Abstract
The versatility of Ca2+ signals derives from their spatio-temporal organization1,2. For Ca2+ signals initiated by inositol trisphosphate (IP3) this requires local interactions between IP3 receptors (IP3R)3,4 mediated by their rapid stimulation and slower inhibition4 by cytosolic Ca2+. This allows hierarchical recruitment of Ca2+ release events as the IP3 concentration increases5. Single IP3R respond first, then clustered IP3R open together giving a local Ca2+ puff, and as puffs become more frequent they ignite regenerative Ca2+ waves1,5-9. We demonstrate, using nuclear patch-clamp recording10, that IP3R are initially randomly distributed with an estimated separation of ~1 μm. Low concentrations of IP3 cause IP3R to aggregate rapidly and reversibly into small clusters of ~4 closely associated IP3R. At resting cytosolic [Ca2+], clustered IP3R open independently, but with lower open probability (Po), shorter open time, and lesser IP3 sensitivity than lone IP3R. Increasing cytosolic [Ca2+] reverses the inhibition caused by clustering, IP3R gating becomes coupled, and the duration of multiple openings is prolonged. Clustering both exposes IP3R to local Ca2+ rises and increases the effects of Ca2+. Dynamic regulation of clustering by IP3 tunes IP3R sensitivity to IP3 and Ca2+, facilitating hierarchical recruitment of the elementary events that underlie all IP3-evoked Ca2+ signals3,5.
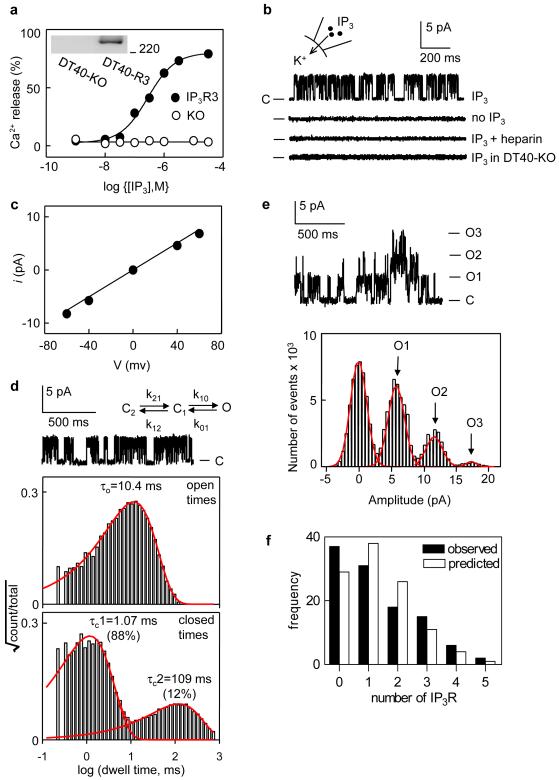
IP3-activated currents recorded from patches excised from the outer nuclear envelope of DT40 cells10 expressing rat IP3R3 are entirely due to IP3R3 (Fig. 1). With 10 μM IP3 in the pipette solution (PS) the single channel open probability (Po) was 0.44 ± 0.05 (n = 6) and the mean open time (τo) was 11.9 ± 1.6 ms. The distribution of closed times (τc) had two components (Fig. 1d). Recordings in the on-nucleus configuration confirmed these results (not shown). The results are consistent with the gating scheme shown in Figure 1d (see Supplementary Methods).
Figure 1. IP3R are randomly distributed.
a, IP3-evoked Ca2+ release from permeabilized DT40-IP3R3 (EC50 = 281 ± 46 nM) and DT40-KO cells (means ± SEM, n ≥ 3). Immunoblot with IP3R3-specific antiserum (10 μg membrane protein/lane, 220-kDa marker shown). b, Currents recorded from excised patches with 10 μM IP3 in PS. No currents were detected without IP3 (n = 20), with heparin (100 μg/ml) and IP3 (n = 15), or with IP3 in DT40-KO cells (n > 30). C denotes closed state. c, i-V relationship for IP3-evoked current (γK = 121 ± 2.8 pS, n = 7). d, Dwell time distribution of single IP3R3 stimulated with 10 μM IP3. Open time distribution of this typical recording is fitted with a single probability density function (pdf) with τo = 10.4 ms (mean = 11.9 ± 1.6 ms, n = 6). The pdf for the τc distribution has two components (1.07 ms, 88% and 109 ms, 12%). Dwell time distributions are consistent with the gating scheme (Supplementary Methods, Supplementary Figs 5, 6). e, Typical all-points current amplitude histogram of an excised patch containing 3 IP3R stimulated with 10 μM IP3; C and O denote closed and open states. f, Observed and predicted numbers of IP3R/patch from 109 patches (mean = 1.34) stimulated with 10-100 μM IP3.
