Abstract
Aims
This study examined the functional role of B-type natriuretic peptide (BNP) in epoxyeicosatrienoic acid (EET)-mediated cardioprotection in mice with targeted disruption of the sEH or Ephx2 gene (sEH null).
Methods and results
Isolated mouse hearts were perfused in the Langendorff mode and subjected to global no-flow ischaemia followed by reperfusion. Hearts were analysed for recovery of left ventricular developed pressure (LVDP), mRNA levels, and protein expression. Naïve hearts from sEH null mice had similar expression of preproBNP (Nppb) mRNA compared with wild-type (WT) hearts. However, significant increases in Nppb mRNA and BNP protein expression occurred during post-ischaemic reperfusion and correlated with improved post-ischaemic recovery of LVDP. Perfusion with the putative EET receptor antagonist 14,15-epoxyeicosa-5(Z)-enoic acid prior to ischaemia reduced the preproBNP mRNA in sEH null hearts. Inhibitor studies demonstrated that perfusion with the natriuretic peptide receptor type-A (NPR-A) antagonist, A71915, limited the improved recovery in recombinant full-length mouse BNP (rBNP)- and 11,12-EET-perfused hearts as well as in sEH null mice. Increased expression of phosphorylated protein kinase C ε and Akt were found in WT hearts perfused with either 11,12-EET or rBNP, while mitochondrial glycogen synthase kinase-3β was significantly lower in the same samples. Furthermore, treatment with the phosphoinositide 3-kinase (PI3K) inhibitor wortmannin abolished improved LVDP recovery in 11,12-EET-treated hearts but not did significantly inhibit recovery of rBNP-treated hearts.
Conclusion
Taken together, these data indicate that EET-mediated cardioprotection involves BNP and PI3K signalling events.
KEYWORDS: B-type natriuretic peptide, Epoxyeicosatrienoic acid, Ischaemia-reperfusion, GSK-3β
1. Introduction
Arachidonic acid (AA), an essential polyunsaturated fatty acid found esterified to membrane phospholipids, can be metabolized by cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 (CYP) epoxygenases to numerous products collectively termed eicosanoids, which have important cellular functions.1,2 Ischaemic events activate cytosolic phospholipase A2, which will release AA from stores of glycerophospholipids.3 AA can then be metabolized by various CYPs to four regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EETs), all of which are biologically active.2 Modulation of EET levels can occur via re-incorporation into phospholipid membranes or by β-oxidation to smaller reactive epoxides;4,5 however, the predominant pathway occurs through metabolism to inactive vicinal diols compounds by epoxide hydrolases.1,6 Two major epoxide hydrolases are found in mammalian tissues—microsomal epoxide hydrolase (mEH) and soluble epoxide hydrolase (sEH or Ephx2).5
Accumulating evidence indicates that EETs have important functional roles in ischaemia-reperfusion injury. They activate various stress response systems, such as the phosphoinositide 3-kinase (PI3K) pathway; enhance membrane ion channel activity, such as sarcolemmal (sarcKATP) and mitochondrial (mitoKATP) ATP-sensitive K+ channels; and improve post-ischaemic recovery of left ventricular function.7–12 Evidence indicates that EET-mediated cardioprotective signals target glycogen synthase kinase-3β (GSK-3β), a key protein involved in cellular function and signalling essential to cardioprotection.13,14 Regulated via phosphorylation, GSK-3β acts as a central convergence point targeting upstream signalling to mitochondria.13,15 Published reports indicate that multiple protective pathways converge upon GSK-3β prior to effecting mitochondrial function.7,13,16 Alteration in the production and/or elimination of EETs may affect steady-state cellular levels of these bioactive eicosanoids in vivo and could potentially influence cardiac function.
B-type or brain natriuretic peptide (BNP) is becoming a valuable biomarker for cardiovascular disease that bears diagnostic, prognostic, and therapeutic importance in congestive heart failure, arrhythmias, and acute myocardial infarction.17,18 Evidence indicates that BNP can attenuate ischaemic-reperfusion injury in animal models but the underlying mechanism is unknown.19,20 Isolated perfused rat heart studies suggest that increased BNP expression at baseline and release of peptide in the coronary effluent during reperfusion are attributed to wall stretch and acute ischaemic injury.21,22 Cardioprotective effects of BNP correlate with elevated cGMP and NO levels, which can be abolished by inhibiting the mitoKATP channel.20,21 The specific involvement of BNP and mitoKATP opening in cardioprotection is largely unknown. The anti-ischaemic profile of natriuretic peptides and correlation to mitoKATP suggest that endogenous BNP may be an attractive target for cardioprotection and may warrant further investigation.
Recently, we reported that mice with the targeted disruption of the Ephx2 gene had enhanced post-ischaemic recovery of left ventricular function, which was mediated by activation of the PI3K pathway and K+ channels.10 In the present study, we demonstrate that EET-mediated cardioprotection involves increased expression of BNP. Moreover, our data suggest a role for PKCε and GSK-3β in integrating EET-mediated effects to the mitochondria. Taken together, these data merge two endogenous cardioprotective mediators, EETs and BNP, provide a novel mechanism for cardioprotection, and suggest a potential target for therapeutic intervention.
2. Methods
For an elaborate Methods section, see Supplementary material online.
