Abstract
Clinical studies have revealed that the D166V mutation in the ventricular myosin regulatory light chain (RLC) can cause a malignant phenotype of familial hypertrophic cardiomyopathy (FHC). It has been proposed that RLC induced FHC in the heart originates at the level of the myosin cross-bridge due to alterations in the rates of cross-bridge cycling. In this report we examine whether the environment of an active cross-bridge in cardiac myofibrils from transgenic (Tg) mice is altered by the D166V mutation in RLC. The cross-bridge environment was monitored by tracking the fluorescence lifetime (τ) of Alexa488-phalloidin labeled actin. The fluorescence lifetime is the averaged rate of decay of a fluorescent species from the excited state, which strongly depends on various environmental factors. We observed that the lifetime was high when cross-bridges were bound to actin and low when they were dissociated from it. The lifetime was measured every 50 msec from the center half of the I-band during 60 sec of rigor, relaxation and contraction of muscle. We found no differences between lifetimes of Tg-WT and Tg-D166V muscle during rigor, relaxation and contraction. The duty ratio expressed as a fraction of time that cross-bridges spend attached to the thin filaments during isometric contraction was similar in Tg-WT and Tg-D166V muscles. Since independent measurements showed a large decrease in the cross-bridge turnover rate in Tg-D166V muscle compared to Tg-WT, the fact that the duty cycle remains constant suggests that the D166V mutation of RLC causes a decrease in the rate of cross-bridge attachment to actin.
Despite significant clinical advances in the treatment of various cardiovascular diseases, mortality rates remain high. Familial hypertrophic cardiomyopathy (FHC) is one pathological manifestation of the heart resulting from its inability to adequately pump blood, thus leading to premature fatigue, dyspnea, hypertrophy and/or cardiac failure (1, 2). FHC is an autosomal dominant disease originating from mutations in genes that encode for the major contractile proteins of the heart, including the ventricular myosin regulatory light chain (RLC) (for review see (3, 4)). It is characterized by ventricular and septal hypertrophy, myofibrillar disarray, abnormal ECG findings and sudden cardiac death (SCD) at a young age (5, 6). Clinical studies have revealed that the D166V mutation in myosin RLC is associated with a malignant FHC disease phenotype (7). Our objective was to examine the molecular determinants of the D166V induced malfunction of the heart. We hypothesize that the D166V mutation of RLC leads to alterations in myosin cross-bridge kinetics and ultimately affects the interaction of the thick and thin filaments during cardiac muscle contraction.
Analysis of the crystal structures, X-ray diffraction patterns and spectroscopic experiments confirmed the original idea of Huxley (8) that during isometric muscle contraction the myosin cross-bridges assume at least two distinct conformations, when they are strongly attached to- and dissociated from- thin filaments (9–11). It is believed that strongly attached cross-bridges produce contractile force. The environment of a strongly attached cross-bridge is different from the environment of a dissociated cross-bridge. In this paper we compare environments of a cross-bridge during isometric contraction of transgenic wild type (Tg-WT) mouse ventricle and transgenic ventricle carrying the D166V point mutation (Tg-D166V). A convenient way to get information about the environment of a cross-bridge is to measure the fluorescence lifetime of a fluorophore attached to actin. The fluorescence lifetime is the average rate of decay of a fluorescent species from its excited state. The fundamental feature of the fluorescence lifetime is that it depends on a variety of environmental factors such as general solvent effects due to the interaction of the dipole of the fluorophore with environment, specific solvent effects due to fluorophore-solvent interactions, formation of internal charge transfer states and viscosity and the dipole-dipole interaction of fluorophores (12). The fluorescence lifetime is generally longer when fluorophores are bound to immobile structures than when they are free in aqueous solution. Lifetime is independent of fluorophore concentration, a critical advantage in systems where photobleaching is significant. These advantages were recognized by Ferenczi and his collaborators who first used fluorescence lifetimes to detect actomyosin states in mammalian muscle sarcomeres (13). In addition to fluorescence lifetime, a meaningful indicator of the state of muscle is its duty cycle. A cross-bridge is in a strongly attached state for a period of time ts, while it remains in a dissociated or a weakly attached state for a period of time, td. The ratio Ψ = ts /(td+ts) is defined as the duty cycle. To measure Ψ one can follow the changes in the environment of a cross-bridge while it undergoes a cycle of binding and dissociation from actin.
From the relative frequency of occurrence of a given lifetime it was estimated that during contraction the lifetimes and duty cycle of Tg-WT and Tg-D166V hearts were not different. Independently performed measurements in skinned papillary muscle fibers showed a large decrease in the cross-bridge dissociation rate (g) in Tg-D166V preparations compared to controls (14). The cross-bridge dissociation rate (g≈1/ts) was calculated by taking the ratio of fiber ATPase/concentration of cross-bridges attached at all levels of force activation. For the duty cycle Ψ to remain constant, with a large decrease in g, one has to observe a parallel decrease in the rate of cross-bridge attachment, f≈1/td. Therefore, our measurements suggest that the rate of attachment of D166V cross-bridges to thin filaments is lower than in WT cross-bridges. This is consistent with prolonged force transients combined with no change in Ca2+ transients observed in Tg-D166V intact papillary muscle compared to Tg-WT fibers (14).
MATERIALS AND METHODS
Chemicals and solutions
Alexa488-phalloidin (AP) was from Molecular Probes (Eugene, OR). All other chemicals including 1-ethyl-3-(3’-dimethylaminopropyl) carbodiimide (EDC), creatine phosphate, creatine phosphokinase, glucose oxidase, catalase and ATP were from Sigma (St Louis, MO). EDTA-rigor solution contained 50 mM KCl, 2 mM EDTA, 1 mM DTT, 10 mM Tris-HCl buffer pH 7.5. Ca2+-rigor solution contained 50 mM KCl, 4 mM MgCl2, 0.1 mM CaCl2, 1 mM DTT, 10 mM Tris-HCl buffer pH 7.5. Mg2+-rigor solution contained 50 mM KCl, 4 mM MgCl2, 1 mM DTT, 10 mM Tris-HCl buffer pH 7.5. Relaxing solution contained 50 mM KCl, 4 mM MgCl2, 5 mM ATP, 2 mM EGTA, 1 mM DTT, 10 mM Tris-HCl buffer pH 7.5. Contracting solution was the same as Ca2+-rigor, except that it contained in addition 5 mM ATP.
Muscle
After euthanasia, the hearts from 6 month old Tg-D166V and Tg-WT mice were quickly removed and rinsed briefly (no more than 30 sec) with ice-cold 0.9% NaCl. Muscle strips from left ventricles and papillary muscles were dissected at 4°C/in a cold room in ice-cold pCa 8 solution (10−8 M [Ca2+], 1mM [Mg2+], 7mM EGTA, 2.5 mM [MgATP2+], 20 mM MOPS, pH 7.0, 20 mM creatine phosphate and 15 units/ml creatine phosphokinase, ionic strength = 150 mM adjusted with potassium propionate) containing 30 mM 2,3-butanedione monoxime (BDM) and 15% glycerol (15). After dissection, muscle strips were transferred to pCa 8 solution mixed with 50% glycerol and incubated for 1 hr on ice. Then the muscle strips were transferred to fresh pCa 8 solution mixed with 50% glycerol and containing 1% Triton X-100 for 24 hr at 4°C. Muscle strips were finally transferred to a fresh batch of pCa 8 solution mixed 1:1 with glycerol and kept at −20°C until used for the preparation of myofibrils (16).
