Abstract
Memory is a ubiquitous phenomenon in biological systems, yet the mechanisms responsible for memory, and how to manipulate it at the subcellular level, remain poorly understood. Subjected to transient stimuli, biological systems can exhibit short early responses and/or prolonged (or permanent) late responses. Experimental evidence suggests that early responses (“short-term memory”) involve posttranslational modification of existing proteins and/or their intracellular relocalization, whereas late responses (“long-term memory”) depend on new protein synthesis. Although this provides an intuitive explanation at the basic molecular level, it does little to clarify the important dynamics that actually maintain memory at the systems level. In this study, we use mathematical modeling to study dynamical mechanisms of biological memory. We first examined the response of four fundamental motifs (positive/negative feedforward and feedback) to external stimuli. Because motifs do not exist in isolation within the cell, we then combined these motifs to form signaling modules to understand how they confer biological memory. These motifs, and different combinations thereof, displayed distinct behavior in response to external stimuli. The principles described in this study have important implications for experimental approaches to identify the mechanisms for biological memory and for the development of therapeutic strategies to modulate signaling network responses in the setting of human disease.
Keywords: computational biology, protein interactions, dynamics
Many biological systems exhibit biphasic responses to a transient stimulus, in which an immediate brief response is followed, often after a delay, by a substantially prolonged response, conferring a form of biological “memory” to signal transduction. For example, in nerve synapses, a single train of stimuli causes a response lasting a few hours, but multiple trains of stimuli can induce long-term potentiation (LTP) of the response which can last >24 hours or indefinitely (1). These early and late LTP responses have been proposed to be the equivalents, at the synaptic level, of short-term and long-term memory underlying the learning behavior (1,2). In heart (Fig.1A), protection against ischemic injury (“heart attack”) can be induced by a brief preconditioning episode of low blood flow, which results in an immediate early phase of protection, lasting a few hours, followed, after a delay of up to 24 hrs, by a late protection phase that lasts for several days (3,4). Similar protection phases have been documented experimentally in the brain (5,6), gut (7), and lung (8). A third example occurs in oocyte development (Fig.1B), in which progesterone induces transient elevation of maturation promoting factor (MPF), followed by a sustained elevation. This sustained level of MPF is maintained until fertilization triggers cell cycling, after which MPF levels oscillate to drive cell cycle progression (9-11). Other examples of biphasic responses in biological systems include allergen-induced early and late asthmatic responses (12,13), biphasic lymphatic responses to intradermal alloantigens (14), fibroblast growth factor-2 (FGF-2) induced membrane protrusion (15), and NMDA receptor-induced early and late gene expression (16).
Fig.1. Memory induced by short stimulus in biological systems.
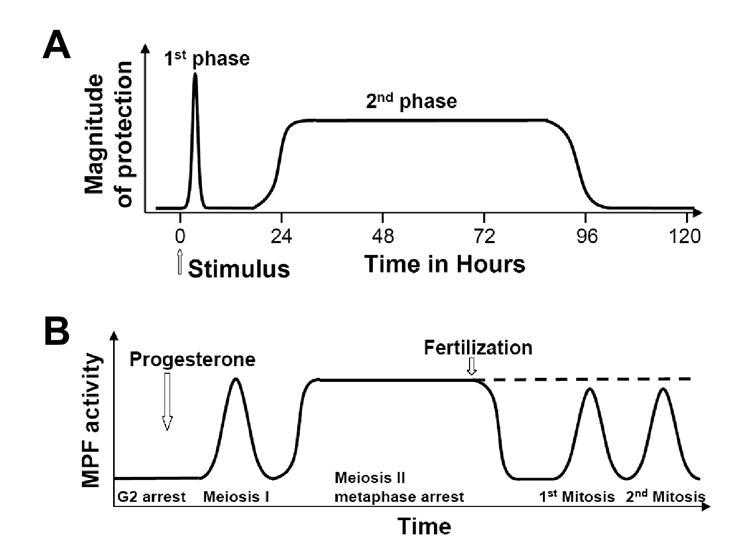
A. Schematic plot of the two phases of protection in heart following short stimulation, such as a short period of NO or CCPA infusion (similar observations made in multiple organ systems, see text). This panel was modified from Yellon and Downey (3). B. Schematic plot of the MPF activity after progesterone stimulation and fertilization in Xenpus oocytes, modified from Murray and Kirschner (9) and Ferrell (10). The dashed line indicates that MPF would maintain high if there is no fertilization.