2.1. Development of rabbit anti-BNP polyclonal antibody
Sequence variability of BNP between species is large and therefore production of a mouse polyclonal antibody was required.23 Rabbit anti-BNP polyclonal antibody was produced using a female rabbit (NZW) immunized with recombinant full-length mouse BNP (rBNP) antigen emulsified with Freunds adjuvant (Sigma) (1:1). Immune serum was collected and ELISA was performed for antibody titre. Anti-BNP polyclonal antibody was then purified using protein-G affinity chromatography column (Sigma, USA) as described.24
2.2. Production of rBNP and anti-BNP antibody
The Escherichia coli codon-optimized BNP cDNA was cloned into plasmid pBM802 in the reading frame with His6 tag at the C-terminal and controlled by the arabinose pBAD promoter to increase protein expression levels in inclusion bodies (Supplementary material online, Figure S1a).25 The pDS18BNP was transformed into E. coli expression host Top10 to generate recombinant BNP (rBNP) protein with a predicted molecular mass of 16 kDa. Immunoblot analysis using anti-His6 MAb detected rBNP protein at the expected mass (Supplementary material online, Figure S1b). Medium scale production was then performed to generate rBNP protein that was subsequently extracted from inclusion bodies, purified, and refolded according to previously published techniques (Supplementary material online, Figure S1c).26 Immunoblot analysis with anti-His6 MAb demonstrated that the refolded rBNP purified protein electrophoresed as a single band and was stable for 7 days in PBS at 4°C (Supplementary material online, Figure S1d). Purified rBNP was used to immunize rabbit for production of the polyclonal antibody against mouse BNP. Immunoblot analysis demonstrated that anti-rabbit mouse BNP polyclonal antibody was able to detect rBNP (Supplementary material online, Figure S1e).
2.3. Animals
A colony of mice with targeted disruption of the Ephx2 gene (sEH null), originating from Darryl Zeldin (NIH/NIEHS) and backcrossed onto a C57BL6 genetic background for more than seven generations, is maintained at the University of Alberta. C57BL6 mice and New Zealand White rabbit were purchased from Charles River Laboratories (Pointe Claire, PQ). All experiments used male and female mice aged 3–5 months, weighing 20–34 g and were treated in accordance with the guidelines of Health Science Laboratory Animal Services (HSLAS), University of Alberta. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.4. Isolated heart perfusions
Hearts were perfused in the Langendorff mode as previously published.14,27 Briefly, hearts were perfused with Krebs–Henseleit buffer for 40 min of baseline and subjected to 20 or 30 min of global no-flow ischaemia followed by 40 min of reperfusion. For some experiments, hearts were perfused with rBNP (10 nmol/L), 11,12-EET (1 µmol/L), NPR-A antagonist A71915 (50–100 nmol/L) (Bachem, USA), PI3K inhibitor wortmannin (200 nmol/L) (Sigma–Aldrich, Oakville, ON) or the putative EET receptor antagonist 14,15-EEZE (1 µmol/L) throughout 40 min of reperfusion. The percentage of left ventricular developed pressure (%LVDP) at 40 min of reperfusion (R40), when compared with baseline LVDP, was taken as a marker for recovery of contractile function. After 40 min of reperfusion, hearts were immediately frozen and stored below −20°C.
2.5. Gene expression
Quantitative real-time PCR (qPCR) analysis of natriuretic peptide precursor type B (NM_008726) expression was performed in hearts from naïve mice and isolated perfused experiments. Some isolated hearts were treated with the PI3K inhibitor, LY294002 (5 µmol/L) or the putative EET receptor antagonist 14,15-EEZE (100 nmol/L) prior to ischaemia. Total RNA was isolated using an RNeasy Midi kit (Qiagen, Valencia, CA, USA) and concentrated using a Microcon YM-30 column (Millipore, Billerica, MA, USA). A formaldehyde agarose gel containing ethidium bromide was used to assess the quality of the RNA. mRNA from individual hearts were treated with DNaseI and then 1 µg was used to prepare cDNA with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). cDNA levels were detected using qPCR with the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Each individual sample was normalized to the RNA expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) within the same heart. Fold expression change was determined by quantitation of cDNA from target (perfused) samples relative to a calibrator sample (naïve) of the same genotype. To determine this normalized value, 2−ΔΔCt values were compared between target and calibrator samples, where ΔCt = target gene (crossing threshold) Ct − GAPDH Ct, and ΔΔCt = ΔCtsEHnull − ΔCtWT.28 All the RNAs from WT and sEH null hearts were made on the same day and also analysed together on the same PCR plate.
2.6. Immunoblot analysis
Protein was resolved on SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted as previously described.27 Cytosolic and mitochondrial fractions were prepared from frozen mouse hearts as described.29 Immunoblots were prepared using cytosolic (100 µg protein) or mitochondrial (25 µg protein) fractions and probed with antibodies to PKCε, phospho-PKCε (pSer729PKCε), GSK-3β, phospho-GSK-3β (pSer9GSK-3β), Akt, phospho-Akt (pSer473Akt), and GAPDH (Cell Signaling Technology Inc., Danvers, MA, USA) and prohibitin (Fitzgerald, Concord, MA, USA). GAPDH and prohibitin were used as loading controls for cytosolic and mitochondrial fractions, respectively. Relative band intensities were assessed by densitometry using Image J (NIH, USA). Protein expression in vehicle-treated controls were taken as 100% and compared with treated group.
