests that it may be of autoimmune origin. The disease develops quite slowly, and often a mouse housed in the same cage with others that have severe keratitis never develops disease (e.g., see Fig. 2C). Moreover, whereas most mice eventually develop keratitis in both eyes, it is not unusual to find that only one eye is affected in a given mouse, even after many weeks of severe inflammation in the other eye. Also, adding a broad-spectrum antibacterial drug (Septra) to the drinking water of B10.TCRδ−/− mice had no effect on either the induction or progression of keratitis, and did not reduce either the incidence or severity (not shown). Furthermore, we were unable to identify any bacteria or fungi in histological sections, whether stained with hematoxylin/eosin (e.g. see Fig. 1D), or with Gomori’s silver stain to reveal fungi or a tissue Gram’s stain to reveal Gram-positive or -negative bacteria (data not shown). CD4+ αβ T cells appear to be critically important to the development of autoimmunity in a number of different systems [reviewed in30], and were well-represented among the infiltrating cells in keratic corneas (Fig. 1E). If the keratitis that develops in B10.TCRδ−/− mice is indeed mediated by autoimmune mechanisms, αβ T cells are then likely to be involved in the pathology, but would in contrast be expected to have a beneficial effect if the disease is instead brought on by an infectious agent. To test for a role for αβ T cells, we depleted B10.TCRδ−/− mice of αβ TCR expression by administering a pan-reactive anti-αβ TCR monoclonal antibody weekly for weeks; this reduced the percentage of cells staining brightly with anti-CD3 to about 1% of the level seen in sham-treated controls, although cells with very low TCR levels were still present (Fig. 4A), and reduced the total number of splenic CD4+ or CD8+ T cells by 5–6 fold, as compared to sham-treated controls (Fig. 4B). As summarized in Fig. 3 (group 2), this also greatly reduced the incidence of keratitis in these mice compared to sham-treated controls. To rule out any non-specific effects resulting from antibody-mediated depletion, we also generated B10.TCRβ−/−δ−/− mice, B10 background mice incapable of producing either αβ or γδ T cells. By 18 weeks of age, the females of this strain had a substantially lower incidence of keratitis (21%; Fig. 3, Group 3) than do B10.TCRδ−/− females (in which the incidence is approximately 80% by 18 weeks). The males of this “double knockout” strain also appeared to be slightly less susceptible to developing keratitis (0%) than are B10.TCRδ−/− males (~15%), although the difference was not significant. These findings indicate a pathological role for αβ T cells in the development of keratitis in B10.TCRδ−/− mice.
Fig. 4.
A, Long-term treatment of B10.TCRδ−/− mice with anti-TCRαβ monoclonal antibody depletes 98–99% of splenic αβ TCRs. Each panel shows flow cytometric profiles of nylon-wool purified spleen T cells from three B10.TCRδ−/− individual females. Histograms with thick lines show results using an anti-CD3 mAb to stain cells from each individual; the thin line histograms show unstained cells as a negative control. An was chosen to detect the αβ TCR because it would not be inhibited by any remaining anti-TCRαβ antibody which might still be bound to the cells. Left panel - Results from mice treated with anti-TCRαβ monoclonal antibody weekly for 12 weeks. Spleens were taken 3 days after the final antibody treatment, and the cells stained with anti-CD3 antibody to reveal any remaining αβ TCR+ cells. Right panel - Examples of spleen T cells from three B10.TCRδ−/− individual females sham-treated weekly at the same time with hamster IgG, prepared and stained in the same way. B, the average number of αβ T cells obtained from the spleen of each mouse shown in panel A was calculated based on the percentages that expressed CD4 or CD8.
Cyclosporin A is a commonly used immunosuppressive drug that works by inhibiting calcineurin, which, via NFAT dephosphorylation, plays an essential role in inducing the expression of cytokine genes needed for T cell proliferation 31, such as IL-2, IL-4, and IL-12. Cyclosporin A has been successfully used to treat autoimmune disease in humans, including eye inflammation in Sjogren’s syndrome 32, and also certain autoimmune diseases induced experimentally in mice [e g. type 1 diabetes 33 and systemic lupus 34]. We therefore tested whether cyclosporin A administration could reduce the development of keratitis in B10.TCRδ−/− females. Although a low dose (~10 mg/kg, not shown) had no effect, injecting 30 mg/kg twice weekly substantially reduced the disease incidence (Fig. 3, Group 4). This strongly implicates T-cell mediated immune mechanisms in the development of keratitis in these mice.