In these biological responses, it has generally been speculated that the immediate, transient response to a stimulus involves posttranslational modification of existing proteins and/or their mobilization between cellular compartments, whereas the late response depends on new RNA and protein synthesis. Although this provides an intuitive explanation for the transience of the early phase and the prolonged nature of the late phase, it does little to clarify important subtleties that often exist in stimulus-response dynamics. For example, in the heart, cardioprotection can be induced pharmacologically by administration of the adenosine A1 receptor antagonist (CCPA), with a single dose resulting in both immediate early and delayed late protection that lasts more than 72 hours in the rabbit (3,4). However, a 72-hour continuous CCPA infusion afforded no sustained protection (17), whereas pulses of CCPA at 48-hour intervals maintained the beneficial response continuously for at least 10 days (18). Therefore, understanding what mechanisms underlie the different forms of memory and how the system responses to additional stimuli is of great importance for therapeutic purposes. Here we hypothesize that signaling network motifs, the building blocks of the signal transduction networks of biological systems, confer different forms of memory responses. We demonstrate through various simulations that these motifs combine to produce distinct emergent behavior, and we suggest experimental approaches to dissect these dynamics in biological systems.
Signaling network motifs and their role in the formation of memory
Signaling networks can exhibit a variety of dynamical motifs, including positive and negative feedback, and positive and negative feedforward (19,20). Due to the complex network topologies that govern the interactions of these motifs, various types of responses are observed experimentally (21-23). A modeling study by Mangan and Alon (19) analyzed the responses of feedforward loop motifs in transcription networks and found that incoherent feedforward loops acted as sign-sensitive accelerators, whereas coherent feedforward loops acted as sign-sensitive delays. Here we show how these signaling motifs and their combinations can confer different memory responses. Fig.2 shows the four types of motifs: positive feedforward (A); positive feedback (B); negative feedforward (C); negative feedback (D). In each motif, the stimulus S directly activates the response protein, R. Time dependence regulating the delay and/or duration of the response R arises from signaling cascades, such as “X1 →…→ XK” in Fig.2, which are activated either by the stimulus S or by the response protein R. The subscript indices, such as K, indicate the length of the cascade. These motifs can either function alone or be combined into signaling modules capable of accomplishing more complex biological functions that arise as emergent properties of the signaling network.
Fig.2. Signaling motifis.
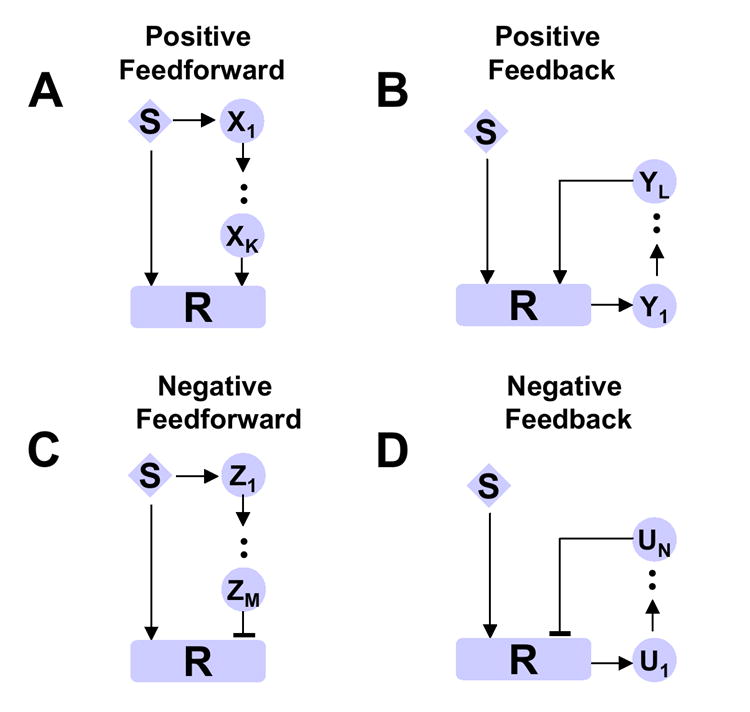
A. positive feedforward; B. positive feedback; C. negative feedforward; D. negative feedback. S represents the stimulus and R the effector protein. Xk (k=1,K), Yl (l=1,L), Zm (m=1,M), and Un (n=1,N) represent the proteins in the signaling cascades.