2.7. Statistical analysis
Values expressed as mean ± SEM. Statistical significance was determined by the unpaired Student's t-test and one-way analysis of variance. To determine whether significant difference exists between the groups, both Duncan's and Newman-Keuls post hoc tests were performed. Values were considered significant if P < 0.05.
3. Results
3.1. Increased BNP expression in sEH null mouse hearts
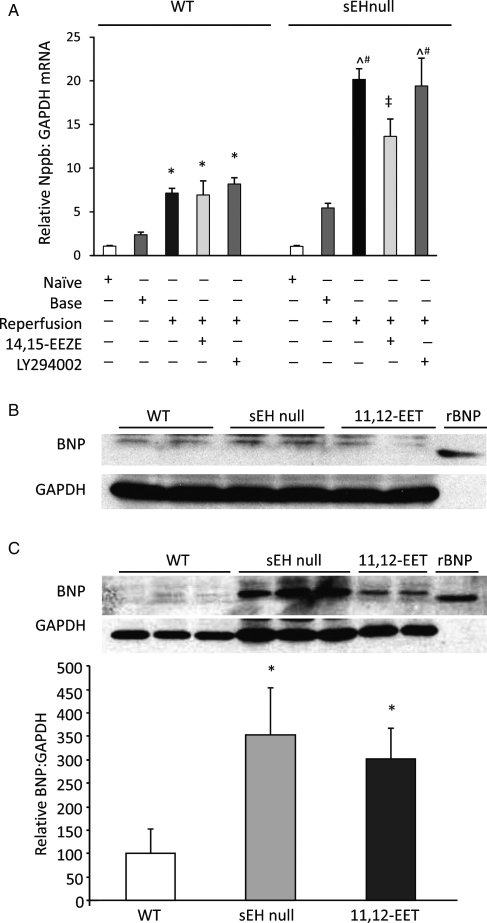
qPCR analysis of preproBNP (Nppb) mRNA revealed no significant differences in cardiac expression between naïve sEH null mice and wild-type (WT) littermates (Figure 1A). Similarly, aerobically perfused WT and sEH null hearts had similar levels of Nppb mRNA (Figure 1A). However, significant differences between WT and sEH null hearts, Nppb mRNA levels were observed following global ischaemia and reperfusion (Figure 1A). To determine if the increased Nppb mRNA levels in sEH null hearts was mediated by EETs, we conducted experiments in the presence of 14,15-EEZE. This putative pan-EET receptor antagonist caused a significant reduction in post-ischaemic Nppb mRNA levels in sEH null hearts but had no effect on WT hearts (Figure 1A). Perfusion of hearts with LY294002, a PI3K inhibitor, did not affect the increased post-ischaemic expression of Nppb mRNA in sEH null or in WT hearts (Figure 1A).
Figure 1.
PreproBNP mRNA and protein expression. (A) qPCR data showing relative expression of Nppb to GAPDH mRNA in non-perfused (naïve) wild-type and sEH null mouse hearts, during 20 min aerobic baseline perfusion (base) and at 40 min post-ischaemic reperfusion (reperfusion). Hearts were administered with vehicle, 14,15-EEZE (1 µmol/L), or LY294002 (5 µmol/L). Values represent mean ± SEM (n = 3). *P < 0.05 vs. naïve WT; ^P < 0.05 vs. vehicle-treated WT; #P < 0.05 vs. naïve sEH null; ‡P < 0.05 vs. vehicle-treated controls of the same genotype. (B) Representative immunoblots showing expression of BNP in WT, sEH null, and 11,12-EET- (1 µmol/L) treated hearts during aerobic baseline. (C) Representative immunoblots and densitometry showing relative abundance of BNP to GAPDH in WT, sEH null, and 11,12-EET- (1 µmol/L) treated hearts at 40 min reperfusion following global ischaemia. Values represent mean ± SEM (n = 6 per group). *P < 0.05 vs. WT.
While immunoblotting with the anti-mouse BNP antibody demonstrated no significant differences in BNP protein expression following aerobic perfusion (Figure 1B), a significant increase in post-ischaemic BNP protein expression was observed in sEH null hearts compared to WT hearts (Figure 1C). In addition, significantly higher expression of BNP was observed in post-ischaemic WT hearts perfused with exogenous 11,12-EET (Figure 1C). Densitometric analysis revealed that BNP expression in sEH null mice hearts was four times higher than in WT hearts while exogenous 11,12- EET-treated WT hearts had two times higher BNP expression than vehicle-treated WT hearts (Figure 1C). EET-mediated increased expression was attenuated by co-treatment with 14,15-EEZE (Supplementary material online, Figure S3).
