Abstract
Background and Aims
Plant evolution is well known to be frequently associated with remarkable changes in genome size and composition; however, the knowledge of long-term evolutionary dynamics of these processes still remains very limited. Here a study is made of the fine dynamics of quantitative genome evolution in Festuca (fescue), the largest genus in Poaceae (grasses).
Methods
Using flow cytometry (PI, DAPI), measurements were made of DNA content (2C-value), monoploid genome size (Cx-value), average chromosome size (C/n-value) and cytosine + guanine (GC) content of 101 Festuca taxa and 14 of their close relatives. The results were compared with the existing phylogeny based on ITS and trnL-F sequences.
Key Results
The divergence of the fescue lineage from related Poeae was predated by about a 2-fold monoploid genome and chromosome size enlargement, and apparent GC content enrichment. The backward reduction of these parameters, running parallel in both main evolutionary lineages of fine-leaved and broad-leaved fescues, appears to diverge among the existing species groups. The most dramatic reductions are associated with the most recently and rapidly evolving groups which, in combination with recent intraspecific genome size variability, indicate that the reduction process is probably ongoing and evolutionarily young. This dynamics may be a consequence of GC-rich retrotransposon proliferation and removal. Polyploids derived from parents with a large genome size and high GC content (mostly allopolyploids) had smaller Cx- and C/n-values and only slightly deviated from parental GC content, whereas polyploids derived from parents with small genome and low GC content (mostly autopolyploids) generally had a markedly increased GC content and slightly higher Cx- and C/n-values.
Conclusions
The present study indicates the high potential of general quantitative characters of the genome for understanding the long-term processes of genome evolution, testing evolutionary hypotheses and their usefulness for large-scale genomic projects. Taken together, the results suggest that there is an evolutionary advantage for small genomes in Festuca.
Keywords: Festuca, fescue, grasses, genome size evolution, chromosome size, base composition, GC content, polyploidy, phylogeny, retrotransposon dynamics, flow cytometry
INTRODUCTION
Beyond polyploidy, genome size differentiation is one of the most important evolutionary processes in plants, and a large number of recent studies have documented significant differences in genome size associated with evolution of taxa at different taxonomic levels, groups of different ecological specialization or geographical origin (e.g. Bureš et al., 2004; Caetano-Anollés, 2005; Leitch et al. 2005; Price et al., 2005; Johnston et al., 2005; Závesky et al., 2005; Bancheva and Greilhuber, 2006; Weiss-Schneeweiss et al., 2006). Monoploid genome size, the total DNA content divided by the ploidy level (Cx-value; Greilhuber et al., 2005), is an important general characteristic of genomes. It is especially useful for comparing genomes of taxa within polyploid complexes.
Another general parameter of the genome is the percentage of guanine and cytosine nucleotides in the genome (GC content). The GC content may reflect significant compositional features of the genome, as indicated by several studies in human and other vertebrates (Zoubak et al., 1996; Bernardi, 2000a,b) and in Prokaryota (Nishio et al., 2003; Musto et al., 2004; Basak and Ghosh, 2005). The GC content also clearly differs among some plant families (Barow and Meister, 2002) and among the nearly completed sequences of Arabidopsis, maize and rice (Arabidopsis Genome Initiative, 2000; Meyers et al., 2001; International Rice Genome Sequencing Project, 2005). However, until now, the GC content has been reported for only about 215 species (Meister and Barow, 2007), and its role in plant evolution, especially in lower taxonomic groups, is still unknown.
The differences found in monoploid genome size and GC content among monophyletic groups in plants provide an opportunity to use both attributes in evolutionary and phylogenetic studies, which until now have been based solely on the sequence data of very limited portions of genomes. Recent molecular studies bring particular advances in the understanding of mechanisms for genome size diversification and genome evolution of some model taxa; however, detailed long-term dynamics of these processes remain still only limitedly known. Advances in this area may come from detailed genome size studies in genera in which the genome size and composition data may be associated with both the results of fine phylogenetic studies and comparative genomic projects (Gregory, 2005).
Grasses (Poaceae, Gramineae) represent such a model group for these studies. Grasses include nearly 11 000 species (Clayton et al., 2002 onwards; http://www.kew.org/data/grasses-db.html) and are the most widespread plant family, dominating in many biomes over the entire globe. Numerous species, such as rice, maize, wheat, barley, sorghum, rye-grass, meadow fescue and tall fescue, are very important human food sources or forage crops and recently have been targets of detailed comparative genomic studies (Gaut, 2002; Feuillet and Keller, 2002; Paterson et al., 2005; Ammiraju et al., 2006).
The evolution of grasses was accompanied by frequent and repeated genome size gain and loss (Kellogg, 1998; Gaut, 2002; Kellogg and Bennetzen, 2004; Caetano-Anollés, 2005) and DNA base composition changes (King and Ingrouille, 1987). To date an approx. 64-fold difference in monoploid genome size (Angiosperm DNA C-value database; Bennett and Leitch, 2004; http://www.rbgkew.org.uk/cval/homepage.html) and 6·2 % variation in GC content are found in grasses; ranging from 41·0 % in Setaria woodii to 47·2 % in Zea mays (cf. Meister and Barow, 2007).
Fescue (Festuca L., Pooideae, Poeae) is the most species-rich and highly diversified genus of grasses. It comprises about 600 species, which are perennial and dominates nearly worldwide in various types of dry, steppe, mountain and alpine grasslands or meadows; some species grow also in forests; some are cultivated as crops or ornamentals (Clayton et al., 2002 onwards). About 70 % of species are polyploid (up to 12x); diploids are restricted mostly to Eurasia (Hunziker and Stebbins, 1987; Dubcovsky and Martínez, 1992; Šmarda and Stančík, 2006), an assumed primary diversification centre of the genus with the highest species diversity (Catalán et al., 2004). The monoploid genome sizes range in Festuca from 1·58 to 4·03 pg (Angiosperm DNA C-value database, with some corrections of ploidy level; Bennett and Leitch, 2004) and seems to be useful for infrageneric classification (Šmarda, 2006; Loureiro et al., 2007).
Within the tribe Poeae, Fetuca belongs to the well-supported subtribe Loliinae. The sister groups of the subtribe Loliinae (Fig. 3) represent subtribes Dactylidinae + Cynosurinae (Catalán et al., 2004). Festuca is formed by two main evolutionary lines (Fig. 3), broad-leaved and fine-leaved species (Torrecilla and Catalán, 2002; Catalán et al., 2004; Torrecilla et al., 2004). The main evolutionary lineages of broad-leaved fescues are assumed to have evolved at the same time (Catalán et al., 2004). One of the derived lineages is represented by Festuca subgen. Schedonorus, which gave rise to the genus Lolium (Pasakinskiene et al., 1998; Torecilla and Catalán, 2002; Catalán et al., 2004). Within fine-leaved fescues, sections Eskia and Dimorphae possess basal positions, while sections Festuca and Aulaxyper represents derived groups, both being the most rapidly evolving and species-rich groups. The members of sections Festuca and Aulaxyper are also assumed to be ancestral to numerous Mediterranean annuals treated as separate genera, e.g. Vulpia, Castellia, Cutandia, Ctenopsis, Micropyrum, Wangenheimia and Psilurus (Catalán et al., 2004; Torrecilla et al., 2004). Over 250 species included within sections Festuca and Aulaxyper used to be divided into species groups according to important morphological characters, which, however, vary among authors. The present low cover of their species-diversity with molecular markers, high intra-specific sequence diversity (Gaut et al., 2000) and reticular relationships does not allow a reliable and detailed synthetic view on their evolution to be presented yet.
Fig. 3.

The evolution of monoploid genome size and GC content within Loliinae. Reduction of both parameters in the derived groups of both main clades of fine- and broad-leaved fescues is of particular interest. Based on the consensus ITS and trnL-F tree by Catalán et al. (2004; modified).
Here, an analysis of monoploid genome size (Cx-value), average chromosome size (C/n-value) and GC content of Eurasian fescues and their close relatives is completed. The data are compared with the existing phylogeny based on ITS and trnL-F sequences and used to outline a possible scenario for genome evolution within this group. It is intended to evaluate the impact of phylogenetic history, polyploidy and speciation rate on Cx- and C/n-values and GC content variation, as well as to discuss the main mechanisms responsible for the observed Cx- and C/n-values and GC content variability.
MATERIALS AND METHODS
Plants
Plants originated predominantly from the wild collections of the authors cultivated in the garden of the Faculty of Education and the Faculty of Science, Masaryk University, Brno, Czech Republic, and in the Botanical Garden ‘Giardino dei Semplici’, Florence University, Florence, Italy. Some Siberian fescues and representatives of the annual Mediterranean relatives were grown in Brno from seed collections by the authors and from seeds provided by the Palermo Botanical Garden, Italy. Some living samples of Portuguese fescues were kindly provided by J. Loureiro and P. Silveira. The origin of the samples is shown in Supplementary Information. The herbarium vouchers of the samples investigated are stored at the herbaria of the Masaryk University in Brno (BRNU), University of Florence (FI), University of Pavia (PAV) and University of Aveiro (AVE).