Preparation of cardiac myofibrils
Myofibrils from Tg-WT and Tg-D166V mouse papillary muscles and left ventricles were prepared from glycerinated fiber bundles stored at −20°C in glycerinating solution. The muscle fibers were first incubated in EDTA rigor solution until they turned white (~1 hr). The fiber bundle was then homogenized using a Heidolph Silent Crusher S homogenizer for 20 sec (with a break to cool after 10 sec) in Mg2+-rigor solution. It was important that the fibers were not homogenized in the EDTA rigor buffer to avoid foaming. All fluorescence lifetime experiments were always performed on freshly prepared myofibrils. Labeled myofibrils (25 µl) were applied to a 20 mm diameter (19 mm × 19 mm) glass bottom coverslip (Menzel-Glaser 20×20 mm #1). The sample was then incubated on the coverslip for 3 min to allow the myofibrils to adhere to the glass. The bottom cover slip was covered with a small (5 mm in diameter) top glass coverslip separated from each other by Avery Hole Reinforcement Stickers. Labeled myofibrils were washed with at least 5 volumes of the Ca2+-rigor solution by applying the solution to the one end of the sandwich and absorbing with #1 filter paper at the other end.
Labeling and estimation of the degree of labeling
To determine the number of fluorescent actins contributing to the signal, the concentration of actin in the muscle was estimated by weighing the muscle before preparation of myofibrils. Actin content was assumed to be 25% of muscle’s dry weight. We estimated that a suspension of myofibrils contained ~100 µM actin. However, this estimation carries ~100% error because of the uncertainty of calculating the dry weight of muscle. Myofibrils were rapidly Vortex-mixed with 100 nM AP and thoroughly washed with rigor solution. Labeling with 100 nM phalloidin gives therefore the Degree of Labeling (DOL) ≈ 100 µM/100 nM = 1000. A typical half-sarcomere (HS) is 1 µm long, 1 µm wide and 0.1 µm thick and therefore its volume is ~0.5 × 10−16 L (17, 18). Taking the concentration of actin in muscle as 0.6 mM (19), we calculate that it contains ~20,000 G-actins.
Sample cross-linking