3.2. NPR-A signalling in EET and rBNP post-ischaemic contractile function
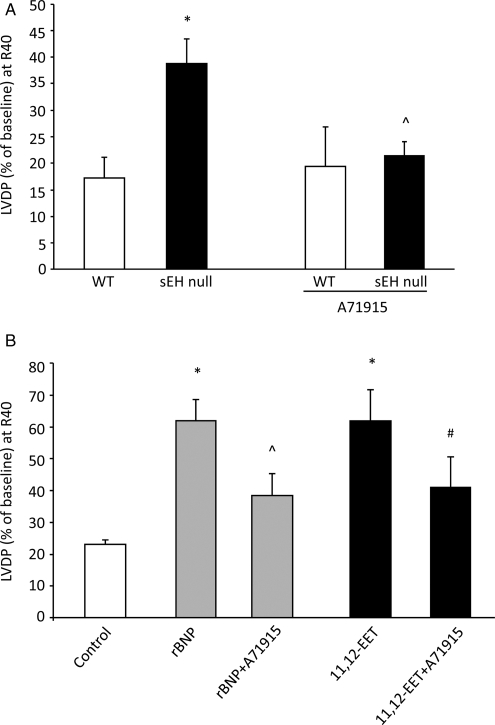
Consistent with our previous observations,14 sEH null and WT mice had normal baseline contractile function as measured by LVDP. Furthermore, sEH null hearts had improved post-ischaemic LVDP recovery following 30 min of global ischaemia and 40 min reperfusion compared with WT hearts (35.6% vs.17.2%, respectively, P < 0.05) (Figure 2A). Likewise, treatment of WT hearts with 11,12-EET improved post-ischaemic LVDP (Figure 2B; see Supplementary material online, Tables S2 and S3).
Figure 2.
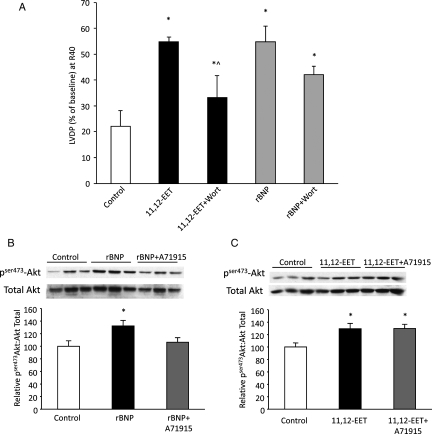
Effect of exogenous rBNP and 11,12-EET on post-ischaemic contractile function of WT hearts. (A) LVDP recovery at 40 min of reperfusion in sEH or WT hearts treated with A71915 (100 nmol/L) or vehicle. Values represent mean ± SEM (n = 4–9 per group). *P < 0.05 vs. WT; ^P < 0.05 vs. vehicle control. (B) LVDP recovery at 40 min of reperfusion as percentage of baseline. Hearts were perfused with exogenous rBNP (10 nmol/L) or 11,12-EET (1 µmol/L) in presence or absence of NPR-A antagonist (A71915) (50 nmol/L). Values represent mean ± SEM (n = 5–9 per group). *P < 0.05 vs. vehicle control; ^P < 0.05 vs. treated with rBNP; #P < 0.05 vs. treated with 11,12-EET.
Recent evidence suggests a role for BNP in the recovery of post-ischaemic cardiac function.19,30,31 The increased BNP expression in sEH null and 11,12-EET-treated hearts suggested the hypothesis that BNP may be a mediator of the cardioprotective effects of EETs. To examine the role of BNP, we first perfused WT mouse hearts with exogenous rBNP (10 nmol/L) to study the effect of our recombinant protein on post-ischaemic contractile function. Hearts treated with exogenous rBNP did not demonstrate significant changes to baseline LVDP (data not shown); however, these BNP-treated hearts showed a significant increase in post-ischaemic functional recovery when compared with vehicle-treated control hearts (62% vs. 23%, respectively, P < 0.05) (Figure 2B). Perfusion with the natriuretic peptide receptor-A (NPR-A) antagonist A71915 attenuated the improved post-ischaemic functional recovery in WT hearts perfused with rBNP (Figure 2B). To further assess the role of BNP in EET-mediated cardioprotection, WT hearts were perfused with 11,12-EET in the presence or absence of A71915. Perfusion with A71915 during ischaemia and reperfusion significantly inhibited, but did not completely reverse, the improved post-ischaemic recovery observed in 11,12-EET-treated hearts (Figure 2B). Moreover, a significant reduction in post-ischaemic functional recovery of sEH null hearts was observed when perfused with A71915 (Figure 2A). In contrast, there was no effect of A71915 treatment on WT hearts (Figure 2A). Together, these data suggest that 11,12-EET-mediated cardioprotection is dependent upon the NPR-A receptor.
