Abstract
Spatial abilities have been associated with many ecologically-relevant behaviors such as territoriality, mate choice, navigation and acquisition of food resources. Differential demands on spatial abilities in birds and mammals have been shown to affect the hippocampus, the region of the brain responsible for spatial processing. In some bird and mammal species, higher demands on spatial abilities are associated with larger hippocampal volumes. The medial and dorsal cortices are the putative reptilian homologues of the mammalian hippocampus, yet few studies have examined the relationship between these brain areas and differential spatial use strategies in reptiles. Further, many studies in birds and mammals compare hippocampal attributes between species that utilize space differently, potentially confounding species-specific effects with effects due to differential behaviors in spatial use. Here, we investigated the relationship between spatial use strategies and medial and dorsal cortical volumes in males of the side-blotched lizard (Uta stansburiana). In this species, males occur in three different morphs, each morph using different spatial niches: large territory holders, small territory holders and non-territory holders with home ranges smaller than the territories of small territory holders. We found that large territory holders had larger dorsal cortical volumes relative to the remainder of the telencephalon compared with non-territorial males, and small territory holders were intermediate. These results suggest that some aspect of holding a large territory may place demands on spatial abilities, which is reflected in a brain region thought partially responsible for spatial processing.
Keywords: dorsal cortex, hippocampus, spatial use, Uta stansburiana
The ability to track the spatial distribution of resources has fitness ramifications in the contexts of territoriality, mate choice, navigation, acquisition of food resources and many other ecologically-relevant behaviors (e.g. Brennan et al. 1990; Godard 1991; Menzel et al. 2000; Shettleworth 1990). Further, differences in spatial ability and demands on spatial ability have been shown to differentially affect brain anatomy, specifically reflected in the hippocampus, one of the brain regions thought to be important in spatial memory processing (O’Keefe & Nadel 1979; Morris et al. 1982). Animals that have a greater reliance on spatially-relevant behaviors tend to have larger hippocampi (Healy & Krebs 1996; Jacobs & Spencer 1994; Krebs et al. 1989; Lucas et al. 2004; Sherry et al. 1989). Therefore, there appears to be a positive relationship between increased demands placed on spatial abilities and enlargement of the hippocampus.
Numerous studies have found a positive relationship between space use and hippocampal morphology. Interspecific comparisons have found that species with higher demands on spatial abilities have larger hippocampal volumes than do closely related species that do not exhibit such demands on spatial abilities. For example, differences in space use in relation to foraging behaviors have been shown to correlate with hippocampal volume; species that rely on spatially diverse resources, rely on spatial memory, and/or engage in active foraging search patterns have larger hippocampi (Krebs et al. 1989; Sherry et al. 1989; Jacobs & Spencer 1994; Day et al. 1999; Lucas et al. 2004). Intraspecific differences in hippocampal formations have also been correlated with differential space use patterns. This pattern has been shown between the sexes and between populations that utilize space differently, especially when one group has a greater reliance on tracking spatially distributed resources than the other group. For example, male meadow voles (Microtus pennsylvanicus) have higher demands on spatial abilities as a result of patrolling large territories compared with females that reside in significantly smaller home ranges. This is reflected by a larger hippocampal volume in male meadow voles than that found in females (Jacobs et al. 1990). Similarly, male cottonmouth snakes (Agkistrodon piscivorous) have larger home ranges than females, which positively relates to the size of the putative reptilian hippocampal homologue (Roth et al. 2006). Also, nest-searching female brown-headed cowbirds have larger hippocampi than males that do not search for host nests (Sherry et al. 1993). Blackcapped chickadees (Poecile atricapillus) from northern populations, which live in harsh environments demanding heavier reliance on memory for food caches have larger hippocampal volumes compared to chickadees from southern populations living in milder environments (Pravosudov & Clayton 2002; Roth & Pravosudov 2009). These studies suggest that a relationship exists between demands on spatial ability and the hippocampal architecture, where higher demands on spatial abilities appear to contribute to increases in hippocampal size.
Although there is support for the relationship between spatial ability demands and hippocampal volume, differences in hippocampal attributes could relate to greater genetic and other differences between species, rather than differences in spatial use per se (e.g. Girvan & Braithwaite 1998). Therefore, a better relationship between hippocampal structure and differential space use could be elucidated by comparing individuals within the same species in the same population, where individuals utilize spatial niches differently. By doing so, effects on the hippocampal structure due to species-specific differences can potentially be minimized, outside of the effects of differential space use.