The number of channels within a patch (1.34 ± 0.13, n = 109) can be estimated reliably from the largest multiple of simultaneous openings to the unitary current level (Fig. 1e, Methods). The distribution of IP3R in a patch is random: it is not significantly different from a Poisson distribution (χ2, p>0.05; Fig. 1f, Supplementary Table 1). Others suggested that IP3R are clustered in the nuclear envelope11,12, but it seems likely that in making repeated recordings from the same nucleus they stimulated nuclei with IP3 before recording and thereby caused IP3R clustering (see below).
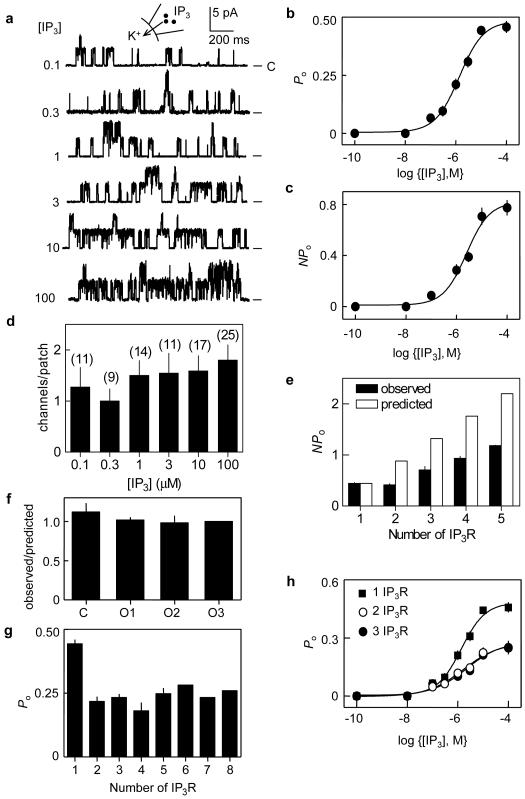
Channel activity (Po, Fig. 2a-c), but not the number of active IP3R (Fig. 2d), increased with IP3 concentration (EC50 = 1.38 ± 0.03 μM for patches with one IP3R). There was more than one IP3R in 57% of active patches, and each opened to the same γ (Fig. 1e, 2a), but NPo (the overall channel activity) was less than expected from the summed behaviour of lone IP3R (Fig. 2e). For multi-IP3R patches, the sensitivity to IP3 of NPo was also significantly reduced (EC50 = 2.47 ± 0.25 μM for patches with 3 IP3R, Fig. 2c, Supplementary Table 2). Do IP3R behave independently in such multi-IP3R patches or do they interact, like some ryanodine receptors13,14? For each of the four states in patches with three IP3R (closed and 1, 2 or 3 simultaneously open IP3R), Po predicted from the binomial distribution matched the observed Po (Fig. 2f, Methods). Similar results were obtained for patches with different numbers of IP3R and for type 1 IP3R (Supplementary Figs 1, 2). At resting cytosolic [Ca2+], therefore, each IP3R within a multi-IP3R patch behaves identically and opens independently.