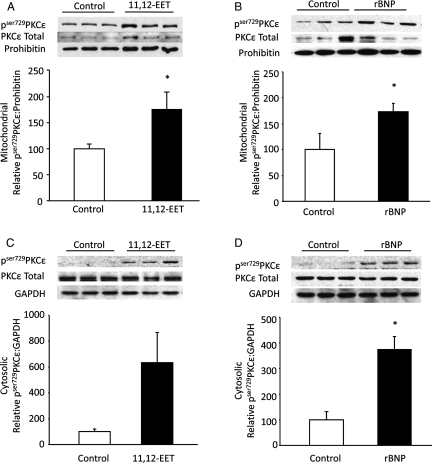
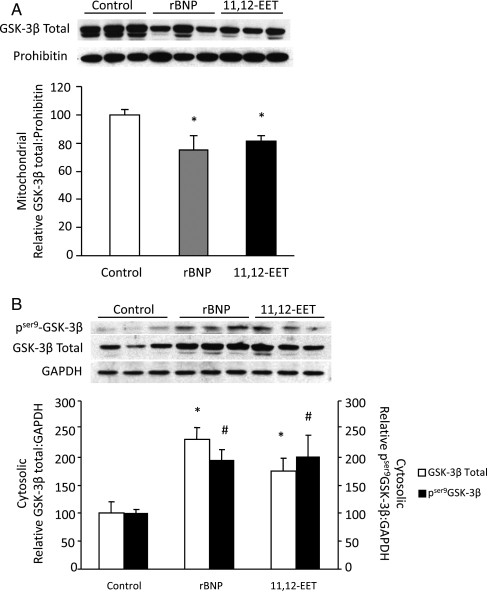
To further assess downstream targets of the NPR-A cascade, we examined the expression of pSer729PKCε in mitochondrial and cytosolic fractions. In 11,12-EET-perfused hearts, significant increases in the phosphorylation of PKCε were observed in mitochondrial fraction while a clear trend towards increase in cytosolic fractions were observed at 40 min of reperfusion (Figure 3A and C). Similar results were obtained from rBNP-perfused hearts with significant increases in mitochondrial and cytosolic pSer729PKCε levels (Figure 3B and D). Evidence suggests that PKCε-mediated cardioprotection involves increased phosphorylation and subsequent inactivation of GSK-3β.13 A significant decrease in mitochondrial total GSK-3β expression was observed in 11,12-EET and rBNP-treated hearts when compared with vehicle-treated controls at R40 (Figure 4A). Conversely, higher expression levels of phosphorylated GSK-3β and total GSK-3β were observed in cytosolic fractions from hearts perfused with 11,12-EET or rBNP (Figure 4B). Together these data indicate that 11,12-EET and BNP trigger phosphorylation of PKCε and this event is associated with phosphorylation and inactivation of GSK-3β.
Figure 3.
Phosphorylated PKCε expression in mitochondria and cytosol fraction of ischaemic-reperfused mouse hearts. (A) Representative immunoblot and densitometry showing pser729PKCε and total PKCε in mitochondrial fractions of 11,12-EET (1 µmol/L) or vehicle-treated WT mouse hearts. Values represent mean ± SEM (n = 6 per group). *P < 0.05 vs. vehicle control. (B) Representative immunoblot and densitometry demonstrating pser729PKCε and total PKCε in mitochondrial fractions of rBNP (10 nmol/L) or vehicle-treated WT mouse hearts. Values represent mean ± SEM (n = 5 per group). *P < 0.05 vs. vehicle control. (C) Representative immunoblot and densitometry of cytosolic fraction of 11,12-EET (1 µmol/L) or vehicle-treated C57BL6 mice hearts showing pser729PKCε and total PKCε expression. Values represent mean ± SEM (n = 6 per group). *P < 0.05 vs. vehicle control. (D) Representative immunoblot and densitometry of cytosolic fraction of rBNP (10 nmol/L) or vehicle-treated WT mouse hearts. pser729PKCε and total PKCε expression. Values represent mean ± SEM (n = 5 per group). *P < 0.05 vs. vehicle control.
Figure 4.
GSK-3β expression in ischaemic-reperfused mouse hearts. (A) Representative immunoblot and densitometry of total GSK-3β in mitochondrial fractions of 11,12-EET (1 µmol/L), rBNP (10 nmol/L), or vehicle-treated hearts. Values represent mean ± SEM (n = 3 per group). *P < 0.05 vs. vehicle control. (B) Representative immunoblot for cytosolic expression of pser9GSK-3β and total GSK-3β in 11,12-EET (1 µmol/L), rBNP (10 nmol/L), or vehicle-treated hearts. Values represent mean ± SEM (n = 3 per group). *P < 0.05 total GSK-3β-treated vs. vehicle control; #P < 0.05 pSer9GSK-3β-treated vs. vehicle control.
3.3. Role of PI3K pathway in EET- and BNP-mediated cardioprotection
As previously documented, EET-mediated cardioprotection involves activation of a PI3K pathway while BNP causes activation of a NPR-A/cGMP/PKG pathway.14,19,30 Partial inhibition of EET-mediated cardioprotection by A71915 (Figure 2B) suggests that EETs may work through multiple pathways. Therefore, we investigated the contribution of PI3K and NPR-A in the EET–BNP-mediated cardioprotection. Hearts from WT mice were perfused with 11,12-EET or rBNP in the presence or absence of the PI3K inhibitor wortmannin. Post-ischaemic functional recovery was significantly inhibited in hearts co-perfused with 11,12-EET and wortmannin but did not return to vehicle control levels (Figure 5A). Interestingly, wortmannin also resulted in a partial inhibition of post-ischaemic functional recovery in hearts co-perfused with rBNP, although these changes were not statistically significant (Figure 5A). To further assess downstream targets in the PI3K cascade, we examined the phosphorylation status of Akt following ischaemia-reperfusion. Expression levels of pSer473Akt were significantly higher in both 11,12-EET- and rBNP-perfused when compared with vehicle control hearts at 40 min of reperfusion (Figure 5B and C). Moreover, the ratio of pSer473Akt to total Akt expression was significantly decreased in WT hearts perfused with both rBNP and A71915 (Figure 5C). These data suggest that EET-mediated signalling down the PI3K pathway does not occur solely via the NPR-A receptor.