Anti-nuclear antibodies (ANA) are commonly found in individuals with some but not all autoimmune diseases, including systemic lupus, scleroderma, Sjogren’s syndrome, and polymyositis35. However, when we analyzed serum from B10.TCRδ−/− keratitic females for the presence of this autoantibody type using a commercial ELISA kit, ANA levels were very low or absent (data not shown).
The greater prevalence of keratitis in female vs. male B10.TCRδ−/− mice is not due to a lack of male hormones
In the majority of autoimmune diseases common in humans, females are substantially more susceptible than are males, including rheumatoid arthritis, systemic lupus, scleroderma, Sjogren’s syndrome, and multiple sclerosis36. It has been postulated that this may be due to the ability of estrogens to boost cytokine production. Alternatively, in a recent report, male hormones were found to increase the levels of peroxisome proliferator–activated receptor (PPAR)α expression, a nuclear receptor involved in the control of inflammatory responses, and PPARα expression was implicated in susceptibility to experimental autoimmune encephalomyelitis in mice37. To test whether male vs. female hormones can explain the greater prevalence of keratitis in B10.TCRδ−/− females, we attempted to “feminize” the male mice by orchiectomizing them at the age of weaning, and treating them with 17β-estradiol. As can be seen (Fig. 3, Group 5), this was not effective, and although the sisters of the treated males raised at the same time in adjacent cages developed keratitis at a high rate, very few of the treated males did. Instead, whether orchiectomized only, or both orchiectomized and 17β-estradiol-treated, the “feminized” males developed keratitis at about the same rate as untreated males (~17%; see Fig. 1B). Though this does not rule out a role for female hormones in keratitis induction, it does imply that higher androgen levels are not responsible for the decreased disease incidence in males.
Repletion of B10.TCRδ−/− females with normal γδ T cells reduces the incidence and severity of keratitis
Although it seems likely that the genetic inactivation of the Cδ gene, because it renders these mice unable to produce γδ T cells, is responsible for the development of keratitis in B10.TCRδ−/− mice, at least one other possibility exists: that another unknown gene on chromosome 14, closely linked to the Cδ gene, which was carried along in the backcross, is actually responsible for the disease. To test this, we therefore attempted to replete B10.TCRδ−/− females with adult female B10-derived γδ T cells, as they neared the age at which keratitis typically begins to develop (7–9 weeks). This indeed reduced the incidence of keratitis substantially (Fig. 3, Group 6), supporting the interpretation that the lack of γδ T cells in B10.TCRδ−/− female mice is the defect that leads to spontaneous keratitis.
However, the disease incidence in B10.TCRδ−/− mice which received adoptively transferred γδ T cells was still higher than 15%, the expected frequency in normal B10 females by 18 weeks of age. Because the disease appears to develop in a manner dependent upon stochastic processes in individual mice, the timing of the repletion of γδ T cells may be critical to whether or not they will be effective. We have previously found that in B6 mice lacking γδ T cells, simply injecting γδ T cells will not reconstitute the mice because the transferred cells disappear within a few days38. However, B6 mice lacking both αβ and γδ T cells allow for the homeostatic expansion of adoptively transferred γδ T cells, because in these hosts, γδ T cells are not outcompeted by endogenous αβ T cells as in TCRδ−/− mice. Retention of adoptively transferred γδ T cells in TCRδ−/− mice that have ongoing inflammation has been more successful, presumably because antigens for the γδ TCR are present, as well as cytokines that stimulate γδ T cell growth and survival38. Because we cannot tell when a mouse is just beginning to develop keratitis, we can only aim for a time near the average age of onset, and thus can probably expect at best to achieve a partial reduction in the overall incidence. Among the adoptively transferred B10.TCRδ−/− females in this experiment that did develop keratitis, the severity was not noticeably less than in sham-treated controls (not shown). The transferred γδ T cells may have disappeared from mice that later went on to develop keratitis, and indeed, we could not detect γδ T cells in the spleens of any of the adoptively transferred mice when the experiment was terminated at 18–19 weeks of age, by flow cytometry (data not shown).