The dynamics of the signaling motifs are distinct. In the feedforward motif (Fig.2A), a short stimulus causes both an early transient response and a delayed prolonged response (Fig.3A), similar to that shown in Fig. 1A. The delay time and the duration of the second response are determined by activation time constant of each step and the length of the cascade shown in Fig.2A. Additional stimuli either further prolong the late response (if the stimulus occurs during the late response period, Fig.3B) or activate a new response (if it is given after the late response period). For the positive feedback motif (Fig.2B), the same short stimulus can cause a similar early transient response while the late prolonged response becomes permanent (Fig.3C), which was proposed by Xiong and Ferrell (11) as the mechanism for oocyte maturation (Fig. 1B). In fact, this permanent response depends on the strength of the positive feedback. If feedback is weak, the steady-state response to a constant stimulus is sigmoidal. As the feedback strength increases, however, the response changes from sigmoidal to bistable, i.e., over a certain range of stimulus strength, the steady-state response becomes bimodal, either low or high. With a bistable system, when the stimulus strength increases from small to large (dashed green arrows in Fig.3D), the response “jumps” to the higher state once a threshold stimulus strength is achieved. However, as the stimulus strength decreases from large to small, the response “jumps” down at a different, lower stimulus strength. This forms a hysteresis loop—a rudimentary manifestation of biological memory. Hysteresis and bistability due to nonlinear protein-protein interactions have been experimentally demonstrated in several biological systems (24-28). If the feedback is even stronger, the response to a stimulus can become irreversible (Fig. 3D), i.e., upon decreasing the stimulus to zero, the system still remains activated, unable to return to the basal low state, which causes the permanent response in Fig.3C. In this setting, however, if a negative feedforward or feedback loop also exists within the system, an irreversible response can be converted to a reversible one, provided the negative feedback or feedforward motifs are sufficiently strong (Fig. 3E). In other words, a sustained bistable response due to positive feedback that appears irreversible may be inactivated if a negative feedback motif is inserted/recruited to counteract the sustained activation.
Fig.3. Responses of netwrok motifs to stimulation.
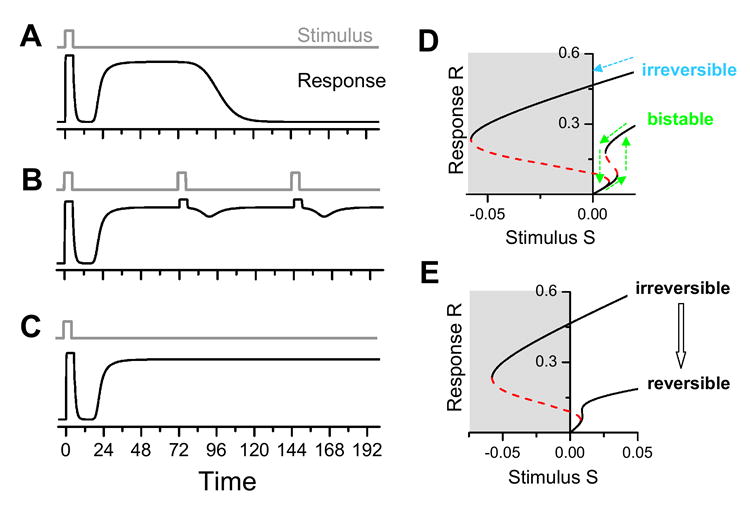
A. Response of the positive feedforward motif to a stimulation pulse. B. Response of the positive feedforward motif (Fig.2A) to multiple stimulation pulses. C. Response of the positive feedback motif (Fig.2B) to a stimulation pulse. D. Steady state response R to the strength of a constant stinulus S for the positive feedback motif, showing bistablity and an irreversible steady state. E. Steady state response to the strength of a constant stimulus S for the positive feedback motif and for a module composed of a positive feedback motif and a negatvie feedback motif, showing the conversion of an irreversible response to a reversible one following addition of a negative feedback motif. In D and E, dashed lines are unstable steady states. The gray areas are negative stimulation strengths which cannot exist in a real system, thereby manifesting as irreversible responses.