In order to prevent shortening during contraction, myofibrils were cross-linked with the water-soluble cross-linker EDC (20). Myofibrils (1 mg/ml) were incubated with 20 mM EDC for 20 min at room temperature in the Ca2+-rigor buffer. The reaction was stopped by adding 20 mM DTT. The lack of shortening was checked under differential contrast (21). In 8 control experiments, the mean ± SD sarcomere length of rigor and contracting myofibrils remained unchanged during contraction. The paired t-test showed that the difference was not statistically significant (t=0.42, P=0.68, 8 degrees of freedom). It has been shown that cross-liked myofibrils are a good in vitro model for muscle fiber ATPase and the kinetics of Ca2+-activated activity (22). The large Pi bursts and kcat values were also the same in cross-linked myofibrils and muscle fibers (20). Those results were confirmed by Lionne et al. (23).
Lifetime measurements in solution
Fluorescence lifetimes were measured by time-domain technique using the FluoTime 200 fluorometer (PicoQuant, Inc.). The sample contained 1 µM Factin labeled with 100 nM Alexa488 dye. The measured concentration of dye was 0.1 µM. The excitation was by a 470 nm laser pulsed diode, and the observation was through a monochromator at 510 nm with supporting 550 nm long wave pass filter. FWHM of pulse response function was 68 ps (measured by PicoQuant, Inc.). Time resolution was better than 10 ps. The intensity decays were analyzed in term of multi-exponential model using FluoFit software (PicoQuant, Inc.).
Lifetime measurements in muscle
Fluorescence lifetimes were measured by the time-domain technique using a MicroTime 200 instrument coupled to an Olympus IX71 microscope (PicoQuant, GmbH, Berlin, Germany). Excitation was achieved using a 470-nm pulsed laser diode, and the observation was made through a 500-nm long wave pass filter. Full-Width-at-Half-Maximum (FWHM) of pulse response function was 68 psec (measured by PicoQuant, Inc.) while the time resolution was better than 10 psec. The intensity decays were analyzed in terms of a multiexponential model using SymPhoTime v. 4.3 software (PicoQuant, Inc.). The intensity average lifetime was calculated as and αi is the fractional contribution of the i-th lifetime, τi. The amplitude average lifetime was calculated as . The lifetimes of free and bound Alexa488-phalloidin in water and glycerol were measured with/in a FluoTime 200 fluorometer (PicoQuant Inc) equipped with a microchannel plate and 470 nm pulsed laser diode (76 ps half-width). This instrument provides an exceptional resolution in the sub-nanosecond range (17, 18).
RESULTS
Preventing shortening of contracting muscle
