Abstract
Presilphiperfolan-8β-ol synthase, encoded by the BcBOT2 gene from the necrotrophic plant pathogen Botrytis cinerea, catalyzes the multistep cyclization of farnesyl diphosphate (2) to the tricyclic sesquiterpene alcohol presilphiperfolan-8β-ol (3), the preursor of the phytotoxin botrydial, a strain-dependent fungal virulence factor. Incubation of (1R)-[1-2H]farnesyl diphosphate (2b) with recombinant presilphiperfolan-8β-ol synthase gave exclusively (5R)-[5α-2H]-3b, while complementary incubation of (1S)-[1-2H]FPP (2c) gave (5S)-[5β-2H]-3c. These results established that cyclization of farnesyl diphosphate involves displacement of the diphosphate group from C-1 with net inversion of configuration and ruled out the proposed intermediacy the cisoid conformer of nerolidyl diphosphate (9) in the cyclization. While not a mandatory intermediate, (3R)-nerolidyl diphosphate was shown to act as a substrate surrogate. Cyclization of [13,13,13-2H3] farnesyl diphosphate (2d) gave [14,14,14-2H3]-3d, thereby establishing that electrophilic attack takes place exclusively on the si face of the 12,13-double bond of 2. The combined results provide a detailed picture of the conformation of enzyme-bound farnesyl diphosphate at the active site of presilphiperfolan-8β-ol synthase.
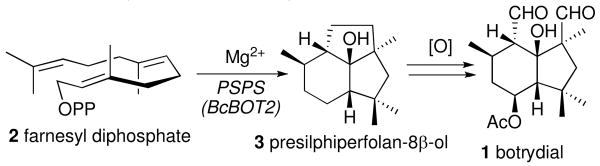
Many terpenoid metabolites serve as virulence factors for filamentous fungi, allowing the phytopathogenic fungus to overcome the natural plant host cell defenses.1 The fungus Botrytis cinerea, a necrotrophic plant pathogen that secretes the sesquiterpene phytotoxin botrydial (1), is the causal agent of the economically important gray mold disease that affects over 200 plant host species worldwide.2 Recently, the botrydial biosynthetic gene cluster in B. cinerea has been identified.3 Among the five clustered genes, the 1.2 kb BcBOT2 gene was shown to encode the sesquiterpene synthase that is responsible for the committed step in the biosynthesis of botrydial. Targeted deletion of the BcBOT2 gene abolished production of botrydial and all related probotryane metabolites, which were fully restored by in trans complementation. Inactivation of the BcBOT2 gene also resulted in strain-dependent loss of virulence. Direct evidence for the biochemical function of BcBOT2 came from the demonstration that recombinant BcBOT2 protein converted farnesyl diphosphate (2, FPP) to the parent tricyclic alcohol presilphiperfolan-8β-ol (3, PSP) (Scheme 1). 3 is converted to botrydial by the action of three cytochrome P450s encoded by the co-regulated genes BcBOT1, BcBOT3, and BcBOT4 as well as by an acetyl transferase (BcBOT5), acting in as yet undetermined sequence.3,4 Several probotryane and botryane metabolites that are likely intermediates linking 3 and botrydial.5
Scheme 1.
Biosynthesis of Botrydial (1).
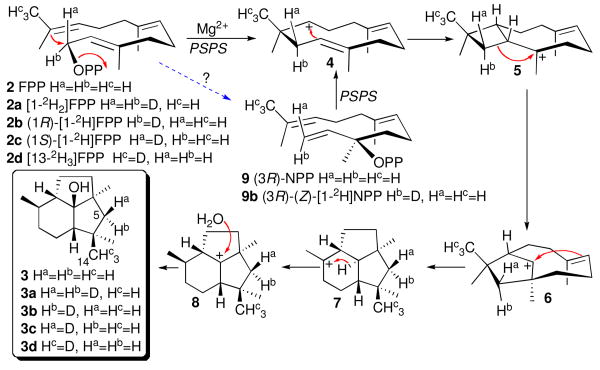
Incorporation experiments in which labeled acetate and mevalonate were fed to cultures of B. cinerea first established the origin of the botrydial carbon skeleton and indicated the operation of a backbone rearrangement as well as a 1,3-hydride shift during the course of FPP cyclization.6 We have recently corroborated this original mechanistic proposal by incubation of recombinant PSP synthase (BcBOT2) with [1,1-2H2]FPP (2a) giving [5,5-2H2]-3a, whose 1H NMR spectrum was identical to that of unlabeled 3 except for the absence of the characteristic doublets at δ 2.19 and 1.23 corresponding to the geminal H-5 methylene protons (Scheme 2).3
Scheme 2.