Emergent forms of memory arising from combinations of motifs into signaling modules
As mentioned above, a positive feedback motif combined with a negative feedback motif can turn a permanent response into one that is transient. Fig.4 shows two such signaling modules and their response to single and multiple stimuli. Fig.4A shows a signaling module composed of a positive feedback motif and a negative feedforward motif. We set the delays from both cascades to be long, with the delay in the negative feedforward cascade longer than the delay in the positive feedback cascade. The difference in delays is sufficient to produce a response similar to that shown diagrammatically in Fig. 1A (second panel in Fig. 4A). In this case, however, an additional stimulus can elicit a response only after a certain time period has passed (i.e. the refractory period; middle panel). If the system is stimulated at an interval shorter than the refractory period, no further responses are elicited (bottom panel) thereby perpetuating the refractoriness. Fig.4B shows a signaling module composed of a positive feedback motif and a negative feedback motif. If the delay in both cascades are long, with the delay in the negative feedback cascade longer than the delay in the positive feedback cascade, the response is similar to that shown in Fig. 1A (top panel in Fig. 4B). However, when subjected to periodic stimuli, the system responds only to stimuli applied outside of the refractory period (bottom panel). A comparison between the modules in Fig. 4A and 4B highlights the implications of the analyses in this study: combination of either a negative feedforward (Fig. 4A) or a negative feedback (Fig. 4B) motif with a positive feedback motif causes emergence of refractory behavior that is essentially indistinguishable under basal conditions. However, repeated stimulation perpetuates this refractoriness indefinitely when the feedforward motif is present—fundamentally changing the behavior of the system—whereas repeated stimulation of the module containing the feedback motif has no such influence to alter the system. If one substitutes the positive feedback with positive feedforward in Fig. 4, the same refractory behaviors would occur.
Fig.4. Responses to stimuli for signaling modules composed of distinct motifs.
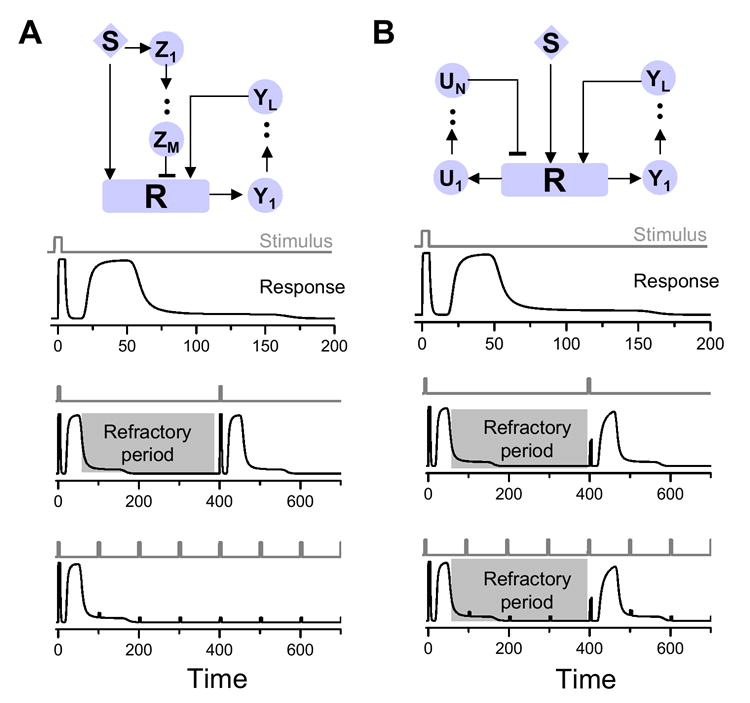
Signaling modules composed of a positive feedback and negative feedforward motif (A) and of a positive feedback and negative feedforward motif (B) and their responses to different stimuli. The gray areas indicate the refractory period.