Genome size and GC content estimations
Genome size and GC content were measured by flow cytometry at the Institute of Botany and Zoology, Masaryk University. Measurements were conducted on two flow cytometers using two different fluorochromes: Cy Flow SL (Partec, Germany) – intercalating propidium iodide (PI) for absolute DNA content estimations; and PA-1 (Partec) – an AT-specific 4′,6-diamidino-2-phenylindole (DAPI) for the calculation of AT : CG ratio and GC content. A two-step procedure (Otto, 1990) was used for sample preparation. A piece of tiller leaf was chopped using a sharp razor blade together with an internal standard in a Petri dish containing 1 mL Otto I buffer (0·1 m citric acid, 0·5 % Tween 20). An additional 1 mL Otto I buffer was added. The crude nuclear suspension was filtered through 50-μm nylon mesh. The filtered suspension was divided into two sample tubes to which either 1 mL of Otto II buffer (0·4 m Na2HPO4·12H2O) supplemented with DAPI, or 1 mL of Otto II buffer with PI + RNase was added. The final concentration of PI and RNase was 50 µg mL−1. The concentration of DAPI was 2·0 µg mL−1. All samples were measured simultaneously on both flow cytometers with Pisum sativum ‘Ctirad’ as the primary internal standard (2C DNA content = 9·09 pg; Doležel et al., 1998; GC content = 38·5 %; Barow and Meister, 2002). When sample peaks overlapped with the peak of the primary standard either in the measurement with PI or DAPI, an alternative internal standard of diploid sample F1229 of Festuca pallens (2n = 14; Šmarda and Kočí, 2003; 2C DNA = 5·059 pg; this work) was used. In that case, results were consequently recalculated to the primary standard Pisum sativum based on the estimated ratio of both standards (F1229/Pisum = 0·5565 for PI; F1229/Pisum = 0·3914 for DAPI). All measurements were repeated three times and the results were averaged. The average coefficient of variance of all peaks in the measurements with PI was 3·48 % and 1·51 % with DAPI.
Monoploid genome size (Cx-value) was calculated according to Greilhuber et al. (2005) as the absolute 2C DNA content (2C-value) of the sample divided by the ploidy level. It is proposed here to include also the average chromosome size (C/n-value), calculated analogically by dividing the somatic total DNA content (2C) by somatic chromosome number (2n). As the basic chromosome number (x = 7) is the same for all the species and genera analysed, Cx-value and C/n-value are linearly correlated and together are referred to as ‘Cx- and C/n-values’ in the text.
When selecting plants for measurements, plants with previously documented chromosome numbers were preferred. In plants where chromosome numbers were not directly known, or any other counted plant was not available for comparison, the most common number reported in the literature, preferably from the same or a geographically close region, was used (for detailed reference list see Supplementary Information). In six taxa that had ambiguous chromosome counts or in 15 taxa that have not yet been investigated karyologically, ploidy level and the chromosome number were approximated from a comparison with genome sizes of the closest relatives.
The GC content was determined from comparison of parallel measurements with the two different fluorochromes, intercalating PI and base-specific DAPI. For the calculation, the ‘Dye Factor’, the proportion of the sample : standard fluorescence ratio with base-specific DAPI to the sample :standard fluorescence ratio with base-unspecific, intercalating PI, was used (Barow and Meister, 2002: eqn 6). The exact values of the GC content were calculated according to Barow and Meister (2002: eqns 7 and 8) using the mathematical approximation, regula falsi method, calculated in an automated Excel sheet available at http://www.sci.muni.cz/botany/systemgr/Data/Festuca/ATGCFlow.xls. The binding length of DAPI was calculated as equal to 4 according to Barow and Meister (2002).
Statistical treatment
Within the whole dataset, the Cx- and C/n-values and GC content of diploids and polyploids were compared by Student's t-test. The respective species pairs used for direct diploid–polyploid comparison are indicated in Supplementary Information. The effect of polyploidy on Cx- and C/n-values and GC content within six groups (F. amethystina group; Festuca sect. Eskia; F. ovina + pallens + glauca + laevigata cluster; F. valesiaca + brevipila cluster; Festuca sect. Bovinae; Vulpia sect. Vulpia) was evaluated by factorial ANOVA. The overall correlation of GC content with monoploid genome size in the dataset and in the data of Ammiraju et al. (2006, tab. 4) was tested by Pearson parametric correlation. The descriptive statistics, statistical tests and graphs were calculated in the Statistica 7·1 program (http://www. statsoft.com).
RESULTS
In total, absolute DNA content, monoploid genome size, average chromosome size and GC content of 129 samples from 115 taxa were completed (Table 1). Within the fine-leaved and broad-leaved fescues, monoploid genome size and average chromosome size varied about 2·52-fold: monoploid genome size ranged from 1·94 pg in the diploid Festuca arvernensis to 4·89 pg in the diploid Festuca drymeia; GC content varied by 3·8 %, from 42·6 % in the diploid Vulpia bromoides to 46·4 % in the diploid Festuca alpestris. Taking into account other Poeae relatives, monoploid genome size varied 3·14-fold (including the smallest genomes of the diploid Sclerochloa dura, Poinae), and the GC content differed by 3·9 % (including the most GC-poor tetraploid, Dactylis polygama).
Table 1.
Ploidy levels, infrageneric classification, 2C DNA content (2C-value), monoploid genome sizes (Cx-value), average chromosome sizes (C/n-value) and GC contents (GC) of the samples analysed
| Species | Ploidy level (x)* | Origin† | Clade‡ | Subgenus§ | Section¶ | Species group# | 2C-value (pg) | Cx-value (pg) | C/n-value (pg) | GC content (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Subtribe Cynosurinae Fr. | ||||||||||
| Cynosurus cristatus L. | 2d | Cz | – | – | – | – | 6·095 | 3·047 | 0·435 | 42·66 |
| Subtribe Dactylidinae Stapf. | ||||||||||
| Dactylis glomerata L. | 4d | Cz | – | – | – | – | 9·042 | 2·260 | 0·323 | 43·53 |
| D. polygama Horv. | 2d | Cz | – | – | – | – | 4·525 | 2·262 | 0·323 | 42·53 |
| Subtribe Loliinae Dumort. | ||||||||||
| Castellia tuberculosa (Moris) Bor | 2g | It | C | – | – | – | 6·313 | 3·156 | 0·451 | 42·93 |
| Festuca acuminata Gaudin | 2d | It | F | Fes | Esk | – | 6·576 | 3·288 | 0·470 | 45·61 |
| F. airoides Lam. | 2d | F | F | Fes | Fes | Ovi | 4·882 | 2·441 | 0·349 | 44·38 |
| F. airoides Lam. | 2d | Bu | F | Fes | Fes | Ovi | 4·939 | 2·469 | 0·353 | 44·33 |
| F. alpestris Roem. et Schult. | 2a | It | F | Fes | Esk | – | 8·892 | 4·446 | 0·635 | 46·41 |
| F. alpina s.l. (alfrediana Foggi et Signorini) | 2b | It | F | Fes | Aul | Hal | 4·587 | 2·294 | 0·328 | 45·43 |
| F. alpina Suter subsp. alpina | 2b | A | F | Fes | Aul | Hal | 4·388 | 2·194 | 0·313 | 44·89 |