Our task was to compare the fluorescence lifetimes of actin in Tg-WT and Tg-D166V myofibrils during rigor, relaxation and contraction. There is no difficulty in measuring lifetime of rigor myofibrils. However, it is impossible to do so during contraction, because myofibrils shorten. Myofibrils often shorten in relaxing solution as well, due to the damage to troponin or tropomyosin, which become ineffective in preventing contraction even in the absence of Ca2+. To measure the lifetime of a relaxed or contracting muscle, it is therefore necessary to prevent myofibrils from shortening. This was done by cross-linking with the water-soluble cross-linker EDC (20). Cross-linking does not affect force development (20, 24). Myofibrils were cross-linked for 20 min at room temperature with 20 mM EDC. The lack of shortening was checked by following the changes in the image in a microscope as described in (21).
Distribution of lifetimes during contraction of Tg-WT muscle
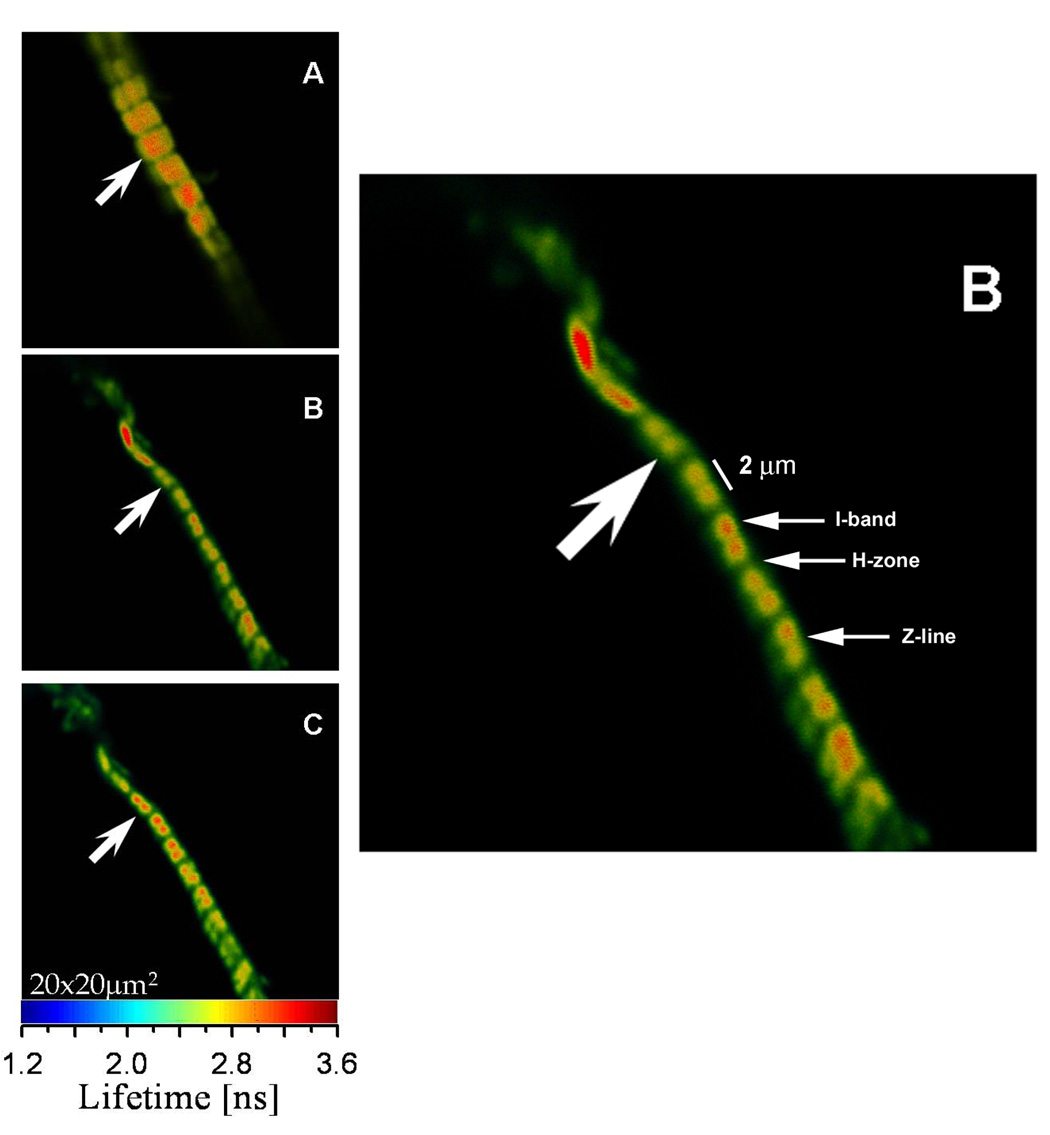
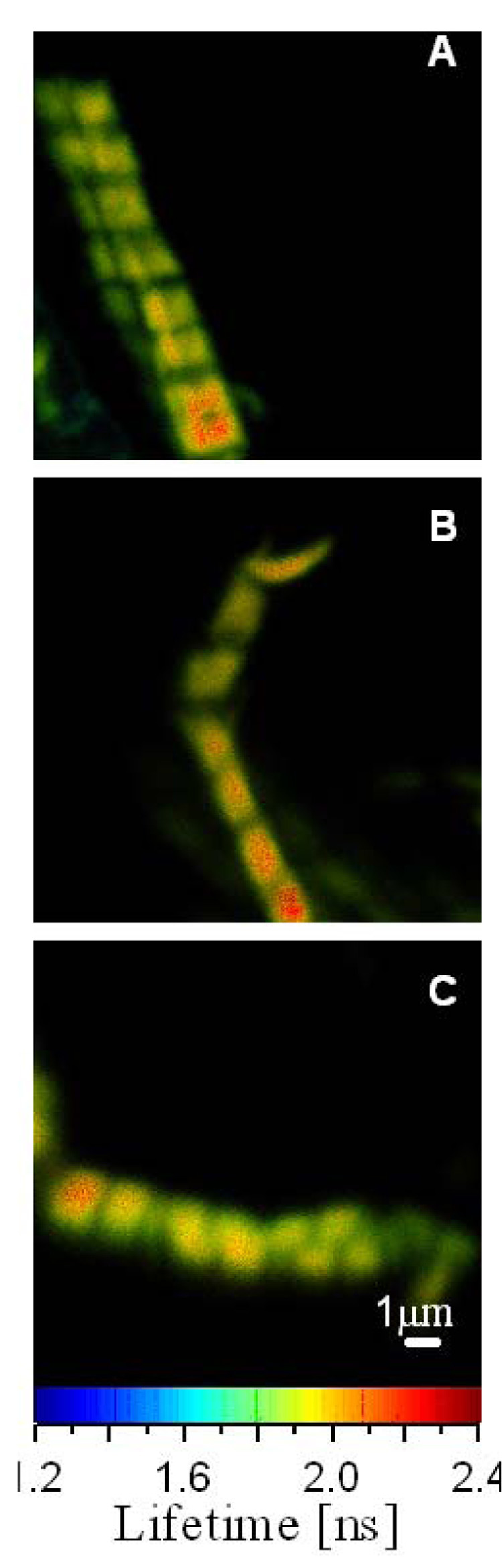
Fig. 1 shows a typical lifetime image of rigor, relaxed and contracting cardiac Tg-WT myofibrils labeled with Alexa488-phalloidin. As shown, the entire I-bands of cardiac myofibrils are fluorescently labeled. This is in contrast to skeletal muscle myofibrils that demonstrate preferential phalloidin binding to the pointed and barbed ends of actin filaments and to the overlap zone (25). This skeletal-specific pattern of phalloidin binding is regulated by nebulin which is absent in cardiac muscle (26).
Fig. 1.
Lifetime images of rigor (A), relaxed (B) and contracted (C) Tg-WT myofibrils of cardiac papillary muscle. The color bar at the bottom is the lifetime scale. The enlarged image of a relaxed myofibril is shown on the right. Myofibrils were cross-linked to avoid shortening. Excitation was at 470 nm, emission was viewed through a 500 nm long-pass filter.
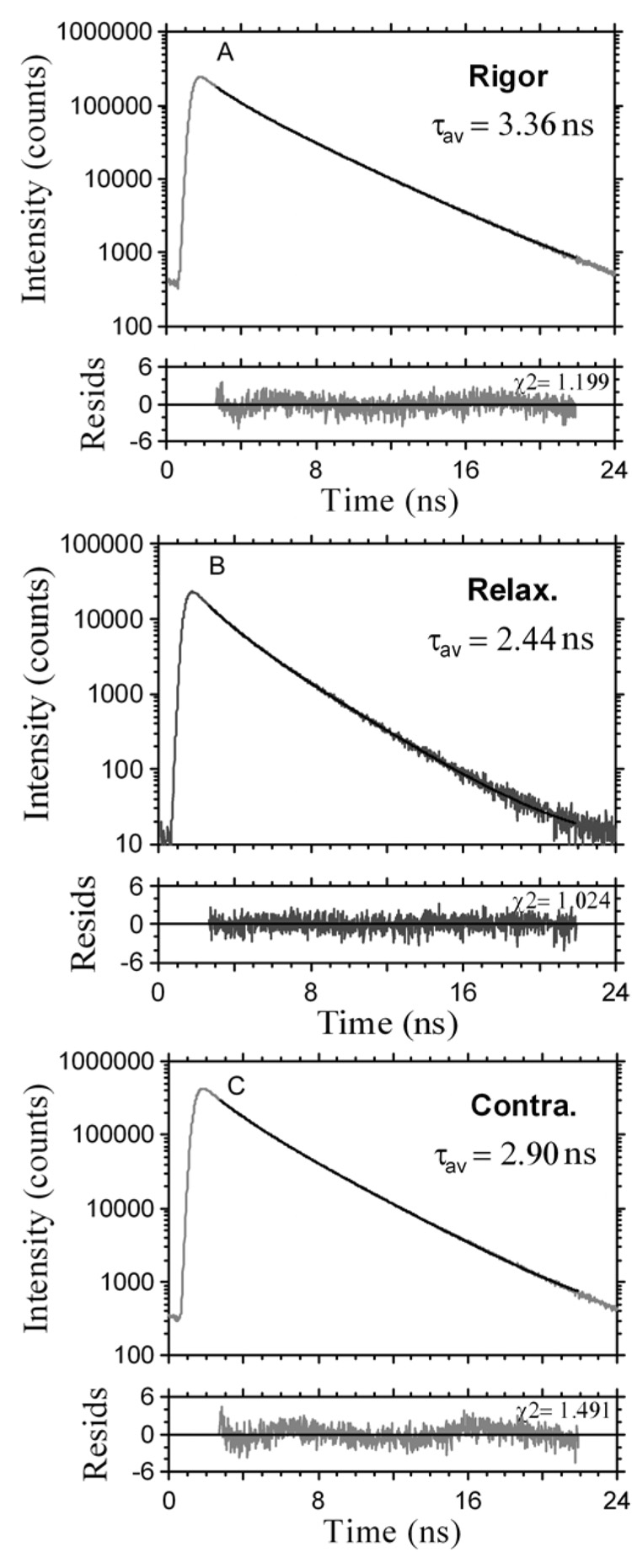
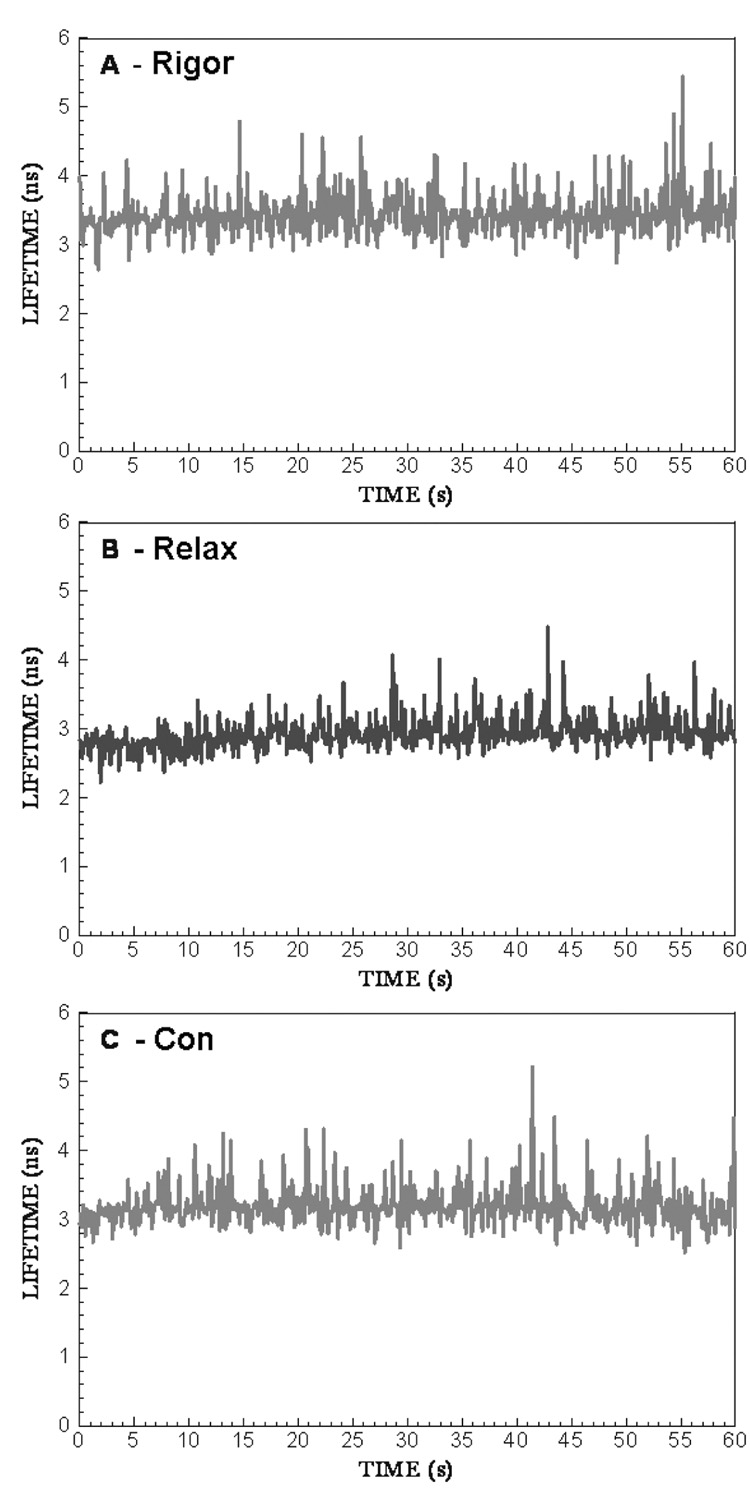
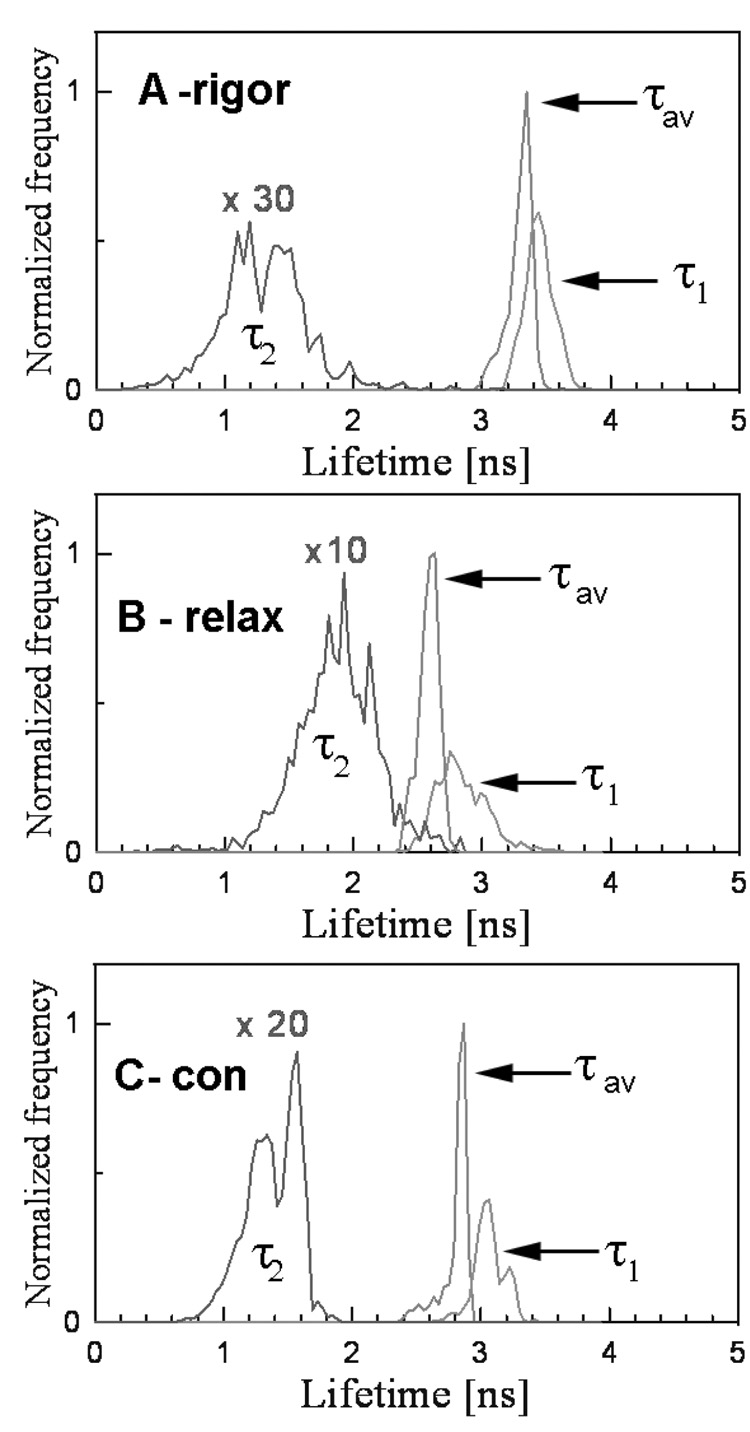
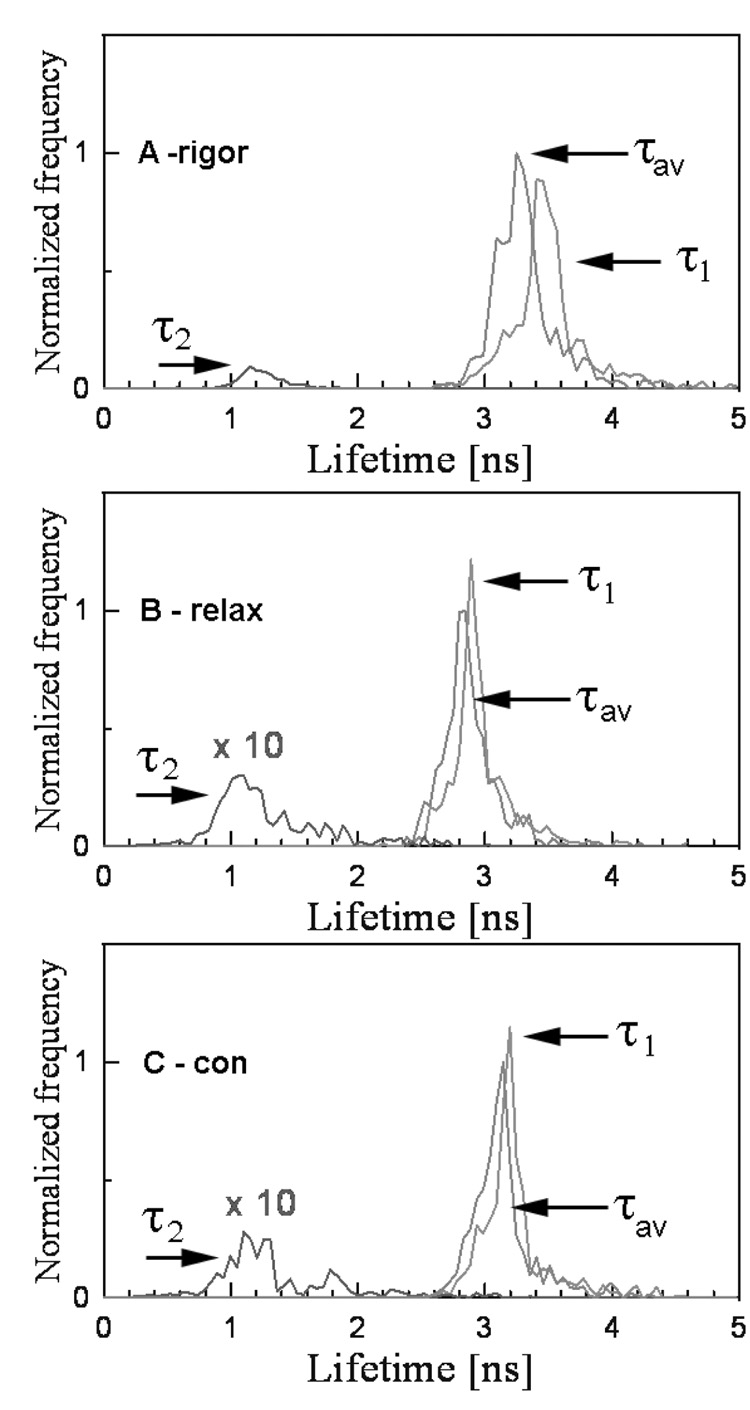
The lifetime was measured by positioning the laser beam at the center half of the I-band pointed to by the arrow. The position of the laser beam was fixed and the data were collected from the area of 0.28 µm2. Fig. 2 shows the lifetime data collected during 60 sec. The fluorescence intensity decay was best fit (black line) with a double exponential function with slow (τ1) and fast (τ2) decaying components. The contributions of slow and