Therefore, the goal of our study was two-fold: 1) to isolate the effects of differential space use on brain attributes, while controlling for species and population differences and 2) to determine if the volumes of the putative reptilian hippocampal homologues (medial and dorsal cortices; Butler 1976; Rodríguez et al. 2002a; Salas et al. 2003) relate to the differential use of space, as has been shown in the hippocampal structures in mammals and birds. Concisely, we wanted to relate space use with particular brain regions know to be involved with spatial processing. To this end, we used the male side-blotched lizard (Uta stansburiana) as our model species.
In this species, the males within the same populations are found in one of three morphs, where each morph appears to utilize space differently (Sinervo & Lively 1996; Sinervo et al. 2000, 2006). The orange morph occupies and defends large territories (~40 m2), the blue morph occupies and defends smaller territories (~23 m2) while the yellow morph does not hold or defend a territory and its home range is smaller than the territories of the other two morphs (~20 m2) (Sinervo & Lively 1996; Zamudio & Sinervo 2000; Sinervo et al. 2000, 2006; Calsbeek & Sinervo 2002). As has been shown with other studies linking hippocampal volume and aspects of ecological space use (Jacobs et al. 1990; Day et al. 1999; Roth et al. 2006), permanent territory patrol and defense likely cause increased demands on spatial use, memory, and processing. Further, large territory holders should have larger demands on spatial use, memory and processing compared with smaller territory holders. We predicted, based on the patterns of territoriality and space use of the three morphs, that the orange morph would have the largest medial and dorsal cortical volumes relative to the rest of the telencephalon due to the increased demand on spatial abilities related to holding and patrolling a large territory. We expected that the yellow morph would have the smallest relative medial and dorsal cortical volumes, as yellow males do not hold or defend territories. Finally, we predicted the blue morph would have intermediate medial and dorsal cortical volumes relative to the orange and yellow morphs, as these males do hold territories but these territories are smaller than those held by orange males.
METHODS
We collected nine orange males, eight blue males and seven yellow males in March of 2008 from Los Baños Grandes area, Merced County, California. Lizards were transported individually in plastic containers (15.24 × 10.16 × 10.16 cm) to the University of Nevada, Reno (travel time ≈ 3.5 hours). Upon arrival, individuals were immediately anesthetised with a lethal overdose of Nembutal (0.05 ml of 50mg/ml Nembutal). The lizards were transcardially perfused with 0.1 M phosphate buffered saline for 10 min followed by 15–20 min perfusion of 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were extracted and post-fixed in 4% paraformaldehyde for 24 hours before cryoprotection. Brains were cryoprotected in 15% sucrose, then 30% sucrose, and finally flash-frozen on dry ice. Brains were stored at −80C until sliced. Brains were sliced on a cryostat (Leica CM 3050S: −20C) in the coronal plane every 40 μm. Every section was mounted and Nissl stained with thionin. Slides were coded, thus tissue slices were measured blind to morph type.
Identification of the brain areas responsible for spatial processing in reptiles is, at present, equivocal. However, two main areas, the medial and dorsal cortex, have been identified in several studies as important in spatial processing, in that lesions to either cortical region can impair spatial abilities (Grisham & Powers 1990; Petrillo et al. 1994; Reiman-Avigan & Schade-Powers 1995; Rodríguez et al. 2002a,b; López et al. 2003). Thus, both areas were measured and analyzed separately in this study The goal of our study was to see whether differential space use specifically affects the medial and dorsal cortical volumes, but not the rest of the brain; therefore, we used the remainder of the telencephalon (i.e., telencephalon minus medial and dorsal cortical volumes) as a control area (as per Krebs et al. 1989; Clayton 2001; Pravosudov & Clayton 2002; Roth et al. 2006).