| F. alpina Suter subsp. alpina | 2b | Sk | F | Fes | Aul | Hal | 4·250 | 2·125 | 0·304 | 45·05 |
| F. altaica Trin. | 4d | Ru | B | Leu | Bre | – | 14·996 | 3·749 | 0·536 | 45·34 |
| F. altissima All. | 2e | Cz | B | Dry | – | – | 8·939 | 4·470 | 0·639 | 45·71 |
| F. amethystina L. subsp. amethystina | 4b | Cz | F | Fes | Aul | Am | 12·971 | 3·243 | 0·463 | 45·03 |
| F. arundinacea Schreb. subsp. arundinacea | 6e | Cr | B | Sch | Bov | – | 17·218 | 2·870 | 0·410 | 44·49 |
| F. arundinacea subsp. uechtritziana (Wiesb.) Hegi | 6g | It | B | Fes | Bov | – | 16·978 | 2·830 | 0·404 | 44·44 |
| F. arvernensis Auquier, Kerguélen et Markgr.-Dann. subsp. arvernensis | 2f | F? | F | Fes | Fes | Lvg | 3 887 | 1·943 | 0·214 | 44·09 |
| F. arvernensis subsp. costei (St-Yves) Auquier et Kerguélen | 4a | It | F | Fes | Fes | Lvg | 9·098 | 2·274 | 0·325 | 44·90 |
| F. auquieri Kerguélen | 4d | F | F | Fes | Fes | Pal | 9·460 | 2·365 | 0·338 | 44·69 |
| F. balcanica (Acht.) Markgr.-Dann. subsp. balcanica | 2g | Bu | F | Fes | Esk | – | 7·412 | 3·706 | 0·529 | 46·02 |
| F. billyii Kerguélen et Plonka | 6e | F | F | Fes | Fes | Lvg | 13·356 | 2·226 | 0·318 | 44·12 |
| F. bosniaca Kumm. et Sendtn. subsp. bosniaca | 2g | Cr | F | Fes | Esk | – | 7·397 | 3·698 | 0·528 | 45·94 |
| F. bosniaca subsp. pirinica (Acht.) Markgr.-Dann. | 2g | Bu | F | Fes | Esk | – | 6·982 | 3·491 | 0·499 | 45·96 |
| F. brevipila R.Tracey | 6c | A | F | Fes | Fes | Bre | 14·088 | 2·348 | 0·335 | 44·79 |
| F. brigantina (Markgr.-Dann.) Markgr.-Dann. | 8a | Pt | F | Fes | Fes | Ovi | 20·122 | 2·515 | 0·359 | 43·88 |
| F. callieri (Hack.) Markgr. | 4e | Rm | F | Fes | Fes | Bre | 9·770 | 2·443 | 0·349 | 44·82 |
| F. calva (Hack.) K.Richt. | 2g | Sl | F | Fes | Esk | – | 7·300 | 3·650 | 0·521 | 45·68 |
| F. carnuntina R.Tracey | 6c | A | F | Fes | Fes | Val | 13·940 | 2·323 | 0·332 | 44·38 |
| F. cinerea Vill. | 4b | F | F | Fes | Fes | Gla | 9·962 | 2·491 | 0·356 | 44·69 |
| F. circummediterranea Patzke s.l. | 4b | It | F | Fes | Fes | Lvg | 9·979 | 2·495 | 0·356 | 44·37 |
| F. circummediterranea Patzke | 2a | It | F | Fes | Fes | Lvg | 5·433 | 2·717 | 0·388 | 44·23 |
| F. csikhegyensis Simonk. | 4a | Ge | F | Fes | Fes | Pal | 9·450 | 2·363 | 0·338 | 45·17 |
| F. dalmatica (Hack.) K.Richt.. | 4d | Hu | F | Fes | Fes | Val | 9·915 | 2·479 | 0·354 | 45·07 |
| F. degenii (St-Yves) Markgr.-Dann. | 4d | F | F | Fes | Fes | Gla | 10·068 | 2·517 | 0·360 | 44·53 |
| F. drymeia Mert. et Koch | 2d | Cz | B | Dry | – | – | 9·781 | 4·890 | 0·699 | 45·44 |
| F. × duernsteinensis Vetter | 4b | Sk | F | Fes | Fes | Ovi | 10·037 | 2·509 | 0·358 | 44·67 |
| F. durandoi var. livida (Hack.) Rivas Ponce et Cebolla | 4b | Pt | B | Fes | Sub | – | 14·662 | 3·666 | 0·524 | 45·85 |
| F. duriotagana Franco et Rocha Afonso | 10a | Pt | F | Fes | Aul | Rub | 20·283 | 2·028 | 0·290 | 43·95 |
| F. duvalii (St-Yves) Stohr | 4d | Ge | F | Fes | Fes | Val | 10·144 | 2·536 | 0·362 | 45·00 |
| F. eggleri R.Tracey | 2d | A | F | Fes | Fes | Ovi | 4·857 | 2·428 | 0·347 | 44·14 |
| F. exaltata C.Presl | 2g | It | B | Dry | – | – | 9·725 | 4·862 | 0·695 | 45·64 |
| F. extremiorientalis Ohwi | 4e | Ru | B | Sub | Par | – | 13·467 | 3·367 | 0·481 | 45·57 |
| F. filiformis Pourr. | 2b | It | F | Fes | Fes | Ovi | 5·013 | 2·507 | 0·358 | 44·15 |
| F. gamisansii subsp. aethaliae Signorini et Foggi | 10a | It | F | Fes | Fes | Lvg | 24·080 | 2·408 | 0·344 | 44·59 |
| F. gautieri subsp. scoparia (A. Kern. et Hack.) Kerguélen | 2d | orn | F | Fes | Esk | – | 6·116 | 3·058 | 0·437 | 45·82 |
| F. gigantea (L.) Vill. | 6e | Cz | B | Sch | Pla | – | 20·752 | 3·459 | 0·494 | 43·68 |
| F. glauca Vill. | 6d | Sp | F | Fes | Fes | Gla | 14·215 | 2·369 | 0·338 | 44·06 |
| F. guestfalica Reichenb. | 4b | Cz | F | Fes | Fes | Ovi | 9·780 | 2·445 | 0·349 | 44·64 |
| F. guinochetii (Bidault) S.Arndt. | 10d | It | F | Fes | Fes | Lvg | 22·918 | 2·292 | 0·327 | 44·26 |
| F. guinochetii (Bidault) S.Arndt. | 10d | It | F | Fes | Fes | Lvg | 22·896 | 2·290 | 0·327 | 44·07 |
| F. hallerii All. | 2e | It | F | Fes | Aul | Hal | 5·040 | 2·520 | 0·360 | 44·94 |
| F. hallerii All. | 2e | It | F | Fes | Aul | Hal | 5·405 | 2·703 | 0·386 | 44·36 |
| F. heteromalla Pourr. | 8f | A | F | Fes | Aul | Rub | 16·386 | 2·048 | 0·293 | 43·63 |
| F. heteropachys (St-Yves) Auquier | 4e | F | F | Fes | Fes | Ovi | 9·837 | 2·459 | 0·351 | 44·69 |
| F. heterophylla Lam. | 4e | It | F | Fes | Aul | Het | 11·322 | 2·830 | 0·404 | 44·05 |
| F. hirtovaginata (Acht.) Markgr.-Dann. | 6g | Bu | F | Fes | Fes | Lvg | 13·978 | 2·330 | 0·333 | 44·07 |
| F. humifusa Brullo & R.Guarino | 2a | It | F | Fes | Fes | Lvg | 5·234 | 2·617 | 0·374 | 44·50 |
| F. inops De Not. | 2b | F | F | Fes | Fes | Gla | 4·679 | 2·340 | 0·334 | 43·82 |
| F. inops De Not. | 2b | It | F | Fes | Fes | Pal | 4·677 | 2·339 | 0·334 | 43·90 |
| F. inops De Not. | 2b | It | F | Fes | Fes | Pal | 4·870 | 2·435 | 0·348 | 44·17 |
| F. laevigata Gaudin | 8a | It | F | Fes | Fes | Lvg | 18·604 | 2·325 | 0·332 | 44·28 |
| F. laevigata Gaudin | 8b | It | F | Fes | Fes | Lvg | 18·709 | 2·339 | 0·334 | 44·03 |
| F. laxa Host | 4e | Sl | F | Fes | Dim | – | 12·934 | 3·234 | 0·462 | 45·84 |
| F. lemanii Bast. | 6d | Ge | F | Fes | Fes | Ovi | 13·960 | 2·327 | 0·332 | 44·41 |
| F. malyschevii E.B.Alexeev | 2g | Ru | F | Fes | Aul | Am | 6·986 | 3·493 | 0·499 | 45·01 |
| F. nigrescens Lam. subsp. nigrescens | 6b | Cz | F | Fes | Aul | Rub | 13·307 | 2·218 | 0·317 | 43·90 |
| F. nigrescens Lam. subsp. nigrescens | 6a | F | F | Fes | Aul | Tri | 13·105 | 2·184 | 0·312 | 44·20 |
| F. nigrescens subsp. microphylla (St-Yves) Markgr.-Dann. | 6e | It | F | Fes | Aul | Rub | 13·225 | 2·204 | 0·315 | 43·96 |
| F. norica (Hack.) K.Richt. | 2d | It | F | Fes | Aul | Am | 6·172 | 3·086 | 0·441 | 45·12 |
| F. ovina L. | 2b | It | F | Fes | Fes | Ovi | 4·825 | 2·412 | 0·345 | 43·84 |
| F. pallens Host | 2a | Sk | F | Fes | Fes | Pal | 5·059 | 2·529 | 0·361 | 45·05 |
| F. paniculata (L.) Schinz et Thell. subsp. paniculata | 2d | It | B | Fes | Sub | – | 7·646 | 3·823 | 0·546 | 46·15 |
| F. picturata Pils | 2d | A | F | Fes | Aul | Vio | 5·809 | 2·905 | 0·415 | 44·44 |
| F. pirinica Markgr.-Dann. | 2d | Bu | F | Fes | Aul | Hal | 5·254 | 2·627 | 0·375 | 45·02 |
| F. polesica Zapał. | 2d | Po | F | Fes | Fes | Pal | 5·196 | 2·598 | 0·371 | 44·44 |
| F. pratensis Huds. subsp. pratensis | 2e | Cz | B | Sch | Bov | – | 6·472 | 3·236 | 0·462 | 44·38 |
| F. psammophila subsp. dominii (Krajina) P.Šmarda | 2a | Cz | F | Fes | Fes | Pal | 4·918 | 2·459 | 0·351 | 44·37 |
| F. psammophila (Čelak.) Fritsch subsp. psammophila | 2b | Po | F | Fes | Fes | Pal | 5·187 | 2·594 | 0·371 | 44·68 |
| F. pseudodalmatica Domin | 4b | Sk | F | Fes | Fes | Val | 9·652 | 2·413 | 0·345 | 44·83 |
| F. pseudovaginata Penksza | 4e | Hu | F | Fes | Fes | Pal | 9·841 | 2·460 | 0·351 | 45·13 |
| F. pseudovaria subsp. winnebachensis (Wallosek) J.Müller | 6e | It | F | Fes | Esk | – | 18·959 | 3·160 | 0·451 | 46·05 |