In B10.TCRδ+/− mice, the number of γδ T cells drops to about half of the normal number (Fig. 5). We wondered whether such a reduction would be sufficient to induce keratitis. However, the incidence of keratitis in female B10.TCRδ+/− mice was low (6%; Fig. 3, Group 7), close to the level we observed among B10 wildtype females (10–15%), indicating that the reduced number of γδ T cells in TCRδ-heterozygous B10-background females is sufficient to prevent disease.
Fig. 5.
Examples of FACS profiles of TCRδ+/+ (top row) vs. TCRδ+/− (bottom row) mice. Nylon-wool enriched peripheral blood cells, each from one individual, were analyzed by twocolor staining for γδ T cells; the frequency as a percentage of the total CD3+ cells is indicated.
Keratitis-promoting genes from the B10 background appear to be neither chromosome-4 nor -13 encoded
Because B10.TCRδ−/− mice are keratitis-susceptible whereas B6.TCRδ−/− mice are not, a gene (or genes) from the B10 background likely is responsible for the tendency of B10.TCRδ−/− mice to develop keratitis. The B10 and B6 strains are very closely related, however. The two largest known differences between these two strains include chromosome 439, 40, which differs at a locus that contains several genes with obvious immunological roles [including interferon-α, Lck, stathmin 1, complement component C1qC, and tumor necrosis factor receptor 2 p7539], and chromosome 13. where two loci have been found to differ between these two strains41. One of the chromosome 13 loci, the TCR-γ locus, can likely be ruled out as having anything to do with keratitis susceptibility, because B10.TCRδ−/− mice cannot in any case produce γδ T cells. However, the other chromosome 13 locus is of interest, as it has been shown to accelerate the incidence of lupus in MHC Class II transgenic mice42. We therefore investigated whether or not inheritance of a B10-derived chromosome 4 or 13 could be correlated with keratitis susceptibility in mixed B10/B6 background mice lacking γδ T cells. To generate these, we crossed B10.TCRδ−/− mice with B6.TCRδ−/− mice to generate F2 offspring. Because the incidence of keratitis is much higher in females, we scored female F2 mice only for keratitis until they reached 18–19 weeks of age. We then looked for correlations between the source of chromosome 4 and/or chromosome 13 (B6 or B10) and the development of keratitis. If only a single (homozygous) gene is needed to confer a keratitic tendency, we expected to see more keratitis in those mice carrying B10 vs. B6 type chromosomes which encode that gene, assuming that crossover events are rare. However, among the 61 F2 females analyzed, no correlation between the inheritance of the B10 version of either chromosome and the development of keratitis was evident (Fig. 6). This may indicate that instead, more than one B10-derived gene is required, or that a gene encoded elsewhere is critical. B6 and B10 also differ in regions of chromosomes 2, 11, and 16 as defined by microsatellites 40, so it is possible that a gene near one of these loci is in fact the critical one.
Fig. 6.
Genotypes for chromosome 4 (gray bars) and chromosome 13 (striped bars) of individual B10.TCRδ−/− × B6.TCRδ−/− F2 females. Results for the 6 F2 females only are shown in the left panel; no association between the development of keratitis and inheritance of a particular genotype is evident for either chromosome. Genotypes of all 61 F2 females obtained are shown in the right panel; for each genotype - B10/B10, B10/B6, and B6/B6 - frequencies close to the predicted Mendelian ratios of 1:2:1 were observed.
Discussion
A study published in 1986 showed that different strains of mice will develop “cloudy” eyes if maintained in dirty cages43. This opacity was found to involve inflammation of the corneal epithelium and stroma, with stromal vascularization. Strain differences in the frequency of opaque eyes were noted in this study, with levels as high as 30% in DBA/2 mice. However, for all strains examined, changing the cages frequently greatly reduced the incidence, sometimes to 0%, and the authors suggested that ammonia from dirty cage litter might damage the eyes and initiate the condition. Frequent cage changes (weekly) are standard protocol in our facility, and increasing this to three times weekly did not affect the disease incidence or severity. Moreover, the keratitis we see in B10.TCRδ−/− mice differs from this previous study in the greater severity of the disease, its exceptionally high incidence, and its prevalence in females. However, the highly variable onset of keratitis among individuals of this strain may indicate that there must first be an initiating event for keratitis to develop, although this is not likely to be chemical damage due to the presence of ammonia. For example, a scratch on the cornea at some point during the life of the mouse might initiate keratitis, but we have yet to test this. Although treating B10.TCRδ−/− mice with a broad-spectrum anti-bacterial agent reduced neither the incidence nor severity of the keratitis (not shown), it is also possible that infection with bacteria or some other microbial agent instead plays a role in initiating or promoting keratitis in these mice.