Cyclization of FPP (2) and of the surrogate substrate (3R)-NPP (9) to 3.
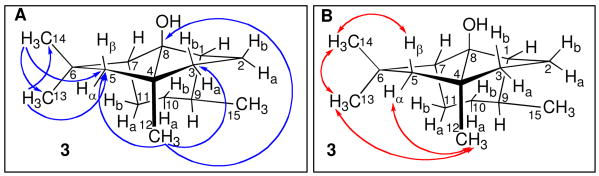
In order to probe the stereochemistry of formation of PSP, we rigorously assigned the 1H and 13C NMR resonances of 3 using a combination of 1H, 13C, 1H-1H COSY, NOESY, HSQC, and HMBC NMR.7 The four methyl groups of 3 were readily identified: a geminal methyl pair (H-13, δ 1.21; C-13, 28.0 ppm and H-14, δ 1.42; C-14, 36.4 ppm) attached to a quaternary carbon (C-6, 48.0 ppm); the bridgehead methyl (H-12, δ 1.16; C-12, 28.1 ppm) attached to a quaternary carbon (C-4, 56.4 ppm); and a secondary methyl (H-15, δ 0.88, d, J = 6.4 Hz; C-15, 21.8 ppm) adjacent to a C-H (H-9, δ 1.29, m; C-9, 37.5 ppm) (Figure 1). The HMBC spectrum revealed correlations between the protons and carbon atoms of the geminal methyl pair as well as cross-peaks between each of the three singlet methyl protons (H-12, H-13, and H-14) and the resonance for the intervening C-5 methylene at 49.4 ppm. The H-12 methyl protons also showed a three-bond HMBC cross-peak to C-8 (96.2 ppm). The remaining five methylene carbons each carried a pair of diastereotopic protons which were identified by characteristic sets of cross-peaks in the HSQC, COSY, and NOESY spectra and individually assigned on the basis of the observed coupling constants and NOESY spectrum. In particular the H-5 methylene protons were unambiguously assigned based on the NOESY cross-peaks between H-14 (δ 1.42) and H-5β (δ 2.19) and between H-12 (δ 1.16) and both H-13 (δ 1.21) and H-5α (δ 1.23).
Figure 1.
Key A) HMBC and B) NOESY correlations for 3.