fast decaying components to the total fluorescence intensity were approximately equal in rigor, relaxed and contracting muscle, and were respectively 0.6 and 0.4. The excellent quality of the fit is demonstrated by a small χ2 value of the fit (the residuals are shown in the bottom figure of each panel). An equivalent strategy was to measure the lifetime 1,200 times every 50 msec for a total of 60 sec. The 50 msec interval is the approximate time necessary to collect enough photons in a sparsely labeled muscle to accurately calculate lifetime. Fig. 3 shows a typical time-course of the intensity averaged lifetime of a single pixel of rigor (A), relaxed (B) and contracting (C) Tg-D166V muscle monitored every 100 msec. It can be seen that the average value of the lifetime remained constant in spite of the fact that photobleaching occurred. The normalized distributions of lifetimes of rigor, relaxed and contracting Tg-WT muscle (1,200 measurements) are plotted in Fig. 4. The intensity averaged lifetimes for Tg-WT muscle were 3.31±0.21, 2.59±0.19 and 2.89±0.10 nsec for rigor, relaxed and contracted muscle, respectively. These data are summarized in Table 1. The average of at least 3 measurements for each myofibrillar preparation was calculated and then the grand average (average of averages) of all measurements performed on 3 different myofibrillar preparations was determined. As shown, an increase in mobility of the fluorophore occurs in the following order: τrig>τcon>τrel for both Tg-WT and Tg-D166V.
Fig. 2.
Intensity decays of fluorescence of pixels in the center half of the I-band pointed to by the arrows in Fig. 1. Black lines – data fitted by two exponentials. Bottom panels: residuals. Data collected for 60 sec, excitation with a 470 nm pulse of light, emission through LP500 filter.
Fig. 3.
The time-course of changes of τ2 of contracting Tg-D166V muscle. A –rigor, B – relaxation, C- contraction. Data obtained every 1 msec, but the points are plotted every 100 msec.
Fig. 4.
Normalized histogram of single-point lifetimes taken every 50 msec during a 60 sec of data collection in rigor (A), relaxed (B) and contracted (C) Tg-WT muscle. The histograms of slow (τ1), fast (τ2) and intensity average (τav) decay times are identified.
Table 1.
Intensity averaged lifetimes of Alexa488-phalloidin labeled actin filaments located in the center half of the I- band in rigor, relaxed and contracted Tg-WT and Tg-D166V myofibrils.