Curiously, B10.TCRδ−/− mice appear to be very “quiet” with this eye disease - they have not been observed pawing or scratching at their diseased eyes, no excess tearing has been evident, and the mice do not appear to be in distress, because when bred, they instead are exceptionally reliable mothers, often raising large litters even after the keratitis has progressed so far that they are effectively blind. This is quite surprising because human patients with keratitis generally find it a painful and distracting condition. Although we did not find evidence of inflammation at other sites in decalcified sections of heads of B10.TCRδ−/− keratitic females (including the lacrimal glands and sebaceous glands; not shown), except in instances in which the inflammation appears to have to have spread to other parts of the eye following corneal rupture, we conjecture that there may also be some nerve destruction that makes the disease relatively painless. The keratitis that develops in B10.TCRδ−/− mice in some ways resembles the pathology seen in diabetes mellitus patients that develop keratitis often along with neuropathies in the eye44, and in pannus, a chronic superficial keratitis that develops as a immune-mediated disease in genetically predisposed dogs45, and might prove useful as a model of these diseases.
The eye is critical for long-term survival in most animals, and for this reason, mechanisms have evolved to keep inflammatory responses that could interfere with vision at a minimum. The eye maintains its “immune privileged” status in several different ways [reviewed in46]. In part, access of peripheral immune cells to the eye is limited by the maintenance of a “blood/ocular barrier,” established by a lack of blood vessels in the normal cornea and by tight junctions in the retinal epithelium. In addition, the presentation of antigens in the eye is limited by the maintenance of resident dendritic cells in the eye in an immature state, and by the lack of lymphatic drainage from the eye. Moreover, high levels of local immunosuppressive factors can be found in the eye, consisting of both cell-bound (complement inhibitors and FasL) and soluble (TGF-β, TSP, PGE2) components. These immune-suppressive mechanisms are thought to bring about the phenomenon of ACAID (anterior chamber-associated immune deviation), nonresponsiveness to antigens which have been experimentally introduced into the anterior chamber of the eye. Instead, the response “deviates” to one of suppression or tolerance. In the last several years, γδ T cells have been found to play a part in immune regulation in the eye. Mice lacking γδ T cells failed to develop ACAID47, 48, and γδ T cells were found to be crucial to corneal graft survival 49. These findings are not surprising in light of the many studies in other systems which suggest that inflammatory or immune regulation is a primary function of γδ T cells [e.g.2, 9–12, 50–55]. Moreover, a recent report showed that γδ T cells predominate among the T cells in the limbal epithelium of eye of the mouse, and are important in neutrophil and platelet recruitment following corneal abrasion56. However, like other lymphocytes, γδ T cells are normally excluded from the cornea, and it seems likely that the lack of this resident population is responsible for the prevalence of keratitis in B10.TCRδ−/− mice. In this study, we found that whereas female B10.TCRδ−/− mice are highly susceptible to the development of autoimmune keratitis, those of the closely related B6.TCRδ−/− strain are not. Thus, depending upon unknown factors, other mechanisms which normally inhibit ocular inflammation may be insufficient to prevent an autoimmune attack on the eye when γδ T cells are absent. Because γδ T cells, like αβ T cells, are themselves not normally present in the cornea, the mechanism by which they prevent the development of keratitis may be indirect, via secretion of soluble factors, or they may act directly to negatively regulate or block corneal infiltration by inflammatory cells, dendritic cells, or αβ T cells recruited to the eye by an inflammatory signal.
Regulatory αβ T cells have been shown to be necessary for the development of ACAID 57. Moreover, a recent study showed that γδ T cells are critical for the development of CD8+ αβ TCR+ regulatory cells in ACAID, and that their ability to produce IL-10 is a necessary component58. We hypothesize that in B10.TCRδ−/− mice, a deficiency in γδ T cell-dependent regulatory T cells allows an autoimmune attack on the cornea to develop. Because B10.TCRβ−/−δ−/− mice are less keratitis-susceptible than B10.TCRδ−/− mice, a lack of regulatory γδ T cells is not sufficient to cause a high incidence of the disease, however. Rather, our results imply that autoaggressive αβ T cells must also be present for keratitis to be prevalent in B10-background mice lacking γδ T cells.