We measured medial cortex, dorsal cortex and remainder of the telencephalon volumes using standard stereological techniques; all volumes were estimated with standard stereological methods (StereoInvestigator, Microbrightfield, Inc.; microscope, Leica M4000B). We optimized our measuring scheme such that our coefficient of error was less than 0.05 for both the medial and dorsal cortex. We measured medial and dorsal cortical volumes by using every third section (average of 12.52 sections per brain) and the remainder of the telencephalon volume by using every fourth section (telencephalon volume minus medial and dorsal cortical volumes). Medial and dorsal cortical volumes and telencephalon volume were measured in their entirety and estimated with the Cavalieri procedure (Gundersen and Jensen 1987). Medial and dorsal cortical volumes were measured with a 200 μm grid; telencephalon volume was measured with a 300 μm grid. The left and right hemispheres were both measured for all volumes and then added to produce the given values. There were no significant differences between left and right medial and dorsal cortical volumes or telencephalon volumes.
Statistics
The data were log-transformed to conform to the assumptions for parametric analyses and Levene’s test on transformed data found no difference in variances among the morphs (dorsal cortex: F2,21 = 3.694, p = 0.051; medial cortex: F2,21 = 0.003, p ≈ 1.0). We tested for differences in telencephalon volume among the three types of morphs with general linear models (GLM) with snout-vent length as the covariate. By doing so, we verified that morph type had no effect on telencephalon volume, when controlled for snout-vent length. We then tested our a priori predictions for separate differences in relative dorsal and medial cortical volumes using GLM, with telencephalon volume as a covariate, followed by planned comparisons. We considered all results to be statistically significant if alpha ≤ 0.05.
Because our a priori predictions were directional, in that orange morphs should have larger medial and cortical volumes relative to the rest of the telencephalon than yellow morphs while blue morphs are intermediate, we also used an ordered heterogeneity test (Rice & Gaines 1994a, b). This test incorporates the complement of the probability from the non-directional heterogeneity test (Pc= 1 − ANOVA p value) and the rank order from a Spearman rank correlation (rs) to permit testing of order within the context of a heterogeneity test (test statistic: rs Pc) (Rice & Gaines 1994a, b).
RESULTS
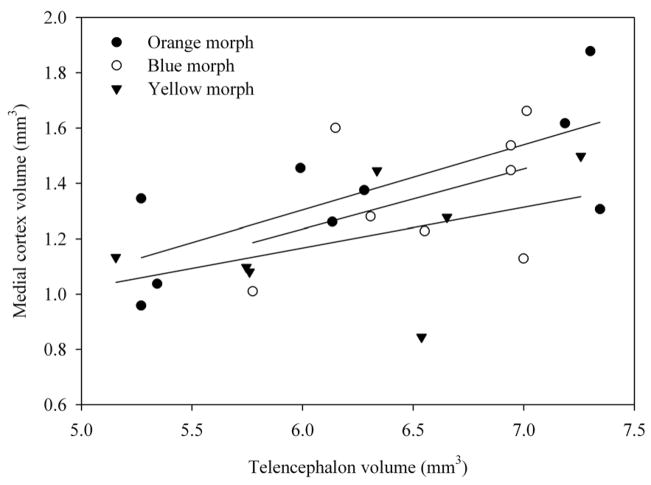
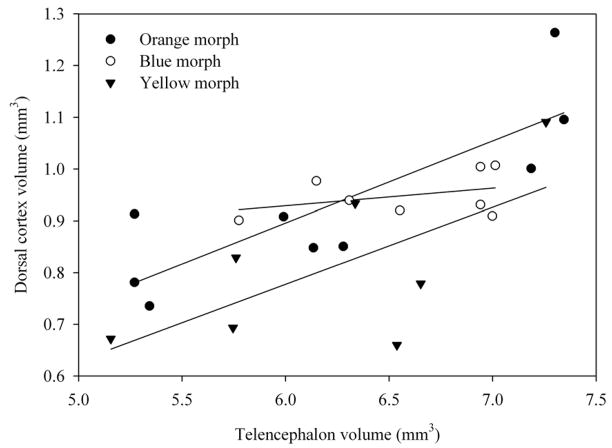
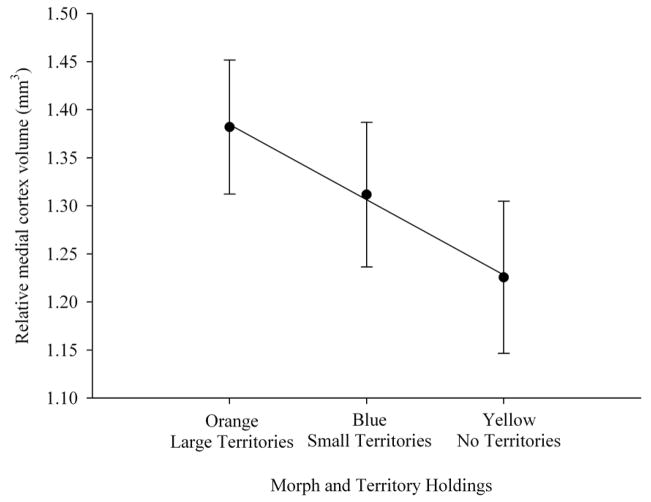
Telencephalon volume relative to snout-vent length was not significantly different among orange, blue and yellow morphs (F2,20 = 0.701, p = 0.508; SVL covariate F1,20 = 2.925, p = 0.011.; Figs. 1, 2, 3). Morph type also did not have a significant association with medial cortex volume relative to the rest of the telencephalon (F2,20 = 1.097, p = 0.353; telencephalon covariate: F1,20 = 9.292, p = 0.006; Fig. 4). However, morph type did have a significant association with relative dorsal cortex volume (F2,20 = 3.557, p = 0.048; Fig. 5; telencephalon covariate: F1,20 = 20.258, p < 0.001). Yellow males had significantly smaller relative dorsal cortices than orange males (p = 0.017) while blue males exhibited a trend towards larger dorsal cortices compared with yellow males (p = 0.07). However, there were no differences in relative dorsal cortical volumes between orange and blue males (p = 0.55).