| F. pseudovina Wiesb. | 2d | Hu | F | Fes | Fes | Val | 4·412 | 2·206 | 0·315 | 44·35 |
| F. pumila Vill. | 2f | A | F | Fes | Esk | – | 6·561 | 3·281 | 0·469 | 45·95 |
| F. pumila Vill. | 2f | It | F | Fes | Esk | – | 6·911 | 3·455 | 0·494 | 46·04 |
| F. riccerii Foggi et Gr.Rossi | 6g | It | F | Fes | Fes | Ovi | 14·052 | 2·342 | 0·335 | 44·53 |
| F. riloensis (Hayek) Markgr.-Dann. | 2d | Bu | F | Fes | Aul | Hal | 5·670 | 2·835 | 0·405 | 44·85 |
| F. robustifolia Markgr.-Dann. | 10a | It | F | Fes | Fes | Gla | 22·286 | 2·229 | 0·318 | 44·16 |
| F. rubra L. subsp. rubra | 6f | Rm | F | Fes | Aul | Rub | 13·684 | 2·281 | 0·326 | 44·07 |
| F. rubra subsp. juncea (Hack.) K.Richt | 8f | Cz | F | Fes | Aul | Rub | 16·971 | 2·121 | 0·303 | 43·99 |
| F. rubra subsp. pruinosa (Hack.) Piper | 6a | Pt | F | Fes | Aul | Rub | 12·885 | 2·147 | 0·307 | 43·77 |
| F. rupicaprina (Hack.) A.Kern. | 2d | A | F | Fes | Aul | Hal | 4·857 | 2·428 | 0·347 | 44·71 |
| F. rupicaprina (Hack.) A.Kern. | 2e | It | F | Fes | Aul | Hal | 4·965 | 2·483 | 0·355 | 45·03 |
| F. rupicola Heuff. | 6a | Cz | F | Fes | Fes | Val | 14·180 | 2·363 | 0·338 | 44·50 |
| F. rupicola Heuff. | 6b | Ru | F | Fes | Fes | Val | 14·536 | 2·423 | 0·346 | 44·69 |
| F. saxatilis Schur | 6e | Rm | F | Fes | Fes | Val | 14·362 | 2·394 | 0·342 | 44·74 |
| F. staroplaninica Velčev | 6g | Bu | F | Fes | Fes | Bre | 13·833 | 2·305 | 0·329 | 44·01 |
| F. staroplaninica Velčev | 6g | Bu | F | Fes | Fes | Bre | 13·200 | 2·200 | 0·314 | 43·57 |
| F. stenantha (Hack.) K.Richt. | 2d | A | F | Fes | Aul | Hal | 5·387 | 2·693 | 0·385 | 44·91 |
| F. stricta Host subsp. stricta | 6c | A | F | Fes | Fes | Bre | 14·050 | 2·342 | 0·335 | 44·60 |
| F. stricta subsp. bauzanina Pils | 8d | It | F | Fes | Fes | Bre | 18·484 | 2·310 | 0·330 | 44·54 |
| F. summilusitana Franco et Rocha Afonso | 10a | Pt | F | Fes | Fes | Gla | 21·830 | 2·183 | 0·312 | 44·34 |
| F. supina Schur | 4e | Rm | F | Fes | Fes | Ovi | 9·917 | 2·479 | 0·354 | 45·12 |
| F. tatrae (Csakó) Degen | 2d | Sk | F | Fes | Aul | Am | 6·998 | 3·499 | 0·500 | 45·09 |
| F. cf. taurica (Hack.) Trautv. | 2g | Bu | F | Fes | Fes | Val | 4·687 | 2·343 | 0·335 | 44·3 |
| F. trichophylla (Gaudin) K.Richt. subsp. trichophylla | 6e | It | F | Fes | Aul | Tri | 13·205 | 2·201 | 0·314 | 43·81 |
| F. trichophylla subsp. asperifolia (St.-Yves) Al-Bermani | 6g | It | F | Fes | Aul | Tri | 13·106 | 2·184 | 0·312 | 44·09 |
| F. tristis Krylov et Ivanitzky | 4g | Ru | B | Leu | Bre | – | 12·917 | 3·229 | 0·461 | 46·38 |
| F. vaginata Willd. | 2b | Hu | F | Fes | Fes | Pal | 4·901 | 2·450 | 0·350 | 44·56 |
| F. valesiaca Gaudin | 2c | Po | F | Fes | Fes | Val | 4·553 | 2·276 | 0·325 | 44·17 |
| F. valida (Uechtr.) Penzés subsp. valida | 4d | Bu | F | Fes | Esk | – | 18·029 | 4·507 | 0·644 | 46·13 |
| F. valida (Uechtr.) Penzés subsp. valida | 4d | Bu | F | Fes | Esk | – | 17·780 | 4·445 | 0·635 | 46·05 |
| F. versicolor subsp. brachystachys (Hack.) Markgr.-Dann | 2c | A | F | Fes | Esk | – | 7·728 | 3·864 | 0·552 | 46·17 |
| F. versicolor subsp. pallidula (Hack.) Markgr.-Dann. | 2g | A | F | Fes | Esk | – | 7·111 | 3·555 | 0·508 | 46·00 |
| F. versicolor Tausch subsp. versicolor | 2b | Cz | F | Fes | Esk | – | 7·343 | 3·672 | 0·525 | 45·65 |
| F. violacea subsp. italica Foggi, Gr.Rossi et Signorini | 2b | It | F | Fes | Aul | Vio | 5·135 | 2·568 | 0·367 | 44·29 |
| F. vivipara (L.) Sm. | 4f | No | F | Fes | Fes | Ovi | 9·922 | 2·481 | 0·354 | 45·14 |
| F. wagneri (Degen, Thaisz et Flatt) Degen, Thaisz et Flatt | 4d | Hu | F | Fes | Fes | Bre | 9·494 | 2·373 | 0·339 | 44·60 |
| F. xanthina Roem. et Schult. | 2d | Rm | F | Fes | Esk | – | 7·980 | 3·990 | 0·570 | 45·46 |
| Lolium multiflorum Lam. | 2e | Cz | B | – | – | – | 5·442 | 2·721 | 0·389 | 43·28 |
| L. perenne L. | 2e | Cz | B | – | – | – | 5·511 | 2·756 | 0·394 | 43·46. |
| L. rigidum Gaudin | 2e | It | B | – | – | – | 5·494 | 2·747 | 0·392 | 43·61 |
| L. temulentum L. | 2e | It | B | – | – | – | 5·717 | 2·858 | 0·408 | 43·51 |
| Vulpia bromoides (L.) S.F.Gray | 2e | It | F | – | Vul | – | 5·861 | 2·930 | 0·419 | 42·59 |
| V. ciliata Dumort. | 4e | It | F | – | Vul | – | 8·289 | 2·072 | 0·296 | 43·35 |
| V. myuros (L.) C.C.Gmel. | 6e | It | F | – | Vul | – | 13·783 | 2·297 | 0·328 | 43·94 |
| V. myuros (L.) C.C.Gmel. | 6d | Cz | F | – | Vul | – | 13·718 | 2·286 | 0·327 | 43·75 |
| V. sicula (C. Presl) Link | 2d | It | F | – | Lor | – | 5·899 | 2·949 | 0·421 | 42·80 |
| Subtribe Poinae Dumort. | ||||||||||
| Poa trivialis L. | 2e | Cz | – | – | – | – | 3·324 | 1·662 | 0·237 | 43·89 |
| Sclerochloa dura (L.) P.Beauv. | 2e | Cz | – | – | – | – | 3·117 | 1·558 | 0·223 | 44·01 |
See Supplementary Information for extended measurement results, details to sample origin, taxonomic classification and references to ploidy level data.
* Superscript letters in ploidy levels indicate the relevance of ploidy level data, as follows. a, b Direct comparison with counted plant: a, the counted plant was measured; b, the results were compared with another counted plant. c–e DNA content of a sample agrees with the expected chromosome number reported in literature from c the same locality, d geographically close region (within the state, or up to ±200 km around) or e geographically far region. f, g Ploidy level derived from comparison of DNA content of related taxa because fseveral sources report different chromosome counts or gno chromosome data are available.
† Origin/locality: A, Austria; Bu, Bulgaria; Cr, Croatia; Cz, Czech Republic; F, France; Ge, Germany; Hu, Hungary; It, Italy; No, Norway; Po, Poland; Pt, Portugal; Rm, Romania; Ru, Russia; Sl, Slovenia; Sk, Slovakia; Sp, Spain; orn, ornamental plant.
‡ Main clades of fescues and fescue-like grasses based on trnL-F and ITS sequences according to Catalán et al. (2004): B, broad-leaved clade; C, Castellia; F, fine-leaved clade (see Fig. 3 for further details).
§ Subgenera (only for Festuca genus): Dry, Festuca subgen. Drymanthele V.I.Krecz. et Bobrov; Fes, Festuca subgen. Festuca; Leu, Festuca subgen. Leucopoa (Griesb.) Tzvel.; Sch, Festuca subgen. Schedonorus (P.Beauv.) Peterm.; Sub, Festuca subgen. Subulatae (Tzvel.) E.B.Alexeev.
¶ Sections (only for Festuca and Vulpia genera): Aul, Festuca sect. Aulaxyper Dumort.; Bov, Festuca sect. Bovinae (Anderss.) Hack.; Bre, Festuca sect. Breviaristatae Krivot.; Dim, Festuca sect. Dimorphae Joch. Müller et Catalán; Esk, Festuca sect. Eskia Willk.; Fes, Festuca L. sect. Festuca; Lor, Vulpia sect. Loretia (Duval-Jouve) Boiss.; Par, Festuca sect. Parviglumae S.Aiken et X.Chen; Pla, Festuca sect. Plantynia (Dum.) Tzvel; Sub, Festuca sect. Subbulbosae (Nyman) Hack.; Vul, Vulpia Gmelin sect. Vulpia.
# Morphological species groups (only for Festuca sect. Festuca and F. sect Aulaxyper): Ame, Amethystina; Bre, Brevipila; Gla, Glauca; Hal, Halleri; Het, Heterophylla; Lvg, Laevigata; Ovi, Ovina; Pal, Pallens; Rub, Rubra; Tri, Trichophylla; Val, Valesiaca; Vio, Violacea.