The conversion of FPP to PSP (3) is proposed to involve initial formation of the humulyl cation (4) which then cyclizes to the 2-epi-caryophyllenyl ion 5 (Scheme 2).3,8,9 Following ring expansion to 6 and further cyclization, the resultant tricyclic cation 7 undergoes a 1,3-hydride shift to generate the presilphiperfolan-8-yl cation (8) which is then quenched by capture of water to give PSP (3). Incubation of (1R)-[1-2H]FPP (2b) with PSP synthase gave exclusively (5R)-[5α-2H]-3b, m/z 223, which displayed a broad singlet for H-5β that was shifted 0.03 ppm upfield to δ 2.16 due to the isotope effect of the geminal D-5α. The disappearance of the characteristic 1H NMR doublet at δ 1.23 for H-5α was also corroborated by the absence of the H-5α/C-5 cross-peak from the HSQC spectrum, while a clear cross-peak for H-5β/C-5 was evident at δ 2.16 and 49.4 ppm. Complementary incubation of (1S)-[1-2H]FPP (2c) gave (5S)-[5β-2H]-3c, which lacked the H-5β doublet at δ 2.19 while exhibiting a cross-peak for H-5β/C-5 at δ 1.18 and 49.4 ppm. These results established that cyclization of FPP involves net inversion of configuration at C-1 (Scheme 2), fully consistent with the known stereochemistry of formation of the intermediate trans,trans-humulyl cation (4) without intervening isomerization of the 2,3-double bond.10
Recently reported quantum chemical calculations on the formation of PSP have examined the energetics of a predicted pathway for formation to PSP (3). The penultimate 1,3-hydride shift was found to be exergonic by 5-6 kcal/mol with an activation barrier of ∼9-13 kcal/mol.11 The authors also proposed a variation in the cyclization mechanism in which FPP would first be rearranged to its tertiary allylic isomer, nerolidyl diphosphate (9, NPP) whose cisoid conformer would undergo cyclization to the corresponding cis,trans-humulyl cation and thence to the trans-fused diastereomer of 5. Although isomerization of FPP to NPP is well-established for cyclizations leading to sesquiterpenes with cis-double bonds, the intermediacy of the cisoid conformer of NPP in the biosynthesis of presilphiperfolan-8-ol is definitely ruled out since this transformation always involves net retention of configuration at C-1 of FPP, as a consequence of coupled syn-1,3-allylic isomerization and anti-SN′ cyclization,12 in contrast to the experimentally observed net inversion of configuration at C-1 of FPP in the conversion to PSP (3).
Although NPP could be therefore be excluded as a mandatory intermediate, we tested (3RS)-NPP as a surrogate substrate of PSP synthase. GC-MS analysis revealed the formation of 3 along with an anomalous product, β-farnesene, that is not observed when FPP is used as the substrate. To determine which enantiomer of NPP undergoes cyclization, we carried out competitive incubation of a 1:1 mixture of (3S)-(Z)-[1-2H]NPP and unlabeled (3RS)-NPP with PSP synthase, resulting in formation of 3 that was devoid of deuterium (>95% d0), as established by selected ion monitoring GC-MS.12 Complementary incubation of a 1:1 mixture of unlabeled (3S)-NPP and (3RS)-(Z)-[1-2H]NPP with PSP synthase gave [2H]-3 whose deuterium content indicated a >90:10 preference for (3R)-NPP over (3S)-NPP.
Preparative-scale incubation with (3RS)-(Z)-[1-2H]NPP (9b) gave exclusively (5R)-[5α-2H]-3b whose 1H NMR spectrum was identical to that of 3b prepared from (1R)-[1-2H]FPP (2b). Cyclization of (3R)-NPP thus involves attack on the si face of the vinyl group, consistent only with anti-SN′ cyclization of the transoid conformer of (3R)-NPP. The intermediacy of the corresponding cisoid conformer of (3R)-NPP is thus rigorously excluded by the labeling data from both (3RS)-(Z)-[1-2H]NPP and chirally deuterated FPP. Although (3R)-NPP is not a mandatory intermediate, ionization of this surrogate substrate generates a transoid allylic cation-pyrophosphate ion pair identical to that derived directly from the natural substrate FPP. Both the absolute configuration and the absolute sense of folding of this surrogate NPP substrate therefore directly reveal the absolute conformation of the natural enzyme-bound FPP that undergoes the cyclization reaction.
Further insight into the stereochemistry of the cyclization came from preparative scale incubation of [13,13,13-2H3]FPP (2d)13 with PSP synthase. The 1H NMR spectrum of the resulting [14,14,14-2H3]-3d lacked the characteristic methyl singlet at δ 1.42 for H-14, thereby establishing that in cyclization of FPP to the humulyl cation 4, electrophilic attack takes place exclusively on the si face of the 12,13-double bond (Scheme 2). Notably the original C-13 methyl of FPP maintains its cis relationship with the vinylic H-10 proton throughout the entire biosynthetic sequence.