Figure 1.
Relationship between telencephalon volume and medial cortex volume in three morphs of male side-blotched lizards.
Figure 2.
Relationship between telencephalon volume and dorsal cortex volume in three morphs of male side-blotched lizards.
Figure 3.
Relative telencephalon volume + SE (least squares means from the model with snout-vent length as the covariate) across three male morph types. Orange males defend large territories (n = 9), blue males defend smaller territories (n = 8), while yellow males do not defend territories and have smaller home ranges than orange and blue males (n = 7). There was not a significant effect of morph on relative telencephalon volume.
Figure 4.
Relative medial cortex volume ± SE (least squares means from the model with telencephalon volume as the covariate) across three male morph types. Orange males defend large territories (n = 9), blue males defend smaller territories (n = 8), while yellow males do not defend territories and have smaller home ranges than orange and blue males (n = 7). There was not a significant effect of morph on relative medial cortex volume, but mean values showed perfect correlation with the morph.
Figure 5.
Relative dorsal cortex volume ± SE (least squares means from the model with telencephalon volume as the covariate) across three male morph types. Orange males defend large territories (n = 9), blue males defend smaller territories (n = 8), while yellow males do not defend territories and have smaller home ranges than orange and blue males (n = 7). Orange males had larger dorsal cortical volumes compared with yellow males (p = 0.017), whereas blue males were intermediate.
Finally, the ordered heterogeneity test indicated that morph type, incorporating predicted directionality of morph effects, was significantly associated with relative dorsal cortex volume (rs Pc = 0.953, p< 0.001). These results show that orange males had larger dorsal cortices relative to the rest of the telencephalon than yellow males, whereas blue males were intermediate between the orange and yellow males. Interestingly, relative medial cortex volume followed the same trend as the dorsal cortex and mean values for the three morphs showed perfect negative correlation with space use (rs = 1.0; Fig. 4), although the ordered heterogeneity test was marginally non-significant (rsPc = 0.647, 0.05 < p < 0.1).
DISCUSSION
We found that the dorsal cortex volume relative to the rest of the telencephalon in male side-blotched lizards differs depending on male morph type. Orange males, which hold large territories and occupy the largest home ranges, had relatively larger dorsal cortical volumes compared with yellow males that do not hold territories and have the smallest home ranges of the three morphs compared. Blue males that hold territories and occupy home ranges that are smaller than those of orange males but larger than the home ranges of yellow males had intermediate relative dorsal cortical volumes compared with those of orange and yellow males. Yellow males, which do not hold territories and have the smallest home ranges compared with orange or blue males, had the smallest dorsal cortical volumes.