Figure 5.
PI3K activation in EET- and BNP-mediated cardioprotection. (A) LVDP recovery at 40 min of reperfusion as percentage of baseline. Hearts treated with exogenous rBNP (10 nmol/L) or 11,12-EET (1 µmol/L) in presence and absence of PI3K antagonist (wortmannin) (200 nmol/L). Values represent mean ± SEM (n = 5–7 per group). *P < 0.05 vs. vehicle control; ^P < 0.05 vs. treated with 11,12-EET. (B) Representative immunoblot and densitometry demonstrating expression of pser473Akt to total Akt in control, 11,12-EET-treated, or 11,12-EET- and A71915-treated C57BL6 mice hearts. Values represent mean ± SEM (n = 3 per group). *P < 0.05 vs. vehicle control. (C) Immunoblot and densitometry demonstrating expression of pser473Akt to total Akt expression in control, rBNP-treated, or rBNP- and A71915-treated hearts. Values represent mean ± SEM (n = 6 per group). *P < 0.05 vs. vehicle control.
4. Discussion
Since the first report of the cardioprotective effects CYP epoxygenase metabolites of arachidonic acid against ischaemia reperfusion injury by Wu et al., there has been considerable interest in investigating this novel protective mechanism.7,14,27 However, important questions remain unanswered regarding the molecular and cellular mechanisms responsible for this protection. Evidence suggests that cardioprotective signalling of EETs involves activation of PI3K/Akt pathway which further prevents ischaemic injury by reducing mitochondrial damage through activation of mitoKATP channels.14 Herein, we demonstrate for the first time, that targeted disruption of the Ephx2 gene and treatment with exogenous 11,12-EET leads to increased BNP expression following ischaemia-reperfusion resulting in improved functional recovery. Moreover, our data suggest a critical role of BNP and GSK-3β in mediating the cardioprotective effect of EETs. Thus, EET-mediated cardioprotection involves two pathways, PI3K/Akt and BNP-PKCε-mediated signalling, which converge on the mitochondria. Taken together, these data suggest that EETs have important intracellular protective effects in the ischaemic heart that limits functional damage attributed to ischaemia-reperfusion injury.
BNP is a cardiac hormone predominantly found in ventricles of heart. Considered an immediate-early gene, it can be both rapidly synthesized and released within 1 h in response to stimuli like stretch, volume, pressure overload and ischaemia.32 Basal expression levels of Nppb mRNA were found to be similar in naïve sEH null and WT hearts, indicating targeted disruption of Ephx2 gene does not alter BNP under normal conditions. Studies in isolated rat hearts demonstrate that increased Nppb mRNA expression was observed during aerobic perfusion, reflecting a stretch-induced activation.33,34 We observed an increased Nppb mRNA levels during aerobic perfusion, which was slightly higher in sEH null hearts compared with WT hearts. This early increased expression did not translate into elevated BNP protein levels. However, the significant increases in Nppb mRNA levels observed in sEH null hearts following ischaemia-reperfusion correlated with large increases in BNP protein levels in sEH null hearts and WT mice perfused with EETs. Consistent with our previous results from sEH null mice, cardiac effects of sEH disruption occurred only after ischaemia, presumably owing to enhanced release of EETs from phospholipid stores.14 A role for EETs in the induction of BNP is suggested by our observation that the putative EET receptor antagonist 14,15-EEZE attenuates the increase in Nppb mRNA in sEH null mice.
Apart from mechanical stimulus, BNP gene expression can be induced in response to various factors such as endothelin-1, β-adrenergic stimulation, and proinflammatory cytokines, like interleukin-1β.35 Interleukin-1β induces BNP gene expression, partially through a Ras, Rac, and p38 kinase pathway, where p38 kinase acts on the MCAT element at position-97 of human BNP gene promoter.35,36 Alternatively, stabilization of Nppb mRNA via an MAPK and PKC-dependent mechanism results in increased levels as demonstrated in neonatal rat cardiomyocytes.37 While the mechanism for EET-mediated activation of BNP is not known, the observation that LY294002 did not affect increased Nppb mRNA in sEH null hearts suggests that the PI3K pathway is not involved. Our results demonstrate both stretch and ischaemia-reperfusion injury increase Nppb mRNA expression in WT hearts, which was markedly enhanced in sEH null hearts. Importantly, increased Nppb mRNA correlated with elevated BNP protein expression in hearts of both sEH null and WT mice perfused with EETs following ischaemia-reperfusion. These results provide further evidence for the cardioprotective effects of EETs and suggest they act as a novel regulator of BNP release.