Figure 2. Lone IP3R are more active than clustered IP3R at resting cytosolic Ca2+.
a, Typical records from patches (2 IP3R/patch) stimulated with IP3 (μM). b, c, Effect of IP3 on Po of patches containing a single IP3R (b) or on NPo of patches with 3 IP3R (c) (n ≥ 4). d, Numbers of IP3R detected in each patch for each IP3 concentration (n = 9-25). e, Predicted (ie NPlone) and observed NPo for patches containing 1-5 IP3R (n ≥ 3; n = 2 for 5-IP3R patch). f, For patches with 3 IP3R, observed/predicted values are shown for the indicated numbers of simultaneous openings (Supplementary equation 4). g, Po as a function of the number of IP3R within a patch after stimulation with 10 μM IP3 (Supplementary equation 5). h, Effect of IP3 on Po for lone IP3R and IP3R within multi-IP3R patches (n ≥ 4).
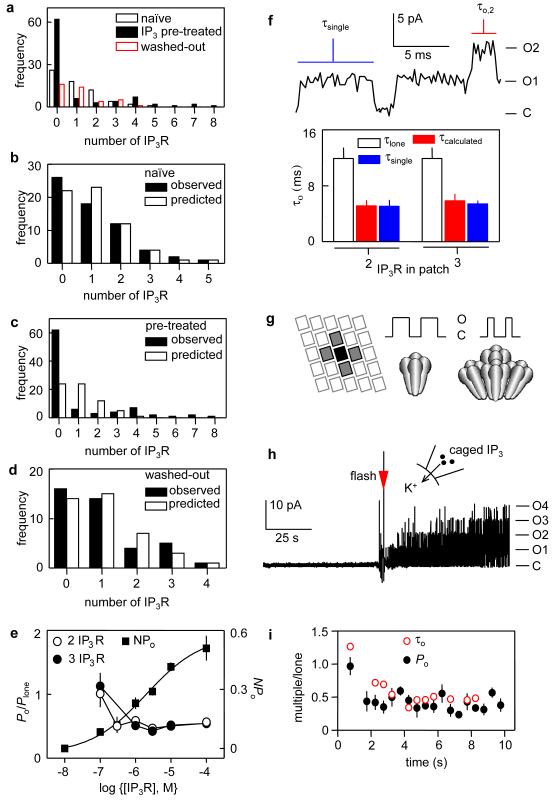
How can randomly distributed IP3R that open independently behave with such uniformity, and yet so differently from lone IP3R, when a patch fortuitously contains several IP3R? Recordings from Xenopus nuclei also suggest that heterogenous behaviour of lone IP3R becomes more uniform when patches contain several IP3R15. We suggest that IP3 causes IP3R to cluster16 and that clustered IP3R are less active. To test this hypothesis, nuclei were bathed in IP3 (10 μM, 2 min) before forming seals for patch-clamp recording. In these paired experiments, the mean number of IP3R per patch was unaffected by IP3-pre-treatment (Supplementary Table 1), confirming that IP3 neither inactivated IP3R nor affected the area of membrane trapped beneath the patch. But the distributions of IP3R were very different before and after IP3 treatment (Fig. 3a). In naïve nuclei IP3R were randomly distributed (Fig. 3b), but their distribution after IP3-pre-treatment differed significantly from the Poisson distribution (p<0.05): many patches had no IP3R, single IP3R were under-represented, and several patches had unusually large numbers of IP3R (Fig. 3c). This clustering of IP3R fully reversed within 8-10 min of removing IP3 (Fig. 3a, d). Po of lone IP3R from naïve nuclei (0.44 ± 0.05, n = 6) was indistinguishable from Po of the only lone IP3R caught within a patch after IP3 pre-treatment (0.41). Po for each IP3R within a cluster was also indistinguishable for recordings from naïve (0.24 ± 0.01, n = 18) and IP3-pre-treated nuclei (0.25 ± 0.01, n = 18). Furthermore, there was no decrease in Po during recordings that outlasted the IP3 pre-treatment (Supplementary Fig. 3). We conclude that clustering, rather than IP3 per se, decreases Po.