As predicted, differences in spatial use tactics through territoriality/home range sizes among the morphs were reflected in an area of the brain thought partially responsible for spatial processing (i.e. the dorsal cortex). Males that hold large territories and occupy large home ranges presumably have increased demands on spatial ability due to increased processing of spatial information, whereas males that do not hold territories, have smaller home ranges, and are more philopatric, as in the yellow morph (Zamudio & Sinervo 2000; Sinervo et al. 2006), likely have comparatively lower demands on processing spatial information. This difference appears to be reflected in the relative volume of the dorsal cortex, as an increase in demands on spatial abilities relates to a larger dorsal cortex relative to the rest of the telencephalon. Such differentiation among morphs in relative dorsal cortical volume may result from flexible differential space use strategies or could be genetically determined, but our study does not allow discrimination between these two explanations because the animals in this study were free-ranging.
We did not, however, find statistically significant differences among morphs in medial cortex volume. This result does not fully coincide with a previous study in lizards, in which both dorsal and medial cortical volumes positively correlated with an increase in foraging activity (Day et al. 1999). Other studies have found that lesions to the medial cortex do result in spatial learning deficits in turtles (Rodríguez et al. 2002a,b; López et al. 2003). Ultimately, the scarcity of information on the importance of the dorsal cortex and the medial cortex in spatial processing in reptiles, as well as the paucity of studies relating spatial memory, ecology and dorsal and medial cortical volumes in reptiles precludes meaningful comparisons across studies. Although our results did not reach statistical significance, medial cortex volume did exhibit the same trend as that found for dorsal cortex volume (Figs. 4 and 5). A larger sample size may potentially clarify the variance surrounding dorsal cortex volumes found in this study, which may lend support that the medial cortex, as well as the dorsal cortex, are involved in spatial processing.
To our knowledge, this is the first study to demonstrate differences in brain anatomy relating to differential behavioral tactics of space use within the same species in a single population of reptiles. The relationship between brain volume and ecology within the same species in a population was also demonstrated in the paper wasp (Polistes dominulus), where the volumes of brain areas dealing with vision and olfaction in queens differed depending on whether the queen was solitary or with other queens (Ehmer et al. 2001). Accounting for species and population differences may be important when these differences confound the relationship between differences in space use and brain anatomy. For instance, hippocampal architecture may be influenced by differential genetic architecture, diet, mating system, ontogeny or dispersal/migration patterns that occur between or within species (e.g. Healy et al. 1998; Hutcheon et al. 2002; Pravosudov et al. 2006). In an interspecific comparison on lizards, Perry & Garland (2002) found that diet affects home range size, while other studies found that different foraging strategies affects hippocampal attributes (Jacobs & Spencer 1994; Day et al. 1999). Although we do not know conclusively if diet or foraging strategy differs between morphs of the side-blotched lizard, these factors may also potentially lead to differences in brain attributes due to differential space use. Evidence also suggests that differences in mating systems may have different effects on hippocampal attributes (Jacobs et al. 1990) and, although all three morphs of side-blotched lizards are polygamous, there are differences in rates of mate acquisition among the morphs. These differences may also lead to differences in hippocampal attributes. Regardless, we suggest that it may be advantageous to employ model species that can potentially control for specific effects that may not be related to space use, especially if these variables may influence hippocampal attributes, as differences in the hippocampal formation may be shaped by differences between species or populations outside of differential spatial processing.
Similarly, sex differences in hippocampal anatomy and function has been shown to be affected by differences in gonadal hormones (e.g. Williams et al. 1990; Roof & Havens 1992; Madeira & Leiberman 1995), outside of effects due to differential space use. While we did use individuals of the same sex in our study, which may have partially mitigated certain non-hormonal reproductive differences mandated by sex, male morphs do have different hormonal profiles, which may also influence space use (DeNardo & Sinervo 1994). Further, individuals may exhibit differences in hormonal sensitivity based on morphotype (Comendant et al. 2003; Knapp et al. 2003). Because of these differences in hormone profiles, space use and other correlated responses such as survival, activity level, immune function, and stress response may be affected and differ among morphs (e.g. Comendant et al. 2003; Knapp et al. 2003; Stapley & Keogh 2004). Although the mechanistic basis of the relationship between space use and the brain was beyond the scope of this study, hormonal underpinnings likely constitute a mechanism underlying the relationship between space use, dorsal cortex volume and potentially other correlated behavioral responses in this species. Future work should consider manipulating hormone levels among these morphs to ascertain the importance of hormone levels on the dorsal and medial cortical volumes, while concurrently controlling for space use.