Can we infer the structure and dynamics of a signaling network from experimental data?
The principles regarding motifs described herein are tools the biologist can employ to understand behavior of the signaling system that would otherwise be opaque to biochemical analyses. One example comes from the field of cardiovascular disease, in particular, pharmacologic strategies to protect against heart attack-induced cell death. As noted above, the drug CCPA (an adenosine agonist) have been shown to induce a biphasic protective response in the heart as shown diagrammatically in Fig.1A. Based on the analyses above, either the single positive forward motif (Fig.3A) or combined ones positive and negative ones, such as the ones shown in Fig.4, can give rise to such a response. However, two early experimental studies (17,18) provide additional information to identify a signaling module driving this protective response. The basic experimental results for CCPA-induced protection are summarized in Fig.5A. A single dose of CCPA is protective either immediately after the removal of stimulus or 48 hours later. A continuous 72 hour administration of this same drug, however, failed to protect the heart immediately following removal of the CCPA (17). Multiple pulses of the drug at 48 hour intervals for 10 days were effective to maintain the protection at 48 hours after the final pulse (18). Based on these observations, we constructed a signaling module as illustrated in Fig. 5B (positive feedforward motif plus negative feedforward motif), in which the delay in the negative feedforward cascade is short. Fig.5C shows the response of this module to different stimulation protocols. For a single brief stimulus (top panel), the typical response imitates the experimental observation as shown in Fig. 1A. For a continuous stimulus (middle panel), however, the prolonged response is inhibited by the negative feedforward motif, but a late prolonged response emerges after the stimulus is terminated. Periodic stimuli (bottom panel) produce transient inhibition of the prolonged response, with a normal prolonged response emerging after the last stimulus. These results agree with the experimental data shown diagrammatically in Fig.5A; moreover, these findings suggest further experimentation that can be carried out to better understand the dynamics of the system. These included investigation of whether protection still exists 48 hours after the continuous 72 hour drug infusion is terminated (middle panel in Fig. 5C) and whether there is an unprotected period between the initial stimulus and 48 hours later in the case of periodic stimulation (bottom panel in Fig. 5C). These questions are suggested by the models put forth in the present study and have direct relevance to determining dosage protocols and windows of vulnerability in patients treated with these drugs.
Fig.5.
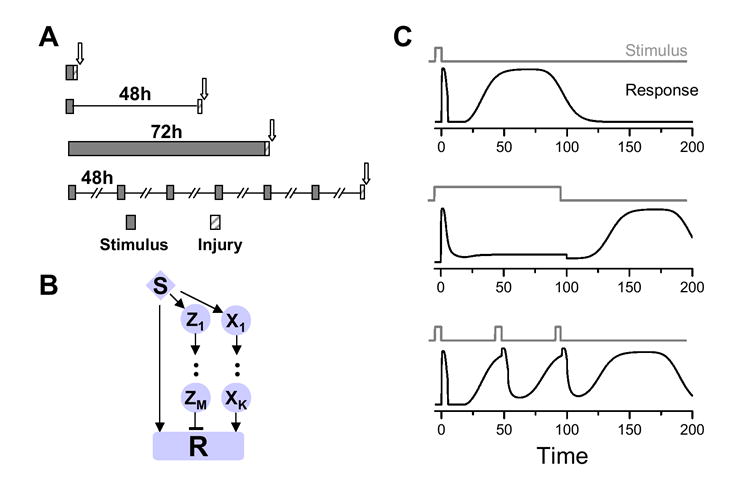
A. Schematic representation of experimental data from cardiac protection studies. Scale is approximate; arrow indicated time point of analysis. B. Signaling module composed of positive feedforward motif and a negative feedforward motif as one potential representation of the module driving the experimental behavior in A. C. Response of signaling module in B to different stimuli.