Figure 3. Reversible clustering of IP3R by IP3.
a, Numbers of IP3R detected in patches from naive nuclei (n = 63), after pre-treatment with bath-applied IP3 (10 μM, ~2 min; n = 88), or the latter after recovery for 8-10 min without IP3 (n = 40). b-d, Observed and predicted numbers of IP3R/patch. e, Effects of IP3 on IP3R clustering and gating. Clustering is reported by Po/Plone for patches with 2 or 3 IP3R, and gating by NPo for patches with 2 IP3R (EC50 = 2.02 ± 0.20 μM). f, τo for patches with 2 or 3 IP3R measured from the duration of single channel openings (blue line, τsingle) or calculated from the duration of openings to the Nth level (red line, τcalculated = N.τo,N). These are compared with τo for lone IP3R (τlone). Typical trace is from a patch with 2 IP3R. g, IP3 drives IP3R into small clusters consistent with arrays (grey) formed by IP3R at high density19. Within a cluster, each IP3R opens independently, but closes more rapidly than a lone IP3R. h, Typical recording from a patch containing 4 IP3R with IP3 released from caged IP3 in PS by flash photolysis (electrical noise caused by the flash is shown). i, From records similar to h (Supplementary Fig. 8), Po (from NPo/N) and τo were measured during each 0.5 s interval after the flash (1.5 s for first interval). The ratio (multi-IP3R patch/lone IP3R) is shown for both τo and Po. Results (means ± SEM) are from 4 (single) and 7 (multiple, with 2-4 IP3R/patch) patches.
The decrease in Po as IP3R cluster is identical whether clustering is evoked by application of IP3 to an isolated patch (Fig. 2e, h) or the entire nucleus (Fig. 3e). Both reduce Po to ~54% that of lone IP3R. The latter condition better replicates the situation in vivo, confirming that results with isolated patches (Figs 1, 2) faithfully report the behaviour of IP3R roaming freely within the nuclear envelope. The effect of cluster size on Po indicates that pairing of IP3R is sufficient to cause the maximal decrease in Po. Additional IP3R can join a cluster, and their activity is attenuated, but IP3R within larger clusters are no more inhibited than pairs of IP3R (Figs 2g, h, Supplementary Table 2). IP3R associate with actin4 and microtubules17, but neither is required for clustering-evoked changes in Po (Supplementary Fig. 4).
To examine the effects of clustering on IP3R gating, we compared mean open time (τo, Supplementary Information) of lone IP3R with τo for single channel openings from patches with several (N) IP3R (blue line in Fig. 3f). These τo should be similar if lone and grouped IP3R behave identically. For multi-IP3R patches, we also measured the duration of events in which all IP3R were simultaneously open (τo,N, red line in Fig. 3f), and from that calculated τo for individual, independently gated IP3R (=Nτo,N). Both analyses gave the same result: τo for IP3R within a cluster was reduced to 47% of that for lone IP3R (Fig. 3f). A similar analysis of closed states confirmed that neither was affected by clustering (Supplementary Fig. 5, Supplementary Table 3). IP3-evoked clustering almost doubles the rate of channel closure (1/τo) and this is alone sufficient (Supplementary Fig. 6, Supplementary Table 4) to account for the decreased Po of clustered IP3R (Fig. 2g). Clustered IP3R open for half as long as lone IP3R (5.4 vs 11.9 ms), and pairing of IP3R is enough to cause the full effect (Fig. 2g). Other regulators of IP3R usually influence τc and so rates of channel opening4. The difference is important because τo will affect the time course of the initial Ca2+ release within elementary events7 and thereby Ca2+-mediated interplay between clustered IP3R. This is confirmed by simulations of intracellular Ca2+ spikes, where the ~50% decrease in τo of clustered IP3R causes the frequency of Ca2+ spiking to decrease by 4-fold (Supplementary Fig. 7).