Further, our results suggest that differential spatial use tactics may correlate with one of the putative reptilian homologues of the mammalian and avian hippocampus. Many previous studies have examined the effects of spatial use, spatial abilities and hippocampal attributes in birds and mammals (e.g. Krebs et al. 1989; Sherry et al. 1989; Jacobs et al. 1990; Sherry et al. 1993; Jacobs & Spencer 1994; Healy & Krebs 1996; Pravosudov & Clayton 2002; Lucas et al. 2004; Pravosudov et al. 2006; Roth & Pravosudov 2009). Many of these studies have found a positive relationship between spatial abilities and hippocampal volume. However, only a few studies have studied whether these effects occur in different taxa, specifically in lizards (Day et al. 1999, 2001). Some studies have found that reptiles can use spatial maps to orient (Holtzman et al. 1999; Zuri & Bull 2000; López et al. 2001; but see Day et al. 2001) and both the dorsal cortex and the medial cortex have been shown to be involved in spatial tasks. In turtles, lesions of the medial cortex have been shown to impair performance on spatial tasks (Rodríguez et al. 2002a,b; López et al. 2003); similarly, dorsal cortex lesions impair spatial mapping in turtles (Grisham & Powers 1990; Petrillo et al. 1994; Reiman-Avigan & Schade-Powers 1995). However, Day et al. (2001) found that medial and dorsal cortical lesions slowed locating a goal, but none of the subjects used a spatially-dependent strategy to locate a goal. We have no data to date on whether differential spatial abilities exist among morphs in side-blotched lizards, but this would be the logical next step in connecting space use, spatial abilities and brain volume among morphs. Because of the limited and conflicting studies to date, future studies addressing spatial abilities in reptiles may be especially fruitful to ascertain if these animals do in fact use spatial strategies, if natural selection has acted on these abilities in particular contexts and whether these abilities are reflected by larger hippocampal homologues. While our study did not address specific mechanisms responsible for causing the differences in brain morphology among the three morphs, it demonstrated an association between a brain region thought to be important in spatial processing and differences in spatial tactics.
Acknowledgments
This research adhered to the ASAB/ABS Guidelines for the Use of Animals in Research and all procedures were approved by the IACUC at the University of Nevada, Reno. We thank the ever stygian T.C. Roth II for comments on earlier versions of this draft. Procedures were approved by University of Nevada, Reno IACUC (#00114). This research was supported by grants from NSF (IOB-0615021) and NIH (MH079892, MH076797) to V.V.P and NSF (LTREB DEB 051597) to B.S. and A. McAdam. Animals were collected under a California Department Fish and Game permit issued to B.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brennan P, Kaba H, Keverne EB. Olfactory recognition: a simple memory system. Science. 1990;250:1223–1226. doi: 10.1126/science.2147078. [DOI] [PubMed] [Google Scholar]
- Butler AB. Telencephalon of the lizard Gekko gecko: some connections of the cortex and dorsal ventricular ridge. Brain, Behavior and Evolution. 1976;13:396–417. doi: 10.1159/000123824. [DOI] [PubMed] [Google Scholar]
- Calsbeek R, Sinervo B. The ontogeny of territoriality during maturation. Oecologica. 2002;132:468–477. doi: 10.1007/s00442-002-0975-8. [DOI] [PubMed] [Google Scholar]
- Clayton NS. Hippocampal growth and maintenance depend on food-caching experience in juvenile mountain chickadees (Poecile gambeli) Behavioral Neuroscience. 2001;115:614–625. doi: 10.1037//0735-7044.115.3.614. [DOI] [PubMed] [Google Scholar]
- Comendant T, Sinervo B, Svensson EI, Wingfield J. Social competition, corticosterone and survival in female lizard morphs. Journal of Evolutionary Biology. 2003;16:948–955. doi: 10.1046/j.1420-9101.2003.00598.x. [DOI] [PubMed] [Google Scholar]
- Day LB. The importance of hippocampus-dependent non-spatial tasks in analyses of homology and homoplasy. Brain, Behavior and Evolution. 2003;62:96–107. doi: 10.1159/000072440. [DOI] [PubMed] [Google Scholar]
- Day LB, Crews D, Wilczynski W. Relative medial and dorsal cortex volume in relation to foraging ecology in congeneric lizards. Brian, Behavior and Evolution. 1999;54:314–322. doi: 10.1159/000006631. [DOI] [PubMed] [Google Scholar]