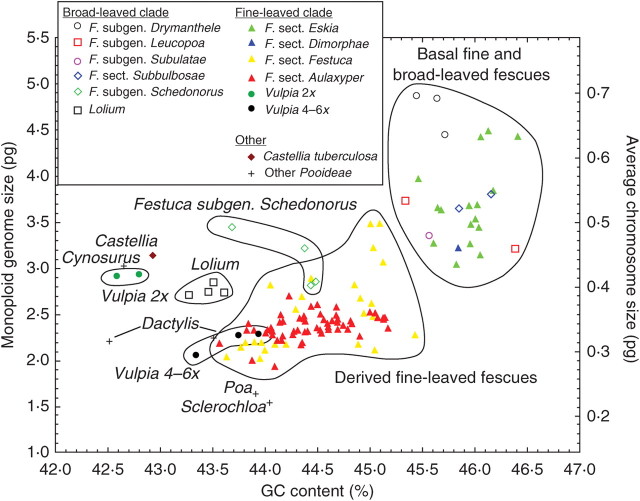
Although Cx-value and GC content were correlated (r = 0·60; P < 0·001), these parameters clearly differed among taxa of all the taxonomic levels analysed. Considerable differences were noted among subtribes and genera, as well as among closely related species groups. Plotting Cx- and C/n-values versus GC content, several clear clusters representing individual evolutionary lineages and species groups were obtained (Figs 1 and 2). The mutual relations of species groups within Loliineae (Figs 1 and 3) agree well with the present phylogeny of the group based on ITS and trnL-F sequence data (Catalán et al., 2004, fig. 3A, maximum parsimony method). Both basal groups of fine-leaved and broad-leaved fescues have apparently high Cx- and C/n-values and GC content compared with the other related genera, which are placed in the left bottom of the diagram (Figs 1 and 4). Within the evolutionarily derived groups, Cx- and C/n-values and GC content considerably decrease. In broad-leaved fescues, the Cx- and C/n-values of the most extreme Festuca subgen. Drymanthele is about twice as high, and the genome contains about 3 % more GC than members of the sister clade including subtribes Dactylidinae and Cynosurinae. An apparent reduction in Cx- and C/n-values and GC content appears in the Festuca subgen. Schedonorus clade, and a further decrease can be seen in its derived lineage represented by genus Lolium (Figs 1, 3 and 4). Within the fine-leaved fescues of Festuca subgen. Festuca, the basal sections Eskia and Dimorphae have the highest Cx- and C/n-values and GC content. As in the broad-leaved clade, the derived groups of fine-leaved fescues, sections Festuca and Aulaxyper, have smaller genomes and lower GC content. The Cx- and C/n-values and GC content pattern within these two sections are shown in detail in Fig. 2. Festuca sect. Aulaxyper appears as four distinct lineages. The Festuca amethystina group possesses a very separate position with the highest Cx- and C/n-values and GC content. The tetraploid F. heterophylla and the diploids of the F. violacea morphological group also occupy isolated positions. The rest of high polyploid taxa of the F. rubra and F.trichophylla group form one cluster (F. rubra + trichophylla) in the bottom left part of the diagram. Compared with F. sect. Aulaxyper, F. sect. Festuca is more homogeneous in its Cx- and C/n-values and GC content pattern. Only three clusters, representing the main morphological groups, are partly separated. Alpine fescues of the F. halleri group have generally the largest and the most GC-rich genomes. The Festuca valesiaca and F. brevipila morphological groups were very similar and form a compact cluster (F. valesiaca + brevipila) in the middle of the Cx- and C/n-values and GC content diagram. Other species are dispersed regularly over the whole Festuca sect. Festuca cluster and are included in one polymorphic cluster (F. ovina + pallens + glauca + laevigata). The main difference in Vulpia is found between the diploids and the polyploids. The diploid species of Vulpia form a separate group together with Castellia tuberculosa, another Mediterranean annual; the Vulpia polyploids are very close to the polyploid taxa of the F. rubra + trichophylla cluster, Festuca sect. Aulaxyper (Figs 1 and 2).
Fig. 1.
A comparison of monoploid genome size and average chromosome size with GC content of the taxa analysed.
Fig. 2.
A comparison of monoploid genome size and average chromosome size with GC content within derived groups of the fine-leaved fescue clade, genus Vulpia, Festuca sect. Festuca, and F. sect. Aulaxyper.
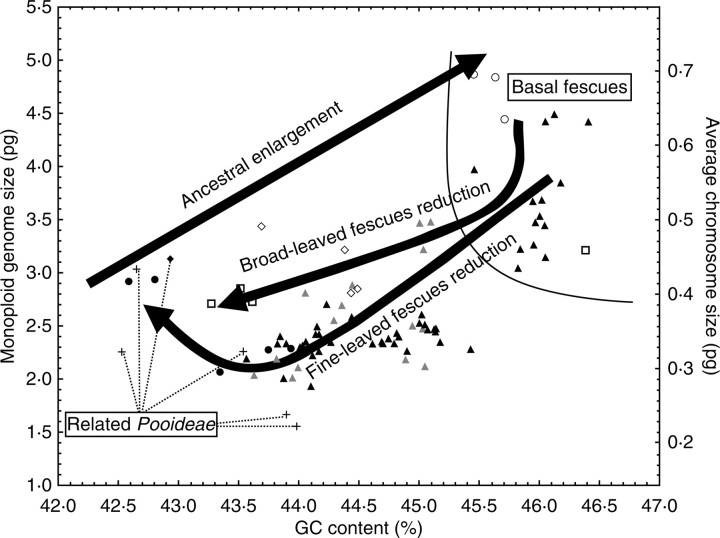
Fig. 4.
The possible scenario of monoploid genome size (Cx-value), average chromosome size (C/n-value), and GC content evolution in Festuca. The divergence of basal fescues from the related Poeae was preceded by about a 2-fold increase in Cx- and C/n-values and considerable GC content enrichment. The subsequent reduction in GC content and Cx- and C/n-values, running parallel in both main evolutionary lineages of fine-leaved and broad-leaved fescues, appears to diverge among the existing species groups. Symbols correspond to those used in Fig. 1.
Within the entire dataset, polyploids had significantly lower Cx- and C/n-values compared with diploids (P < 0·001). This was because polyploids are much more common in the derived evolutionary groups of fine-leaved fescues that generally have lower Cx- and C/n-values than basal, predominantly diploid groups. No difference was found in GC content of polyploids and diploids (P > 0·05). The effect of polyploidy within separate species groups became insignificant for Cx- and C/n-values (P > 0·05) and was only marginally significant for GC content (P = 0·023). Although the Cx- and C/n-values in polyploids were lower within Festuca sect. Bovinae and genus Vulpia, a reverse trend appeared in the Festuca sect. Eskia. Polyploids in all groups had a very similar to a slightly higher GC content compared with diploids; the statistical significance of this parameter was caused mainly by a shift towards higher GC content in polyploid taxa of Vulpia sect. Vulpia.
As by comparison of distant taxa within a section or a wider species group the effect of polyploidy on Cx- and C/n-values and GC content may be biased, the core effect of polyploidy on Cx- and C/n-values and GC content was analysed by only comparing pairs of closely related diploid and polyploid taxa. This comparison revealed two different patterns (Fig. 5): (1) polyploids derived from a parent with a large genome size and high GC content had smaller Cx- and C/n-values and only slightly deviated from the parental GC content; (2) polyploids derived from parents with a small genome and low GC content generally had a markedly increased GC content and slightly higher Cx- and C/n-values.
Fig. 5.
A comparison of monoploid genome size, average chromosome size and GC content evolution in close diploid–polyploid species pairs or taxa groups. The arrows run from diploid to polyploid. The two different trends observed (different types of arrow) may indicate the limitation of genome evolution of polyploids by parental monoploid genome size or by allopolyploid (interrupted arrows) versus autopolyploid (solid arrows) formation of polyploids. Symbols correspond to those used in Fig. 1. Pairs: 1, Vulpia (2x) → Vulpia (4–6x); 2, Festuca circummediterranea (2x) → F. circummediterranea s.l. (4x); 3, F. violacea group (2x) → F. rubra group (6–10x); 4, F. pratensis → F. arundinacea; 5, F. tatrae → F. amethystina; 6, F. pallens → F. csikhegyensis; 7, F. paniculata → F. durandoi; 8, F. versicolor subsp. brachystachys → F. pseudovaria subsp. winnebachensis; 9, Dactylis polygama → D. glomerata; 10, F. ovina → F. guestfalica; 11, F. inops → F. degenii; 12. F. airoides → F. supina; 13, F. valesiaca → F. pseudodalmatica; 14, F. arvernensis subsp. arvernensis → F. arvernensis subsp. costei; 15, F. vaginata → F. pseudovaginata.
DISCUSSION
Evolutionary, phylogenetic and taxonomic implications
Ancient Cx- and C/n-values and GC content expansion and subsequent reduction
The pattern observed among separate sections and species in Cx- and C/n-values and GC content corresponded well with their phylogenetical relationships based on ITS and trnL-F sequence data (Catalán et al., 2004; Fig. 3). The comparison of flow cytometry data with the present phylogeny also reveals the long-term cyclic character of Cx- and C/n-values and GC content evolution in fescues. Both basal fine-leaved and basal broad-leaved fescues actually had apparently high Cx- and C/n-values and GC content compared with the other related Poeae genera (Fig. 4), indicating that their divergence must have been associated with a process effectively increasing their genome size and GC content. In the most extreme case, Festuca subgen. Drymanthele, Cx- and C/n-values were about twice as high, and the genome was about 3 % GC richer than in subtribes Dactylidinae and Cynosurinae, sister to Festuca genus (Fig. 3). Similarly high Cx- and C/n-values and GC content in the basal groups of both fine-leaved and broad-leaved fescues indicate that this increase was probably due to a unique, ancient event preceding their divergence. Following the ancient Cx- and C/n-values expansion and GC enrichment, the fescue genome must have undergone substantial reverse reduction running parallel in both broad-leaved and fine-leaved fescues, and numerous species groups appear to separate during this reduction process. The most dramatic reductions of Cx- and C/n-values and GC content were particularly associated with the derived evolutionary lineages. Within the broad-leaved fescues, a high reduction appeared in Festuca subgen. Schedonorus, and a further decrease was apparent in its derived lineage, Lolium. Within fine-leaved fescues, a marked decrease of Cx- and C/n-values and GC content was found in sections Festuca and Aulaxyper, and a further reduction accompanied the evolution of Vulpia, probably a derived lineage of Festuca sect. Aulaxyper (Ainscough et al., 1986; Torrecilla et al., 2004), in which Cx- and C/n-values and GC content were similar to those of related Poeae. As indicated by the high substitution rate of ITS and trnL-F sequences (Torrecilla et al., 2004) and high intra-specific ITS sequence diversity (Gaut et al., 2000), Festuca sect. Festuca, F. sect. Aulaxyper, and the genus Vulpia belong to the most rapidly evolving groups, and the reduction process seems to be closely associated with the rapid genome evolution within these groups. Some taxa of these groups also exhibit considerable intra-specific genome size variability (Šmarda, 2006; Šmarda and Bureš, 2006; Šmarda et al., 2007) which, together with the observations described above, indicates that that the reduction process is probably ongoing and evolutionarily recent.