Within a patch, cluster size is limited to the number of IP3R fortuitously caught beneath the patch-pipette, but for nuclei pre-treated with bath-applied IP3 the clusters are larger (Fig. 3c). This demonstrates that a maximal concentration of IP3 causes >93% of IP3R to cluster (85/91 IP3R from 88 nuclei pre-treated with IP3) and the average cluster contains 4.25 ± 0.38 IP3R (Methods). Inhibition of IP3R within a cluster is not caused by feedback inhibition4 from Ca2+ passing through neighbouring IP3R. Both BS and PS have the same [Ca2+] and are buffered with BAPTA, the inhibition occurs at positive (Fig. 2) and negative holding potentials (Supplementary Discussion), and clustered IP3R open independently (Fig. 2f). Because permeating ions cannot regulate neighbouring IP3R under our recording conditions, inhibition must be mediated by contacts between IP3R. From this, we estimate that the average separation of IP3R falls from ~1 μm to ~20 nm after clustering, and that clusters are ~2 μm apart (Supplementary Discussion). These spacings concur with confocal measurements suggesting that a Ca2+ puff originates from a cluster ~50 nm wide and that clusters are ~3 μm apart18. When expressed at high densities, IP3R19 and ryanodine receptors20 form arrays with each tetrameric receptor contacting four others. We speculate that IP3-evoked clusters (of 4.25 ± 0.38 IP3R) exploit similar contacts and so, with single IP3R, form the fundamental units of Ca2+ signalling (Fig. 3g).
IP3-evoked clustering is complete within seconds of stimulation with a maximal concentration of IP3 (Supplementary Fig. 3). To resolve the time course, we used photolysis of caged IP3 rapidly to increase the IP3 concentration bathing IP3R trapped beneath the patch-pipette. IP3R were initially quiescent and then rapidly activated when IP3 was photo-released (Fig. 3h). Irrespective of the number of IP3R caught within a patch, τo was initially similar for all IP3R (~10 ms). It then remained stable for many minutes for lone IP3R (11.4 ± 0.5 ms), but τo fell within 2.5 s to 5.8 ± 0.3 ms for patches containing more than one IP3R (Fig. 3i, Supplementary Fig. 8). Using τo to report IP3R clustering suggests that clustering is complete within 2.5 s of IP3 addition. A similar analysis of Po suggests a half-time for clustering of ~1.5-2 s (Fig. 3i). Our evidence that clustering does not require the cytoskeleton and measurements of IP3R3 mobility21,22 suggest that diffusion alone may be sufficient to allow IP3R3 clustering within a few seconds (Supplementary Discussion).
We can define the IP3 sensitivity of clustering by measuring the extent to which Po of each IP3R within a multi-IP3R patch (Po = NPo/N, Supplementary Abbreviations) falls below Po of an identically stimulated lone IP3R (Plone). This demonstrates that IP3R clustering (EC50 < 300 nM) is ~10-times more sensitive to IP3 than channel opening (EC50 = 2.02 μM, Fig. 3e). Steady-state exposure to low IP3 concentrations that evoke Ca2+ puffs5,7 would, by assembling IP3R clusters, allow both generation of puffs and loss of Ca2+ blips23.
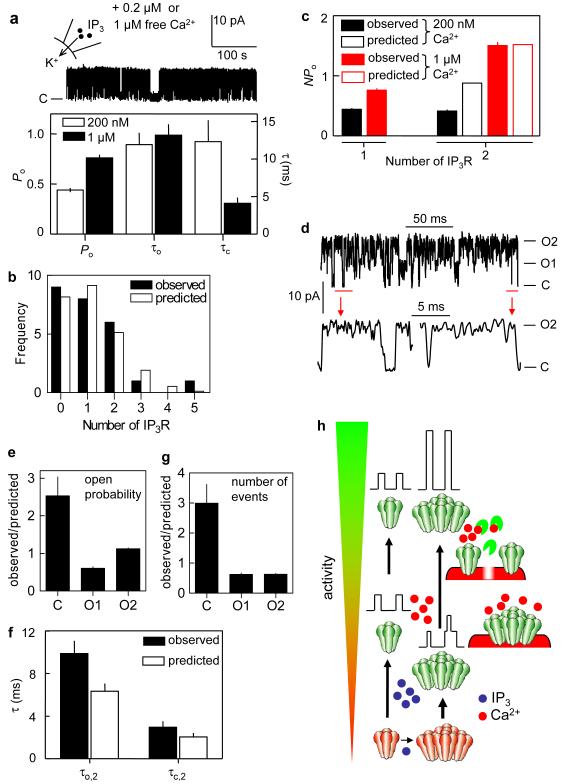
Clustering moves IP3R (~1 μm apart) from being insulated from their neighbours by Ca2+-buffering to domains (~20 nm apart) where they will instantly experience high local [Ca2+] whenever a neighbour opens24 (Supplementary Fig. 7). Hitherto (Figs 1-3), we prevented such interactions by using K+ as charge-carrier and recording at a free [Ca2+] (200 nM) that mimics a resting cell. Subsequent experiments include 1 μM free [Ca2+] with IP3 in PS to simulate the [Ca2+] near open IP3R. For simplicity we use K+ as charge-carrier. With 1 μM [Ca2+] in PS, IP3R activity was increased: Po for lone IP3R almost doubled, as τc decreased (Fig. 4a)4. Neither the number of IP3R/patch (1.12 ± 0.24) nor their random distribution (Fig. 4b) was affected