| Muscle | Tg-WT | Tg-D166V |
|---|---|---|
| τrig (nsec) | 3.31±0.21 | 3.30±0.20 |
| τrel (nsec) | 2.59±0.19 | 2.81±0.18 |
| τcon (nsec) | 2.89±0.10 | 3.15±0.19 |
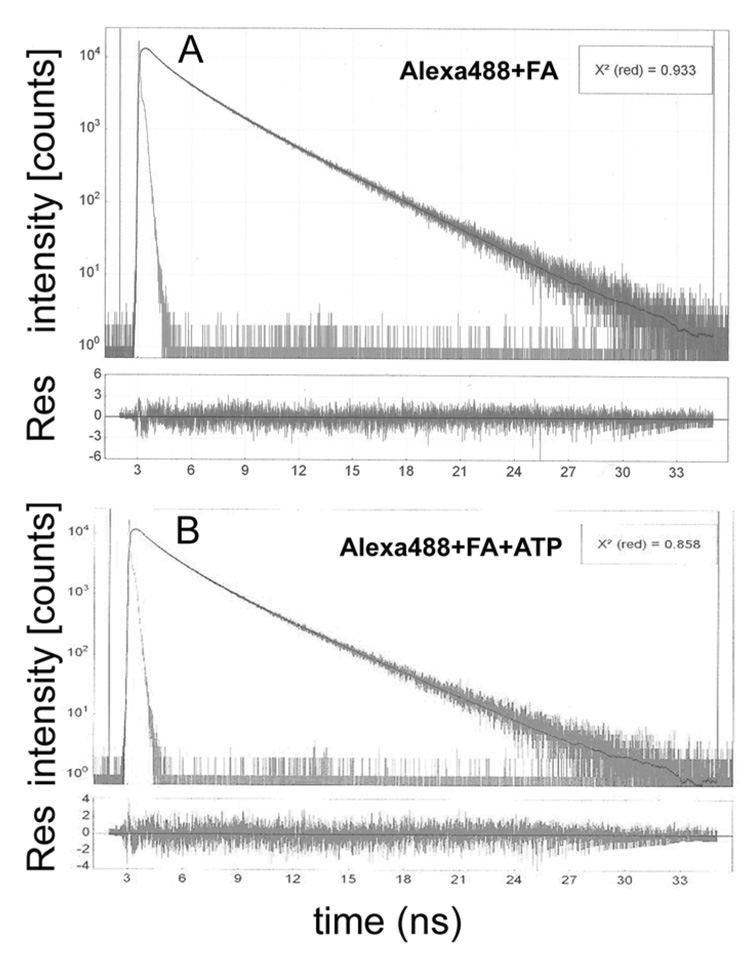
In order to find out whether the lifetime of actin in relaxed muscle is influenced by myosin cross-bridges, we compared lifetimes of purified actin in which every tenth monomer was labeled with Alexa488, with the lifetime of WT muscle in relaxation. The results summarized in Fig. 5 show that the lifetime of Alexa488-phalloidin labeled purified actin is similar in solution and in muscle. In solution, like in muscle, decay of fluorescence could be best fitted by long (3.527 ± 0.015 ns) and short (1.518 ± 0.019 ns) components, comprising ~60% and ~40% of the total fluorescence intensity, respectively (in addition to the long and short lifetimes, the data was best fitted by taking into account additional very short lifetime of ~0.3 ns. This was not considered in this analysis because it contributed only 5–6% to the total intensity). The average intensity weighted lifetime was 2.732 ns. Addition of ATP quenched fluorescence somewhat, the long (3.248 ± 0.017 ns) and short (1.453 ± 0.015 ns) components comprising now 54% and 40% of the total fluorescence intensity, respectively. The average intensity weighted lifetime decreased to 2.355 ns. These results are consistent with the 2.59 ns lifetime of Alexa488-phalloidin labeled myofibrils under relaxing conditions and support the view that the increase in the lifetime under contracting and rigor conditions to 2.89 ns and 3.31 ns, respectively, is caused by the actin-bound myosin cross-bridges.
Fig. 5.
A – (top panel): The decay of fluorescence intensity following pulse excitation of F-actin (FA) labeled with Alexa488. B – (top panel): The decay of fluorescence intensity following pulse excitation of F-actin (FA) labeled with Alexa488 in the presence of 2 mM ATP‥ The residuals (RES) of the best two-exponent fit (black line) are shown in the bottom panels. The goodness of fit is characterized by the small value of χ2. Excitation at 470 nm, emission at 510 nm. 1 µM F-actin + 100 nM Alexa488-phalloidin in rigor buffer (A) and in rigor buffer + 2 mM ATP (B).
The duty cycle was calculated assuming that 100% of cross-bridges were bound to actin in rigor and that 0% during relaxation. Thus, under ideal conditions the distribution of lifetimes during contraction should be biphasic: it should assume the values characteristic of either relaxation or rigor. In practice, the distribution was smeared: the observed (apparent) lifetime τcon was intermediate between rigor and relaxation τcon = (Nrel·τrel + Nrig·τrig)/ (Nrel + Nrig), where Nrel and Nrig are the number of times that relaxed and rigor lifetimes appeared during the experiment. We think that this is because the peaks corresponding to the averaged values of τrig and τrel cannot be resolved. It is therefore reasonable to assume that the duty cycle is Ψ = (τcon-τrel)/(τrig-τrel)×100%. From the intensity averages in Fig. 4 the duty cycle Ψ = (τcon-τrel)/(τrig-τrel)×100% for Tg-WT muscle was calculated as 45%. Only in some cases the distribution of τ2 was biphasic (e.g. τ2 trace in Fig. 4C). The lifetime τ2 always behaved oppositely to τ1: whereas for τ1 τrig>τcon>τrel, the opposite was true for 2, i.e. τrig<τcon<τrel. From the τ2 curves in Fig 4C it can be seen that some contraction lifetimes are characteristic of rigor and some are characteristic of relaxation. The ratio of the two was estimated as 1.5, suggesting that the duty cycle during contraction of Tg-WT muscle is (1+1/1.5)−1 ≈ 60%.
Distribution of lifetimes during contraction of Tg-D166V muscle
Fig. 6 shows a typical lifetime image of a rigor, relaxed and contracting cardiac Tg-D166V myofibril. As before, the decay could be best fit with a double exponential function. The fractional contributions of slow and fast decaying components were approximately constant at 0.6 and 0.4. The normalized histograms are plotted in Fig. 7. The intensity averaged lifetimes and their variations were τrig= 3.30 ± 0.20, τrel= 2.81 ± 0.18 nsec and τcon= 3.15 ± 0.19 nsec for 60 sec data collected from muscle in rigor, relaxation and contraction, respectively. These data are summarized in Table 1. The differences between WT and mutated muscle were not statistically significant (trig=0.062, trel=1.57, tcon=1.605, P<0.184 in all cases). The duty cycle Ψ = (τcon-τrel)/(τrig-τrel) × 100% was 69%.
Fig. 6.
Lifetime images of rigor (A), relaxed (B) and contracted (C) Tg-D166V myofibril. Note the change in the lifetime scale. Excitation was at 470 nm, emission was viewed through a 500 nm long-pass filter.
Fig. 7.
Normalized histogram of single-point lifetimes taken every 50 msec during 60 sec of data collection in rigor (A), relaxed (B) and contracted (C) Tg-D166V muscle. The slow (τ1), fast (τ2) and intensity average (τav) decay times are identified.
DISCUSSION
In this study we compared the fluorescence lifetimes of Alexa488 labeled actin molecules in Tg-WT and Tg-D166V muscle myofibrils.
Single-point lifetime
Lifetime was measured 1,200 times every 50 msec for a total of 60 sec from an area fixed at the center half of the I-band of rigor, relaxed and contracting muscle. Lifetime was measured from the frequency distributions. The number of actin monomers contributing to the signal was determined by DOL and by the size of the sampled area. The simple calculation outlined in the Materials & Methods suggests that there are ~20,000 G-actins in a typical HS. Taking DOL of 1000 and the concentration of actin in muscle as 0.6 mM, we calculate that each HS contains ≈ 20 fluorophores. The sampled area is diffraction limited by a 1.2 NA water objective and 470 nm laser wavelength, giving a diameter of the spot from which data are collected as ~0.3 µm. Thus, the microscope resolves (π. 0.302) = 0.28 µm2 area. There are approximately 13 such areas in HS, each containing on average 20/13=1.6 fluorophores. The phalloidin binding site is located at the contact region between three actin subunits. The observed changes of lifetime therefore reflect collective response to cross-bridge binding of ~5 actin monomers. It is therefore not surprising that we did not observe step-wise photobleaching, which is characteristic of single molecule observations. It should be noted that there is a problem with calculating number of observed molecules from concentration of myofibrils, because of the serious experimental difficulty in determining the dry weight of the mouse ventricle (see Materials & Methods). Therefore the most reliable way to estimate how many fluorophores are contributing to the change of lifetime in response to cross-bridge turnover is from the rate of arrival of photons. Typically, we observed ~16,000 photons arrive in 1 sec. We estimate that ~4,000 photons arrive from a single molecule in 1 sec (based on data reported in (17)). We therefore estimate that in a single-point measurement on average ~4 fluorophores contribute to the signal, in approximate agreement with the theoretical calculation above.