Phylogenetic and taxonomic consequences
The differences in Cx- and C/n-values and GC content pattern found among several species and taxa groups as well as known trends of their evolution show that it is possible to use these parameters for the delimitation of taxa, and formulation of and testing evolutionary hypotheses. Preliminary results from an analysis of Cx- and C/n-values and GC content with easily accessible methods also offer an opportunity to survey main evolutionary processes in large species groups, which is essential for appropriate sample selection for future large-scale genomic projects, as has been already shown in Oryza and Gossypium (Hawkins et al., 2006; Piegu et al., 2006).
The high Cx- and C/n-values and GC content in basal fine-leaved fescues, sections Eskia and Dimorphae, are associated with the particular presence of the broad-leaved syndrome (flat leaves, sclerenchyma girders, extravaginal shoots and convolute to supervolute vernation; cf. Catalán et al., 2004), which is otherwise typical of all broad-leaved fescues and all other Poeae. The loss of this syndrome, formation of fine, narrow leaves, and the reduction in Cx- and C/n-values and GC content seem to follow the evolution of ‘modern’ fine-leaved fescues of sections Festuca and Aulaxyper.
The partial differentiation of the three main clusters of Festuca sect. Festuca in Cx- and C/n-values and CG content pattern (Fig. 2) gives additional support to our hypothesis that they might have developed at different evolutionary centres: (1) the F. halleri group in the alpine zone of the Alps and related south European high mountains; (2) the F. valesiaca + brevipila cluster in steppes and mountains of southern Siberia and the Middle East; and (3) the F. ovina + pallens + glauca + laevigata cluster in steppes and mountains of the southern and south-western Mediterranean.
Festuca amethystina may have a basal position within Festuca sect. Aulaxyper as indicated by the partial presence of the broad-leaved syndrome (sclerenchyma girders), and considerably higher Cx- and C/n-values and GC content, which are similar to basal fine- and broad-leaved fescues (Figs 1 and 2). The main gradient in the rest of the taxa of Festuca sect. Aulaxyper seems to be between diploids and polyploids, similarly as in Vulpia. The difference between Vulpia diploids and polyploids agrees with the main differences found in the ITS and trnL-F sequences (Catalán et al., 2004; Torrecilla et al., 2004), but it disagrees with the morphological data. Stace (2005) considered this to be a result of allopolyploidy and reticular evolution that may also have served as a reason for the decrease in Cx- and C/n-values typical of allopolyploids observed (see below).
Evolutionary implications
Studying highly diversified genera of Macaronesian flora, Suda et al. (2005) argued for the advantage of smaller genome in rapid adaptive evolution. Similarly, Knight et al. (2005) argued that large genomes have evolutionary and ecological constraints and showed that genera with small genomes are likely to be species-richer and over-represented in extreme environmental conditions. These hypotheses correspond well with the situation found in Festuca where the smallest and very GC-poor genomes are found in sections Festuca and Aulaxyper, which are (a) the most rapidly diverging fescue groups (Gaut et al., 2000) harbouring the most of species-diversity found within the genus, and (b) groups that have large ecological amplitude with numerous species dominating in extreme habitats of dry xeric steppes and alpine grasslands.
GC content and genome size variability
It is assumed that the reason why the ancient Cx- and C/n-values and GC content increase in basal fescues is that it may have been caused by GC-rich retrotransposon proliferation. Polyploidization or hybridization are unlikely as (a) diploids are common in both basal and derived fescues groups and (b) the present linkage map of meadow fescue (Festuca pratensis) is highly collinear with all model grasses, showing only a minimum of duplicated segments (Alm et al., 2003), which regularly accompany diploidized polyploid genomes (Ahn and Tanksley, 1993; Gaut and Doebley, 1997; Ilic et al., 2003) or homoploid hybrids (Rieseberg et al., 1995). Different mechanisms of transposable element proliferation and deletion are considered major reasons for the present genome size variability in grasses (SanMiguel and Bennetzen, 1998; Li et al., 2004) as well as in angiosperms as a whole (Kumar and Bennetzen, 1999; Bennetzen, 2002; Bennetzen et al., 2005; Vitte and Panaud, 2005). Transposable elements form a large portion of grass genomes (Meyers et al., 2001; Li et al., 2004; Messing et al., 2004; Haberer et al., 2005; Messing and Dooner, 2006; Paux et al., 2006), even in the smallest grass genome of rice (about 35 % of the genome; International Rice Genome Sequencing Project, 2005). Similarly to polyploidy, massive retrotransposon amplifications may rapidly increase genome size during a relatively short evolutionary period (SanMiguel and Bennetzen, 1998; SanMiguel et al., 1998; Bennetzen et al., 2005; Hawkins et al., 2006; Piegu et al., 2006). The genome of Oryza australiensis was increased >2-fold during the last three million years by a massive amplification of three retrotransposons, RIRE1, Kangourou and Wallabi (Piegu et al., 2006), recently accounting for about 60 % of its genome. GC contents of RIRE1, Kangourou and Wallabi retrotransposons (Noma et al., 1997; Piegu et al., 2006) are 44·6 %, 50·0 % and 50·9 %, respectively, which is considerably more, compared with the average GC content of the whole genome (43·6 %) or even genes (45·3 %) of the related Oryza sativa (International Rice Genome Sequencing Project, 2005). The assumed increase in GC content of O. australiensis was recently confirmed by partial sequencing of its genome within Oryza Map Alignment Project (Ammiraju et al., 2006). We believe that the retrotransposon-driven evolution of the Oryza genus (Uozu et al., 1997; Ma et al., 2004; Ammiraju et al., 2006) may be analogous to the evolution of fescues, and that the ancient Cx- and C/n-values and GC content expansion and reverse reduction (Fig. 4) may reflect a long-term dynamics of GC-rich retrotransposon proliferation and removal. This assumption serves also as a possible solution for a considerable correlation of Cx- and C/n-values with GC content, which was similarly to Festuca found also by comparing the GC contents and monoploid genome sizes in 12 wild rice species (cf. data of Ammiraju et al., 2006, tab. 4; r = 0,91; P < 0·001).
The 42·5–46·4 % GC content found in fescues and related genera is in accordance with results from studies in other grasses, indicating that the GC content of grasses is apparently the highest within the whole of the angiosperms and gymnosperms (Carels and Bernardi, 2000; Barow and Meister, 2002; Kuhl et al., 2004; Meister and Barow, 2007). One of the reasons for a higher GC content in grasses may be explained by the presence of grass genes that are extremely GC rich compared with other angiosperms (Carels and Bernardi, 2000; Meyers et al., 2001; Wong et al., 2002; Kuhl et al., 2004) and use therefore a modified codon coding (Kuhl et al., 2004). The fact that the GC content of genes positively correlates with the GC content of its surrounding, forming a GC-rich isochore structures (Bernardi, 2000a; Eyre-Walker and Hurst, 2001; Zhang and Zhang, 2004), further explains the potential impact of a gene's GC content on the overall genome GC content, in spite of the relatively low density of genes in most plant genomes. Beyond the phylogenetic differences in gene structure, the reasons for the GC content variability in grasses may be found further (a) in the composition and amount of transposable elements that can strongly vary in GC content; e.g. from about 28–34 % in MITE elements, common in rice, to over 60 % in Huck type elements in maize (Meyers et al., 2001; Turcotte et al., 2001), or (b) by differences in the proportion of coding and non-coding DNA, as genes and gene-rich regions are generally much more GC rich than non-coding ones (cf. Arabidopsis Genome Initiative, 2000; Meyers et al., 2001; International Rice Genome Sequencing Project, 2005).
Diploid–polyploid comparison
The two kinds of polyploid formation (Fig. 5) seem not to be phylogenetically conditioned, as similar trends occur in advanced fine-leaved fescue groups and closely related genus Dactylis. Beyond the limitation of polyploid Cx- and C/n-values and GC content by parental genome constitution, the reason for these two different patterns gives also allopolyploid versus autopolyploid formation of polyploids (Fig. 5; patterns 1 and 2, respectively).
In some taxa (groups) examined here, polyploids originating from diploids with large and GC-rich genomes may be assumed to be allopolyploids. This assumption is supported by the qualitative morphological differences within compared pairs. In the diploid F. pratensis and hexaploid F. arundinacea, the allopolyploid origin of the latter was proved also by the rDNA restriction pattern (Pasakinskiene et al., 1998). The putative hybrid origin of some polyploid Vulpia species is discussed by Stace (2005) and a recent hybrid origin of polyploid taxa of the F. rubra group is hypothesized by Kerguélen and Plonka (1989). The decrease in Cx- and C/n values in allopolyploids observed here agrees with the genome downsizing and rapid non-genic DNA elimination documented in wheat hybrids, assumed to be the general mechanism of successful allopolyploid formation (Feldman et al., 1997; Ozkan et al., 2001, 2003; Shaked et al., 2001).