The proposed intermediacy of the cis-fused caryophyllenyl cation 5 follows directly from the conformation of the FPP precursor as well as the assumption that formation of the cyclobutyl carbinyl cation either takes place much faster than conformational reorganization of the trans-trans-humulyl cation 4 or is in fact synchronous with the generation of 4. The conformation of the remaining 6,7-double bond of FPP is also readily deduced from the configuration of the derived H-1 bridgehead proton of 3, resulting in an intricately detailed picture of the folding of the acyclic FPP substrate at the active site of the presilphiperfolanol synthase.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM30301 to DEC.
Footnotes
Supporting Information Available: Experimental procedures, NMR assignments for presilphiperfolan-8β-ol, GC-MS and NMR data, and supplemental Figures and Tables. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Keller NP, Turner G, Bennett JW. Nat Rev Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]; Yu JH, Keller NP. Annu Rev Phytopathol. 2005;43:437–458. doi: 10.1146/annurev.phyto.43.040204.140214. [DOI] [PubMed] [Google Scholar]
- 2.a) Williamson B, Tudzynski B, Tudzynski P, van Kan JA. Mol Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]; b) Choquer M, Fournier E, Kunz C, Levis C, Pradier JM, Simon A, Viaud M. FEMS Microbiol Lett. 2007;277:1–10. doi: 10.1111/j.1574-6968.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 3.Pinedo C, Wang CM, Pradier JM, Dalmais B, Choquer M, Le Pecheur P, Morgant G, Collado IG, Cane DE, Viaud M. ACS Chem Biol. 2008;3:791–801. doi: 10.1021/cb800225v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siewers V, Viaud M, Jimenez-Teja D, Collado IG, Gronover CS, Pradier JM, Tudzynski B, Tudzynski P. Mol Plant-Microbe Interact. 2005;18:602–612. doi: 10.1094/MPMI-18-0602. [DOI] [PubMed] [Google Scholar]
- 5.Collado IG, Sanchez AJ, Hanson JR. Nat Prod Rep. 2007;24:674–686. doi: 10.1039/b603085h. [DOI] [PubMed] [Google Scholar]
- 6.Bradshaw APW, Hanson JR, Nyfeler R, Sadler IH. J Chem Soc, Perkin Trans. 1982;1:2187–2192. [Google Scholar]
- 7.The previously reported NMR assignments for the quaternary methyl groups, C-12, -13, and -14 and the geminal H-5 methylene protons of 3 from Eriophyllum staechadifolium (lizard tail) (ref. 8) are incorrect
- 8.a) Bohlmann F, Zdero C, Jakupovic J, Robinson H, King RM. Phytochemistry. 1981;20:2239. [Google Scholar]; b) Coates RM, Ho Z, Klobus M, Wilson SR. J Am Chem Soc. 1996;118:9249–9254. [Google Scholar]
- 9.Hinkley SFR, Perry NB, Weavers RT. Phytochemistry. 1994;35:1489–1494. [Google Scholar]
- 10.a) Cane DE, Oliver JS, Harrison PHM, Abell C, Hubbard BR, Kane CT, Lattman R. J Am Chem Soc. 1990;112:4513–4524. [Google Scholar]; b) Harrison PHM, Oliver JS, Cane DE. J Am Chem Soc. 1988;110:5922–5923. [Google Scholar]
- 11.Wang SC, Tantillo DJ. Org Lett. 2008;10:4827–4830. doi: 10.1021/ol801898v. [DOI] [PubMed] [Google Scholar]
- 12.Cane DE, Ha HJ. J Am Chem Soc. 1986;108:3097–3099. [Google Scholar]; Cane DE, Ha HJ. J Am Chem Soc. 1988;110:6865–6870. [Google Scholar]; Cane DE, Tandon M. J Am Chem Soc. 1995;117:5602–5603. [Google Scholar]
- 13.Cane DE, Salaski EJ, Prabhakaran PC. Tetrahedron Lett. 1990;31:1943–1944. [Google Scholar]; The sample of [13,13,13-2H3]FPP (2e) contained ∼10% of the isomeric [12,12,12-2H3]FPP.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.