- Day LB, Crews D, Wilczynski W. Effects of medial and dorsal cortex lesions on spatial memory in lizards. Behavioural Brain Research. 2001;118:27–42. doi: 10.1016/s0166-4328(00)00308-9. [DOI] [PubMed] [Google Scholar]
- DeNardo DF, Sinervo B. Effects of steroid hormone interaction on activity and home range size of male lizards. Hormones and Behaviors. 1994;28:273–287. doi: 10.1006/hbeh.1994.1023. [DOI] [PubMed] [Google Scholar]
- Ehmer B, Reeve HK, Hoy RR. Comparison of brain volumes between single and multiple foundresses in the paper wasp Polistes dominulus. Brain, Behavior and Evolution. 2001;57:161–168. doi: 10.1159/000047234. [DOI] [PubMed] [Google Scholar]
- Girvan JR, Braithwaite VA. Population differences in spatial learning in three-spined sticklebacks. Proceedings of the Royal Society of London, B. 1998;265:913–918. [Google Scholar]
- Godard R. Long-term memory of individual neighbours in a migratory songbird. Nature. 1991;350:228–229. [Google Scholar]
- Grisham W, Schade-Powers A. Function of the dorsal and medial cortex of turtles in learning. Behavioral Neuroscience. 1989;103:991–997. doi: 10.1037//0735-7044.103.5.991. [DOI] [PubMed] [Google Scholar]
- Grisham W, Powers AS. Effects of dorsal and medial cortex lesion on reversals in turtles. Physiology & Behavior. 1990;47:43–49. doi: 10.1016/0031-9384(90)90040-b. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficacy of systematic sampling in stereology and its predictions. Journal of Microscopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Healy SD, Krebs JR. Food storing and the hippocampus in Paridae. Brain Behavior and Evolution. 1996;47:195–199. doi: 10.1159/000113239. [DOI] [PubMed] [Google Scholar]
- Healy SD, Gwinner E, Krebs JR. Hippocampal volume in migratory and non-migratory warblers: effects of age and experience. Behavioral Brain Research. 1998;81:61–68. doi: 10.1016/s0166-4328(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Holtzman DA, Harris TW, Aranguren G, Bostock E. Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata. Animal Behaviour. 1999;57:51–60. doi: 10.1006/anbe.1998.0971. [DOI] [PubMed] [Google Scholar]
- Hutcheon JM, Kirsch JAW, Garland T., Jr A comparative analysis of brain size in relation to foraging ecology and phylogeny in the Chiroptera. Brain, Behavior and Evolution. 2002;60:165–180. doi: 10.1159/000065938. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Spencer WD. Natural space-use patterns and hippocampal size in kangaroo rats. Brain, Behavior and Evolution. 1994;44:125–132. doi: 10.1159/000113584. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Sciences USA. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Hews DK, Thompson CW, Ray LE, Moore MC. Environmental and endocrine correlates of tactic switching by nonterritorial male tree lizards (Urosaurus ornatus) Hormones and Behavior. 2003;43:83–92. doi: 10.1016/s0018-506x(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proceedings of the National Academy of Sciences USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JC, Gómez Y, Rodríguez F, Broglio C, Vargas JP, Salas C. Spatial learning in turtles. Animal Cognition. 2001;4:49–59. [Google Scholar]
- López JC, Vargas JP, Gómez Y, Salas C. Spatial and non-spatial learning in turtles: the role of medial cortex. Behavioural Brain Research. 2003;143:109–120. doi: 10.1016/s0166-4328(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Brodin A, de Kort SR, Clayton NS. Does hippocampal size correlate with the degree of caching specialization? Proceedings of the Royal Society of London B. 2004;271:2423–2429. doi: 10.1098/rspb.2004.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Leiberman AR. Sexual dimorphism in the mammalian limbic system. Progress in Neurobiology. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Menzel R, Brandt R, Gumbert A, Komischke B, Kunze J. Two spatial memories for honeybee navigation. Proceedings of the Royal Society of London B. 2000;267:961–968. doi: 10.1098/rspb.2000.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impared in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Perry G, Garland T. Lizard home ranges revisited: effects of sex, body size, diet, habitat, and phylogeny. Ecology. 2002;83:1870–1885. [Google Scholar]
- Petrillo M, Ritter CA, Schade-Powers A. A role for acetylcholine in spatial memory in turtles. Physiology & Behavior. 1994;56:135–141. doi: 10.1016/0031-9384(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Pravosudov VV, Clayton NS. A test of the adaptive specialization hypothesis: population differences in caching, memory and the hippocampus in black-capped chickadees (Poecile atricapilla) Behavioral Neuroscience. 2002;116:515–522. [PubMed] [Google Scholar]