The diploid–polyploid pairs with small and GC-poor genomes are morphologically closer than those of diploid–polyploid pairs with large genomes. In the F. inops–degenii, F. airoides–supina, F. ovina–guestfalica and F. valesiaca–pseudodalmatica pairs, polyploids differ only in some quantitative morphological characteristics from diploids, and in the latter two pairs, mixed populations are frequently found (P. Šmarda, unpubl. res.). It is likely that these pairs reflect a diploid–autopolyploid relationship. Experimental evidence for the autotetraploid origin of Dactylis glomerata from France was reported by Lumaret et al. (1989) and a diploid-autotetraploid relationship may also be suspected for the Dactylis polygama–glomerata pair in the present study. Although it is not clear whether all the pairs discussed represent diploid–autopolyploid relationships, autopolyploidy may explain the relatively small Cx- and C/n-value deviation in the polyploids observed here. It is unlikely that autopolyploidy itself results in large shifts in the proportion of bases, and it is assumed that these shifts are a consequence of another process. A small increase in genome size and the positive shift in GC content in autopolyploids may be explained, for instance, by post-hybridization amplification of GC-rich transposable elements, assumed above to be the main reason for the large-scale variation in Cx- and C/n-values and GC content within the whole Festuca genus.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are indebted to all colleagues who helped them collect the material. Special thanks are addressed to Joao Loureiro and Paulo Silveira (University of Aveiro) for providing the karyologically investigated plants from Portugal, Gilberto Parolo (University of Pavia) for providing plants from the Alps and to the Palermo Botanical Garden for seeds of some Mediterranean species. This study was supported by the Czech Ministry of Education (projects MSM0021622416 and LC06073) and partly by the Czech Academy of Sciences (project GA AV ČR IAA6163303).
LITERATURE CITED
- Ahn S, Tanksley SD. Comparative linkage maps of the rice and maize genomes. Proceedings of the National Academy of Sciences of the USA. 1993;90:7980–7984. doi: 10.1073/pnas.90.17.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainscough MM, Barker CM, Stace CA. Natural hybrids between Festuca and species of Vulpia section Vulpia. Watsonia. 1986;16:143–151. [Google Scholar]
- Alm V, Fang C, Busso CS, Devos KM, Vollan K, Grieg Z, Rognli OA. A linkage map of meadow fescue (Festuca pratensis Huds.) and comparative mapping with other Poaceae species. Theoretical and Applied Genetics. 2003;180:25–40. doi: 10.1007/s00122-003-1399-5. [DOI] [PubMed] [Google Scholar]
- Ammiraju JSS, Luo MZ, Goicoechea JL, Wang WM, Kudrna D, Mueller C, et al. The Oryza bacterial artificial chromosome library resource: construction and analysis of 12 deep-coverage large-insert BAC libraries that represent the 10 genome types of the genus Oryza. Genome Research. 2006;16:140–147. doi: 10.1101/gr.3766306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Bancheva S, Greilhuber J. Genome size in Bulgarian Centaurea s.l. (Asteraceae) Plant Systematics and Evolution. 2006;257:95–117. [Google Scholar]
- Barow M, Meister A. Lack of correlation between AT frequency and genome size in higher plants and the effect of nonrandomness of base sequences on dye binding. Cytometry. 2002;47:1–7. doi: 10.1002/cyto.10030. [DOI] [PubMed] [Google Scholar]
- Basak S, Ghosh TC. On the origin of genomic adaptation at high temperature for prokaryotic organisms. Biochemical and Biophysical Research Communications. 2005;330:629–632. doi: 10.1016/j.bbrc.2005.02.134. [DOI] [PubMed] [Google Scholar]
- Bennett MD, Leitch IJ. Angiosperm DNA C-values database. 2004 www.rbgkew.org.uk/cval/homepage.html. (release 5·0, December 2004)
- Bennetzen JL. Mechanisms and rates of genome expansion and contraction in flowering plants. Genetica. 2002;115:29–36. doi: 10.1023/a:1016015913350. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL, Ma J, Devos KM. Mechanisms of recent genome size variation in flowering plants. Annals of Botany. 2005;95:127–132. doi: 10.1093/aob/mci008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G. Isochores and the evolutionary dynamics of vertebrates. Gene. 2000;a 241:3–17. doi: 10.1016/s0378-1119(99)00485-0. [DOI] [PubMed] [Google Scholar]
- Bernardi G. The compositional evolution of vertebrate genomes. Gene. 2000;a 259:31–43. doi: 10.1016/s0378-1119(00)00441-8. [DOI] [PubMed] [Google Scholar]
- Bureš P, Wang Y-F, Horová L, Suda J. Genome size variation in Central European species of Cirsium (Compositae) and their natural hybrids. Annals of Botany. 2004;94:353–363. doi: 10.1093/aob/mch151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G. Evolution of genome size in the grasses. Crop Science. 2005;45:1809–1816. [Google Scholar]
- Carels N, Bernardi G. Two classes of genes in plants. Genetics. 2000;154:1819–1825. doi: 10.1093/genetics/154.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán P, Torrecilla P, López Rodríguez JA, Olmstead RG. Phylogeny of festucoid grasses of subtribe Loliinae and allies (Poeae, Pooideae) inferred from ITS and trnL-F sequences. Molecular Phyogenetics and Evolution. 2004;31:517–541. doi: 10.1016/j.ympev.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Clayton WD, Harman KT, Williamson H. onwards. World Grass Species, Descriptions, Identification and Information Retrieval. [(accessed 3 January 2007)];2002 http://www.kew.org/data/grasses–db.html .
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, et al. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Annals of Botany. 1998;82(Suppl. A):17–26. [Google Scholar]
- Dubcovsky J, Martínez A. Distribución geográfica de los niveles de ploidía en Festuca. Parodiana. 1992;7:91–99. [Google Scholar]
- Eyre-Walker A, Hurst LD. The evolution of isochores. Nature Reviews Genetics. 2001;2:549–555. doi: 10.1038/35080577. [DOI] [PubMed] [Google Scholar]
- Feldman M, Liu B, Segal G, Abbo S, Levy AA, Vega JM. Rapid elimination of low-copy DNA sequences in polyploid wheat, a possible mechanism for differentiation of homoeologous chromosomes. Genetics. 1997;147:1381–1387. doi: 10.1093/genetics/147.3.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet C, Keller B. Comparative genomics in the Grass family: molecular characterisation of grass genome structure and evolution. Annals of Botany. 2002;89:3–10. doi: 10.1093/aob/mcf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut BS. Evolutionary dynamics of grass genome. New Phytologist. 2002;154:15–28. [Google Scholar]
- Gaut BS, Doebley JF. DNA sequence evidence for the segmental allotetraploid origin of maize. Proceedings of the National Academy of Sciences of the USA. 1997;94:6809–6814. doi: 10.1073/pnas.94.13.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut BS, Tredway LP, Kubik C, Gaut RL, Meyer W. Phylogenetic relationships and genetic diversity among members of the Festuca-Lolium complex (Poaceae) based on ITS sequence data. Plant Systematics and Evolution. 2000;224:33–53. [Google Scholar]
- Gregory TR. Synergy between sequence and size in large-scale genomics. Nature Reviews Genetics. 2005;6:699–708. doi: 10.1038/nrg1674. [DOI] [PubMed] [Google Scholar]
- Greilhuber J, Doležel J, Lysák MA, Bennett MD. The origin, evolution and proposed stabilisation of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer G, Young S, Bharti AK, Gundlach H, Raymond C, Fuks G, et al. Structure and architecture of the maize genome. Plant Physiology. 2005;139:1612–1624. doi: 10.1104/pp.105.068718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JS, Kim H-R, Nason JD, Wing RA, Wendel JF. Differential lineage-specific amplification of transposable elements is responsible for genome size variation in Gossypium. Genome Research. 2006;16:1252–1261. doi: 10.1101/gr.5282906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker JH, Stebbins GL. Chromosomal evolution in the Gramineae. In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME, editors. Grass systematics and evolution. Washington, DC: Smithsonian Institution Press; 1987. pp. 178–187. [Google Scholar]
- Ilic K, SanMiguel PJ, Bennetzen JL. A complex history of rearrangement in an orthologous region of the maize, sorghum and rice genomes. Proceedings of the National Academy of Sciences of the USA. 2003;100:12265–12270. doi: 10.1073/pnas.1434476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature. 2005;436:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- Johnston JS, Pepper AE, Hall AE, Chen ZJ, Hodnett G, Drabek J, Lopez R, Price HJ. Evolution of genome size in Brassicaceae. Annals of Botany. 2005;95:229–235. doi: 10.1093/aob/mci016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. Relationships of cereal crops and other grasses. Proceedings of the National Academy of Sciences of the USA. 1998;95:2005–2010. doi: 10.1073/pnas.95.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA, Bennetzen JL. The evolution of nuclear genome structure in seed plants. American Journal of Botany. 2004;91:1709–1725. doi: 10.3732/ajb.91.10.1709. [DOI] [PubMed] [Google Scholar]
- Kerguélen M, Plonka F. Les Festuca de la Flore de la France (Corse complice) Buletin de la Société Botanique du Centre-Ouest. 1989;10:1–368. [Google Scholar]
- King GJ, Ingrouille MJ. Genome heterogenity and classification of the Poaceae. New Phytologist. 1987;107:633–644. [Google Scholar]