Acknowledgements
Dr. Melanie Gubbles is thanked for her help with orchiectomies, Dr. Rebecca Tucker for her advice on microsatellite PCR and providing the method for preparing tail DNA, Barry Silverstein for mouse photography, and Dr. Deming Sun for valuable advice on experiments.
Support for this project was provided by NIH grants 1R03 EY15840 and 2R01 AI044920 to RLO.
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hayday AC. γδ T cells: A right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Fu Y-X, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, et al. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 3.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Matsuzaki G, Nomoto K. The protective role of T cell receptor Vγ1+ T cells in primary infection with Listeria monocytogenes. Immunol. 1999;96:29–34. doi: 10.1046/j.1365-2567.1999.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore TA, Moore BB, Newstead MW, Standiford TJ. γδ T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 6.Tam S, King DP, Beaman BL. Increase of γδ T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides. Infec & Immun. 2001;69:6165–6171. doi: 10.1128/IAI.69.10.6165-6171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, et al. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 9.Mukasa A, Hioromatsu K, Matsuzaki G, O'Brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- 10.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 11.Skeen MJ, Rix EP, Freeman MM, Ziegler HK. Exaggerated proinflammatory and Th1 responses in the absence of γδ T cells after infection with Listeria monocytogenes. Infec & Immun. 2001;69:7213–7223. doi: 10.1128/IAI.69.12.7213-7223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR. Evidence for the opposing roles of different γδ T cell subsets in macrophage homeostasis. Eur J Immunol. 2006;36:1729–1738. doi: 10.1002/eji.200635959. [DOI] [PubMed] [Google Scholar]
- 14.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, et al. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycininduced lung injury. Inflammation. 2008 doi: 10.1007/s10753-008-9062-6. In press. [DOI] [PubMed] [Google Scholar]
- 15.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, et al. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 16.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Clarke AR, Hooper ML, Tonegawa S. Creation of a large genomic deletion at the T-cell antigen receptor β-subunit locus in mouse embryonic stem cells by gene targeting. Proc Natl Acad Sci USA. 1991;88:3083–3087. doi: 10.1073/pnas.88.8.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, et al. Distribution and leukocyte contacts of γδ T cells in the lung. J Leuk Biol. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 19.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 20.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, et al. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 21.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, et al. Subset-specific, uniform activation of Vγ6/Vδ1+ γδ T cells elicited by inflammation. J Leuk Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 23.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–588. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman T, LeCorre R, Lefrancois L. A T-cell receptor γδ-specific monoclonal antibody detects a Vγ5 region polymorphism. Immunogen. 1992;35:65–68. doi: 10.1007/BF00216631. [DOI] [PubMed] [Google Scholar]
- 25.Tomonari K. A rat antibody against a structure functionally related to the mouse T cell receptor/T3 complex. Immunogen. 1988;28:455–458. doi: 10.1007/BF00355379. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien RL, Fu Y-X, Cranfill R, Dallas A, Reardon C, Lang J, et al. Heat shock protein Hsp-60 reactive γδ cells: A large, diversified T lymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 28.Goodman T, Lefrancois L. Intraepithelial lymphocytes: Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr DJJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on Herpes Simplex Virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasler P. Biological therapies directed against cells in autoimmune disease. Springer Semin Immun. 2006:443–456. doi: 10.1007/s00281-006-0013-8. [DOI] [PubMed] [Google Scholar]
- 31.Weiner DC, Shenolikar S. Analysis of protein phosphorylation. Curr Prot Mol Biol. 2003 18.10-11-18-10.13. [Google Scholar]
- 32.Coaccioli S, Giuliani M, Puxeddu A. The therapy of Sjogren's syndrome: a review. Clin Ter. 2007;158:453–456. [PubMed] [Google Scholar]