Following ischaemia, rapid gene expression and de novo peptide synthesis occurs which results in increased plasma levels of BNP in acute myocardial infarction patients.32 BNP plasma concentrations are biomarkers for heart disease providing a valuable diagnostic tool for cardiac patients.38 However, the role of BNP in the pathophysiology of ischaemia-reperfusion injury and whether the heart is a target remains to be elucidated. BNP can exert autocrine or paracrine effects by binding with natriuretic peptide receptor type-A (NPR-A), which activates particulate guanylyl cyclase (pGC), thereby increasing formation of cGMP leading to activation of PKG.39 PKG can then activate PKC, particularly PKCε, which will translocate to mitochondria interacting with various mitochondrial membrane proteins, such as mitoKATP channels.13,40 Translocation of PKCε to mitochondria and activation of mitoKATP channels are believed to protect the myocardium from ischaemia-reperfusion injury, with a net effect of protecting mitochondrial function.13,29 D'souza et al.31 demonstrated that perfusion of rat hearts with endogenous BNP induces protection against reperfusion injury involving pGC and activation of KATP channels. We established that EET-mediated cardioprotection in CYP2J2 transgenic and sEH null mice involve activation of K+ channels; however, the exact intracellular signal remains unknown.14,27 In the present study, we observed increased levels of PKCε in mitochondrial fractions of hearts perfused with 11,12-EET or rBNP, which correlated with improved post-ischaemic functional recovery. Furthermore, administration of NPR-A antagonist, A71915, attenuated the improved post-ischaemic recovery of sEH null hearts and hearts perfused with rBNP peptide, but only partial inhibition was observed with exogenous EETs. Taken together, our experiments suggest that EET-mediated cardioprotection, at least in part, involves NPR-A activation of intracellular pathways that target the mitochondria.
GSK-3β is a constitutively active protein which is an important regulator of cellular function and a key enzyme in response to myocardial ischaemia-reperfusion injury.13 Inhibition of GSK-3β, either by increased phosphorylation or pharmacological inactivation, is known to reduce cell death caused by ischaemia-reperfusion injury. Increased expression of GSK-3β in mitochondria following ischaemia-reperfusion has been reported in cardiomyocytes and isolated heart experiments.41 The significance and mechanism of GSK-3β translocation has not been determined but evidence suggests a role for PI3K and PKC kinases.41 In addition, the specific effect of increased mitochondrial GSK-3β is not completely known but potentially involves modulation of function. Juhaszova et al.13 hypothesized that cardioprotective signalling pathways converge onto GSK-3β resulting in the prevention of mitochondrial permeability transition (mPTP). Recent work by Das and coworkers42 reports that inhibition of GSK activity induces dephosphorylation of VDAC, which subsequently preserves ATP levels, prevents calcium overloading, and oxidant stress independent of mPTP opening. Phosphorylation of GSK-3β by upstream kinases like Akt and PKC inactivate GSK-3β preventing myocardial damage from ischaemia-reperfusion injury.43 Previously, we demonstrated that cardioprotection in sEH null mice involves activation of PI3K followed by phosphorylation of GSK-3β.14 Interestingly, our current results demonstrate a significant reduction in active GSK-3β in mitochondrial fractions of both 11,12-EET and rBNP-treated hearts when compared with controls. In addition, we observe increased levels of active Akt (pser473Akt) and inactive GSK-3β (pser9GSK-3β) in cytosolic fractions, we predict that EETs and BNP either prevent the translocation of GSK-3β to the mitochondria or enhance the translocation from the mitochondria. In addition, our data suggest that BNP-mediated effects on GSK-3β do not involve PI3K/Akt pathway but activation of Akt via the NPR-A receptor. In contrast, EET-mediated events involve both PI3K/Akt and enhanced release of BNP. These data are consistent with evidence suggesting that inhibition of GSK-3β is a key component in integrating protective stimuli with mitochondria.7,13,16 However, the relative contribution of each pathway (PI3K/Akt or NPR-A) to the regulation of GSK-3β is unknown and requires further investigation.
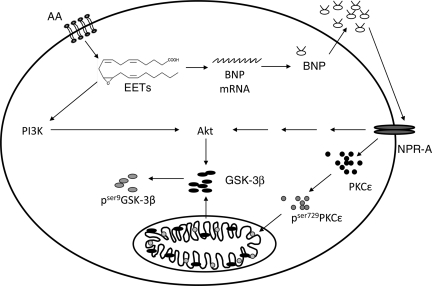
We propose that enhanced post-ischaemic functional recovery attributed to EETs is mediated through two different signalling pathways—PI3K/Akt and BNP/NPR-A signalling. Figure 6 shows a schematic of the proposed mechanisms, whereby EETs can trigger the release of BNP to act in an autocrine manner or activate the PI3K pathway. Together the data in the current manuscript provide further evidence for the beneficial effects of EETs and suggest a novel mechanism for BNP in cardioprotection.
Figure 6.
Schematic diagram showing pathways of EET-mediated improved left ventricular function. We propose that the enhanced post-ischaemic functional recovery attributed to EETs is mediated through two different signalling pathways—PI3K/Akt and BNP/NPR-A signalling. First, EETs can activate the PI3K/Akt pathway which will inactivate GSK-3β. Or second, EETs can activate the BNP/NPR-A pathway, which in turn can either activate Akt independent of PI3K, or activate PKCε.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: None declared.
Funding
J.M.S. is the recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research. This work was supported by Canadian Institutes of Health Research Grant (J.M.S., MOP79465) and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES025034), USPHS NIH (J.R.F., GM31278), and the Robert A. Welch Foundation (J.R.F.).