Human patients are normally heterozygous for FHC mutations, so their myosin containing thick filaments are composed of wild type myosin heads interspersed with FHC mutant heads. Therefore, it is essential to have a technique capable of monitoring lifetimes of a few molecules. The advantage of the present approach is its ability to avoid averaging over ensembles of molecules with different kinetics and the ability to unambiguously determine the behavior of a “healthy” vs. “diseased” single myosin-cross-bridge. This is not critical in the current study since Tg-D166V myocardium contains more than 90 % of FHC molecules making the comparison between kinetics of diseased (Tg-D166V) vs. healthy (Tg-WT) cross-bridges achievable.
It was assumed that during time ts the cross-bridge bound to actin in the thin filament has lifetime τrig and that during time td the cross-bridge is free from actin and has lifetime τrel. This is because the environment and mobility of the fluorophore are significantly different when a cross-bridge is bound to or free from actin. It was observed that τrig> τrel. This difference is not expected to result from fluorescence quenching by amino acids of a cross-bridge when it is bound to actin during rigor, because quenching causes a decrease in lifetime. The most likely explanation for the observation that τrig> τrel is that binding of cross-bridges stabilizes fluorophores attached to actin. Mobility of the dye may lead to a non-radiative depopulation of the excited state resulting in a decrease of lifetime. To check this hypothesis, we compared the lifetimes of mobile and immobilized Alexa488-phalloidin. The fluorophore was dissolved in water or 100% glycerol to assure its mobility or immobility, respectively. The intensity decay curves were best fit by a sum of 3 exponentials. The amplitude averaged lifetimes in water and glycerol were 2.479±0.037 and 2.904±0.013 nsec, respectively, confirming the suggestion that the observed increase in lifetime during rigor is due to immobilization of the dye. This reasoning implies that the only factor contributing to a change in lifetime is binding of a cross-bridge to actin and not binding of ATP to myosin. This is because binding of ATP makes no difference to lifetime of fluorescein attached to myosin (27).
The accuracy of single point measurement was <26%. This low accuracy is perhaps due to the fact that myofibrils did not remain completely still during relaxation, and consequently data were not collected from a single position. In relaxation it is important that all troponin and tropomyosin molecules be fully functional. If even a small fraction of them is damaged, the muscle may oscillate due to contraction of sarcomeres containing non- functional regulatory proteins. This is unlikely to happen during contraction because Ca2+ and ATP are both present. Ishiwata et al. found that the myosin II motors show non-linear auto-oscillation, named SPOC (SPontaneous Oscillatory Contraction). For cardiac muscle it occurs when the activation level is intermediate between those of contraction and relaxation (17, 28). In our experiments, however, pCa was fixed at 4. In addition, we have considered three possible explanations which could give rise to artifacts and concluded that none is likely:
It is impossible that lifetime is influenced by photobleaching because lifetime does not depend on intensity of fluorescence.
It is unlikely that measurements of lifetime are influenced by the dissociation of phalloidin from thin filaments because in a control experiment we first labeled 1 mg/ml (3 µM actin) of cardiac myofibrils with excess of unlabeled phalloidin. After brief incubation, we added 4 µM of fluorescent phalloidin in an attempt to displace bound toxin with fluorescent one. If the off-rate were fast, myofibril would have now become fluorescent. This was not the case, suggesting that the off-rate is slow. This is consistent with the fact that the dissociation of phalloidin from skeletal muscle actin is known to be slow (4.8 × 10−4 sec−1, (29)), i.e. phalloidin spends on the average 2,000 sec on actin before dissociating.
It is impossible that Trp fluorescence of myofibrils contributes to the signal because Trp has practically no absorption at 470 nm and unlabeled myofibrils had no autofluorescence whatsoever (probably because the sample was only ~100 nm thick).
The low accuracy of measurements did not allow us to conclude that Ψ is statistically different in Tg-WT and Tg-D166V muscle. If it were possible to measure single-point lifetime of the same I-band in rigor, relaxed and contracted heart muscle, the accuracy of the measurement of one type of muscle (Tg-WT or Tg-D166V) would have been <1%. Unfortunately, such an experiment proved impossible. The exchange of bathing solution created a flow that often moved the myofibril beyond the field-of-view of the microscope. This means that in order to determine the absolute value of lifetimes, the measurements have to be done on a different I-band in a different myofibril. It should also be noted that from the records such as shown in Fig. 4 it is possible, in principle, to measure the absolute amount of time cross-bridge spends attached and dissociated from actin (i.e. not only Ψ). At present, this signal is too noisy. Future work is needed to improve it. Also, in the future it should be possible to design an experimental chamber to allow irrigation of myofibril with relaxing and contracting solutions so gently as to avoid any displacement.
Measuring changes of lifetime of these actins reflects changes of lifetime of a cross-bridge. Indeed, it has been shown that a 3-actin complex and a cross-bridge rotate in synchrony (30). Labeling actin has an important advantage in that it can be labeled specifically and stoichiometrically with fluorescent phalloidin. Further, phalloidin does not alter the enzymatic properties of muscle (31, 32) and does not impair the regular structure of the myofibril.
Implications for heart muscle
In a parallel study, skinned and intact papillary muscle fibers from Tg-D166V mice were examined using a Guth Muscle Research System and the effects of the D166V mutation compared to Tg-WT and NTg (non-transgenic) mice (14). A large increase in the Ca2+ sensitivity of force and ATPase (ΔpCa50 higher than 0.25) measured simultaneously under isometric conditions was determined in skinned muscle fibers from Tg-D166V mice compared to control NTg and Tg-WT mice. This D166V mediated increase in the Ca2+ sensitivity of force and ATPase was shown to be due to a large decrease in the cross-bridge dissociation rate (g), expressed as the ratio of fiber ATPase/concentration of cross-bridges attached at all levels of force activation. Since g≈1/ts, where ts corresponds to time when a cross-bridge is attached to actin and generates force, this implies that ts would have to increase in Tg-D166V muscle to result in a decreased g. As proposed by Huxley (8), the transition from the non-force-generating states to the force-generating states in muscle can be characterized by the cross-bridge attachment rate (f≈1/td) and the rate of the cross-bridge return to the non-force-generating states (g≈1/ts). In the present study we show that the duty cycle Ψ defined as a ratio of ts /(td+ts) was the same in Tg-WT and in Tg-D166V myofibrils. Since the duty cycle Ψ = ts /(td+ts) was not changed and ts was predicted to increase in Tg-D166V muscle, this implies that in order for Ψ to remain constant, td would have to also increase and consequently the rate of cross-bridge attachment f would have to decrease.
Our results of no D166V mediated change in Ψ and predicted increases in ts (≈1/g) and td (≈1/f) suggest that the rates of cross-bridge attachment and detachment are most likely decreased in Tg-D166V muscle compared to control Tg-WT. This hypothesis is supported by the measurements of prolonged force transients in Tg-D166V intact papillary muscle fibers compared to Tg-WT fibers (14). The slow force relaxation rate of the fibers could potentially result in diastolic dysfunction of the D166V mutated myocardium. Abnormal diastolic filling of the heart could also lead to a decreased stroke volume causing systolic dysfunction. These changes if severe enough would ultimately result in compensatory hypertrophy and could lead to sudden cardiac death as observed in the individuals harboring the D166V mutation (7).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr S. Chen (UNTHSC) for help with the statistical treatment, Jingsheng Liang (Univ. of Miami) for preparing glycerinated muscle strips and Michelle Jones (Univ. of Miami) for her help in preparing the manuscript for publication.