- Knight CA, Molinari NA, Petrov DA. The large genome constraint hypothesis, evolution, ecology and phenotype. Annals of Botany. 2005;95:177–190. doi: 10.1093/aob/mci011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl JC, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, et al. A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. The Plant Cell. 2004;16:114–125. doi: 10.1105/tpc.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bennetzen JL. Plant retrotransposons. Annual Review of Genetics. 1999;33:479–532. doi: 10.1146/annurev.genet.33.1.479. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Soltis DE, Soltis PS, Bennett MD. Evolution of DNA amounts across land plants (Embryophyta) Annals of Botany. 2005;95:207–217. doi: 10.1093/aob/mci014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang P, Fellers JP, Friebe B, Gill BS. Sequence composition, organisation and evolution of the core Triticeae genome. The Plant Journal. 2004;40:500–511. doi: 10.1111/j.1365-313X.2004.02228.x. [DOI] [PubMed] [Google Scholar]
- Loureiro J, Kopecký D, Castro S, Santos C, Silveira P. Flow cytometric and cytogenetic analyses of Iberian Peninsula Festuca spp. Plant Systematics and Evolution. 2007;269:89–105. [Google Scholar]
- Lumaret R, Bowman CM, Dyer TA. Autopolyploidy in Dactylis glomerata L. – further evidence from studies of chloroplast DNA variation. Theoretical and Applied Genetics. 1989;78:393–399. doi: 10.1007/BF00265302. [DOI] [PubMed] [Google Scholar]
- Ma J, Devos KM, Bennetzen JL. Analyses of LTR-retrotransposon structures reveal recent and rapid genomic DNA loss in rice. Genome Research. 2004;14:860–869. doi: 10.1101/gr.1466204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A, Barow M. DNA base composition of plant genomes. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells: analysis of genes, chromosomes and genomes. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2007. pp. 177–215. [Google Scholar]
- Messing J, Dooner H. Organisation and variability of the maize genome. Current Opinion in Plant Biology. 2006;9:157–163. doi: 10.1016/j.pbi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Messing J, Bharti AK, Karlowski WM, Grundlach H, Kim HR, Yu Y, et al. Sequence composition and genome organisation of maize. Proceedings of the National Academy of Sciences of the USA. 2004;101:14349–14354. doi: 10.1073/pnas.0406163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers BC, Tungey SV, Morgante M. Abundance, distribution and transcriptional activity of repetitive elements in the maize genome. Genome Research. 2001;11:1660–1676. doi: 10.1101/gr.188201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto H, Naya H, Zavala A, Romeo H, Alvarez-Valín F, Bernardi G. Correlations between genomic GC levels and optimal growth temperatures in prokaryotes. FEBS Letters. 2004;573:73–77. doi: 10.1016/j.febslet.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Nakamura Y, Kawarabayasi Y, Usuda Y, Kimura E, Sugimoto S, et al. Comparative complete genome sequence analysis of the amino acid replacements responsible for thermostability of Corynebacterium efficiens. Genome Research. 2003;13:1572–1579. doi: 10.1101/gr.1285603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Nakajima R, Ohtsubo H, Ohtsubo E. RIRE1, a retrotransposon from wild rice Oryza australiensis. Genes and Genetic Systems. 1997;72:131–140. doi: 10.1266/ggs.72.131. [DOI] [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z, editors. Methods in cell biology. Vol. 33. New York, NY: Academic Press; 1990. pp. 105–110. Flow cytometry. [DOI] [PubMed] [Google Scholar]
- Ozkan H, Levy AA, Feldman M. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops-Triticum) group. The Plant Cell. 2001;13:1735–1747. doi: 10.1105/TPC.010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan H, Tuna M, Arumuganathan K. Nonadditive changes in genome size during allopolyploidisation in the wheat (Aegilops-Triticum) group. Journal of Heredity. 2003;94:260–264. doi: 10.1093/jhered/esg053. [DOI] [PubMed] [Google Scholar]
- Pasakinskiene I, Anamthawat-Jonsson K, Humphreys MW, Paplauskiene V, Jones RN. New molecular evidence on genome relationships and chromosome identification in fescue (Festuca) and ryegrass (Lolium) Heredity. 1998;81:659–665. [Google Scholar]
- Paterson AH, Freeling M, Sasaki T. Grains of knowledge: genomics of model cereals. Genome Research. 2005;15:1643–1650. doi: 10.1101/gr.3725905. [DOI] [PubMed] [Google Scholar]
- Paux E, Roger D, Badaeva E, Gay G, Bernard M, Sourdille P, Feuillet C. Characterizing the composition and evolution of homoeologous genomes in hexaploid wheat through BAC-end sequencing on chromosome 3B. The Plant Journal. 2006;48:463–474. doi: 10.1111/j.1365-313X.2006.02891.x. [DOI] [PubMed] [Google Scholar]
- Piegu B, Guyot R, Picault N, Roulin A, Saniyal A, Kim H, et al. Doubling genome size without polyploidization, dynamics of retrotransposition-driven genomic expansion in Oryza australensis, a wild relative of rice. Genome Research. 2006;16:1262–1269. doi: 10.1101/gr.5290206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price HJ, Dillon SL, Hodnett G, Rooney WL, Ross L, Johnston JS. Genome evolution in the genus Sorghum (Poaceae) Annals of Botany. 2005;95:219–227. doi: 10.1093/aob/mci015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg LH, Van Fossen C, Desrochers AM. Hybrid speciation accompanied by genomic reorganisation in wild sunflowers. Nature. 1995;375:313–316. [Google Scholar]
- SanMiguel P, Bennetzen JL. Evidence that a recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Annals of Botany. 1998;82:37–44. [Google Scholar]
- SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL. The paleontology of intergene retrotransposons of maize. Nature Genetics. 1998;20:43–45. doi: 10.1038/1695. [DOI] [PubMed] [Google Scholar]
- Shaked H, Kashkush K, Ozkan H, Feldman M, Levy AA. Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. The Plant Cell. 2001;13:1749–1759. doi: 10.1105/TPC.010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šmarda P. DNA ploidy levels and intraspecific DNA content variability in Romanian fescues (Festuca L, Poaceae), measured in fresh and herbarium material. Folia Geobotanica. 2006;41:417–432. [Google Scholar]
- Šmarda P, Bureš P. Intraspecific DNA content variability in Festuca pallens on different geographical scales and ploidy levels. Annals of Botany. 2006;98:665–678. doi: 10.1093/aob/mcl150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šmarda P, Kočí K. Chromosome number variability in Central European members of the Festuca ovina and F. pallens groups (sect Festuca) Folia Geobotanica. 2003;38:65–95. [Google Scholar]
- Šmarda P, Stančík D. Ploidy level variability in South American fescues (Festuca L, Poaceae), use of flow cytometry in up to 5½-year-old caryopses and herbarium specimens. Plant Biology. 2006;8:73–80. doi: 10.1055/s-2005-872821. [DOI] [PubMed] [Google Scholar]
- Šmarda P, Horová L, Bureš P. Random distribution pattern and non-adaptivity of genome size in a highly variable population of Festuca pallens. Annals of Botany. 2007;100:141–150. doi: 10.1093/aob/mcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stace CA. Plant taxonomy and biosystematics – does DNA provide all the answers? Taxon. 2005;54:999–1007. [Google Scholar]
- Suda J, Kyncl T, Jarolímová V. Genome size variation in Macaronesian angiosperms: forty percent of the Canarian endemic flora completed. Plant Systematics and Evolution. 2005;252:215–238. [Google Scholar]
- Torrecilla P, Catalán P. Phylogeny of broad-leaved and fine-leaved Festuca lineages (Poaceae) based on nuclear ITS sequences. Systematic Botany. 2002;27:241–251. [Google Scholar]
- Torrecilla P, López-Rodríguez JA, Catalán P. Phylogenetic relationships of Vulpia and related genera (Poeae, Poaceae) based on analysis of ITS and trnL-F sequences. Annals of the Missouri Botanical Garden. 2004;91:124–158. [Google Scholar]
- Turcotte K, Srinivasan S, Burelu T. Survey of transposable elements from rice genomic sequences. The Plant Journal. 2001;25:169–179. doi: 10.1046/j.1365-313x.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- Uozu S, Ikehashi H, Ohmido N, Ohtsubo H, Ohtsubo E, Fukui K. Repetitive sequences: cause for variation in genome size and chromosome morphology in the genus Oryza. Plant Molecular Biology. 1997;35:791–799. doi: 10.1023/a:1005823124989. [DOI] [PubMed] [Google Scholar]
- Vitte C, Panaud O. LTR retrotransposons and flowering plant genome size: emergence of the increase/decrease model. Cytogenetic and Genome Research. 2005;110:91–107. doi: 10.1159/000084941. [DOI] [PubMed] [Google Scholar]
- Weiss-Schneeweiss H, Greilhuber J, Schneeweiss GM. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. American Journal of Botany. 2006;93:148–156. doi: 10.3732/ajb.91.3.439. [DOI] [PubMed] [Google Scholar]
- Wong GK-S, Wang J, Tao L, Tan J, Zhang J-G, Passey DA, Yu J. Compositional gradients in Gramineae genes. Genome Research. 2002;12:851–856. doi: 10.1101/gr.189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závesky L, Jarolímová V, Štěpánek J. Nuclear DNA content variation within the genus Taraxacum (Asteraceae) Folia Geobotanica. 2005;40:91–104. [Google Scholar]
- Zhang R, Zhang C-T. Isochore structures in the genome of plant Arabidopsis thaliana. Journal of Molecular Evolution. 2004;59:227–238. doi: 10.1007/s00239-004-2617-8. [DOI] [PubMed] [Google Scholar]
- Zoubak S, Clay O, Bernardi G. The gene distribution of the human genome. Gene. 1996;174:95–102. doi: 10.1016/0378-1119(96)00393-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.