- 33.Formby B, Miller N, Garret R, Peterson CM. Effects of Low-Dose cyclosporine prophylaxis in non-obese diabetic mice. J Pharm Exp Therapeut. 1987;241:1106–1111. [PubMed] [Google Scholar]
- 34.Blank M, Ben-Bassat M, Schoenfeld Y. The effect of cyclosporin A on early and late stages of experimental lupus. Arthritis Rheum. 1992 Nov;35(11):1350–1355. doi: 10.1002/art.1780351116. 1992; 35:1350–1355. [DOI] [PubMed] [Google Scholar]
- 35.Kavanaugh A, Tomar R, Reveile J, Solomon DH, HH H. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nculear antigens. Arch Patho Lab Med. 2000;124:71–81. doi: 10.5858/2000-124-0071-GFCUOT. [DOI] [PubMed] [Google Scholar]
- 36.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 37.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, et al. Peroxisome proliferator-activated receptor (PPAR)α expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.French JD, Roark CL, Born WK, O’Brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc Natl Acad Sci USA. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClive PJ, Huang D, Morahan G. C57BL/6 and C57BL/10 inbred mouse strains differ at multiple loci on chromosome 4. Immunogen. 1994;39:286–288. doi: 10.1007/BF00188793. [DOI] [PubMed] [Google Scholar]
- 40.Slingsby JH, Hogarth MB, Simpson E, Walport MJ, Morley BJ. New microsatellite polymorphisms identified between C57BL/6, C57BL/10, and C57BL/KsJ inbred mouse strains. Immunogen. 1996;43:72–75. doi: 10.1007/BF00186607. [DOI] [PubMed] [Google Scholar]
- 41.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogen. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 42.Rozzo SJ, Vyse TJ, K M, Izui S, Kotzin BL. Enhanced susceptibility to lupus contributed from the nonautorimmune C57BL/10 but not C57BL/6, genome. J Immunol. 2000;164:5515–5521. doi: 10.4049/jimmunol.164.10.5515. [DOI] [PubMed] [Google Scholar]
- 43.Van Winkle T, Balk MW. Spontaneous corneal opacities in laboratory mice. Lab Animal Sci. 1986;36:248–255. [PubMed] [Google Scholar]
- 44.Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26:S75–S81. doi: 10.1097/ICO.0b013e31812f6d8e. [DOI] [PubMed] [Google Scholar]
- 45.Nell B, Walde I, Billich A, Vit P, Meingassner JG. The effect of topical pimecrolimus on keratoconjunctivitis sicca and chronic superficial keratitis in dogs: result from an exploratory study. Vet Ophthalmol. 2005;8:39–46. doi: 10.1111/j.1463-5224.2005.04062.x. [DOI] [PubMed] [Google Scholar]
- 46.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leuk Biol. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 47.Xu Y, Kapp JA. γδ T cells in anterior chamber-induced tolerance in CD8+ CTL responses. Invest Ophthalmol Vis Sci. 2002;43:3473–3479. [PubMed] [Google Scholar]
- 48.Xu Y, Kapp JA. γδ T cells are critical for the induction of anterior chamber-associated immune deviation. Immunol. 2001;104:142–148. doi: 10.1046/j.0019-2805.2001.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skelsey ME, Mellon J, Niederkorn JY. γδ T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–4333. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 50.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, et al. T-cell αβ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci, USA. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, et al. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 52.Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 53.Hahn YS, Taube C, Jin N, Takeda K, Park J-W, Wands JM, et al. Vγ4+ γδ T cells regulate allergic airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 54.Dalton JE, Howell G, Pearson J, Scott P, Carding SR. Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol. 2004;173:3660–3667. doi: 10.4049/jimmunol.173.6.3660. [DOI] [PubMed] [Google Scholar]
- 55.Kirby AC, Newton DJ, Carding SR, Kaye PM. Pulmonary dendritic cells and alveolar macrophages are regulated by γδ T cells during the resolution of S. pneumoniae-induced inflammation. J Pathol. 2007;212:29–37. doi: 10.1002/path.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Burns AR, Rumbaut RE, Smith CW. γδ T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epitheial repair after corneal abrasion. Am J Pathol. 2007;171:838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunol. 1990;71:383–389. [PMC free article] [PubMed] [Google Scholar]
- 58.Ashour HM, Niederkorn JY. γδ T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J Immunol. 2006;177:8331–8337. doi: 10.4049/jimmunol.177.12.8331. [DOI] [PubMed] [Google Scholar]