Conclusions
Biological memory emerges from the formation of motifs within the topology of a signaling network. These motifs have distinct properties that, when combined to form modules, impart dynamic behavior to the network that transcends what is imparted from structure alone. Although memory is observed in countless biological systems and the specific components of corresponding signaling networks may vary greatly from system to system, we reason they may share the same basic dynamics for the formation of memory. In other words, we hypothesize that the signaling motifs shown in Fig. 2 are the basic building blocks for the formation of biological memory. As revealed in this study, this memory can be accompanied by distinct types of refractoriness depending on which signaling motifs are combined. The dynamics of this refractoriness lead to completely different behavior of the system following repetitive or continuous stimuli: such insights are directly relevant to strategies for the modulation of signaling networks therapeutically.
Appendix
Differential equations were developed based on the schemes shown in Fig. 2, which were presented in general as:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where Xk is the activity of the kth signaling protein in the X-cascade, as are Yl in the Y-cascade, Zm in the Z-cascade, and Un in the U-cascade, respectively. RT=1 is the total response protein concentration in the system and R is the active fraction. S is the stimulus strength, ranging from 0 to 1. X0=Z0=S (feedforward loop, for a and c in Fig. 1) and Y0=U0=R (feedback loop, for b and d in Fig. 1). For each step in the cascades, we used second-order Hill functions, which can be resulted by multiple phosphorylation (29-31) or other biological processes (32-34). K, L, M, and N are the lengths of the corresponding cascades of Eqs.1-4. Eqs. 1-5 were integrated using the fourth order Runge-Kutta method with a time step 0.002. The default parameter set is: α=β=γ=σ=0.28, τx= τy=τz=τu=6, v1= v2= v3=5, v4=1, and v5= v6=80. Unless otherwise specified, the default parameters were used in all simulations.
When different from control parameters listed in the previous paragraph, the parameters used for simulation were as follows according to Figure: Figs.3A-C, K=L=M=N=10; Fig.3D, L=2, v2=0.35 (bistable) and 1 (irreversible); Fig.3E, L=2, N=2, v2=1 and v5=5; Fig.4A, L=M=10, τz= 3τy=18; Fig.4B, L=N=10, τu= 3τy=18; and Fig.5C, K=10 and M=3.