- Pravosudov VV, Kitaysky AS, Omanska A. The relationship between migratory behaviour, memory and the hippocampus: an intraspecific comparison. Proceedings of the Royal Society B. 2006;273:2641–2649. doi: 10.1098/rspb.2006.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman-Avigan M, Schade-Powers A. The effects of MK-801 injections and dorsal cortex lesions on maze learning in turtles (Chrysemys picta) Psychobiology. 1995;23:63–68. [Google Scholar]
- Rice W, Gaines SD. Extending nondirectional heterogeneity tests to evaluate simply ordered alternative hypotheses. Proceedings of the National Academy of Sciences, USA. 1994a;91:225–226. doi: 10.1073/pnas.91.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W, Gaines SD. The ordered-heterogeneity family of tests. Biometrics. 1994b;50:746–752. [Google Scholar]
- Rodríguez F, López JC, Vargas JP, Broglio C, Gómez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: Insights from reptiles and teleost fish. Brain Research Bulletin. 2002a;57:499–503. doi: 10.1016/s0361-9230(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C, Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. Journal of Neuroscience. 2002b;22:2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Research. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Roth ED, Lutterschmidt WI, Wilson DA. Relative medial and dorsal cortex volume in relation to sex differences in spatial ecology of a snake population. Brain, Behavior and Evolution. 2006;67:103–110. doi: 10.1159/000089183. [DOI] [PubMed] [Google Scholar]
- Roth TC, Pravosudov VV. Hippocampal volume and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proceedings of the Royal Society B. 2009 doi: 10.1098/rspb.2008.1184. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas C, Broglio C, Rodríguez F. Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain, Behavior and Evolution. 2003;62:72–82. doi: 10.1159/000072438. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Vaccarino AL, Buckenham K, Herz RS. The hippocampal complex of food storing birds. Brain Behavior & Evolution. 1989;34:308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Forbes MRL, Khurgel M, Ivy GO. Females have a larger hippocampus than males in the brood-parasitic brown-headed cowbird. Proceedings of the National Academy of Sciences, USA. 1993;90:7839–7843. doi: 10.1073/pnas.90.16.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettleworth SJ. Spatial memory in food-storing birds. Philosophical Transactions of the Royal Society of London B. 1990;329:143–151. [Google Scholar]
- Sinervo B, Lively CM. The rock-paper-scissors game and the evolution of alternative male strategies. Nature. 1996;380:240–243. [Google Scholar]
- Sinervo B, Zamudio KJ. The evolution of alternative reproductive strategies: fitness differential, heritability, and genetic correlation between the sexes. Journal of Heredity. 2001;98:198–205. doi: 10.1093/jhered/92.2.198. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Miles DB, Frankino WA, Klukowski M, DeNardo DF. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Hormones and Behavior. 2000;38:222–233. doi: 10.1006/hbeh.2000.1622. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Calsbeek R, Comendant T, Both C, Adamopoulou C, Clobert J. Genetic and maternal determinants of effective dispersal: the effect of sire genotype and size at birth in side-blotched lizards. American Naturalist. 2006;168:88–99. doi: 10.1086/505765. [DOI] [PubMed] [Google Scholar]
- Stapley J, Keogh JS. Exploratory and antipredator behaviours differ between territorial and nonterritorial male lizards. Animal Behaviour. 2004;68:841–846. [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation of spatial memory. Behavioral Neuroscience. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Zamudio KR, Sinervo B. Polygyny, mate-guarding, and posthumous fertilization as alternative male mating strategies. Proceedings of the National Academy of Sciences, USA. 2000;97:14427–14432. doi: 10.1073/pnas.011544998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuri I, Bull CM. The use of visual cues for spatial orientation in the sleepy lizard (Tiliqua rugosa) Canadian Journal of Zoology. 2000;78:515–520. [Google Scholar]