by Ca2+, but the interplay between IP3R was altered. Whereas clustering reduced the overall activity of IP3R (NPo) at resting [Ca2+] (Fig. 2e), the inhibition was reversed by increased [Ca2+], such that the collective activity of a pair of IP3R (NPo) was the same as that predicted from the summed activity of two lone IP3R (Fig. 4c). This did not result from disaggregation of clusters because at increased [Ca2+], IP3R no longer opened independently. In patches with two IP3R (open-channel noise prevented analysis of larger clusters), open probabilities did not fit the binomial distribution (Fig. 4e): double open and closed events were over-represented (Supplementary Fig. 9). Furthermore, there were many examples of IP3R opening and closing directly to and from states with both IP3R open (Fig. 4d). For paired IP3R, the double openings were prolonged by 50% (Fig. 4f), but 47% less frequent than expected (Fig. 4g). The overall increase in Po for double openings was therefore small (12%) and counteracted by a 39% decrease in the probability of only one IP3R being open and a 116% increase in the probability of both being closed (Fig. 4e). Clustered IP3R exposed to increased [Ca2+] do not therefore behave independently. Their gating is coupled13,14: they are more likely to open and close together, and their simultaneous openings are prolonged (Supplementary Fig. 9). Coupled gating is not caused by local increases in cytosolic [Ca2+], and must instead result from physical coupling of IP3R. Under physiological conditions, clustered IP3R are more likely to experience increased [Ca2+] (because their neighbours may release it), and they are also tuned to respond most to it. By suppressing IP3R activity at resting [Ca2+], clustering increases the impact of a subsequent local increase in [Ca2+] (Supplementary Fig. 7). Within a cluster, increased Ca2+ increases Po (as it does for lone IP3R), but it also reverses the inhibition evoked by clustering and it causes coupled gating. These interactions exaggerate the effect of Ca2+ within a cluster (Fig. 4h). We conclude that IP3 dynamically regulates the assembly and behaviour of Ca2+ puff sites. IP3 rapidly drives IP3R into small clusters, wherein their IP3 and Ca2+ sensitivities are re-tuned to exaggerate Ca2+-mediated recruitment of IP3R and allow hierarchical recruitment of Ca2+ release events (Fig. 4h, Supplementary Fig. 7)5,7.
Figure 4. Clustering retunes Ca2+ regulation of IP3R.
a-e, Patches were stimulated with PS containing 10 μM IP3 and (unless otherwise stated) 1 μM Ca2+. a, Typical recording and summary data (n = 5-6) from lone IP3R show that increasing Ca2+ increases Po by reducing τc. b, Observed and expected numbers of IP3R/patch. c, Observed and predicted NPo for patches containing 1 or 2 IP3R and stimulated with 10 μM IP3 in PS containing 200 nM or 1 μM Ca2+ (n = 5-6). d, Typical recording from a patch with 2 IP3R, enlarged (red) to highlight transitions directly between closed (C) and double open (O2) states. e, Observed and predicted Po for closed (C) and single (O1) or double openings (O2) for patches with 2 IP3R (n = 6, Supplementary equations 4, 5). f, Observed and expected durations of events when both IP3R are simultaneously open (τo,2) or closed (τc,2) for patches with 2 IP3R (n = 6, Supplementary equations 6, 7). g, Observed and predicted numbers of transitions to each of the 3 states in a patch with 2 IP3R (n = 6)26. h, At resting [Ca2+], IP3 drives IP3R into small clusters wherein IP3R gate independently, but with reduced Po and IP3 sensitivity. Ca2+ reverses the inhibition imposed by clustering, openings within a cluster are more synchronized, and simultaneous openings are prolonged. Clustering primes IP3R to respond by repressing their activity, and then allowing Ca2+ to unleash the coordinated gating of clustered IP3R (Supplementary Fig. 7).