Acknowledgments
This study was supported by funds from NIH, AHA, UCLA Department of Medicine, and Laubisch and Kawata Endowments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 2.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchida A, Liu GS, Wilborn WH, Downey JM. Pretreatment with the adenosine A1 selective agonist, 2-chloro-N6-cyclopentyladenosine (CCPA), causes a sustained limitation of infarct size in rabbits. Cardiovasc Res. 1993;27:652–656. doi: 10.1093/cvr/27.4.652. [DOI] [PubMed] [Google Scholar]
- 5.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordet R, Deplanque D, Maboudou P, Puisieux F, Pu Q, Robin E, Martin A, Bastide M, Leys D, Lhermitte M, Dupuis B. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. J Cereb Blood Flow Metab. 2000;20:1190–1196. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 8.Du ZY, Hicks M, Winlaw D, Spratt P, Macdonald P. Ischemic preconditioning enhances donor lung preservation in the rat. J Heart Lung Transplant. 1996;15:1258–1267. [PubMed] [Google Scholar]
- 9.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell JE., Jr Xenopus oocyte maturation: new lessons from a good egg. BioEssays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Xiong W, Ferrell JE., Jr A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DA, McGrath JL, O’Connor BJ, Barnes PJ. Allergen-induced early and late asthmatic responses are not affected by inhibition of endogenous nitric oxide. Am J Respir Crit Care Med. 1998;158:99–106. doi: 10.1164/ajrccm.158.1.9709091. [DOI] [PubMed] [Google Scholar]
- 13.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su M, Young AJ, He C, West CA, Swanson SJ, Mentzer SJ. Biphasic response of the regional lymphatics in the normal lymphocyte transfer reaction. Transplantation. 2001;72:516–522. doi: 10.1097/00007890-200108150-00026. [DOI] [PubMed] [Google Scholar]
- 15.Fera E, O’Neil C, Lee W, Li S, Pickering JG. Fibroblast growth factor-2 and remodeled type I collagen control membrane protrusion in human vascular smooth muscle cells: biphasic activation of Rac1. J Biol Chem. 2004;279:35573–35582. doi: 10.1074/jbc.M400711200. [DOI] [PubMed] [Google Scholar]
- 16.Hong SJ, Li H, Becker KG, Dawson VL, Dawson TM. Identification and analysis of plasticity-induced late-response genes. Proc Natl Acad Sci U S A. 2004;101:2145–2150. doi: 10.1073/pnas.0305170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchida A, Thompson R, Olsson RA, Downey JM. The anti-infarct effect of an adenosine A1-selective agonist is diminished after prolonged infusion as is the cardioprotective effect of ischaemic preconditioning in rabbit heart. J Mol Cell Cardiol. 1994;26:303–311. doi: 10.1006/jmcc.1994.1039. [DOI] [PubMed] [Google Scholar]
- 18.Dana A, Baxter GF, Walker JM, Yellon DM. Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J Am Coll Cardiol. 1998;31:1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 19.Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma’ayan A, Jenkins SL, Neves S, Hasseldine A, Grace E, Dubin-Thaler B, Eungdamrong NJ, Weng G, Ram PT, Rice JJ, Kershenbaum A, Stolovitzky GA, Blitzer RD, Iyengar R. Formation of regulatory patterns during signal propagation in a Mammalian cellular network. Science. 2005;309:1078–1083. doi: 10.1126/science.1108876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15:221–231. doi: 10.1016/s0955-0674(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 22.Lauffenburger DA. Cell signaling pathways as control modules: complexity for simplicity? Proc Natl Acad Sci U S A. 2000;97:5031–5033. doi: 10.1073/pnas.97.10.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–917. doi: 10.1038/43199. [DOI] [PubMed] [Google Scholar]
- 24.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 25.Pomerening JR, Sontag ED, Ferrell JE., Jr Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 26.Becskei A, Seraphin B, Serrano L. Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. EMBO J. 2001;20:2528–2535. doi: 10.1093/emboj/20.10.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha W, Moore J, Chen K, Lassaletta AD, Yi CS, Tyson JJ, Sible JC. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci U S A. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 29.Huang CYF, Ferrell JE. Ultrasensitivity in the Mitogen-Activated Protein Kinase Cascade. Proc Natl Acad Sci USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu Z, Weiss JN, MacLellan WR. Regulation of the mammalian cell cycle: a model of the G1-to-S transition. Am J Physiol Cell Physiol. 2003;284:C349–364. doi: 10.1152/ajpcell.00066.2002. [DOI] [PubMed] [Google Scholar]
- 31.Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldbeter A, Koshland DE., Jr An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci U S A. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian H. Amplifying signal transduction specificity without multiple phosphorylation. Biophys J. 2003;84:1410–1411. doi: 10.1016/S0006-3495(03)74955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian H. Thermodynamic and kinetic analysis of sensitivity amplification in biological signal transduction. Biophys Chem. 2003;105:585–593. doi: 10.1016/s0301-4622(03)00068-1. [DOI] [PubMed] [Google Scholar]