References
- 1.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 3.Saluja I, Song D, O'Regan MH, Phillis JW. Role of phospholipase A2 in the release of free fatty acids during ischemia-reperfusion in the rat cerebral cortex. Neurosci Lett. 1997;233:97–100. doi: 10.1016/s0304-3940(97)00646-0. [DOI] [PubMed] [Google Scholar]
- 4.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, et al. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 6.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 7.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, et al. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724–H735. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, et al. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006;575:627–644. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seubert JM, Goralski K, Sinal CJ, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circulation (Suppl.) 2004;110:808. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 12.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 13.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 16.Hanlon PR, Fu P, Wright GL, Steenbergen C, Arcasoy MO, Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19:1323–1325. doi: 10.1096/fj.04-3545fje. [DOI] [PubMed] [Google Scholar]
- 17.Silver MA. The natriuretic peptide system: kidney and cardiovascular effects. Curr Opin Nephrol Hypertens. 2006;15:14–21. doi: 10.1097/01.mnh.0000199008.49176.37. [DOI] [PubMed] [Google Scholar]
- 18.Krupicka J, Janota T, Kasalova Z, Hradec J. Natriuretic peptides—physiology, pathophysiology and clinical use in heart failure. Physiol Res. 2008 doi: 10.33549/physiolres.931461. [DOI] [PubMed] [Google Scholar]
- 19.Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev. 2007;12:279–291. doi: 10.1007/s10741-007-9029-y. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.D'souza SP, Baxter GF. B Type natriuretic peptide: a good omen in myocardial ischaemia? Heart. 2003;89:707–709. doi: 10.1136/heart.89.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenhunen O, Szokodi I, Ruskoaho H. Posttranscriptional activation of BNP gene expression in response to increased left ventricular wall stress: role of calcineurin and PKC. Regul Pept. 2005;128:187–196. doi: 10.1016/j.regpep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992;10:907–912. [PubMed] [Google Scholar]
- 24.Shahhosseini S, Guttikonda S, Bhatnagar P, Suresh MR. Production and characterization of monoclonal antibodies against shope fibroma virus superoxide dismutase and glutathione-S-transferase. J Pharm Pharm Sci. 2006;9:165–168. [PubMed] [Google Scholar]
- 25.Das D, Suresh MR. Copious production of SARS-CoV nucleocapsid protein employing codon optimized synthetic gene. J Virol Methods. 2006;137:343–346. doi: 10.1016/j.jviromet.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das D, Jacobs F, Feldmann H, Jones SM, Suresh MR. Differential expression of the Ebola virus GP(1,2) protein and its fragments in E. coli. Protein Expr Purif. 2007;54:117–125. doi: 10.1016/j.pep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 30.D'souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–129. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.D'souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, et al. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284:H1592–H1600. doi: 10.1152/ajpheart.00902.2002. [DOI] [PubMed] [Google Scholar]
- 32.Hama N, Itoh H, Shirakami G, Nakagawa O, Suga S, Ogawa Y, et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation. 1995;92:1558–1564. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 33.Kudoh S, Akazawa H, Takano H, Zou Y, Toko H, Nagai T, et al. Stretch-modulation of second messengers: effects on cardiomyocyte ion transport. Prog Biophys Mol Biol. 2003;82:57–66. doi: 10.1016/s0079-6107(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 34.Tokola H, Hautala N, Marttila M, Magga J, Pikkarainen S, Kerkela R, et al. Mechanical load-induced alterations in B-type natriuretic peptide gene expression. Can J Physiol Pharmacol. 2001;79:646–653. [PubMed] [Google Scholar]
- 35.He Q, Wang D, Yang XP, Carretero OA, LaPointe MC. Inducible regulation of human brain natriuretic peptide promoter in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H368–H376. doi: 10.1152/ajpheart.2001.280.1.H368. [DOI] [PubMed] [Google Scholar]
- 36.He Q, LaPointe MC. Interleukin-1beta regulation of the human brain natriuretic peptide promoter involves Ras-, Rac-, and p38 kinase-dependent pathways in cardiac myocytes. Hypertension. 1999;33:283–289. doi: 10.1161/01.hyp.33.1.283. [DOI] [PubMed] [Google Scholar]
- 37.Hanford DS, Glembotski CC. Stabilization of the B-type natriuretic peptide mRNA in cardiac myocytes by alpha-adrenergic receptor activation: potential roles for protein kinase C and mitogen-activated protein kinase. Mol Endocrinol. 1996;10:1719–1727. doi: 10.1210/mend.10.12.8961280. [DOI] [PubMed] [Google Scholar]
- 38.Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide: diagnostic role in stable coronary artery disease. Exp Clin Cardol. 2006;11:99–101. [PMC free article] [PubMed] [Google Scholar]
- 39.Potthast R, Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptors. Peptides. 2005;26:1001–1008. doi: 10.1016/j.peptides.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Kim MY, Kim MJ, Yoon IS, Ahn JH, Lee SH, Baik EJ, et al. Diazoxide acts more as a PKC-epsilon activator, and indirectly activates the mitochondrial K(ATP) channel conferring cardioprotection against hypoxic injury. Br J Pharmacol. 2006;149:1059–1070. doi: 10.1038/sj.bjp.0706922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, et al. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:910–913. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.