METHODS SUMMARY
Nuclei from DT40-IP3R3 cells25 were used for patch-clamp recording from excised patches10.
Supplementary Material
Acknowledgements
Supported by The Wellcome Trust (CWT), The Biotechnology and Biological Sciences Research Council (CWT), a scholarship from the Jameel Family Trust (TUR), and the IRTG “Genomics and Systems Biology of Molecular Networks” of the Deutsche Forschungsgemeinschaft (MF). We thank S. Dedos for help with DT40 cells, D. Prole and B. Billups for advice, and T. Kurosaki for providing DT40KO cells.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 3.Marchant J, Callamaras N, Parker I. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 6.Horne JH, Meyer T. Elementary calcium-release units induced by inositol trisphosphate. Science. 1997;276:1690–1694. doi: 10.1126/science.276.5319.1690. [DOI] [PubMed] [Google Scholar]
- 7.Marchant JS, Parker I. Role of elementary Ca2+ puffs in generating repetitive Ca2+ oscillations. EMBO J. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuai J, Rose HJ, Parker I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys. J. 2006;91:4033–4044. doi: 10.1529/biophysj.106.088880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sneyd J, Falcke M. Models of the inositol trisphosphate receptor. Prog. Biophys. Mol. Biol. 2005;89:207–245. doi: 10.1016/j.pbiomolbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Dellis O, Dedos S, Tovey SC, Rahman T-U, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 11.Mak D-O,D, Foskett JK. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionescu L, Cheung KH, Vais H, Mak DO, White C, Foskett JK. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ. Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 14.Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- 15.Mak D-O,D, Foskett JK. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- 16.Tateishi Y, et al. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J. Biol. Chem. 2005;280:6816–6822. doi: 10.1074/jbc.M405469200. [DOI] [PubMed] [Google Scholar]
- 17.Bourguignon LY, Iida N, Jin H. The involvement of the cytoskeleton in regulating IP3 receptor-mediated internal Ca2+ release in human blood platelets. Cell Biol. Int. 1993;17:751–758. doi: 10.1006/cbir.1993.1136. [DOI] [PubMed] [Google Scholar]
- 18.Dargan SL, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama E, et al. Native structure and arrangement of inositol-1,4,5-trisphosphate receptor molecules in bovine cerebellar Purkinje cells as studied by quick-freeze deep-etch electron microscopy. EMBO J. 1996;15:4844–4851. [PMC free article] [PubMed] [Google Scholar]
- 20.Yin CC, Blayney LM, Lai FA. Physical coupling between ryanodine receptor-calcium release channels. J. Mol. Biol. 2005;349:538–546. doi: 10.1016/j.jmb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Fukatsu K, Bannai H, Zhang S, Nakamura H, Inoue T, Mikoshiba K. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J. Biol. Chem. 2004;279:48976–48982. doi: 10.1074/jbc.M408364200. [DOI] [PubMed] [Google Scholar]
- 22.Ferreri-Jacobia M, Mak D-O,D, Foskett JK. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J. Biol. Chem. 2005;280:3824–3831. doi: 10.1074/jbc.M409462200. [DOI] [PubMed] [Google Scholar]
- 23.Sun X-P, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J. Physiol. 1998;509:67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcke M. Reading patterns in living cells - the physics of Ca2+ signaling. Adv. Phys. 2004;53:255–440. [Google Scholar]
- 25.Boehning D, Joseph SK, Mak D-O,D, Foskett JK. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys. J. 2001;81:117–124. doi: 10.1016/s0006-3495(01)75685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prole DL, Lima PA, Marrion NV. Mechanisms underlying modulation of neuronal KCNQ2/KCNQ3 potassium channels by extracellular protons. J. Gen. Physiol. 2003;122:775–793. doi: 10.1085/jgp.200308897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.