Abbreviations
- FHC
Familial Hypertrophic Cardiomyopathy
- RLC
Regulatory Light Chain of Myosin
- SCD
Sudden Cardiac Death
- DOL
Degree of Labeling
- Ψ
Duty Cycle
- Tg-WT
Transgenic Wild Type
- Tg-D166V
Transgenic D166V RLC Mutant
- τ1
slow component of fluorescence decay
- τ2
fast component of fluorescence decay
- τrig
average fluorescence lifetime of actin in rigor muscle
- τrel
average fluorescence lifetime of actin in relaxed muscle
- τcon
average fluorescence lifetime of actin in contracting muscle
- HS
Half-Sarcomere
- BDM
2,3-butanedione monoxime
- FWHM
Full-Width-at-Half-Maximum
Footnotes
This work was supported by NIH-AR048622 to Julian Borejdo, NIH-HL071778 to Danuta Szczesna-Cordary, the Polish Ministry of Science and Higher Education grant 17/MOB/2007/0 to Rafal Luchowski and Texas ETF grant to the Center of Commercialization of Fluorescence Technologies
REFERENCES
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell. Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Lips DJ, deWindt LJ, van Kraaij DJW, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur. Heart J. 2003;24:883–896. doi: 10.1016/s0195-668x(02)00829-1. [DOI] [PubMed] [Google Scholar]
- 3.Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003;3:187–197. doi: 10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- 4.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J. Cardiovasc. Electrophysiol. 2008;19:104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ. The young competitive athlete with cardiovascular abnormalities: causes of sudden death, detection by preparticipation screening, and standards for disqualification. Card. Electrophysiol. Rev. 2002;6:100–103. doi: 10.1023/a:1017903709361. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 7.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet J-P, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M EUROGENE Heart Failure Project. Hypertrophic Cardiomyopathy: Distribution of Disease Genes, Spectrum of Mutations, and Implications for a Molecular Diagnosis Strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 8.Huxley AF. A hypothesis for the mechanism of contraction of muscle. Prog. Biophys. Biophys. Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 9.Reedy MC. Visualizing myosin's power stroke in muscle contraction. J. Cell Sci. 2000;113(Pt 20):3551–3562. doi: 10.1242/jcs.113.20.3551. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney HL, Houdusse A. The motor mechanism of myosin V: insights for muscle contraction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004;359:1829–1841. doi: 10.1098/rstb.2004.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi Y, Shuman H, Goldman YE. Coupling between phosphate release and force generation in muscle actomyosin. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2004;359:1913–1920. doi: 10.1098/rstb.2004.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer: 2006. [Google Scholar]
- 13.Garcia DI, Lanigan P, Webb M, West TG, Requejo-Isidro J, Auksorius E, Dunsby C, Neil M, French P, Ferenczi MA. Fluorescence lifetime imaging to detect actomyosin states in mammalian muscle sarcomeres. Biophys. J. 2007;93:2091–2101. doi: 10.1529/biophysj.106.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerrick WGL, Kazmierczak K, Xu Y, Jones M, Wang Y, Szczesna-Cordary D. Malignant D166V-FHC mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice. FASEB J. 2008 doi: 10.1096/fj.08-118182. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szczesna-Cordary D, Guzman G, Zhao J, Hernandez O, Wei J, Diaz-Perez Z. The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J. Cell. Sci. 2005;118:3675–3683. doi: 10.1242/jcs.02492. [DOI] [PubMed] [Google Scholar]
- 16.Szczesna-Cordary D, Guzman G, Ng SS, Zhao J. Familial hypertrophic cardiomyopathy-linked alterations in Ca2+ binding of human cardiac myosin regulatory light chain affect cardiac muscle contraction. J. Biol. Chem. 2004;279:3535–3542. doi: 10.1074/jbc.M307092200. [DOI] [PubMed] [Google Scholar]
- 17.Muthu P, Talent JM, Gryczynski I, Borejdo J. Cross-Bridge Duty Cycle in Isometric Contraction of Skeletal Myofibrils. Biochemistry. 2008;47:5657–5667. doi: 10.1021/bi7023223. [DOI] [PubMed] [Google Scholar]
- 18.Muthu P, Gryczynski I, Gryczynski Z, Talent JM, Akopova I, Borejdo J. Decreasing photobleaching by silver nanoparticles on metal surfaces: application to muscle myofibrils. J. Biomed. Opt. 2008;13:014023. doi: 10.1117/1.2854120. [DOI] [PubMed] [Google Scholar]
- 19.Bagshaw CR. Muscle Contraction. London: Chapman & Hall; 1982. [Google Scholar]
- 20.Herrmann C, Sleep J, Chaussepied P, Travers F, Barman T. A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry. 1993;32:7255–7263. doi: 10.1021/bi00079a023. [DOI] [PubMed] [Google Scholar]
- 21.Borejdo J, Muthu P, Talent J, Akopova I, Burghardt TP. Rotation of actin monomers during isometric contraction of skeletal muscle. J. Biomed. Opt. 2007;12:014013. doi: 10.1117/1.2697286. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann C, Lionne C, Travers F, Barman T. Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry. 1994;33:4148–4154. doi: 10.1021/bi00180a007. [DOI] [PubMed] [Google Scholar]
- 23.Lionne C, Iorga B, Candau R, Travers F. Why hoose myofibrils to study muscle myosin ATPase? J. Muscle Res. Cell Motil. 2003;24:139–148. doi: 10.1023/a:1026045328949. [DOI] [PubMed] [Google Scholar]
- 24.Borejdo J, Shepard A, Dumka D, Akopova I, Talent J, Malka A, Burghardt TP. Changes in orientation of actin during contraction of muscle. Biophys. J. 2004;86:2308–2317. doi: 10.1016/S0006-3495(04)74288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczesna D, Lehrer SS. The binding of fluorescent phallotoxins to actin in myofibrils. J. Muscle Res. Cell Motil. 1993;14:594–597. doi: 10.1007/BF00141556. [DOI] [PubMed] [Google Scholar]
- 26.Ao X, Lehrer SS. Phalloidin unzips nebulin from thin filaments in skeletal myofibrils. J. Cell Sci. 1995;108:3397–3403. doi: 10.1242/jcs.108.11.3397. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre R, Gonsoulin F, Cheung HC. Interaction of fluorescently labeled myosin subfragment 1 with nucleotides and actin. Biochemistry. 1986;25:6827–6835. doi: 10.1021/bi00370a015. [DOI] [PubMed] [Google Scholar]
- 28.Ishiwata S, Shimamoto Y, Suzuki M, Sasaki D. Regulation of muscle contraction by Ca2+ and ADP: focusing on the auto-oscillation (SPOC) Adv. Exp. Med. Biol. 2007;592:341–358. doi: 10.1007/978-4-431-38453-3_29. [DOI] [PubMed] [Google Scholar]
- 29.De La Cruz E, Pollard TD. Transient kinetic analysis of rhodamine phalloidin binding to actin filaments. Biochemistry. 1994;33:14387–14392. doi: 10.1021/bi00252a003. [DOI] [PubMed] [Google Scholar]
- 30.Shepard AA, Dumka D, Akopova I, Talent J, Borejdo J. Simultaneous measurement of rotations of myosin, actin and ADP in a contracting skeletal muscle fiber. J. Muscle Res. Cell Motil. 2004;25:549–557. doi: 10.1007/s10974-004-5073-6. [DOI] [PubMed] [Google Scholar]
- 31.Bukatina AE, Fuchs F, Watkins SC. A study on the mechanism of phalloidin-induced tension changes in skinned rabbit psoas muscle fibres. J. Muscle Res. Cell Motil. 1996;17:365–371. doi: 10.1007/BF00240934. [DOI] [PubMed] [Google Scholar]
- 32.Prochniewicz-Nakayama E, Yanagida T, Oosawa F. Studies on conformation of F-actin in muscle fibers in the relaxed state, rigor, and during contraction using fluorescent phalloidin. J. Cell Biol. 1983;97:1663–1667. doi: 10.1083/jcb.97.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.